Abstract
Kisspeptin is the most potent stimulator of LH release. There are two kisspeptin neuronal populations in the rodent brain: in the anteroventral periventricular nucleus (AVPV) and in the arcuate nucleus. The arcuate neurons coexpress kisspeptin, neurokinin B, and dynorphin and are called KNDy neurons. Because estradiol increases kisspeptin expression in the AVPV whereas it inhibits KNDy neurons, AVPV and KNDy neurons have been postulated to mediate the positive and negative feedback effects of estradiol on LH secretion, respectively. Yet the role of KNDy neurons during the positive feedback is not clear. In this study, ovariectomized rats were microinjected bilaterally into the arcuate nucleus with a saporin-conjugated neurokinin B receptor agonist for targeted ablation of approximately 70% of KNDy neurons. In oil-treated animals, ablation of KNDy neurons impaired the rise in LH after ovariectomy and kisspeptin content in both populations. In estradiol-treated animals, KNDy ablation did not influence the negative feedback of steroids during the morning. Surprisingly, KNDy ablation increased the steroid-induced LH surges, accompanied by an increase of kisspeptin content in the AVPV. This increase seems to be due to lack of dynorphin input from KNDy neurons to the AVPV as the following: 1) microinjections of a dynorphin antagonist into the AVPV significantly increased the LH surge in estradiol-treated rats, similar to KNDy ablation, and 2) intra-AVPV microinjections of dynorphin in KNDy-ablated rats restored LH surge levels. Our results suggest that KNDy neurons provide inhibition to AVPV kisspeptin neurons through dynorphin and thus regulate the amplitude of the steroid-induced LH surges.
Kisspeptin has been identified as a neuropeptide that controls the maturation and reproductive function in mammals, including humans (1–5). It is the most potent stimulator of LH secretion acting through direct activation of GnRH neurons (6, 7). It also mediates the positive and negative feedback effects of steroids on GnRH release (8). Loss of function mutations in kisspeptin cause hypogonadotropic hypogonadism, a rare genetic condition that impairs gonadotropin secretion, resulting in failure to reach puberty and infertility (9, 10).
There are two kisspeptin neuronal populations in the rodent brain, located in the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (ARC) (11). Neurons of the ARC population are called KNDy neurons because they coexpress kisspeptin, neurokinin B, and dynorphin (12). KNDy neurons project to several brain hypothalamic regions including GnRH neurons in the preoptic area (13) as well as to GnRH terminals in the median eminence (14, 15). KNDy neurons form an interconnected network through neurokinin B signaling (12, 16) that synchronizes bursts of electrical activity which correlates with LH secretion (17, 18). For that reason, these neurons have been postulated to be a key component of the GnRH pulse generator that governs pulsatile secretion of GnRH and LH.
Both kisspeptin populations express steroid receptors, but whereas estradiol (E2) increases kisspeptin mRNA expression in the AVPV, it decreases kisspeptin expression in KNDy neurons (19). It has been suggested that AVPV neurons are responsible for mediating the positive feedback effects of steroids on LH secretion, whereas KNDy neurons are involved in the negative feedback (16). Interestingly, mutations in the genes encoding neurokinin B or its receptor also causes hypogonadotropic hypogonadism and failure to reach puberty (20), suggesting that KNDy neurons are important mediators of reproductive function.
Recently a method was developed for the selective ablation of KNDy neurons (21). This method uses intra-ARC microinjections of the molecular neurotoxin saporin conjugated to the selective neurokinin B receptor agonist [MePhe7] neurokinin B (Nk3-SAP). In the present study, we use this method to examine the effect of ablation of KNDy neurons on LH secretion in ovariectomized rats with or without steroid replacement. Although KNDy neurons have not been thought to contribute to the positive feedback effect of steroids, ablation of approximately 70% of KNDy neurons increased the magnitude of the LH surges in OVE rats. We postulate that this surprising increase is due to lack of dynorphin input from KNDy neurons to the AVPV.
Materials and Methods
Animals
Adult female Wistar rats weighing 250–300 g were used in experiments 1 and 2, approved by the Ethics Committee for Research Involving Animals of the Dentistry School of Ribeirão Preto, University of São Paulo (Comissão de Ética no Uso de Animais number 2013.1.475.58.5). Adult female Sprague Dawley rats weighing 250–300 g (Charles River) were used in experiments 1, 3, and 4, approved by the Florida State University Animal Care and Use Committee. Different rat strains were used because the experiments were performed in two different locations, and the standard rat strain was used in each place. Rats were grouped housed under conditions of controlled lighting (lights on from 6:00 am to 6:00 pm) and temperature (22ºC ± 0.5ºC). Standard rat chow and water were provided ad libitum.
Experimental design
Experiment 1: temporal effects of ablation of KNDy neurons on kisspeptin morphology in ovariectomized rats
Ovariectomized rats were placed in a stereotaxic apparatus and submitted to two bilateral microinjections of 10 ng per 100 nL PBS of either blank-saporin (SAP) or Nk3-SAP (n = 5–7 rats). Rats were transcardially perfused 1, 2, or 3 weeks after the microinjections. The brains were immunohistochemically processed for kisspeptin in the ARC and the AVPV. Morphological analysis was performed by quantification of the number of kisspeptin cell bodies and kisspeptin fiber density. Another set of ovariectomized Sprague Dawley animals was injected with blank-SAP or Nk3-SAP (n = 5–7 rats) to confirm the ablation protocol in our laboratory at Florida State University. Rats were transcardially perfused 7–10 days after the microinjections. The brains were immunohistochemically processed for kisspeptin, neurokinin B, and tyrosine hydroxylase in the ARC.
Experiment 2: Effects of ablation of KNDy neurons on the LH surge induced by gonadal steroids in ovariectomized rats
The ovariectomized rats were placed in a stereotaxic apparatus and submitted to two bilateral microinjections of 10 ng per 100 nL PBS of either blank-SAP or Nk3-SAP (n = 5–7 rats) and were implanted with capsules containing either oil (OVO group) or E2 (OVE group). After 1 week of recovery, rats were submitted to jugular vein cannulation. Blood samples of 300 μL were taken in the morning (10:00 am) and hourly from 3:00 until 6:00 pm for LH measurements. All animals were killed at 4:00 pm of the following day. Kisspeptin levels were measured in microdissections of the median eminence (ME) and medial preoptic area (POA) by RIA.
Experiment 3: effects of dynorphin blockade in the AVPV of OVE rats
Ovariectomized rats received an E2 capsule and were placed in a stereotaxic apparatus and implanted with a guide cannula in the AVPV. After 1 week of recovery, the rats were submitted to jugular vein cannulation. Blood samples of 300 μL were taken every 2 hours from 8:00 am until 8:00 pm on the next 2 days to measure LH levels. Animals received an intra-AVPV injection of saline (first day) and the κ-opioid antagonist norbinaltorphimine (second day) around 3:00 pm (n = 7–10 rats).
Experiment 4: effects of dynorphin microinjections in the AVPV of OVE rats after ablation of KNDy neurons
Ovariectomized rats were placed in a stereotaxic apparatus and submitted to two bilateral microinjections of 10 ng per 100 nL PBS of either blank-SAP or Nk3-SAP (n = 6–7 rats) and implanted with a guide cannula in the AVPV. Rats also received capsules containing E2 (OVE group). After a 1-week recovery, rats were submitted to jugular vein cannulation. Blood samples of 300 μL were taken every 2 hours from 8:00 am until 8:00 pm on the next 2 days. Animals received an intra-AVPV injection of saline (first day) and dynorphin (second day) around 3:00 pm.
Anesthetic and antibiotic treatment
Ovariectomy, stereotaxy, and transcardial perfusion were performed under ketamine (80 mg/kg, ip) and xylazine (10 mg/kg, ip) anesthesia. For jugular vein cannulation, rats were anesthetized with buffered tribromoethanol (250 mg/kg, ip, pH 7.4) in experiments 1 and 2. For experiments 3 and 4, the surgeries were performed under isoflurane/oxygen gas mixture; isoflurane was set at 2.5%–3% and the oxygen flow to 2.0 L/min. After surgeries, rats were treated with pentabiotic (Fort Dodge; 0.2 mL/rat, im) and analgesic (Flunixin meglumine; 2.5 mg/kg, sc) in experiments 1 and 2, and with a single sc injection of an antiinflammatory analgesic (Metacam; Boehringer Ingelheim Vetmedica; 1 mg/kg) in experiments 3 and 4.
Ovariectomy and hormonal treatment
All animals were ovariectomized bilaterally and allowed to recover for at least 1 week. In experiments 2–4, steroidal replacement immediately followed the stereotaxic surgery. Animals from the OVE group received two SILASTIC brand capsules: SILASTIC brand laboratory tubing, size 0.058 in. (inner diameter) × 0.077 (outer diameter) (Dow Corning), each one containing 400 μg per 8 μL of 17β-estradiol (Sigma) diluted in corn oil, implanted sc in the dorsolateral region, one in each side. These capsules provided constant and gradual hormone release for 30 days, simulating the physiological levels of proestrus (±40 pg/mL) (22). Animals from the OVO group were implanted with two SILASTIC brand capsules containing corn oil (vehicle).
Stereotaxic surgeries and microinjections
Rats were positioned in a stereotaxic instrument (David Kopf) with the incisor bar at −3.3 mm. A 26-gauge bilateral guide cannula (C235G-1.0/SP; Plastics One) was placed to target the dorsolateral border of the ARC. Rostral coordinates were as follows: 2.4 mm posterior to bregma, ±0.5 mm lateral, and 9.8 mm ventral to skull surface. The caudal coordinates were as follows: 3.5 mm posterior to bregma, ±0.5 mm lateral, and 9.7 mm ventral to skull surface. Each rat was submitted to two consecutive bilateral injections of 10 ng per 100 nL PBS of either NK3-SAP or blank-SAP (number KIT-63; Advanced Targeting Systems) at the rostral and the ventral coordinates. Injections were performed via a 33-gauge stainless steel needle (C235I/SP; Plastics One) with an injection pump (KDS100; KD Scientific Inc) set to dispense 0.05 μL solution/min. The syringe was left in place for 5 minutes after the injection before the withdrawal of the needle. The combined surgical procedures were performed over a period of 45–60 minutes. The incision was closed and the animals were returned to their home cages. Animals from experiments 3 and 4 had the same guide cannula implanted to target the AVPV (rostral coordinates: 0.2 mm posterior to bregma, ±0.5 mm lateral, and 8.6 mm ventral to the skull surface). The cannula, protected by a dummy (C235DC/SPC; Plastics One), was attached to the bone with stainless steel screws and acrylic cement. At approximately 3:00 pm of the experiment day, animals received an intra-AVPV injection of sterile 0.9% NaCl on the first day and norbinaltorphimine (0.5 μg per 100 nL; Tocris Bioscience) or dynorphin A (1–17) (1 μg per 100 nL; 24 297; Anaspec Inc) on the second day. The small volume (100 nL) injected over 1 minute spreads for approximately 0.12 mm3 around the injection site (23), not diffusing into the ventricle or the other brain regions. All of the brains were histologically processed, and only the animals with a successful cannula placement were included in our study.
Jugular vein cannulation and blood samples
All animals were allowed to recover for 7–10 days after stereotaxic microinjections. Under isoflurane anesthesia, a catheter (Micro-Renathane; MRE-040, 0.040 in. OD × 0.025 in. inner diameter; Braintree Scientific) was inserted through the external jugular vein into the right atrium, fitted sc, and exteriorized at the back of the animal, as previously described (24). After the surgery, the catheter tube was filled with gentamycin sulfate (Alexis) to prevent bacterial growth and to maintain catheter patency (25). On the morning of the first day of the experiment, an extension of the catheter tubing filled with saline was connected to the jugular catheter, and the rats were left undisturbed in their cages. Blood samples of 300 μL were withdrawn into plastic heparinized syringes, and the same volume of sterile saline (0.9% NaCl; Teknova) was injected through the catheter immediately after the removal of each blood sample.
Perfusion and immunohistochemistry
Rats were deeply anesthetized with ketamine and xylazine and transcardially perfused with PBS, followed by ice-cold 4% paraformaldehyde. Frontal sections of 30 μm were cut into four series between approximately −1.8 and −4.1 mm from bregma (26) and sections submitted to immunoperoxidase kisspeptin single-labeling immunohistochemistry, as previously described (27). No staining was observed after preabsorbtion with kisspeptin-10 (27) or omission of the primary antibody (data not shown). Briefly, sections were incubated with the antikisspeptin rabbit antibody for 40 hours at 4ºC and biotinylated antirabbit goat IgG for 90 minutes at room temperature (see Table 1 for antibody specifications and dilutions) and avidin-biotin complex solution at 1:100 for 1 hour (Elite avidin-biotin complex kit; Vector Laboratories). A solution of nickel sulfate (25 mg/mL), 3,3′-diaminobenzidine-HCl (0.2 mg/mL) and 0.03% H2O2 was used as the chromogen. Kisspeptin, neurokinin B, and tyrosine hydroxylase was visualized using fluorescent immunohistochemistry in another set of animals. Sections were rinsed in PBS, incubated in blocking solution for 1 hour, and then incubated with the primary antibody for 40 hours at 4ºC and appropriate secondary antibody for 2 hours at room temperature (Table 1). Sections were mounted, allowed to dry, and coverslipped using with mounting media (Aqua Polymount; Polysciences). Omission of the primary antibodies resulted in no labeling (data not shown). The images were acquired using a Leica microscope attached to a cooled charge-coupled device camera (Andor Technology USA) and analyzed using Nikon analysis software (NIS Elements AR 3.2). The number of kisspeptin-, neurokinin B-, and tyrosine hydroxylase-positive cell bodies and the kisspeptin-positive fiber densities were performed on a single-labeled series of sections though the ARC and calculated using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Table 1.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|
| Kisspeptin | Antikisspeptin antibody | Millipore, AB9754 | Rabbit, polyclonal | 1:15 000 (DAB) |
| 1:7500 (fluorescence) | ||||
| Rabbit IgG | Biotinylated goat anti-rabbit IgG antibody | Vector Laboratories, BA1000 | Goat, polyclonal | 1:600 |
| Rabbit IgG | Alexa Fluor 555 donkey anti-rabbit IgG Antibody | Life Technologies, A-31572 | Donkey, polyclonal | 1:2000 |
| Neurokinin B | Anti-neurokinin B antibody | Novus, NB300-201 | Rabbit, polyclonal | 1:8000 |
| Rabbit IgG | Alexa Fluor 488 donkey antirabbit IgG antibody | Life Technologies, A-21206 | Donkey, polyclonal | 1:2000 |
| Tyrosine hydroxylase | Anti-TH antibody | Millipore, MAB318 | Mouse, polyclonal | 1:30 000 |
| Mouse IgG | Alexa Fluor 488 donkey antimouse IgG antibody | Life Technologies, A-21202 | Donkey, polyclonal | 1:2000 |
Abbreviations: DAB, 3,3′-diaminobenzidine-HCl; TH, tyrosine hydroxylase.
Brain microdissections
The ME was dissected with fine scissors using a dissecting microscope immediately after decapitation. The POA (including the AVPV) was dissected using the punch technique, as previously described (28). Using a 2.2-mm diameter needle, a punch of the POA was obtained from a coronal section extending from approximately −0.12 to 1.42 mm from bregma (26), as previously described (29). Dissections of the POA and ME were submitted to ultrasonic disruption in 100 or 500 μL of homogenization solution (0.15% HCl; 25% ethanol), respectively. The homogenates were centrifuged for 20 minutes at 12 000 × g. Protein content was determined in the remaining pellet by the Bradford method (30). In the supernatant, kisspeptin concentrations were measured by a RIA as described below. Kisspeptin concentrations were corrected by the protein content of each sample, and kisspeptin content was expressed as nanograms per microgram.
Radioimmunoassay
Blood samples were centrifuged at 1200 × g for 15 minutes at 4°C; the plasma was separated and frozen at −20°C until assayed. Plasma LH was determined by a RIA with kits provided by the National Hormone and Peptide Program (Harbor-UCLA, Torrance, California). 125I was purchased from PerkinElmer Life Sciences, and LH was radioiodinated by the chloramine-T method. The antiserum and reference preparation for LH were antirat LH-S10 and LH-RP3, respectively. The lower limit of detection was 0.08 ng/mL and the intra- and interassay coefficients of variation were less than 4% and 12%, respectively. Brain levels of kisspeptin were assayed by a RIA directed against rodent kisspeptin (31). The 125I-kisspeptin-10 label was prepared using the chloramine-T method and purified by reverse-phase HPLC on a C18 column (Nucleosil 120-3-C18; 4.6 × 150 mm; Macherey-Nagel), using a linear gradient from 15% to 40% B phase in 90 minutes at a flow rate of 1.0 mL/min. This purification was performed at 30°C with the following mobile phase system: phase A: water/trifluoroacetic acid (0.05%) and phase B: acetonitrile/trifluoroacetic acid 0.05%. The eluate was monitorated at 220 nm. All samples were assayed in the same RIA. The lower limit of detection was 0.07 ng/mL and the intraassay coefficient of variation was 2.24%.
Statistical analysis
Data are presented as mean ± SEM. Data were analyzed by a one- or two-way ANOVA followed by the Bonferroni post hoc test. Differences among steroid treatment within the same experimental group were further determined by a Student's t test. Kisspeptin content differences were analyzed by a Student's t test. P < .05 was considered statistically significant.
Computational modeling
A mathematical model was developed to help with the interpretation of experimental data. The model contains the following dimensionless variables: A, the activity level of the KISS neurons in the AVPV; K, the activity level of KNDy neurons; and L, the LH concentration.
Here the activity level reflects the mean neuronal activity of the population that leads to neurotransmitter release. The time course of the neuronal activity of the AVPV subpopulation is described by the following differential equation:
| (1) |
The steroidal environment is simulated by parameter E: E = 0 represents an OVO animal and E = 1.5 represents an OVE animal. The parameter TA determines the activity level in the absence of E2. C (t) is a function that reflects circadian control of AVPV activity; it is equal to 3 between 2:00 pm and 7:00 pm and zero at other times. Because sensitivity to E2 depends on a circadian signal (32), we multiply these two factors. The term in (1) represents the intrinsic level of activity of the AVPV kisspeptin neurons, where the first term, TAE, is a basal level of activity and the second, , reflects inhibition by KNDy neurons. The term − qA provides decay in activity level toward baseline.
The time course of activity of KNDy neurons is described by the following differential equation:
| (2) |
where the first term, includes inhibition of KNDy activity by E2, and the second term, − qK again reflects a decay to a baseline level.
Because kisspeptin indirectly stimulates LH secretion through GnRH release, we simplify the model by combining the effects of GnRH and LH. The variable L denotes the release of LH, which is indirectly affected by both kisspeptinergic subpopulations. The dynamics of the dimensionless LH concentration are described by the following equation:
| (3) |
where the first two terms, aLAA2 and aLKK2 reflect stimulation of LH secretion by AVPV and KNDy. The third term, , represents stimulatory input from a neural population other than KNDy neurons that is inhibited by E2. The final term, − qlL, provides decay toward baseline.
All parameter values are given in Table 2. The differential equations were solved numerically using the Python programming language. Numerical integration was carried out using the odeint function of the Scypy library with adaptive time step. To ensure that the model is in the stable oscillatory regime, transient effects were discarded. The computer code can be downloaded as freeware from http://www.math.fsu.edu/∼bertram/software/pituitary.
Table 2.
Parameters of the Model and Their Interpretations
| Parameter | Value | Interpretation |
|---|---|---|
| TAe | 1.2 | Tonic level of AVPV activity affected by estodial |
| TA | 0.5 | Tonic level of AVPV activity independent of estodial |
| E | 1.5 | E2 concentration (E = 0 for OVX group) |
| q | 0.3 | Time constant for decay of kisspeptin |
| ql | 0.5 | Time constant for decay of GnRH/LH |
| ake | 0.7 | Strength of E2 inhibition on KNDy (ake for KNDy ablation) |
| bke | 3.0 | Half-activation constant for KNDy by E2 |
| aak | 0.75 | Strength of AVPV inhibition by KNDy (aak for NOR-BNI experiment) |
| bak | 0.3 | Half-activation constant of AVPV by KNDy |
| ale | 1.5 | Strength of E2 inhibition |
| ble | 0.3 | Half-activation constant for GnRH/LH by E2 |
| ala | 0.4 | Strength of GnRH/LH stimulation by AVPV |
| alk | 10 | Strength of GnRH/LH stimulation by KNDy |
Results
Partial KNDy ablation decreases the number of kisspeptin and neurokinin B cell bodies and fiber density in the ARC, preserving tyrosine hydroxylase cell number
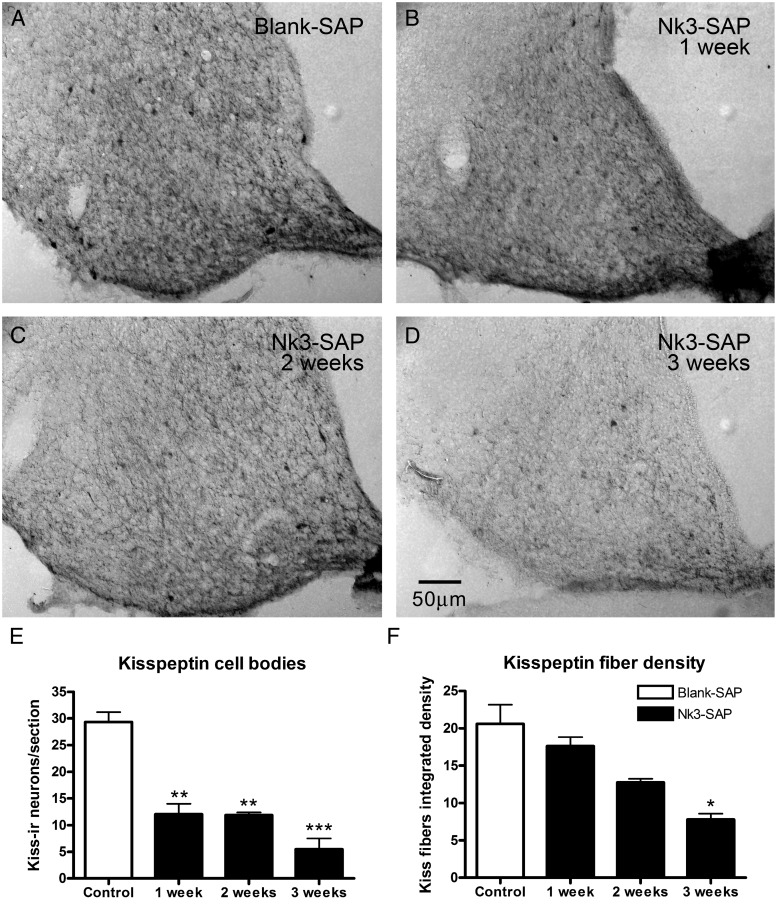
This experiment aims to determine the time required after saporin (Nk3-SAP) injections to obtain a satisfactory ablation of KNDy neurons. Figure 1 shows photomicrographs of the coronal brain sections, illustrating kisspeptin immunoreactivity at several days after the NK3-SAP injections. Although maximal fiber density ablation is achieved only 3 weeks after the microinjections (Figure 1F, P < .05), the number of KNDy neurons is significantly decreased within 1 week of the toxin injection (Figure 1E, P < .001). Because there was no statistical difference between the number of KNDy neurons ablated and the preliminary experiments also did not find further differences regarding the effect of partial KNDy ablation on LH levels after 1, 2, or 3 weeks, all subsequent experiments were then performed 7–10 days after the injection. In the female rat, the AVPV does not display kisspeptin cell bodies unless axonal transport is inhibited (33), so we analyzed the kisspeptin fiber density in the AVPV after the toxin microinjection and found no significant effect of the toxin (data not shown).
Figure 1.
Photomicrographs of coronal brain sections showing kisspeptin immunoreactivity in the arcuate region of rats submitted to microinjection of blank-SAP (A) or Nk3-SAP after 1, 2, or 3 weeks (B–D). Quantification of the number of kisspeptin cell bodies (E) and fiber density (F) in the arcuate region of the same animals. Data are shown as mean ± SEM. *, P < .05, **, P < .01, ***, P < .001 compared with control.
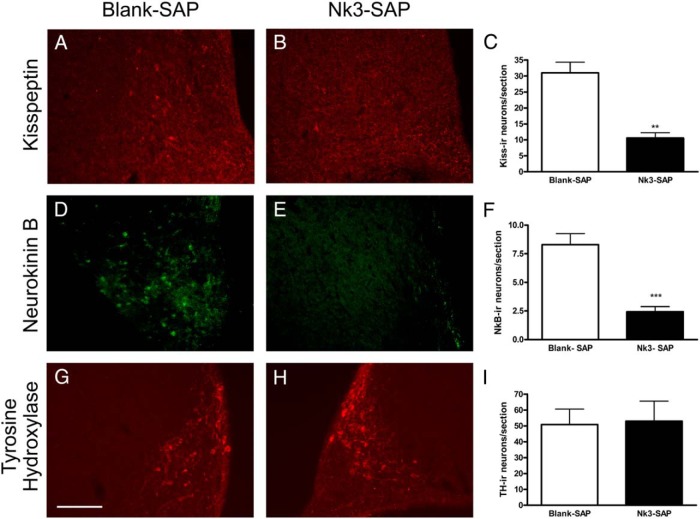
To confirm the ablation protocol, another set of animals was injected with blank-SAP or Nk3-SAP and perfused after 7–10 days. Analysis of kisspeptin and neurokinin B immunoreactivity showed that the number of cell bodies for both neuropeptides was significantly decreased (65% and 75% for kisspeptin and neurokinin B, respectively; Figure 2). Therefore, the average ablation of KNDy neurons was of 70%. A previous study showed that arcuate NK3-SAP injections ablate neurokinin receptor-expressing KNDy neurons, with the preservation of proopiomelanocortin (POMC), neuropeptide Y (NPY), and GnRH neurons (21). No change in the expression of tyrosine hydroxylase (Figure 2) or nonspecific cell loss (Supplemental Figure 1) near the microinjection site was observed in KNDy-ablated animals.
Figure 2.
Photomicrographs of coronal brain sections showing kisspeptin, neurokinin B, and tyrosine hydroxylase immunoreactivity in the arcuate region of rats submitted to microinjection of blank-SAP (A, D, and G) or Nk3-SAP (B, E, and H) after 7–10 days. Quantification of the number of kisspeptin (C), neurokinin B (F), and tyrosine hydroxylase (I) cell bodies in the arcuate region of the same animals. Data are shown as mean ± SEM. **, P < .01, ***, P < .001 compared with control. Scale bar 100 μm.
Partial ablation of KNDy neurons impairs the rise in LH after ovariectomy
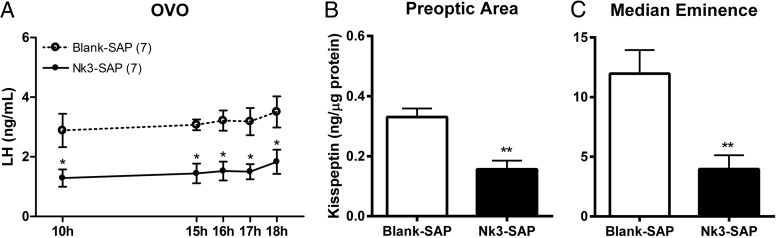
As expected, OVO rats present constant and elevated LH levels due to the loss of steroidal negative feedback after the removal of the ovaries. Selective partial ablation of KNDy neurons of OVO rats reduces LH levels at all time points (Figure 3A, P < .05) but do not seem to diminish them to preovariectomy levels, usually below 0.5 ng/mL during most of the cycle (34). Partial KNDy neuron ablation also reduced kisspeptin content in the POA (Figure 3B, P < .05) and ME (Figure 3C, P < .001) of OVO rats. This reflects a reduced kisspeptin content in the region of AVPV cell bodies (POA) and kisspeptin terminals (ME).
Figure 3.
Plasma LH levels (A) and kisspeptin content in the POA (B) and ME (C) from OVO rats submitted to microinjection of blank-SAP (n = 7) or Nk3-SAP (n = 7) into the arcuate nucleus. Blood samples were taken 7–10 days after neurotoxin injection, and brain punches were collected the following day at 4:00 pm. *, P < .05, **, P < .01 compared with blank-SAP.
Partial ablation of KNDy neurons increases the steroid-induced LH surge
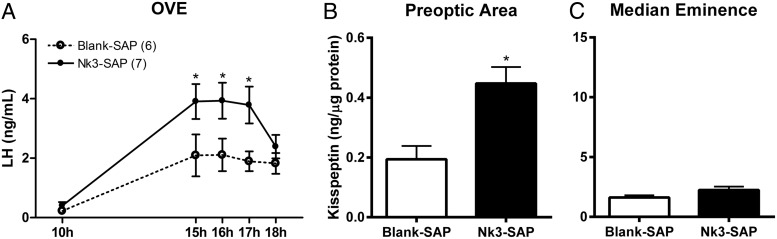
E2 replacement decreased LH levels during the morning and induced an afternoon LH surge in OVE rats. Selective partial ablation of KNDy neurons did not modify basal levels of LH during the morning but increased the peak LH levels in the afternoon of OVE rats (Figure 4A, P < .05). This increased LH secretion was accompanied by an increase in kisspeptin content in the POA area of the OVE rats (Figure 4B, P < .05). Partial ablation of KNDy neurons did not modify the already reduced kisspeptin content in the ME of OVE rats (Figure 4C).
Figure 4.
Plasma LH levels (A) and kisspeptin content in the POA (B) and ME (C) from OVE rats submitted to microinjection of blank-SAP (n = 6) or Nk3-SAP (n = 7) into the ARC. Blood samples were taken 7–10 days after neurotoxin injection and brain punches were collected the following day at 4:00 pm. *, P < .05 compared with blank-SAP.
Dynorphin mediates the postulated inhibitory effect of KNDy neurons on kisspeptin neurons of the AVPV
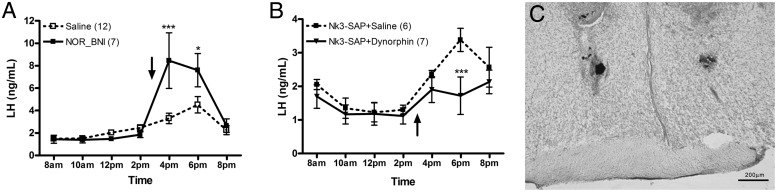
We hypothesize that KNDy neurons directly inhibit kisspeptin neurons of the AVPV, and that ablation of KNDy neurons removes this inhibitory input. Since dynorphin is one of the three neurotransmitters released by KNDy neurons, we hypothesized that inhibition between KNDy and AVPV is mediated by dynorphin. Thus, removal of this inhibitory input to AVPV by KNDy ablation would amplify the LH surge. To test this hypothesis, we microinjected the selective κ-opioid antagonist norbinaltorphimine (NOR-BNI) directly into the AVPV area of the OVE rats. This manipulation significantly increases the magnitude of the LH surge, similar to the effect of partial KNDy ablation in OVE rats (Figure 5A, P < .001). The effect was rapid, significantly increasing the LH level at the following time point (1 h later). We next microinjected dynorphin directly into the AVPV of animals previously subjected to KNDy neuron ablation to determine whether this dynorphin could compensate for the loss of KNDy neurons. Indeed, dynorphin replacement into the AVPV of KNDy-ablated animals greatly reduced the size of the LH surge in the ablated OVE rats (Figure 5B, P < .001). This effect was slower, not reaching significance until the second time point after injections (3 h later). This could indicate that the effect of dynorphin is indirect, acting on other neurons, which in turn inhibit kisspeptin neurons. Only animals with correct cannula placement in the AVPV (Figure 5C) were included in the study.
Figure 5.
Plasma LH levels from OVE rats injected with saline (n = 10) or NOR-BNI (n = 7) into the AVPV. *, P < .05; ***, P < .001 (A). Arrows indicate time of the injection. Plasma LH levels from OVE rats injected with saline (n = 6) or dynorphin (n = 7) into the AVPV of KNDy-ablated rats. B, Photomicrograph of coronal brain section illustrating the microinjection placement in the AVPV (C).
An inhibitory pathway regulates the amplitude of the LH surge
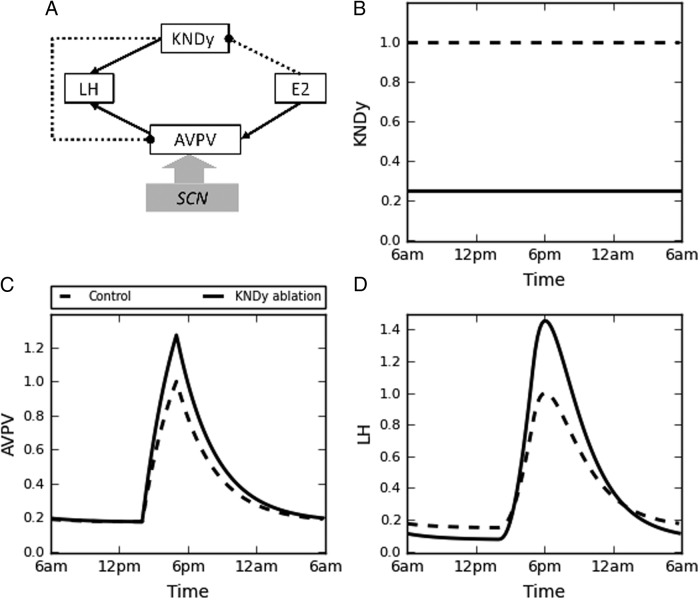
Our hypothesis for the mechanism underlying the enhancement of the LH surge in KNDy-ablated animals is illustrated in Figure 6A. Most importantly, the diagram includes an inhibitory pathway connecting KNDy neurons to kisspeptin neurons of the AVPV. The network diagram has been translated into a mathematical model, and results of computer simulations shown in Figure 6. We simulated the partial KNDy ablation as a reduction in maximum activity level of KNDy subpopulation (by 75% of its original value of 0.7. To better visualize the effect of KNDy ablation, we plotted all variables normalized with respect to control, nonablated simulations (value 1). Consistent with our experimental findings, Figure 6B shows a reduction, but not elimination, of KNDy neuron activity as a result of neurotoxin application. Given that kisspeptin protein expression in the arcuate is greatly reduced by E2 (19), this reduction of KNDy neuron activity due to the partial ablation would have little effect on the LH surge if only the kisspeptin-mediated stimulatory effects of KNDy neurons were considered. However, if, as proposed, there is an inhibitory pathway from the KNDy neurons to the kisspeptin neurons of the AVPV, then the reduction in the KNDy neuron tone caused by the partial ablation would increase the activity of the AVPV neurons via disinhibition (Figure 6C). Because these stimulate GnRH neurons, the result is an increased LH surge (Figure 6D).
Figure 6.
Mathematical modeling simulation of the LH secretion in OVE rats including predictions generated by our simulations. A, Connections within the network model. Solid lines with pointed arrows represent stimulatory connections, whereas dashed lines with blunted arrows represents inhibitory connections. Model simulation results of KNDy neuron activity (B), AVPV kisspeptin neuron activity (C), and the LH level (D) with (solid lines) or without (dashed lines) ablation of KNDy neurons. SCN, suprachiasmatic nucleus.
Discussion
We have shown that KNDy neurons limit the size of the E2-induced LH surge through inhibitory actions on AVPV kisspeptin neurons. We have also demonstrated that this action is mediated by dynorphin. Even though E2 down-regulates kisspeptin in KNDy neurons, we nevertheless find an estrogen-dependent regulatory role for dynorphin (19). Our results thus suggest that KNDy neurons are involved in the E2-positive feedback actions on LH secretion via dynorphin.
Partial KNDy neuron ablation increases the steroid-induced LH surge in OVE rats
Partial ablation of KNDy neurons significantly increases the E2-induced LH surge as well as kisspeptin content in the AVPV area. This result strongly suggests that KNDy neurons are important modulators of the GnRH/LH surges. Current literature suggests that KNDy neurons are a critical component of the hypothalamic pulse generator driving the pulsatile secretion of GnRH (16, 35). This modulation of GnRH pulse frequency may be due to kisspeptin release from the KNDy neurons in the ARC because both intra-ARC and intra-POA administrations of kisspeptin were able to induce increases of LH plasma in OVE rats (36). In addition, specific knockdown of kisspeptin in the ARC decreased LH pulse frequency and the number of regular estrous cycles, suggesting that KNDy neurons may provide a tonic stimulatory drive to GnRH neurons (37).
Our results show that partial ablation of KNDy neurons results in an increased E2-induced LH surge. This suggests that KNDy neurons are important for regulating the size of the preovulatory surge. In female rats, there are more neurons expressing both neurokinin B and kisspeptin in the caudal KNDy neurons compared with males. Also, although the number of kisspeptin immunoreactive cells increases after gonadectomy in males and females, steroid replacement increased kisspeptin fiber density in that region exclusively in female rats (38). In the sheep, KNDy neurons are activated during both the surge and pulsatile LH secretion (39). This suggests a potential role of steroid modulation of KNDy neurons during the E2-positive feedback in different species. Although the percentage of colocalization of kisspeptin and neurokinin B in female rats is very high (40), the ARC also contains a population of neurokinin B-only neurons that is more prominent in the male (38). Whereas our saporin injection likely eliminated this subpopulation, our finding that the modulatory effect on LH secretion is mediated by dynorphin argues against a major role for these neurokinin B-only neurons in the amplification of the LH surge after partial ablation.
In our experiment, the increased E2-induced LH surge was accompanied by a robust increase in kisspeptin content in the POA of KNDy-ablated animals compared to nonablated animals. This result is most likely the reason for the larger LH surge because the POA region contains the AVPV kisspeptin-producing neurons, crucial for the positive feedback actions of steroids driving the GnRH and LH surges (41). It is worth noting that the ablation of KNDy neurons increased LH levels only at surge times, when AVPV kisspeptin expression and activation are maximal (32). E2 stimulates kisspeptin mRNA expression in the AVPV along with the LH surge in the afternoon. Conversely, the blockade of kisspeptin action in the AVPV by a local injection of a kisspeptin antibody abolishes the proestrous LH surge in rats (42). Although our OVE paradigm does not completely mimic the proestrous surge, we observed the same results of increased LH surges when performing pilot experiments in the OVE and ovariectomized rats treated with estradiol and progesterone (OVEP) rats. Similar findings of an increased LH surge in the OVEP rats were also recently demonstrated by the group of Rance and colleagues (43).
Ultrastructural studies demonstrate that kisspeptin influences GnRH secretion, at least partly, through actions on GnRH terminals in the ME (14). Both population of kisspeptin neurons project to the median eminence and are in close contact to the GnRH terminals (44). It is likely that those kisspeptin terminals arise mainly from the ARC population (45), as evidenced by the coexpression of neurokinin B and dynorphin in this area (46). Our results support the importance of the ME because partial ablation of the KNDy neurons did not influence the already reduced kisspeptin levels in the ME of the OVE animals. Because steroids inhibit kisspeptin expression in the KNDy neurons but not in the AVPV (19) and our results show that E2 treatment reduces the kisspeptin content in the ME, it is likely that kisspeptin terminals found in the ME are likely to originate in the KNDy neurons. Whereas it has been shown that the GnRH terminals at the median eminence express neurokinin B receptors (47), saporin destruction of processes comes from actions in the cell body. Specifically, for a peptide-toxin to kill cells, the neurotoxin must act at the cell nucleus. Because saporin is not axonally transported, it is very unlikely that GnRH neurons were destroyed with this microinjection. This has been specifically demonstrated in a recent study showing that intra-ARC injections of NPY-SAP disrupted NPY receptor expressing neurons in the ARC but did not affect circuitry in their vicinity (48).
Dynorphin inhibits AVPV neurons during the steroid-induced LH surge
Given that KNDy neurons project to AVPV neurons (49) and fibers from ARC kisspeptin neurons come into direct contact with kisspeptin neurons of the AVPV (44), we hypothesized that KNDy neuron ablation eliminates an inhibitory factor on AVPV kisspeptin neurons. Dynorphin is the ideal candidate because it exerts its actions mainly through κ-opioid receptors (KORs), which belong to the Gi (inhibitory) protein-coupled, seven-transmembrane receptor superfamily (50). There are dynorphin fibers (51) and KOR mRNA expression in the preoptic area (52) but apparently not in GnRH neurons (53). About 20% of the kisspeptin neurons in the ARC express KORs (16), but it is yet to be determined whether AVPV kisspeptin neurons do. Although the projections described from KNDy to AVPV neurons originate from kisspeptin neurons (49), virtually all kisspeptin neurons in the ARC coexpress neurokinin B and dynorphin (16). Also, dynorphin fibers were observed in the AVPV region of proestrus rats, so we believe this effect is likely exerted indirectly through kisspeptin AVPV neurons.
Early studies suggest that a decrease in dynorphin inhibitory input to the POA is crucial to generate the LH surge (54, 55). Our results complement this finding by showing that although reduced, the dynorphin tone in the AVPV is still sufficient to reduce the size the LH surge. Thus, when the KORs are antagonized, the LH surge gets larger (Figure 4A). This result corroborates previous studies in which perfusion of the POA with NOR-BNI advanced the proestrous LH surge (54). Because our NOR-BNI injection was performed at the time of the surge, we do not know whether it would have advanced the surge if injected earlier. Recent results suggest that dynorphin reduces kisspeptin release in the ARC, therefore being involved in the E2-negative feedback regulation of pulsatile LH release (56). Also, activation of neurokinin B receptors in the ARC seems to inhibit GnRH via dynorphin (57). Our studies suggest an additional role for dynorphin in regulating the size of the E2-induced LH surge. Of particular interest is the fact that the NOR-BNI injection immediately facilitated LH secretion in OVE rats, whereas an injection of dynorphin decreased the LH surge only 3 hours after its administration to KNDy-ablated OVE animals. This can be due the fact that endogenous LH (and GnRH) release had already started before dynorphin injection. Alternatively, this may point toward an indirect effect of dynorphin in other nonkisspeptin neurons in the AVPV, such as tyrosine-hydroxylase-, encephalin-, or dynorphin-immunoreactive neurons (58).
Partial KNDy neuron ablation impaired the rise in LH after ovariectomy but did not influence the negative feedback of steroids in OVE rats
In rodents, gonadal steroids negatively regulate GnRH secretion during most of the estrous cycle, except in the afternoon of proestrus when rising steroid levels stimulate GnRH release (59). Because KNDy neurons are inhibited by E2 (19), this interaction was proposed to mediate the negative feedback of gonadal steroids on GnRH and LH secretion (60). Our results show that approximately 70% of KNDy neurons were ablated after neurotoxin injection. Interestingly, this was accompanied by a similar proportion decrease in the kisspeptin content in the POA and ME as well as in the LH plasma levels in the OVO rats. Although we did not measure the LH secretion before ovariectomy, the LH levels usually range from about 0.5 ng/mL during most of the estrous cycle up to 40 ng/mL during the proestrus preovulatory surge (34). Thus, although partial ablation of KNDy neurons reduced the postovariectomy LH increase, the LH levels are still higher (Figure 3) than the levels usually observed during most of the estrous cycle. Partial ablation of the KNDy neurons did not influence the ability of E2 to suppress LH secretion during the morning (Figure 4), suggesting that KNDy neurons may not be involved in the E2-induced negative feedback in LH secretion.
Direct kisspeptin signaling to GnRH neurons is needed for the E2-negative feedback effect on LH secretion because global and GnRH neuron-selective mutations of the kisspeptin receptor G protein-coupled receptor 54 abolished any post-OVX increase in LH (61, 62). One possibility is that E2 suppresses LH secretion simply by inhibiting KNDy neuron activity. In that case, KNDy neurons would already be inhibited by the presence of E2 and ablation of KNDy neurons would not influence the E2-induced decrease in LH. However, specific ablation of E2 receptors in the ARC (63) or in kisspeptin neurons (64) did not impair the acute E2-negative feedback effect on LH secretion. Also, the only published evidence that KNDy neurons mediate E2-negative feedback on LH secretion is the inhibition of these neurons by E2. There is no increase in c-Fos expression (65) or in the spontaneous firing rate (66–68) in KNDy neurons after ovariectomy. Also, previous ablation of KNDy neurons (21) or selective deletion of E2 receptors in kisspeptin neurons did not modify basal LH levels but attenuated the LH increase only after ovariectomy (69). This suggests that despite KNDy neurons being important, other E2-sensitive neurons contribute to the LH increase after the ovariectomy. Those neurons could be located in the AVPV because there are several nonkisspeptin neurons expressing the E2 receptor in that region that project to GnRH neurons (70). Other candidates include POMC and/or gonadotropin-inhibitory hormone (GnIH) neurons. Both POMC and GnIH neurons express E2 receptor-α (71, 72) and project to GnRH neurons (73, 74). POMC-specific deletion of estrogen receptor-α inhibits the negative feedback regulation of estrogens and impairs fertility in females (75). Selective ablation of leptin receptors in γ-aminobutyric acid secreting neurons decreased the expression of kisspeptin mRNA in KNDy and AVPV kisspeptin neurons, maintaining the negative feedback of gonadal steroids but compromising the increase of LH after the ovariectomy (76). GnIH inhibits LH synthesis and release in rats (77) and GnIH neurons show increased c-FOS expression after E2 treatment, suggesting that they may partially mediate the E2-negative feedback on LH secretion (72). Although our saporin microinjections ablated about 70% of the KNDy neurons, there were still some neurons remaining. Because it was recently demonstrated that only a small number of GnRH neurons are required to maintain reproductive function (78), another possibility is that the few intact KNDy neurons are still able to partially increase LH levels after ovariectomy.
Interestingly, the impairment of the LH increase observed after partial ablation of KNDy neurons in OVO animals was accompanied by a decrease in kisspeptin content not only in the ME but also in the POA. This observation is surprising because the expression of kisspeptin in AVPV neurons is decreased after ovariectomy (20). One possibility is that KNDy neurons provide some sort of tonic excitatory input to AVPV neurons through kisspeptin or neurokinin B that is impaired after ablation of KNDy neurons. Neurokinin B has recently emerged as a critical player in the generation of kisspeptin pulses, mainly acting on KNDy neurons. However, neurokinin B receptors are expressed in the POA of rodents (79) and ewes (80). Central injections of senktide (a neurokinin B agonist) elicit LH secretion in OVE rats (81) and in ewes (82), when administered intra-POA. This increase was proposed to be dependent on kisspeptin/G protein-coupled receptor 54 signaling in the prepubertal male monkey (83), suggesting that neurokinin B is upstream of kisspeptin in the signaling pathway that controls GnRH release and that both are important to sustain LH secretion.
In conclusion, we demonstrated that KNDy neurons seem to be important to control the magnitude of the surges, acting through dynorphin as a suppressor of AVPV kisspeptin neurons. We also showed that although KNDy neurons are important to the post-OVX increase in LH levels, it seems that they are not involved in the E2-induced negative feedback in LH secretion. The kisspeptin content changes described in the AVPV and ME suggest that both kisspeptin populations are involved in the post-OVX increase and E2-induced surges in rodents.
Acknowledgments
We thank Ruth Cristancho Gordo, Natali Dallo, and Yan Barrucceli for technical assistance and Charles Badland for art assistance.
This work was supported by National Institutes of Health Grant DK-43200 and Fundação de Amparo à Pesquisa do Estado de São Paulo Grants 2011/51610-8 and 2011/12642-9. Veronika V. Pogrebna was supported in part by a grant to Washington and Lee University from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program (Grant # 52007570).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- E2
- estradiol
- GnIH
- gonadotropin-inhibitory hormone
- KNDy
- kisspeptin, neurokinin B, and dynorphin
- KOR
- κ-opioid receptor
- ME
- median eminence
- Nk3-SAP
- saporin conjugated to selective neurokinin B receptor agonist [MePhe7]neurokinin B
- NOR-BNI
- norbinaltorphimine
- NPY
- neuropeptide Y
- OVE
- ovariectomized rats implanted with capsules containing E2
- OVO
- ovariectomized rats implanted with capsules containing oil
- POA
- preoptic area
- POMC
- proopiomelanocortin
- SAP
- saporin.
References
- 1. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 2. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320(2):383–388. [DOI] [PubMed] [Google Scholar]
- 3. Smith JT, Saleh SN, Clarke IJ. Seasonal and cyclical change in the luteinizing hormone response to kisspeptin in the ewe. Neuroendocrinology. 2009;90(3):283–291. [DOI] [PubMed] [Google Scholar]
- 4. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102(6):2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhillo WS, Murphy KG, Bloom SR. The neuroendocrine physiology of kisspeptin in the human. Rev Endocr Metab Disord. 2007;8(1):41–46. [DOI] [PubMed] [Google Scholar]
- 6. Han S-K, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 8. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 11. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673–682. [DOI] [PubMed] [Google Scholar]
- 12. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeo S-H. Neuronal circuits in the hypothalamus-controlling GnRH release: the neuroanatomical projections of kisspeptin neurons. Exp Physiol. 2013;98(11):1544–1549. [DOI] [PubMed] [Google Scholar]
- 14. Uenoyama Y, Inoue N, Pheng V, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;23(10):863–870. [DOI] [PubMed] [Google Scholar]
- 15. Kalló I, Vida B, Deli L, et al. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24(3):464–476. [DOI] [PubMed] [Google Scholar]
- 16. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
- 19. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 20. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cordellini MF, Piazzetta G, Pinto KC, et al. Effect of different doses of estrogen on the nigrostriatal dopaminergic system in two 6-hydroxydopamine-induced lesion models of Parkinson's disease. Neurochem Res. 2011;36(6):955–961. [DOI] [PubMed] [Google Scholar]
- 23. James TA, Starr MS. Effects of the rate and volume of injection on the pharmacological response elicited by intranigral microapplication of drugs in the rat. J Pharmacol Methods. 1978;1(3):197–202. [Google Scholar]
- 24. Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974;36(3):391–392. [DOI] [PubMed] [Google Scholar]
- 25. Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10(2):84–94. [DOI] [PubMed] [Google Scholar]
- 26. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed Elsevier Academic Press; 2007. [Google Scholar]
- 27. Araujo-Lopes R, Crampton JR, Aquino NS, et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155(3):1010–1020. [DOI] [PubMed] [Google Scholar]
- 28. Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. [DOI] [PubMed] [Google Scholar]
- 29. Helena CV, Szawka RE, Anselmo-Franci JA. Noradrenaline involvement in the negative-feedback effects of ovarian steroids on luteinising hormone secretion. J Neuroendocrinol. 2009;21(10):805–812. [DOI] [PubMed] [Google Scholar]
- 30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 31. Kinsey-Jones JS, Beale KE, Cuenco J, et al. Quantification of rat kisspeptin using a novel radioimmunoassay. PLoS One. 2014;9(5):e97611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. [DOI] [PubMed] [Google Scholar]
- 34. Helena CV, Franci CR, Anselmo-Franci JA. Luteinizing hormone and luteinizing hormone-releasing hormone secretion is under locus coeruleus control in female rats. Brain Res. 2002;955(1–2):245–252. [DOI] [PubMed] [Google Scholar]
- 35. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X-F, Kinsey-Jones JS, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beale KE, Kinsey-Jones JS, Gardiner JV, et al. The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology. 2014;155(3):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Overgaard A, Ruiz-Pino F, Castellano JM, Tena-Sempere M, Mikkelsen JD. Disparate changes in kisspeptin and neurokinin B expression in the arcuate nucleus after sex steroid manipulation reveal differential regulation of the two KNDy peptides in rats. Endocrinology. 2014;155(10):3945–3955. [DOI] [PubMed] [Google Scholar]
- 39. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005;146(10):4431–4436. [DOI] [PubMed] [Google Scholar]
- 43. Krajewski-Hall SJ, Mittelman-Smith MA, McMullen NT, Rance NE. Ablation of arcuate KNDy neurons amplifies the LH surge in steroid-primed, ovariectomized rats. Paper presented at the 43rd Annual Meeting of the Society of Neuroscience; 2013, Washington, DC (Abstract 274.01). [Google Scholar]
- 44. Hoong Yip S, Boehm U, Herbison AE, Campbell RE. Conditional viral tract-tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–2594. [DOI] [PubMed] [Google Scholar]
- 45. Xu Z, Kaga S, Mochiduki A, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J. 2012;59(2):161–171. [DOI] [PubMed] [Google Scholar]
- 46. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 48. Bugarith K, Dinh TT, Li A-J, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146(3):1179–1191. [DOI] [PubMed] [Google Scholar]
- 49. Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. [DOI] [PubMed] [Google Scholar]
- 50. Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the κ opioid receptor. Science. 1982;215(4531):413–415. [DOI] [PubMed] [Google Scholar]
- 51. Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol. 1988;276(3):442–459. [DOI] [PubMed] [Google Scholar]
- 52. Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11(7):308–314. [DOI] [PubMed] [Google Scholar]
- 53. Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of μ and κ opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport. 1997;8(14):3167–3172. [DOI] [PubMed] [Google Scholar]
- 54. Smith MJ, Gallo RV. The effect of blockade of κ-opioid receptors in the medial preoptic area on the luteinizing hormone surge in the proestrous rat. Brain Res. 1997;768(1–2):111–119. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Q, Gallo RV. Presence of κ-opioid tone at the onset of the ovulatory luteinizing hormone surge in the proestrous rat. Brain Res. 2003;980(1):135–139. [DOI] [PubMed] [Google Scholar]
- 56. Mostari P, Ieda N, Deura C, et al. dynorphin-κ opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. [DOI] [PubMed] [Google Scholar]
- 58. Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16(9):3035–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Neill J, Plant T, Pfaff D, Challins J.eds. Physiology of Reproduction. 3rd ed Academic Press; 2006:2327–2388. [Google Scholar]
- 60. Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–1158. [DOI] [PubMed] [Google Scholar]
- 61. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21(12):1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeo S-H, Clarkson J, Herbison AE. Kisspeptin-Gpr54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice. Neuroendocrinology. 2014;100(2–3):191–197. [DOI] [PubMed] [Google Scholar]
- 63. Yeo S-H, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155(8):2986–2995. [DOI] [PubMed] [Google Scholar]
- 64. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in female mice require estrogen receptor α (ERα) in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. [DOI] [PubMed] [Google Scholar]
- 66. De Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153(11):5384–5393. [DOI] [PubMed] [Google Scholar]
- 67. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155(7):2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C, Boehm U. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology. 2015;1(1):32–38. [DOI] [PubMed] [Google Scholar]
- 71. De Souza FSJ, Nasif S, López-Leal R, Levi DH, Low MJ, Rubinsten M. The estrogen receptor α colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol. 2011;660(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kriegsfeld LJ, Mei DF, Bentley GE, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103(7):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449(1–2):167–176. [DOI] [PubMed] [Google Scholar]
- 74. Gibson EM, Humber SA, Jain S, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martin C, Navarro VM, Simavli S, et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J Neurosci. 2014;34(17):6047–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murakami M, Matsuzaki T, Iwasa T, et al. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199(1):105–112. [DOI] [PubMed] [Google Scholar]
- 78. Herbison AE, Porteous R, Pape J-R, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mileusnic D, Lee JM, Magnuson DJ, et al. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89(4):1269–1290. [DOI] [PubMed] [Google Scholar]
- 80. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dungan Lemko HM, Naderi R, Adjan V, et al. Interactions between neurotensin and GnRH neurons in the positive feedback control of GnRH/LH secretion in the mouse. Am J Physiol Endocrinol Metab. 2010;298(1):E80–E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26(11):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]