Abstract
Loss of circulating 17β-estradiol (E2) that occurs during menopause can have detrimental effects on cognitive function. The efficacy of hormone replacement therapy declines as women become farther removed from the menopausal transition, yet the molecular mechanisms underlying this age-related switch in E2 efficacy are unknown. We hypothesized that aging and varying lengths of E2 deprivation alters the ratio of alternatively spliced estrogen receptor (ER)β isoforms in the brain of female rats. Further, we tested whether changes in global transcriptional activity and splicing kinetics regulate the alternative splicing of ERβ. Our results revealed brain region-specific changes in ERβ alternative splicing in both aging and E2-deprivation paradigms and showed that ERβ could mediate E2-induced alternative splicing. Global transcriptional activity, as measured by phosphorylated RNA polymerase II, was also regulated by age and E2 in specific brain regions. Finally, we show that inhibition of topoisomerase I resulted in increased ERβ2 splice variant expression.
Hormone replacement therapy (HT) has become routine in abrogating the negative effects associated with the decline in circulating 17β-estradiol (E2) in women postmenopause. The efficacy of HT in mediating these negative effects is temporally dependent, as inferred from data obtained in the Women's Health Initiative and Women's Health Initiative Memory Study. Specifically, HT was beneficial or neutral for most parameters measured (cognition, memory, cardiovascular) in younger postmenopausal women, whereas older women who were 10 or more years postmenopause experienced adverse effects, including cardiovascular and coronary disease, stroke, cognitive impairment, and dementia (1–6). Other studies have suggested the idea of a therapeutic window in which HT is beneficial, known as the “timing hypothesis,” pointing to age-related adjustments that occur during and after this critical period of declining E2 levels (5, 7–14). Although the benefits of HT on mood in postmenopausal women are controversial, recent studies have yielded optimism about the beneficial effects of HT, especially on mood and anxiety (15–18). However, the mechanisms by which the molecular environment of the aging brain changes during this period in response to E2 withdrawal remain unknown. We hypothesized that differential effects of E2 could be due to changes in alternative splicing of the estrogen receptor (ER).
The physiological effects of E2 are mediated primarily through ERα and ERβ. These receptors act as transcriptional regulators for genes that are functionally important for a variety of processes in the brain, including memory, reproduction, and stress (19–25). Similar to many other proteins, ERβ is subject to posttranscriptional alternative splicing. There are several identified ERβ splice variants that naturally occur in both humans and in rodents (26–30). ERβ1 is the wild-type isoform that is primarily expressed throughout the human (human ER [hER]β1) and rat (rat ER [rER]β1) brain. This isoform also has the highest affinity for E2, the major circulating estrogen during the reproductively competent period of the lifespan (31). At least 4 hERβ splice variants are expressed in the human brain, and 5 splice variants of rERβ have been described in the rat brain (26, 32). The physiological relevance of these ERβ splice variants has been highlighted in a recent study where increased expression of the rat dominant negative ERβ2 isoform diminished the effectiveness of HT in ovariectomized (OVX) female rats (33). This study also revealed that withdrawal of E2 over time might increase the expression of ERβ2, further implicating the actions of these receptors when circulating E2 levels reach the nadir that occurs with menopause. Thus, the increase in alternative splicing of ERβ may negatively impact E2-regulation of important processes due to the structural and functional differences that occur in these variants.
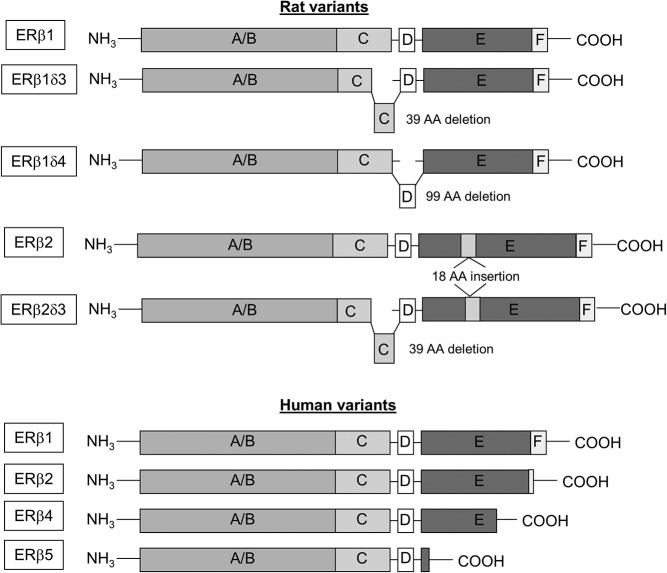
Alternative splicing of ERβ in humans differs from rats: hERβ variants are the result of truncations from the C-terminal end of the mRNA transcript, whereas rERβ splice variants arise from insertions and deletions within the mRNA transcript (Figure 1) (26–28). Nevertheless, both human and rodent ERβ splice variants are functionally different from the wild-type ERβ, can form heterodimers with the wild-type, and ultimately alter downstream E2-mediated signaling pathways (32, 34–36). More importantly, these splice variants have also been shown by our laboratory to be constitutively active in the absence of ligand binding, suggesting they might be particularly important postmenopause. Ligand-independent effects of ERβ impact a variety of genes that are regulated by ERs, including arginine vasopressin and corticotrophin-releasing factor (34, 35, 37, 38), 2 hypothalamic hormones important for regulating stress and anxiety (39). In addition to the hypothalamus, the dorsal and ventral hippocampus are important brain regions that have been shown to express ERβ splice variants. The dorsal hippocampus is important for spatial and verbal memory, whereas the ventral hippocampus is important for fear conditioning and affective processes (40). Memory impairment, overall decline in cognitive function, and increased anxiety are all possible negative effects that coincide with loss of circulating E2 in postmenopausal women (1, 2, 18, 20, 41, 42). Therefore, expression of these alternatively spliced receptors during menopause, a time when E2 is no longer being produced endogenously by the ovaries, may have a variety of implications within many different brain regions, including the hypothalamus and hippocampus.
Figure 1.
Diagram of rat and human ERβ splice variants. Structural depiction comparing the differences between rat and human splice variants of ERβ. A/B, AF-1 domain; C, DNA-binding domain; D, hinge domain, E, AF-2/ligand-binding domain; F, undefined.
Alternative gene splicing contributes to increased biodiversity of proteins that can be expressed from a limited set of genes within our genome, because over 95% of multiexonic genes are alternatively spliced (43). Gene transcripts that contain multiple coding exons can undergo splicing that may exclude these exonic sequences from the final mRNA transcript to be translated (44). Recent evidence has suggested that alternative splicing events increase with age in the brain (45). These alternative splicing events occur through mechanisms that involve either cis-sequences encoded within the pre-mRNA transcript, or by trans-acting splicing factors, such as RNA binding proteins (46, 47). The rate at which alternative splicing occurs can also influence exon inclusion events (48, 49). For instance, interference of RNA polymerase II (RNAPII) elongation rates resulted in inhibition-dependent changes in alternative splicing, whereby exons that were located 3′ downstream of short intronic sequence were included within the mature mRNA transcript (50). It is becoming widely accepted that alternative splicing events likely occur at the site of transcription, and in cooperation with transcription, and that these coordinated actions between trans-acting factors and RNAPII are heavily involved in determining the fate of pre-mRNA transcripts (51, 52).
The purpose of this study was to determine how E2 deprivation, as occurs during menopause, affects the alternative splicing of ERβ in the aging brain. We hypothesized that ERβ2 expression would increase in a tissue-specific manner due to age-related and E2 deprivation-related changes in alternative splicing, and treatment with E2 would abrogate these effects. To test this hypothesis, Fisher 344 rats were OVX at 3 and 18 months of age to study the effects of aging (1-wk OVX) and E2 deprivation (1- to 12-wk OVX) followed by acute E2 treatment (Figure 2). Moreover, changes in the expression or activity of RNAPII have not been previously demonstrated in aged animals, or in relation to E2 treatment. Therefore, we also assessed expression of RNAPII and its activity in these animals to determine how this ubiquitous enzyme might influence alternative splicing. Lastly, we assessed how RNAPII interference may specifically affect ERβ2 alternative splicing in a hypothalamic neuronal cell line. Our data revealed that alternative splicing events are influenced by aging and E2 deprivation in a brain region-specific manner. We also observed that interference of RNAPII increased expression of ERβ2 and that expression and activity of RNAPII changes with aging and E2 deprivation in a brain region-specific manner.
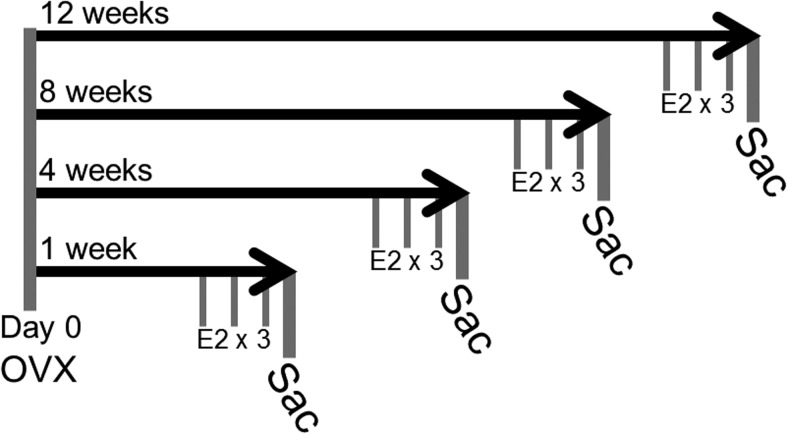
Figure 2.
Diagram of the E2-deprivation paradigm. Animals were OVX at day 0 and then separated into 4 deprivation groups (n = 16–20/deprivation group) that were subjected to increasingly longer periods of hormone deprivation (1, 4, 8, and 12 wk). After deprivation, animals were treated with either vehicle (safflower oil) or E2 (2.5 μg/kg) by sc injection once daily for 3 consecutive days (n = 6–10/treatment group). Animals were killed 24 hours after the last treatment.
Materials and Methods
Animals and deprivation paradigm
Female Fischer 344 rats (3 and 18 mo of age) were obtained from the National Institutes of Health aging colony (Taconic). In this strain of rat, these ages equate to young adult (3 mo) and postmenopausal (18 mo) ages relative to humans based on calculations from survival curves, reduced proestrous cycles and estradiol concentrations, and increased cycle durations in 18-month-old Fischer 344 rats (53–56). Importantly, Fischer 344 female rats had an average lifespan of 26–29 months of age; therefore, an 18-month-old rat would have lived 62%–69% of its life expectancy. By contrast, women in the United States have an average life expectancy of 81.2 years (57). Therefore, a 55-year-old woman is at 67% of her total life expectancy, which is within the same range (62%–69%) as an 18-month-old Fisher 344 rat. All animal protocols were approved by the Institutional Animal Care and Use Committee at Loyola University Chicago, permit number 2009018. Groups of ovarian-intact animals at all ages 3, 18, 19, 20, and 21 months of age were used to compare with OVX animals in experiments 1 and 2 (n = 6/age group).
Experiment 1. Comparison of ERβ2 expression in young (3 mo) and aged (18 mo) female rats
Animals were bilaterally OVX under vaporized isoflurane anesthesia, as previously described (58). One week (7 d) after OVX, animals were administered a sc injection of vehicle (safflower oil) or 2.5-μg/kg E2 (catalog E8875; Sigma) (n = 6–10/treatment group) based on previous studies (58). Treatments were administered once daily for 3 consecutive days. Animals were humanely euthanized 24 hours after the last treatment, trunk blood was collected, and brains were rapidly removed and flash frozen for tissue processing.
Experiment 2. Effects of varying lengths of E2 deprivation on ERβ2 and RNAPII expression, and RNAPII activity in aged female rats
Aged (18 mo) female rats were OVX as described above and then separated into 4 groups that would undergo increasingly longer periods of time without E2: 1, 4, 8, and 12 weeks (n = 16–20/deprivation group) (Figure. 2) (10, 11, 53). After the assigned E2-deprivation period, animals received a sc injection of either vehicle (safflower oil) or 2.5-μg/kg E2 (catalog E8875; Sigma) (n = 6–10/treatment group). Treatments were administered once daily for 3 consecutive days. Animals were humanely euthanized 24 hours after the last treatment, trunk blood was collected, and brains were rapidly removed and flash frozen for tissue processing. No significant differences in body weights were observed between treatment groups (Table 1).
Table 1.
Body Weights (g) of Fischer 344 Rats Before OVX Procedure and After Final Treatment
| Pre-op Weight (g) | Final Weight (g) | |||
|---|---|---|---|---|
| Treatment | Vehicle | E2 | Vehicle | E2 |
| 1 week | 285.4 ± 4.8 | 278.5 ± 6.8 | 280.8 ± 4.7 | 274.0 ± 6.8 |
| 4 weeks | 268.5 ± 5.4 | 273.8 ± 9 | 277.8 ± 3.9 | 282.0 ± 8.9 |
| 8 weeks | 272.0 ± 3.6 | 272.8 ± 5.8 | 298.2 ± 7.3 | 301.2 ± 4.8 |
| 12 weeks | 280.5 ± 7.5 | 285.9 ± 6.7 | 312.7 ± 6.5 | 318.3 ± 4.2 |
There were no statistically significant differences in body weight between treatment groups.
Experiment 3. Effects of ERβ and ERα selective agonists on ERβ2 expression in aged (18 mo) female rats
Aged (18 mo) female rats were OVX as described above. One week (7 d) after OVX, animals were administered a sc injection of vehicle (safflower oil), 2.5-μg/kg E2 (catalog E8875; Sigma), 1-mg/kg diarylproprionitrile (DPN) (catalog 1494; Tocris), or 0.5-mg/kg propyl pyrazole triol (PPT) (catalog 1426; Tocris) (n = 6–10/treatment group). These doses were based on previous studies demonstrating selective activation of their respective receptors (59–62). Treatments were administered once daily for 3 consecutive days. Animals were humanely euthanized 24 hours after the last treatment, trunk blood was collected, and brains were rapidly removed and flash frozen for tissue processing.
E2 concentration assay and vaginal cytology
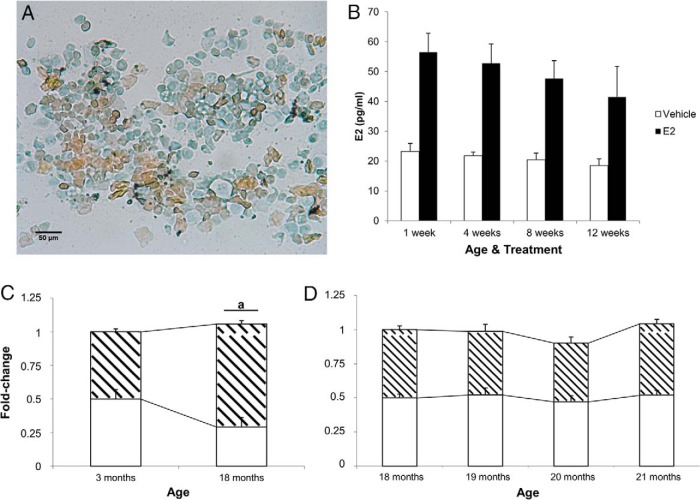
Trunk blood was collected and centrifuged at 4500 rpm for 8 minutes at 4°C. The plasma samples first underwent a liquid-liquid extraction using diethyl ether to eliminate interfering compounds in the plasma as previously described (63). After diethyl ether extraction, samples were reconstituted using buffer contained in the E2 high-sensitivity ELISA kit (AD 901 174; Enzo Life Sciences). The ELISA kit was then used to determine the concentration of circulating E2 in the ovarian-intact (n = 6/age), OVX-vehicle, and OVX-E2-treated (n = 6/age per treatment) animals according to manufacturer's specifications. Absorbance was measured on a BioTek Synergy HT plate reader. The sensitivity of the assay was 14.0 pg/mL. Inter- and intrassay coefficients of variance were 4.6% and 3.8%, respectively. Cross-reactivity with other endogenous estrogens were 17.8% for estrone and 0.9% for estriol; 18-month animals before OVX had low circulating E2 levels (35.0 ± 7.1 pg/mL; n = 6). These low circulating E2 levels corresponded with consistent diestrous-like vaginal cytology as assessed daily for 2 weeks before killing (Figure 3A). Circulating E2 levels remained low in 18-month-old animals treated with vehicle 1 week after OVX (23.2 ± 2.7 pg/mL; n = 6). E2 treatment increased circulating E2 levels in OVX animals (56.5 ± 6.3 pg/mL; n = 6), which is within physiological range of women who receive HT during postmenopause (17–75 pg/mL) (64, 65). Treatment with E2 increased circulating levels consistently within this range throughout the deprivation paradigm (Figure 3B). E2-treated 3-month-old OVX animals (66.7 ± 8.2 pg/mL; n = 8) had levels similar to diestrous-intact animals at the same age (69.4 ± 15.2 pg/mL; n = 6).
Figure 3.
Vaginal cytology, E2 plasma concentrations, and alternative splicing of ERβ in intact animals. A, Representative image (×20) from vaginal smears that were obtained from 18-month-old female rats daily for 7 days before OVX (n = 6). Cells were stained with Papanicolaou stain before imaging. Orange G (orange) stains keratinized squamous epithelial cells. Eosin azure (blue) staining cells represent nonkeratinized squamous epithelial cells, neutrophils, and red blood cells (if present). B, Concentration (pg/mL) of plasma E2 in vehicle- and E2-treated animals during hormone-deprivation paradigm. E2 concentration was analyzed by E2 high-sensitivity ELISA kit; 18-month animals before OVX had low circulating E2 levels (35.0 ± 7.1 pg/mL; n = 6). C, Total ERβ (white region) and ERβ2 (hatched region) mRNA expression was measured in the hypothalamus of intact (non-OVX) 3 and (D) 18- to 21-month-old female rats. a, statistically significant difference (P < .05) in total ERBETA as determined by paired t test. Data are expressed as mean ± SEM.
Quantitative RT-PCR
Flash frozen brains were sectioned at 200 μm on a freezing microtome and regions of interest were microdissected using 0.75-mm Palkovit's brain punch tool (Stoelting Co). The hypothalamus (−0.8 to −3.8 mm relative to bregma), dorsal hippocampus (−2.30 to −4.16 mm relative to bregma), and ventral hippocampus (4.30 to 6.04 mm relative to bregma) were all microdissected for RNA and protein isolation. Brains were split sagittally for tissue collection where 1 hemisphere was used for RNA isolation and the other for protein isolation in a nonbiased manner. RNA isolation was performed on sonicated tissue samples using TRIzol reagent (15596–026; Invitrogen) according to the manufacturer's specifications. All RNA samples were quantified using Nanodrop spectrophotometry and analyzed for quality by visualization of the RNA on 1.0% agarose gel. cDNA was reverse transcribed from 1.0-μg RNA using High Capacity cDNA Reverse Transcription kit (4368814; Applied Biosystems) according to manufacturer's specifications. Quantitative reverse-transcription polymerase chain reaction (RT-qPCR) for ERβ was performed with TaqMan Gene Expression MasterMix (4369016; Applied Biosystems) and TaqMan custom fluorescein amidite-probes that are specific for total ERβ and ERβ2 splice variants on an Eppendorf Realplex4. Hypoxanthine-guanine phosphoribosyltransferase was used as a reference gene to check loading and normalize data, and was measured using TaqMan Gene Expression Assay Rn01527840_m1. Total ERβ was measured by TaqMan Gene Expression Assay Rn00562610_m1. The next custom probes sequences were designed and used to detect rERβ splice variants: ERβ2, TCCTCAGAAGACCCTCAC, ERβ1d3 and ERβ2d3, ATTCAAGGATCCAGGAGA, and ERβ1d4, GTCAAGTGTGGCTTTGTG. The next primer sequences were used to detect the custom probes listed previously: ERβ2 forward, AGCCTGTTGGACCAAGT and reverse, GCACTCTTCATCTGCGCAAC; ERβd3 and ERβd4 forward, GAGAGACACTGAAGAGGAAGC and reverse, TCACGGAACCTTGACGTCGTC. RT-qPCR for RNAPII was performed with Fast Start Universal SYBR Green Master Mix (catalog 04913914001; Roche) according to manufacturer's specifications. RNAPII primer sequences were designed and produced (Integrated DNA Technologies) in the next sequences: forward, GCTGGACCTACTGGCATGTT and reverse, ACCATAGGCTGGAGTTGCAC. Data were analyzed by delta delta threshold cycle method as described previously (66).
Cell culture experiments
ERβ-positive hypothalamic-derived cells (GT1–7) were used for all in vitro experiments (generously provided by Dr Pamela Mellon, University of San Diego, La Jolla, CA) (67). Cells were maintained in DMEM 50:50 F12 media containing glucose, L-glutamine, sodium pyruvate, and 10% fetal bovine serum. Cells were grown to confluency (70%–80%) and media replaced with DMEM 50:50 F12 containing 10% dextran charcoal-stripped fetal bovine serum for steroid-free conditions 48 hours before experiments. Cells were treated with either vehicle (dimethyl sulfoxide) (D128; Fisher Scientific), 32-ng/μL camptothecin (C9911; Sigma) alone, or camptothecin plus 100nM E2 for 6 hours (n = 3 plates/treatment group). All cell line experiments were done in triplicate to ensure reproducibility. Cells were then collected for RNA isolation, cDNA synthesis, and RT-qPCR as described above.
Protein isolation and Western blotting
Hypothalamic, dorsal hippocampal, and ventral hippocampal tissue isolated from aged female rat brains were placed in T-PER Tissue Protein Extraction Reagent (78510; Thermo Fisher Scientific) supplemented with 7× Protease and Phosphatase Inhibitor cocktail (88668; Thermo Fisher Scientific). Tissue was sonicated and insoluble material, including DNA, was pelleted and excluded from the soluble portion of the extracts. A total of 10-μg protein was reduced in 4× Laemmli buffer (161–0747; Bio-Rad) at 95°C for 5 minutes and run on 10% SDS-PAGE gel. RNAPII expression and phosphorylation were blotted with Pol II N-20 (sc-899; Santa Cruz Biotechnology, Inc) and phospho-Pol II 8A7 (sc-13583; Santa Cruz Biotechnology, Inc) antibodies, respectively (Table 2). These antibodies detect the largest subunit of RNAPII that binds DNA and conveys catalytic activity along with the second largest subunit that forms the active center of the RNAPII enzyme (68, 69). Blots were imaged using a ChemiDoc (Bio-Rad) and quantified. Both RNAPII bands (2 largest subunits) were quantified together using Image Lab 3.0 Software (Bio-Rad). Phosphorylated RNAPII (p-RNAPII) and RNAPII expression were both normalized to β-actin expression and then calculated as a ratio (p-RNAPII to RNAPII) to determine activity of RNAPII relative to its expression (70).
Table 2.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| RNAPII | Pol II (N-20) | Santa Cruz Biotechnology, Inc, sc-899 | Rabbit; polyclonal | 1:1000 |
| p-RNAPII | p-Pol II (8A7) | Santa Cruz Biotechnology, Inc, sc-13583 | Mouse; monoclonal | 1:500 |
| β-Actin | β-Actin (13E5) | Cell Signaling, 4970 | Rabbit; monoclonal | 1:1000 |
RNAPII expression and phosphorylation were blotted with N-20 (sc-899; Santa Cruz Biotechnology, Inc) and 8A7 (sc-13583; Santa Cruz Biotechnology, Inc) antibodies, respectively. These antibodies detect the largest subunit of RNAPII that binds DNA and conveys catalytic activity along with the second largest subunit that forms the active center of the RNAPII enzyme. Blots were imaged using a ChemiDoc (Bio-Rad) and quantified. Both RNAPII bands (2 largest subunits) were quantified together using Image Lab 3.0 Software (Bio-Rad). p-RNAPII and RNAPII expression were both normalized to β-actin expression and then calculated as a ratio (p-RNAPII to RNAPII) to determine activity of RNAPII relative to its expression.
Statistical analysis
Significant interactions were assessed by two-way ANOVA with age per treatment as factors (experiment 1, young vs aged), or time per treatment (experiment 2, E2-deprivation paradigm) using Systat 13 software (Systat Software, Inc), followed by Tukey's honestly significant-difference post hoc analysis to determine significant differences among groups where P < .05. Different letters and/or symbols denote statistically significant differences between groups. A separate Tukey's honestly significant-difference post hoc test was used within groups that showed a statistically significant main effect of age and/or treatment. A one-way ANOVA followed by Tukey's honestly significant-difference post hoc analysis was conducted to determine significant difference (P < .05) between selective ER agonists (DPN and PPT; experiment 3) where treatment was the main effect in the hypothalamus of 18-month animals and in vitro studies. All data are presented as mean ± SEM.
Results
Changes in ERβ splice variant expression at different ages in ovarian-intact and OVX animals
Total ERβ and ERβ2 splice variant expression was quantified in the hypothalamus of young (3 mo) and aged (18–21 mo) ovarian-intact animals. There was a statistically significant increase in ERβ2, but not total ERβ, expression between 3- and 18-month-old animals (Figure 3C). By contrast, no statistically significant differences were observed in aged ovarian-intact animals beyond 18 months of age (Figure 3D). We also compared ovarian-intact with OVX vehicle-treated animals in each age group and found no statistically significant differences (data not shown).
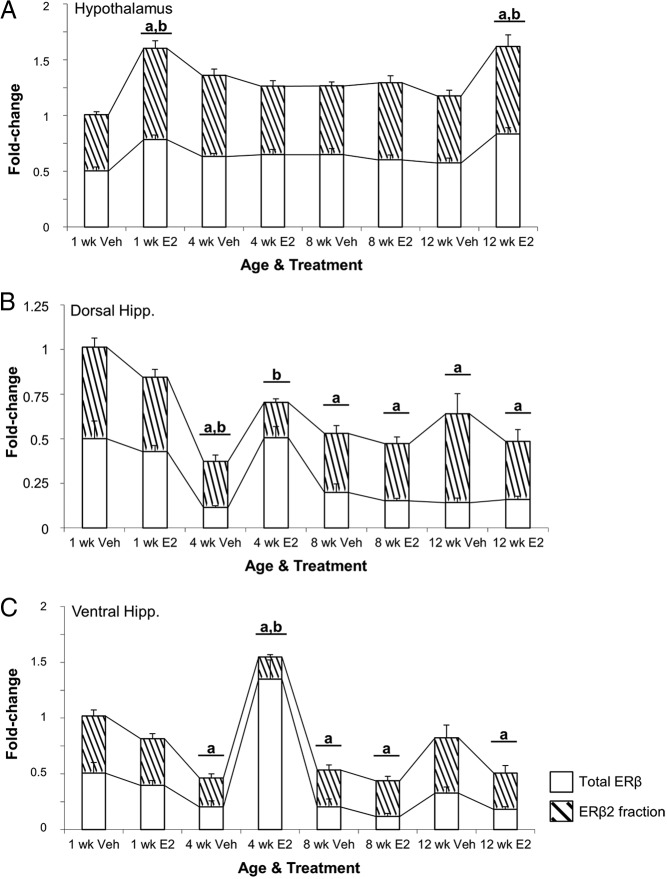
Next, we quantified total ERβ and all known ERβ splice variants in young (3 mo) and aged (18 mo) OVX vehicle-treated and OVX-E2-treated animals in the hypothalamus and hippocampus. The data revealed a statistically significant main effect of age (F1,18 = 7.838, P < .015) on ERβ2 expression in the hypothalamus (Figure 4A, hatched region) but not the dorsal or ventral hippocampus (Figure 4, B and C). In addition, ERβ2 expression was not affected by E2 treatment in the hypothalamus of 3-month-old animals (Figure 4A). By contrast, ERβ2 expression was significantly decreased in 18-month-old vehicle-treated animals compared with 3-month-old animals, yet it was significantly increased after E2 treatment. Moreover, E2 treatment significantly decreased total ERβ expression only in the dorsal hippocampus and there were no differences in 18-month-old animals between treatment groups (Figure 4B). The other previously identified rERβ splice variants, ERβ1d3, ERβ1d4, and ERβ2d3, were undetectable in either age group for all brain regions tested (data not shown).
Figure 4.
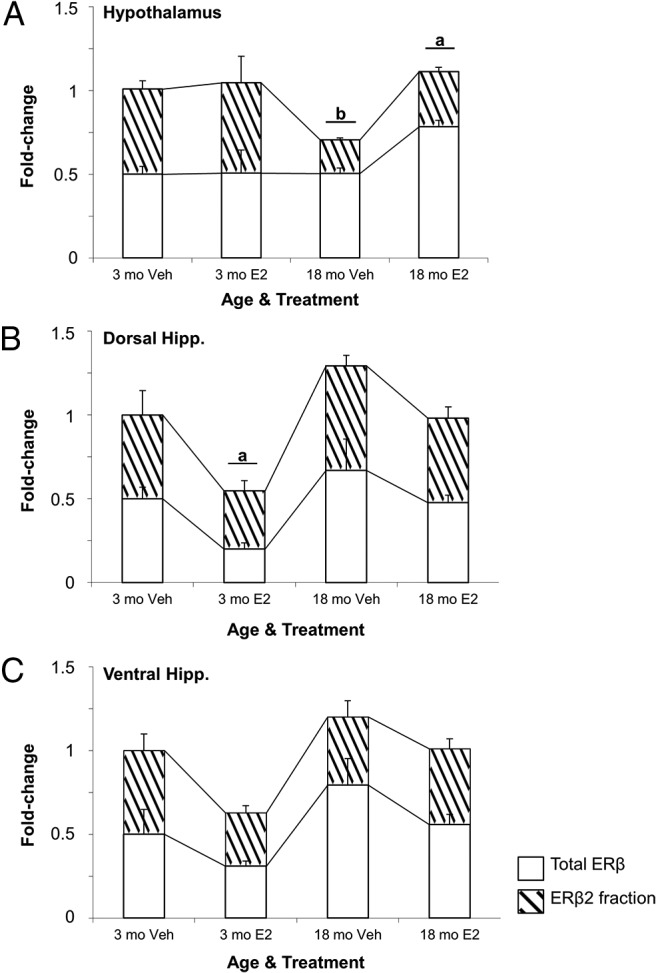
Comparison of total ERβ and the fraction that represents ERβ2 (hatched region) mRNA expression in the young and aged female rat brain. ERβ2 expression was measured in the (A) hypothalamus, (B) dorsal hippocampus, and (C) ventral hippocampus at 1 week after OVX in 3- and 18-month-old female rats (n = 6/age per treatment group). Animals were OVX and 1 week later treated with either vehicle (safflower oil) or 2.5-μg E2 (dissolved in safflower oil). a, statistically significant difference (P < .05) in total ERβ; b, statistically significant difference (P < .05) in ERβ2 as determined by Tukey's honestly significant-difference test after two-way ANOVA. Data are expressed as mean ± SEM.
Brain region-specific changes in ERβ splice variant expression after varying lengths of E2 deprivation in aged female rats
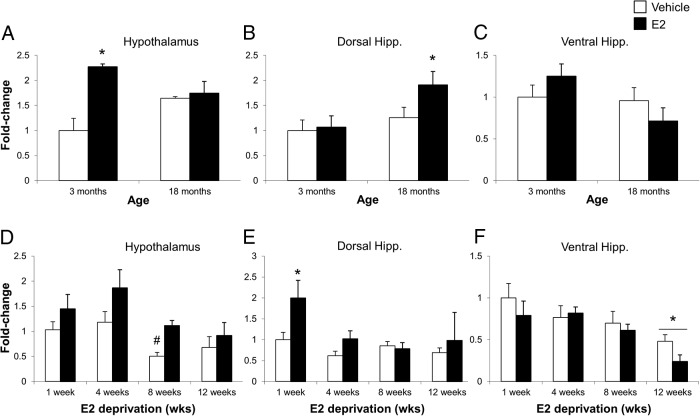
To assess the effects of varying lengths of E2 deprivation in the aged brain, 18-month-old animals were OVX followed by administration of vehicle or 2.5-μg/kg E2 once daily for 3 consecutive days at varying time points (Figure 2). Two-way ANOVA revealed a significant interaction between the length of E2 deprivation and subsequent E2 treatment in the hypothalamus (F3,40 = 3.943, P < .015) (Figure 5A). There was also a significant main effect of length of E2-deprivation alone (ie, vehicle treatment) on ERβ2 expression in both the dorsal and ventral hippocampus (F3,42 = 7.714, P < .001 and F3,41 = 8.409, P < .001, respectively) (Figure 5, B and C), as well as a significant main effect of treatment in the dorsal hippocampus (F1,42 = 5.115, P < .05) (Figure 5B), although no statistically significant interaction was revealed in these brain regions. Treatment with E2 significantly increased ERβ2 at the 1-week deprivation time point in the hypothalamus (Figure 5A), consistent with our earlier-described experiment comparing 3- and 18-month animals (Figure 4A). E2 treatment also significantly increased ERβ2 expression in the hypothalamus at 12, but not 4 or 8, weeks of deprivation (Figure 5A).
Figure 5.
Expression of total ERβ and ERβ2 (hatched region) mRNA in the brain of aged (18-mo-old) female rats after varying periods of E2 deprivation. ERβ2 mRNA expression was measured in the (A) hypothalamus, (B) dorsal hippocampus, and (C) ventral hippocampus at 1, 4, 8, and 12 weeks after OVX (n = 6–10/age per treatment group). Animals were treated with either vehicle (safflower oil) or 2.5-μg E2 (dissolved in safflower oil). a, statistically significant difference (P < .05) in total ERβ; b, statistically significant difference (P < .05) in ERβ2 as determined by Tukey's honestly significant-difference test after two-way ANOVA. Data are expressed as mean ± SEM.
Analysis of total ERβ expression revealed a significant interaction between E2 deprivation and subsequent E2 treatment in both the dorsal and ventral hippocampus (F3,42 = 12.932, P < .001 and F3,41 = 33.585, P < .001, respectively) (Figure 5, B and C). In addition, a significant main effect of E2 treatment on total ERβ expression was observed in the hypothalamus (F1,40 = 6.378, P < .016). Significant increases in total ERβ expression with treatment mirrored the pattern observed for ERβ2 expression at 1 and 12 weeks of deprivation (Figure 5A). We observed a significant decrease in ERβ2 expression in both the dorsal and ventral hippocampus after E2 treatment at 4 weeks, but expression levels returned to baseline (ie, 1 wk after OVX) beyond this time point (Figure 5, B and C). Total ERβ expression, on the other hand, decreased after 4 weeks of deprivation in both dorsal and ventral hippocampus, and remained lowly expressed through the remainder of the deprivation paradigm, whereas administration of E2 at 4 weeks in the ventral hippocampus resulted in a significant increase in total ERβ expression (Figure 5C).
E2-induced increases in alternative ERβ splice variant expression is mediated primarily by ERβ
The effects mediated by E2 on ERβ alternative splicing could be mediated by either ERα or ERβ, because both are expressed in each of the brain regions tested (71). Acute administration of the ERβ-specific agonist DPN or the ERα-specific agonist PPT was given to 18-month-old female OVX rats to determine which ER mediated the observed increases in ERβ2. Administration of all 3 ligands significantly increased total ERβ expression; however, only E2 and DPN significantly increased the expression of ERβ2 (Figure 6). These results suggest that ERβ could play a role in mediating E2-induced changes in alternative splicing.
Figure 6.
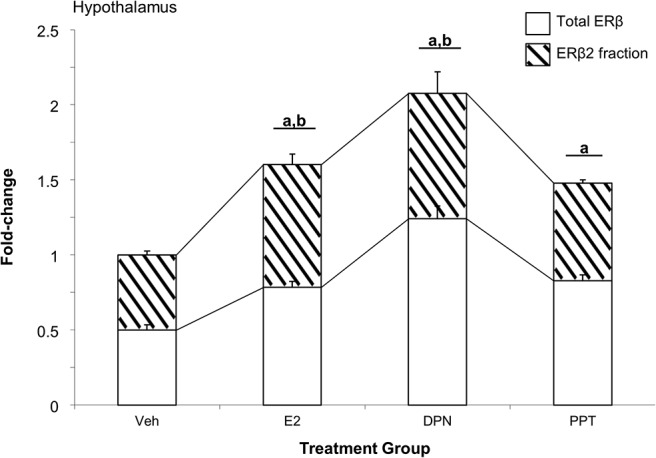
The ERβ-selective agonist DPN increases ERβ alternative splicing. Total ERβ and ERβ2 (hatched region) mRNA expression was measured in the hypothalamus of aged female rats that were OVX and 1 week later treated with either vehicle, E2, 1-mg/kg DPN, or 0.5-mg/kg PPT once daily for 3 consecutive days (n = 6–8/treatment group). a, statistically significant difference (P < .05) in total ERβ; b, statistically significant difference (P < .05) in ERβ2 as determined by Tukey's honestly significant-difference test after one-way ANOVA.
ERβ2 expression is increased after inhibition of topoisomerase 1 (TOP1)
The ERβ2 splice variant is characterized by a 54-bp “insertion” located in the ligand-binding domain (see Figure 1). This additional nucleotide sequence is encoded from a region in the ERβ1 intron that lies between exons 5 and 6 and is flanked by weak splice sites. Inhibition of TOP1 can slow down splicing kinetics, resulting in alternative exon inclusion by allowing the spliceosome machinery more time to recognize weak splice sites in shorter intronic elements flanking the exon (49, 50). In these experiments, splicing kinetics were slowed through inhibition of TOP1and ERβ2 expression levels were quantified. Hypothalamic-derived neuronal cells (GT1–7) were treated with the TOP1 inhibitor camptothecin (32 ng/μL) in the presence or absence of E2 after a period of steroid hormone deprivation. Our results revealed a significant main effect of treatment (F3,12 = 3.544, P < .05) on ERβ2 expression. Specifically, inhibition of TOP1 significantly increased ERβ2 expression and cotreatment with E2 blocked this effect (Figure 7).
Figure 7.
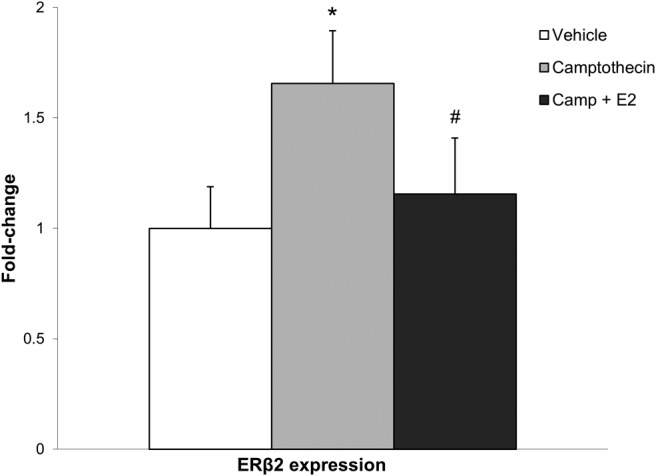
Camptothecin treatment in hypothalamic GT1–7 cells increases ERβ2 expression. ERβ2 mRNA expression was measured in the GT1–7 cell line after 6 hours of treatment with 32-ng/μL camptothecin or camptothecin + 10nM E2 or vehicle (dimethyl sulfoxide) (n = 3). *, Statistically significant difference (P < .05) compared to vehicle; #, statistically significant difference (P < .05) between groups as determined by Tukey's honestly significant-difference test after one-way ANOVA. Data are expressed as mean ± SEM.
Brain region-specific changes in RNAPII in aged female rats
We hypothesized that increased RNAPII expression and/or activity would coincide with our observed increases in age-related alternative splicing of ERβ. Our results revealed a statistically significant interaction between age and treatment in the hypothalamus (F1,18 = 8.317, P < .01) (Figure 8A), but not in the dorsal or ventral hippocampus (Figure 8, B and C). Further, E2 treatment increased RNAPII mRNA expression in the 3-month animals, whereas neither age nor E2 affected RNAPII mRNA expression in the 18-month animals. A significant main effect of age on RNAPII expression was observed in the dorsal hippocampus (F1,18 = 4.825, P < .05) (Figure 8B), which was further increased by E2 treatment in aged, but not young, animals (Figure 8B).
Figure 8.
Effects of age and varying periods of E2 deprivation on RNAPII mRNA expression in the brain of young and aged female rats. RNAPII expression was measured in the (A) hypothalamus, (B) dorsal hippocampus, and (C) ventral hippocampus at 1 week after OVX in 3- and 18-month-old animals (n = 6/age per treatment group). RNAPII expression was also measured in the (D) hypothalamus, (E) dorsal hippocampus, and (F) ventral hippocampus at 1, 4, 8, and 12 weeks after OVX (n = 6–10/age per treatment group). Animals were treated with either vehicle (safflower oil) or 2.5-μg E2 (dissolved in safflower oil). *, statistically significant difference (P < .05) between groups; #, statistically significant difference within groups as determined by Tukey's honestly significant-difference test after two-way ANOVA. Data are expressed as mean ± SEM.
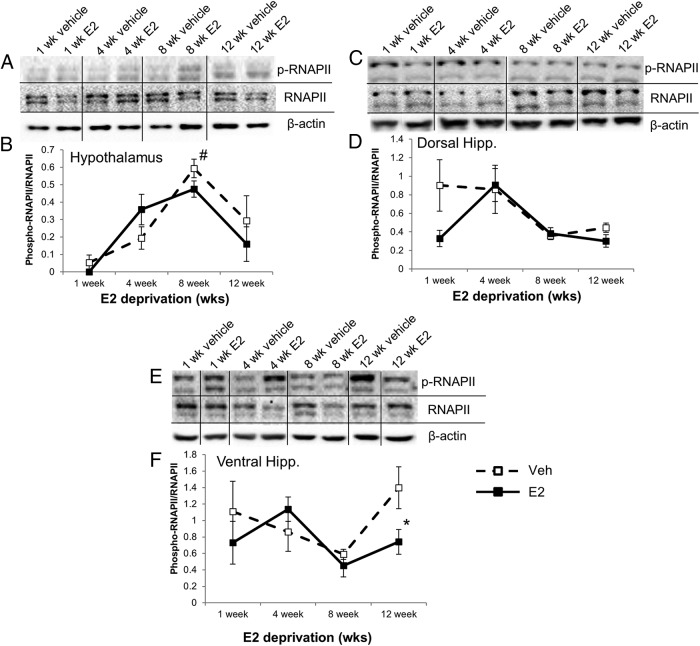
Aged animals subjected to our E2-deprivation paradigm showed a statistically significant main effect of E2-deprivation alone (ie, vehicle treatment) and a main effect of subsequent E2 treatment on RNAPII expression in the hypothalamus (F3,40 = 4.591, P < .01 and F1,40 = 8.504, P < .01, respectively) and dorsal hippocampus (F3,44 = 3.541, P < .05 and F1,44 = 4.222, P < .05, respectively) (Figure 8, D and E). Conversely, in the ventral hippocampus there was a significant main effect of deprivation alone (F3,42 = 6.316, P < .001) but not E2 treatment (Figure 8F). In the dorsal hippocampus, E2 treatment significantly increased RNAPII expression at 1-week deprivation only (Figure 8E). E2 treatment resulted in a significant decrease of RNAPII in the ventral hippocampus of animals deprived of E2 for 12 weeks (Figure 8F) with or without E2 treatment.
Next, we measured RNAPII activity using Western blotting by probing for p-RNAPII and comparing these levels with total RNAPII protein expression in the animal paradigm described previously. The data revealed a significant main effect of deprivation in all 3 brain regions (F3,40 = 3.634, P < .05, hypothalamus; F3,44 = 4.467, P < .01, dorsal hippocampus; F3,42 = 3.341, P < .05, ventral hippocampus). In the hypothalamus, there was a significant difference between the 1-week and 8-week vehicle-treated animals, because p-RNAPII expression was nearly undetectable at the 1-week time point for both vehicle- and E2-treated animals (Figure 9, A and B). The ventral hippocampus had a significant decrease in p-RNAPII expression after treatment with E2 at 12 weeks when compared with the vehicle-treated animals at the same time point (Figure 9, E and F).
Figure 9.
Effects of varying periods of E2 deprivation on RNAPII activity in aged female rats. p-RNAPII and total RNAPII protein levels were measured by Western blotting in the (A and B) hypothalamus, (C and D) dorsal hippocampus, and (E and F) ventral hippocampus at 1, 4, 8, and 12 weeks after OVX (n = 6–10/age per treatment group). After E2 deprivation, animals were treated with either vehicle (safflower oil) or 2.5-μg E2 (dissolved in safflower oil). *, statistically significant difference (P < .05) between groups; #, statistically significant within groups as determined by Tukey's honestly significant-difference test after two-way ANOVA. Representative blot images combined from several gels used to quantify Western blottings by densitometry.
Discussion
This study presents a quantitative descriptive analysis of the expression of the ERβ splice variant, ERβ2, in the rat hypothalamus, dorsal hippocampus, and ventral hippocampus. The expression levels of ERβ2 mRNA were compared with regards to aging and E2 deprivation in young animals, and in an aged surgically induced menopausal animal model designed to mimic E2 replacement therapy occurring at varying time points after menopause. Our studies demonstrate the novel finding that age and E2 deprivation altered the expression of ERβ2 in a brain region-specific manner. The brain regions analyzed all expressed ERβ2 and E2 signaling is physiologically important in these regions for mediating the stress response, neurogenesis, and synaptic plasticity (33, 34, 72–74). We also present a quantitative analysis of RNAPII expression and demonstrate that age and E2 treatment altered RNAPII mRNA expression levels, as well as the amount of p-RNAPII protein, suggesting changes in RNAPII enzymatic activity. Age-dependent changes in RNAPII mRNA expression levels and/or enzymatic activity might impact global alternative splicing patterns. Indeed, we provide evidence that ERβ2 expression levels were increased after inhibition of TOP1, suggesting that changes in splicing kinetics could contribute to age-related changes in alternative splicing. Together, these data suggest a putative molecular mechanism regulating ERβ alternative splicing in the brain, although more mechanistic studies are required to validate a causal relationship. Overall, these data contribute to our overall understanding of ERβ alternative splicing in the aging female brain.
We used a surgically induced menopause and E2 replacement paradigm to address the hypothesis that aging and/or long periods of ovarian hormone deprivation alters the expression of alternatively spliced ERβ variants, which could provide a mechanistic explanation for decreased E2 efficacy in older women. Specifically, ERβ2 has been shown to antagonize the actions of ERβ1 and has a much lower binding affinity for E2; both of these factors could decrease the efficacy of E2 replacement therapy after menopause. We were able to achieve clinically relevant levels of E2 after administration in our female OVX rats that were similar to those observed in postmenopausal patients that received HT (64, 65). Interestingly, E2 administration had brain region-specific effects in the hypothalamus and hippocampus: treatment in the hypothalamus resulted in significant increases in ERβ alternative splicing, whereas treatment in the dorsal and ventral hippocampus resulted in significant decreases. The significant increases in the hypothalamus of ERβ2 after E2 treatment also corresponded with significant increases in total ERβ expression. Therefore, increased availability of ERβ pre-mRNA due to increased transcription could allow for increased alternative splicing through cotranscriptional events (48, 52). These data suggest that any assessments of E2 efficacy will differ depending on the physiological endpoint measured, because each of these brain regions regulates distinct physiological functions. In particular, gene expression within the hypothalamus has recently been shown to adapt to changes in circulating E2 levels, and the increase observed in ERβ2 expression, along with its ability to act in a ligand-independent manner, may underlie this neuronal adaptation to fluctuating circulating E2 levels throughout the lifespan (34, 35, 75). Another important recent study investigated E2 deprivation of 9-month-old Sprague-Dawley rats that were administered E2 treatment either 6 or 180 days (ie, 15 mo old) after OVX. They demonstrated a decrease in E2 efficacy on hippocampal neurogenesis and neuroprotection that coincided with increased expression levels of ERβ2, suggesting that ERβ2 was responsible for the observed decreased E2 efficacy (33). Our data suggest that in aged Fisher 344 rats this increase in ERβ2 does not persist in the hippocampus but instead becomes much more evident in the hypothalamus, a brain region with direct anatomical connections to the hippocampus. Wang et al (33) also showed that E2 treatment blocked the observed increase in ERβ2 expression in animals that were deprived of E2 for only 6 days, but E2 was ineffective after 180 days after OVX. Our results are consistent with these, because we also did not observe a significant effect of E2 after longer periods of E2 deprivation (>8 wk). Collectively, these data and those from previous studies suggest that ERβ2 is the predominant variant expressed in the hippocampus and hypothalamus as the time of E2 deprivation lengthens (41, 76, 77). Future studies into specific hypothalamic nuclei and hippocampal subgroups may further reveal brain region-specific changes occurring during E2 deprivation.
We have shown that E2 can regulate ERβ alternative splicing in a brain region-specific manner; however, whether E2 was acting through ERα or ERβ was unclear. Previous studies have shown age-related changes in the expression levels of ERα within these specific brain regions, whereas there are conflicting reports regarding the expression of ERβ (71, 78, 79). These data led us to predict that E2 effects in aged animals would most likely be mediated by ERβ or ERα. Indeed, both DPN and PPT significantly increased total ERβ, although only DPN significantly increased the expression of ERβ2. These results suggest that ERβ mediated the observed E2-induced increases in alternative splicing in aged animals. It is possible that the lack of a PPT effect was due to lower expression levels of ERα in aged animals; therefore, we are unable exclude the possibility that ERα could mediate E2-induced alternative splicing in younger animals.
To our knowledge, no studies to date have investigated the mechanisms regulating ERβ alternative splicing in the brain or any other tissue. In these studies, we investigated 2 potential mechanisms to explain age-related changes in alternative splicing of ERβ. First, taking advantage of a novel compound called camptothecin, a TOP1 inhibitor, we tested the idea that the weak splice site near the short, 54bp intronic sequence of ERβ2 would increase if transcription kinetics were slowed. Camptothecin has been used previously in several studies to investigate how the rate of transcription can affect alternative splicing (50, 80). Therefore, we treated hypothalamic-derived GT1–7 cells with camptothecin and observed a statistically significant increase in ERβ2 expression. Contrary to what we predicted based on the data obtained in vivo in our aged animals, concomitant E2 and camptothecin treatment attenuated the observed increase in ERβ alternative splicing. These data suggested that E2 might be able to overcome RNAPII stalling, whether by up-regulating expression of RNAPII to compensate for the stalled enzymes or increasing activation of RNAPII via phosphorylation. Therefore, we measured RNAPII mRNA expression and protein phosphorylation levels in our animal model. Our results demonstrated a clear age and E2-dependent effect on RNAPII mRNA expression and p-RNAPII levels, but they were not consistently correlated with observed changes in ERβ2. This suggests a complex regulatory system regulating ERβ2 alternative splicing and these results provide a descriptive analysis from which future experiments can be based.
Our model, along with the previous studies outlined in this manuscript, raise important limitations to consider. The model we used to study menopause and the timing hypothesis differ in some aspects with other similar studies (33, 75). Importantly, rodents do not undergo a menopausal transition that is similar to humans and, therefore, most studies have relied on using a surgically induced menopause model, or a chemically induced delayed depletion of ovarian follicles. Chemically induced ovarian failure, as reported previously, is a valuable model to study the menopausal transition; however, in these studies, we chose the surgical model in order to precisely measure the length of time after complete ovarian E2 deprivation (81). In addition, there is some debate regarding the correct age in rodents to begin manipulations so that they will provide a good comparison with the human menopausal condition and yield generalizable results. These discrepancies are likely due to strain-specific differences in rats, which is an important consideration when comparing various reports in the literature. For instance, Sprague-Dawley rats have an average cycle cessation at 8–12 months, whereas the Fisher 344 rat strain undergoes cycle cessation at 16–18 months (53, 55). Also, the predominant postcyclic vaginal state in Sprague-Dawley rats is persistent estrous, resulting in high estrogen levels. By contrast, Fisher 344 rats typically exhibit repeated pseudopregnancy with low estrogen but higher progesterone (54). We were also able to correlate an approximate age of our animals with a human age (an 18-mo-old rat equals a 55-y-old human) by using previously reported data on aging in Fischer 344 rats) (53–56). Interestingly, a recent study by Yin et al (75) demonstrated that changes in the brain occur well before the onset of menopause and that hypothalamic neuronal networks are highly adaptable to fluctuations in circulating E2 levels. We propose that this adaptability could be the result of altered alternative ERβ splicing and subsequent changes in the ratio of alternatively spliced ERβ isoforms.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01AG033605.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DPN
- diarylproprionitrile
- E2
- 17β-estradiol
- ER
- estrogen receptor
- hER
- human ER
- HT
- hormone replacement therapy
- OVX
- ovariectomized
- PPT
- propyl pyrazole triol
- p-RNAPII
- phosphorylated RNAPII
- rER
- rat ER
- RNAPII
- RNA polymerase II
- RT-qPCR
- quantitative reverse-transcription polymerase chain reaction
- TOP1
- topoisomerase 1.
References
- 1. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. [DOI] [PubMed] [Google Scholar]
- 2. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2959–2968. [DOI] [PubMed] [Google Scholar]
- 3. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 4. Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19(7):791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63(2):231–237. [DOI] [PubMed] [Google Scholar]
- 6. Shufelt CL, Merz CN, Prentice RL, et al. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women's Health Initiative Observational Study. Menopause. 2014;21(3):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams JK, Anthony MS, Honoré EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15(7):827–836. [DOI] [PubMed] [Google Scholar]
- 8. Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004;82(2):391–397. [DOI] [PubMed] [Google Scholar]
- 9. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14(3 pt 1):373–384. [DOI] [PubMed] [Google Scholar]
- 10. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7(1–3):163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maki PM, Dennerstein L, Clark M, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69(1):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST). Am J Obstet Gynecol. 2005;192(2):387–393. [DOI] [PubMed] [Google Scholar]
- 16. Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. [DOI] [PubMed] [Google Scholar]
- 17. MacLennan AH, Henderson VW, Paine BJ, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13(1):28–36. [DOI] [PubMed] [Google Scholar]
- 18. Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. [DOI] [PubMed] [Google Scholar]
- 20. Sherwin BB. Estrogenic effects on memory in women. Ann NY Acad Sci. 1994;743:213–230; discussion 230–211. [DOI] [PubMed] [Google Scholar]
- 21. Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci USA. 2001;98(21):12278–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor β in the brain: from form to function. Brain Res Rev. 2008;57(2):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu F, Day M, Muñiz LC, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–343. [DOI] [PubMed] [Google Scholar]
- 24. Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor β knockout, mice. Behav Neurosci. 2008;122(5):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89(4):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor β missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142(5):2039–2049. [DOI] [PubMed] [Google Scholar]
- 27. Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor β splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res Mol Brain Res. 2000;80(2):260–268. [DOI] [PubMed] [Google Scholar]
- 28. Moore JT, McKee DD, Slentz-Kesler K, et al. Cloning and characterization of human estrogen receptor β isoforms. Biochem Biophys Res Commun. 1998;247(1):75–78. [DOI] [PubMed] [Google Scholar]
- 29. Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor β gene. Mol Cell Endocrinol. 1997;132(1–2):195–199. [DOI] [PubMed] [Google Scholar]
- 30. Lu B, Leygue E, Dotzlaw H, Murphy LJ, Murphy LC, Watson PH. Estrogen receptor-β mRNA variants in human and murine tissues. Mol Cell Endocrinol. 1998;138(1–2):199–203. [DOI] [PubMed] [Google Scholar]
- 31. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 32. Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)- β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci USA. 2006;103(35):13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang JM, Hou X, Adeosun S, et al. A dominant negative ERβ splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PLoS One. 2012;7(3):e33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-β mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148(7):3371–3382. [DOI] [PubMed] [Google Scholar]
- 35. Mott NN, Pak TR. Characterization of human oestrogen receptor β (ERβ) splice variants in neuronal cells. J Neuroendocrinol. 2012;24(10):13111321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogawa S, Inoue S, Watanabe T, et al. The complete primary structure of human estrogen receptor β (hER β) and its heterodimerization with ER α in vivo and in vitro. Biochem Biophys Res Commun. 1998;243(1):122–126. [DOI] [PubMed] [Google Scholar]
- 37. Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146(1):147–155. [DOI] [PubMed] [Google Scholar]
- 38. Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-α and -β recruitment to a 3′,5′-cyclic adenosine 5′-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149(1):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. [DOI] [PubMed] [Google Scholar]
- 40. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherwin BB. Cognitive assessment for postmenopausal women and general assessment of their mental health. Psychopharmacol Bull. 1998;34(3):323–326. [PubMed] [Google Scholar]
- 42. Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217(1):17–22. [DOI] [PubMed] [Google Scholar]
- 43. Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40(12):1413–1415. [DOI] [PubMed] [Google Scholar]
- 44. Krämer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. [DOI] [PubMed] [Google Scholar]
- 45. Tollervey JR, Wang Z, Hortobágyi T, et al. Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res. 2011;21(10):1572–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3(3):195–205. [DOI] [PubMed] [Google Scholar]
- 47. Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582(14):1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G. Multiple links between transcription and splicing. RNA. 2004;10(10):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de la Mata M, Alonso CR, Kadener S, et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol Cell. 2003;12(2):525–532. [DOI] [PubMed] [Google Scholar]
- 50. Ip JY, Schmidt D, Pan Q, et al. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011;21(3):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17(3):251–256. [DOI] [PubMed] [Google Scholar]
- 52. Kornblihtt AR. Coupling transcription and alternative splicing. Adv Exp Med Biol. 2007;623:175–189. [DOI] [PubMed] [Google Scholar]
- 53. vom Saal FS, Finch C. Reproductive senescence: phemonoma and mechanisms in mammals and selected vertebrates. In: Knobil E, Neill J, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1988:2535–2399. [Google Scholar]
- 54. Eldridge JC, Wetzel L, Tisdel M, Stevens J. Pattern of reproductive aging in female rats can affect mammary tumor incidence. In: Li JJ, ed. Hormonal Carcinogenesis II. New York, NY: Springer; 1996;467–470. [Google Scholar]
- 55. Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119(3):821–830. [DOI] [PubMed] [Google Scholar]
- 56. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. [DOI] [PubMed] [Google Scholar]
- 57. Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the United States, 2013. NCHS Data Brief. 2014;(178):1–8. [PubMed] [Google Scholar]
- 58. Mott NN, Pinceti E, Rao YS, et al. Age-dependent effects of 17β-estradiol on the dynamics of estrogen receptor β (ERβ) protein-protein interactions in the ventral hippocampus. Mol Cell Proteomics. 2014;13(3):760–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology. 2003;144(7):3159–3166. [DOI] [PubMed] [Google Scholar]
- 60. Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology. 2002;143(11):4172–4177. [DOI] [PubMed] [Google Scholar]
- 61. Oyola MG, Portillo W, Reyna A, et al. Anxiolytic effects and neuroanatomical targets of estrogen receptor-β (ERβ) activation by a selective ERβ agonist in female mice. Endocrinology. 2012;153(2):837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor α within the hypothalamus. Neuroscience. 2009;159(2):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pak TR, Lynch GR, Ziegler DM, Lunden JB, Tsai PS. Disruption of pubertal onset by exogenous testosterone and estrogen in two species of rodents. Am J Physiol Endocrinol Metab. 2003;284(1):E206–E212. [DOI] [PubMed] [Google Scholar]
- 64. Schmidt G, Andersson SB, Nordle O, Johansson CJ, Gunnarsson PO. Release of 17-β-oestradiol from a vaginal ring in postmenopausal women: pharmacokinetic evaluation. Gynecol Obstet Invest. 1994;38(4):253–260. [DOI] [PubMed] [Google Scholar]
- 65. Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90(1):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 67. Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5(1):1–10. [DOI] [PubMed] [Google Scholar]
- 68. Li OT, Chan MC, Leung CS, et al. Full factorial analysis of mammalian and avian influenza polymerase subunits suggests a role of an efficient polymerase for virus adaptation. PLoS One. 2009;4(5):e5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McKenna NJ, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95(20):11697–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Adon AM, Zeng X, Harrison MK, et al. Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells. Mol Cell Biol. 2010;30(3):694–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- 72. Walf AA, Frye CA. Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86(2):407–414. [DOI] [PubMed] [Google Scholar]
- 73. Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78(3):523–529. [DOI] [PubMed] [Google Scholar]
- 74. Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor β splice variant protein (ERβ2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505(3):249–267. [DOI] [PubMed] [Google Scholar]
- 75. Yin W, Maguire SM, Pham B, et al. Testing the critical window hypothesis of timing and duration of estradiol treatment on hypothalamic gene networks in reproductively mature and aging female rats. Endocrinology. 2015;156(8):2918–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carlson LE, Sherwin BB. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23(6):583–603. [DOI] [PubMed] [Google Scholar]
- 77. Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55(1):2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-β mRNA in the female rat brain. Brain Res. 2007;1155:34–41. [DOI] [PubMed] [Google Scholar]
- 79. Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor α (ER α) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466(3):409–421. [DOI] [PubMed] [Google Scholar]
- 80. Darzacq X, Shav-Tal Y, de Turris V, et al. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol. 2007;14(9):796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]