Abstract
Mutations in the gene encoding the thyroid hormone (TH) transporter, monocarboxylate transporter 8 (MCT8), cause mental retardation in humans associated with a specific thyroid hormone phenotype manifesting high serum T3 and low T4 and rT3 levels. Moreover, these patients have failure to thrive, and physiological changes compatible with thyrotoxicosis. Recent studies in Mct8-deficient (Mct8KO) mice revealed that the high serum T3 causes increased energy expenditure. The TH analog, diiodothyropropionic acid (DITPA), enters cells independently of Mct8 transport and shows thyromimetic action but with a lower metabolic activity than TH. In this study DITPA was given daily ip to adult Mct8KO mice to determine its effect on thyroid tests in serum and metabolism (total energy expenditure, respiratory exchange rate, and food and water intake). In addition, we measured the expression of TH-responsive genes in the brain, liver, and muscles to assess the thyromimetic effects of DITPA. Administration of 0.3 mg DITPA per 100 g body weight to Mct8KO mice brought serum T3 levels and the metabolic parameters studied to levels observed in untreated Wt animals. Analysis of TH target genes revealed amelioration of the thyrotoxic state in liver, somewhat in the soleus, but there was no amelioration of the brain hypothyroidism. In conclusion, at the dose used, DITPA mainly ameliorated the hypermetabolism of Mct8KO mice. This thyroid hormone analog is suitable for the treatment of the hypermetabolism in patients with MCT8 deficiency, as suggested in limited preliminary human trials.
Mutations in the monocarboxylate transporter 8 (MCT8, SLC16A2) gene, located on the X chromosome, occur in the setting of Allan-Herndon-Dudley syndrome (AHDS), identified in 1944 for its severe psychomotor retardation in males (1). It was in 2004 that the gene defect and associated thyroid function test abnormalities were recognized, consisting of high serum T3, low rT3, low T4, and normal to slightly elevated TSH (2, 3). AHDS manifests with truncal hypotonia and poor head control, progressive spastic quadriplegia, lack of speech, and poor communication skills. Moreover, most patients demonstrate failure to thrive and nutritional deficiency with weight below the third centile requiring gastric tube feeding to avert malnutrition. Whereas the neurological impairment causing difficulties with feeding could contribute to the inability to gain weight, our group has recently demonstrated that in the Mct8-deficient mouse (Mct8KO), the high serum T3 increases energy expenditure, resulting in failure to maintain weight despite adequate calorie intake. This hypermetabolic state is generated by excess of thyroid hormone (TH) in tissues that are not predominantly Mct8 dependent for TH transport, including the liver, adipose tissue, and skeletal muscle (4). The coexistence of TH deficiency in the brain and TH excess in some peripheral tissues (5, 6) renders treatment of this condition challenging. Ideally, treatment of the syndrome should correct the central nervous system hypothyroidism and the peripheral tissue hyperthyroidism by supplying a TH analog that enters cells independently of the MCT8 transporter. In this respect, the TH analog diiodothyropropionic acid (DITPA) exerts thyromimetic actions while maintaining a normal metabolic activity (7, 8). It enters the cells independently of Mct8 and when given in high doses to Mct8KO mice rendered hypothyroid, it corrected the biochemical effects of TH deprivation in the central nervous system without producing thyrotoxic effects in the peripheral tissues as assessed in the liver of the same mice (9).
Taking these observations into consideration, DITPA (2.1–2.4 mg/kg·d) was given to four children with MCT8 deficiency for 26–40 months. This treatment normalized the serum T3 and TSH and increased the serum T4 and rT3 to low normal or slightly below normal levels, without any associated adverse effects. In addition, DITPA caused modification of markers of TH action, such as the decline in SHBG in all four subjects, the heart rate in three subjects, and ferritin in one of four subjects as well as the increase in cholesterol levels in two of four subjects. Significant weight gain was observed in a pair of twins (10). Despite these improvements, direct evidence that DITPA reduces the hypermetabolism of MCT8-deficient patients is lacking owing to the difficulties in performing metabolic studies in affected individuals.
To circumvent this limitation, we conducted experiments using Mct8KO mice as a model to determine the effect of DITPA on whole-body energy homeostasis. The effects of DITPA on thyroid function tests, and central and peripheral tissues were also determined. Results demonstrated that DITPA was able to partially reverse the hypermetabolism characteristic of Mct8 deficiency.
Materials and Methods
Experimental animals
The University of Chicago Institutional Animal Care and Use Committee approved the procedures performed in the mice. Animals were housed under controlled temperature (22°C), light (6:00 am to 6:00 pm), and dark (6:00 pm to 6:00 am) conditions. They were allowed ad libitum access to water and standard chow. Wt and Mct8KO male mice were littermates and were generated as described previously (5, 11). Only males were used because AHDS manifests in the hemizygous state. Experiments were performed using different groups of 10- to 12 week-old C57BL/6J mice. All animal groups contained at least seven animals.
Metabolic cage experimental protocol
A baseline blood sample was obtained from the tail vein of each mouse before the initiation of experiments. Mice were then placed individually in an eight-cage combined, open circuit indirect calorimetry system (LabMaster system; TSE System) referred to as metabolic cages in which they were kept for 18 days. The experimental protocol and the timeline of testing procedures are illustrated in Supplemental Figure 1. Mice were adapted to the environment for 48h (d 1 and 2) before starting the baseline recording period that ended after 5 days (d 3–7). Then mice were given ip injections of 0.3 mg DITPA per 100 g body weight per day for 10 days (d 8–17) and data were recorded. Only data from the last 4 consecutive days before (d 3–7) and during DITPA (d 14–17) treatment were used for the analysis. As in humans with MCT8 deficiency (10), DITPA was given without suppression of endogenous TH synthesis. Moreover, the dose of DITPA used in this study was physiological for adult male Wt mice and slightly higher than that given to children with MCT8 deficiency (10). On day 18, mice received a DITPA injection in the morning and after 6 hours were terminally bled and euthanized by decapitation. Collected tissues were immediately frozen on dry ice and stored at −80ºC.
Measurement of serum concentrations of TSH and iodothyronines
Serum TSH was measured by RIA and total T4, T3, rT3, and DITPA were measured by liquid chromatography followed by tandem mass spectrometry as detailed previously (9, 12).
Indirect calorimetry
Indirect calorimetry was recorded and metabolic parameters, ie, food and water intake, total energy expenditure (TEE), respiratory exchange ratio (RER), and glucose consumption were calculated as described elsewhere (4).
Measurement of tissue mRNAs
To study gene expression, brain, liver, and muscle (soleus and gastrocnemius) were collected on day 18 from Wt and Mct8KO mice treated with DITPA (Supplemental Figure 1). Tissues obtained from age-matched Wt and Mct8KO mice not treated with DITPA served as controls. Total RNA was extracted and mRNAs were measured by quantitative PCR as previously described (4). The sequences of primers used to measure hairless (Hr), uncoupling protein-3 (Ucp3), and the major isoforms of myosin heavy-chain IId (Mhc IId) and I (Mhc I) in muscle tissues, deiodinase type 1 (Dio1), glutathione S-transferase (Gst2α), malic enzyme 1 (Me1), and mitochondrial glycerol-3-phosphate dehydrogenase (Gpd2) in liver and Hr and deiodinase type 3 (Dio3) in brain are provided in Supplemental Table 1. The housekeeping gene, RNA polymerase II (RpII), was used as internal control.
Statistical analysis
All results are expressed as mean ± SEM. Statistical analysis was performed using an unpaired two-tailed Student's t test (comparison in two groups) and a one-way ANOVA followed by a Newman-Keuls multiple comparison posttest (comparison among multiple groups). P ≥ .05 was considered not to be significant (NS).
Results
Effect of DITPA on serum TSH and TH concentrations (Figure 1)
Figure 1.
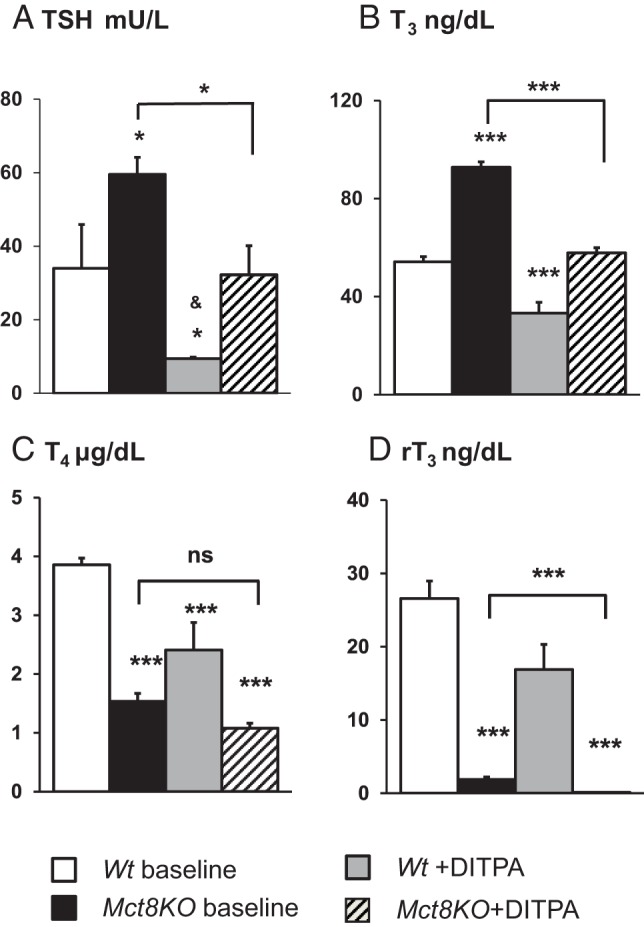
Effect of DITPA on serum TSH and TH concentrations of Wt and Mct8KO mice. Data are expressed as mean ± SE. Genotypes are indicated. Significant differences compared with Wt baseline animals are indicated above the bars. Comparisons of baseline vs DITPA-treated Mct8KO mice are indicated by horizontal lines. Asterisks represent P values by ANOVA. *, P < .05; **, P < .01; ***, P < .001; NS, not significant (P ≥ .05). &,TSH values below the limit of detection (<10 mU/L).
Serum TSH and TH concentrations were measured before placing adult mice in metabolic cages (baseline). As expected, Mct8KO mice demonstrated high serum T3, low T4 and rT3, and slightly elevated TSH (11) when compared with Wt animals. DITPA significantly lowered the TSH (P < .05) and T3 and rT3 (P < .001) in Mct8KO mice compared with same-genotype untreated animals. TSH and T3 of the DITPA-treated Mct8KO mice reached levels similar to those of the Wt animals at baseline (P = NS) (Figure 1, A and B), whereas the mean serum T4 and rT3 levels did not normalize relative to baseline (P < .001) (Figure 1, C and D). Of note, whereas the same serum concentrations of DITPA were achieved in both genotypes (Supplemental Figure 2), DITPA reduced all three iodothyronines in the Wt animals (P < .001 for T4 and T3; P < .01 for rT3), at least in part due to a suppression of the TSH (P < .05).
Metabolic state during DITPA treatment (Figure 2)
Figure 2.
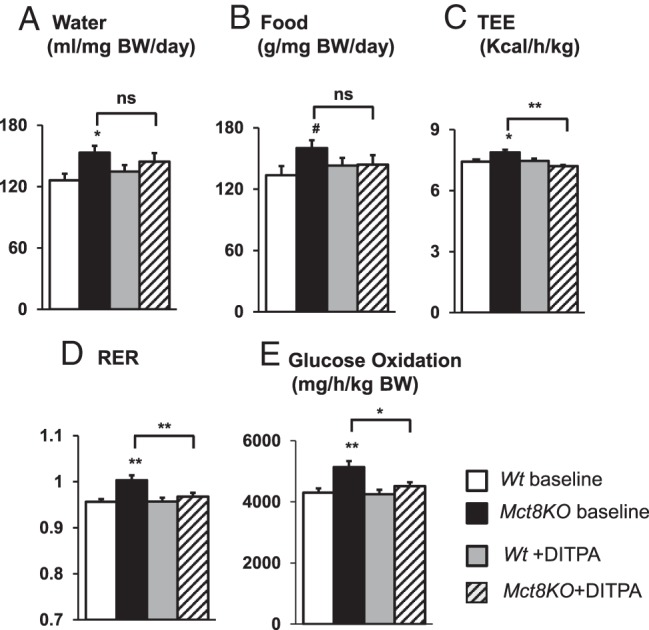
Metabolic cage study of Wt and Mct8KO mice. A, Water intake. B, Food intake. C, TEE. D, RER. E, Glucose oxidation. Genotypes are indicated. Symbols indicating statistical significances are as indicated in the legend to Figure 1. #, P < .05 is calculated by Student's t test compared with Wt baseline animals.
As previously shown (4), metabolic parameters, ie, food and water intake, TEE, RER, and glucose oxidation, had a clear diurnal rhythm in all animal groups, with the highest values registered during the dark phase (6:00 pm to 6:00 am), corresponding to the period of activity for mice (not shown). Data from the dark phase were used as representative of the metabolic state of the two groups of animals.
At baseline, Mct8KO mice had water and food intake, TEE, RER, and glucose oxidation higher than those in Wt littermates, confirming the hypermetabolic state previously reported (4). DITPA-treated Mct8KO mice showed reduced levels of most of those metabolic parameters compared with the same genotype group at baseline (P < .01 for TEE and RER, P < .05 for glucose oxidation). Furthermore, levels of food and water intake, TEE, RER, and glucose oxidation became similar to those of Wt animals at baseline (P = NS for all parameters).
Body weights were not different at baseline between the two genotypes (Wt, 25.8 ± 0.7 g, and Mct8KO, 24.7 ± 1.1 g) and did not change significantly after DITPA treatment (Wt, 25.3 ± 0.6 g, and Mct8KO, 24.6 ± 1.2 g).
TH-dependent gene expression (Figure 3 and Supplemental Figure 3)
Figure 3.
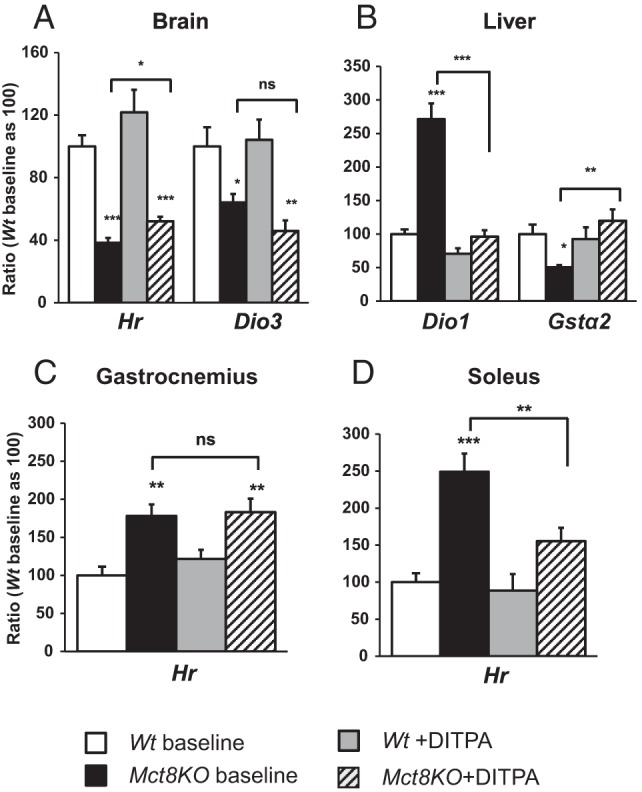
Effect of DITPA treatment on TH-dependent gene expression. A, Brain. B, Liver. C, Gastrocnemius. D, Soleus. Genotypes are indicated. Symbols indicating statistical significances are as indicated in the legend to Figure 1.
As previously reported (5, 6), Mct8KO mice showed at baseline the effects of central TH deficiency and TH excess in peripheral tissues. Indeed, in the brain, the expression of the genes Hr and Dio3, positively regulated by TH, was lower in Mct8KO mice by 60% (P < .001) and 40% (P < .05), respectively, compared with the Wt animals. In contrast, in the liver of the Mct8KO mice, the expression of the positively regulated Dio1, Me1, and Gpd2 genes was 2.7-fold, 1.7-fold, and 1.4-fold (P < .01) higher, respectively, and the negatively regulated Gstα2 gene was 50% lower, than Wt littermates. Similarly, in muscles (gastrocnemius and soleus, respectively) the expression of Hr was increased by 1.8-fold (P < .01) and 2.5-fold (P < .001) compared with the Wt animals. Moreover, the expression of Ucp3 and Mhc IId in the soleus was 1.2-fold and 2.0-fold (P < .01) higher in Mct8KO than Wt animals, whereas Mhc I, a gene negatively regulated by TH, was 24% lower (P = NS) (data not shown).
In the brain of the Mct8KO mice, when compared with the same genotype at baseline, DITPA significantly increased Hr gene expression by 1.4-fold (P < .05) but had no significant effect on Hr expression in Wt mice (P = NS). DITPA had no significant effect on Dio3 gene expression in either Mct8KO or Wt mice (P = NS). In muscles of Mct8KO mice, DITPA did not significantly change (P = NS) Hr gene expression in gastrocnemius but lowered it by 40% (P < .01) in soleus when compared with the same genotype at baseline and reached levels similar to those of Wt animals at baseline (P = NS). However, DITPA produced no significant changes in Ucp3, Mhc IId, and Mhc I of the soleus of either Mct8KO or Wt mice. In the liver of Mct8KO mice, DITPA significantly decreased Dio1, Me1, and Gpd2 gene expression by 65%, 62%, and 51%, respectively (P < .001) and significantly increased Gstα2 gene expression by 2.4-fold (P < .01) compared with levels before treatment. When compared with Wt animals at baseline, DITPA-treated Mct8KO mice showed similar levels of Dio1, Me1, and Gstα2 gene expression (P = NS), whereas the Gpd2 mRNA was significantly lower (P < .05). However, DITPA produced a significant decline (P < .001) in the expression of these same genes in the Wt animals.
Discussion
We previously demonstrated that DITPA enters cells independently of Mct8 and shows a thyromimetic action (9). We now provide evidence that DITPA improves the hypermetabolism associated with Mct8 deficiency. The current novel findings are that DITPA normalizes serum T3 concentrations and metabolic parameters in Mct8KO mice.
That DITPA decreases serum TSH in adult Mct8KO mice not pretreated with MMI was demonstrated previously (9). In the present study, we demonstrate, in addition, that DITPA treatment reduces the serum T3 levels of Mct8KO mice to nonthyrotoxic levels. The mechanisms explaining this reduction of T3 levels are as follows: 1) the suppressive effect of DITPA on TSH diminishes the thyroidal production of both T4 and T3 through the TSH-cAMP signaling pathway (13), and this effect was observed in both Mct8KO and Wt mice; and 2) DITPA availability to the central and peripheral tissues reduces the generation of T3 by deiodination through a decrease in both Dio1 and deiodinase type 2 (Dio2) activity, as shown previously (9) and confirmed in the present work by the reduction of Dio1 gene expression in liver (Figure 3B). The important decrease of serum T4 (Figure 1C) despite the reduction in T3 generation through the decrease of Dio1 expression is likely due to the decreased MCT8-dependent secretion of T4 from the thyroid gland, increased metabolism in the kidneys, and loss of TH in the urine (14, 15). The fact that DITPA was not able to reduce TSH in Mct8KO mice to the same level as that of Wt-treated animals (P < .05), despite the same serum concentrations of DITPA (Supplemental Figure 2), might be due to the additive effect of DITPA to the already higher T4 concentration in the Wt animals.
Given the recent demonstration that the high T3 levels are responsible for the hypermetabolic state of the Mct8 deficiency (4), with the normalization of T3 by DITPA treatment, we expected a normalization of the metabolic parameters in the Mct8KO animals. Indeed, water and food intake, TEE, RER, and glucose oxidation all normalized to the level of Wt mice at baseline. The normalization of those metabolic parameters was not associated with a significant difference in body weight between Mct8KO-treated mice and Wt animals at baseline. These results further confirm the previous findings showing a low effect of DITPA on metabolic activity compared with TH (16, 17) and demonstrate that the beneficial effect of DITPA on the hypermetabolism associated with Mct8 deficiency is mainly due to the reduction of T3 levels (18).
Contrary to the amelioration of the hypermetabolism, the reduction of serum T3 levels was accompanied by a less uniform response of TH-dependent gene expression in the three tissues analyzed. The thyromimetic effect of DITPA was evident in the brain of Mct8KO-treated mice in which Hr gene expression increases when compared with untreated animals of the same genotype. However, it did not reach the levels observed in Wt mice at baseline. In muscles of DITPA-treated Mct8KO mice compared with untreated animals, Hr gene expression did not change in the gastrocnemius, whereas it significantly decreased in the soleus and reached levels similar to those of Wt animals at baseline. Of note, soleus predominantly contains slow fibers and is known to be more TH responsive compared with gastrocnemius, which contains mostly fast fibers (19). However, no thyromimetic effect was observed in the TH-responsive genes Ucp3, MhcIId, and MhcI, examined in the soleus. These differences in the DITPA thyromimetic gene expression effect among tissues could be attributed to either a different mechanism of the analog's action or to a different distribution of TH receptor isoforms (20) and/or coactivators and inhibitors that modulate transcription. Another possibility could be that the decrease in serum T3 outweighs the effects of administered DITPA on peripheral tissues. These hypotheses also could be applied to the liver, in which the expression of all three genes positively regulated by TH (Dio1, Me1, and Gpd2) decreases, and Gst2α, negatively regulated, increases under DITPA treatment. Overall, DITPA at the lower dose of 0.3 mg per 100 g body weight used in this work was able to ameliorate the hepatic thyrotoxicosis of Mct8 deficiency with minimal effect on brain hypothyroidism but normalization of serum TSH concentration.
Notwithstanding the short treatment period, the present study provides new insights on the effects of DITPA in Wt animals. Indeed, in DITPA-treated Wt mice, despite the significant reduction of serum TSH and TH levels (Figure 1), no marked changes were observed either in terms of metabolic parameters or regarding the thyroid status of central and peripheral tissues compared with untreated Wt mice. Should these results be extended to humans, especially in view of a prenatal treatment of MCT8 deficiency (21), the present study suggests that DITPA could be used in heterozygous women carrying a MCT8-deficient fetus, to the benefit of the fetus (see reference 21) without producing hypermetabolism in the mother.
In conclusion, changes in T3 concentrations, metabolic parameters, and effects on TH-dependent gene expression in liver were indicative of peripheral euthyroidism in Mct8KO mice treated with DITPA. Therefore, our study provides direct evidence that DITPA is beneficial for the treatment of the metabolic manifestations of AHDS. Unfortunately, there is no obvious benefit from DITPA use in terms of effect on TH-responsive genes in the murine brain as previously demonstrated, unless the compound was given prenatally (21) or potentially given in a higher dose.
Acknowledgments
We thank Teresa Marchinkowski for measurements of the serum DITPA and iodothyronine levels in the DITPA-treated animals by liquid chromatography followed by tandem mass spectrometry.
Current address for A.M.F.: Istituto Oncologico Veneto (IOV-Istituto di Ricovero e Cura a Carattere Scientifico), Via Gattamelata, 64–35128 Padova, Italy.
Current address for R.E.W.: Department of Medicine, University of Miami Miller School of Medicine, 1120 NW 14th Street, R-761, Room 310, Miami, Florida 33136.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
This work was supported in part by Grants R37DK15070 and DK020595 from the National Institutes of Health, the Sherman Family and the Seymour J. Abrams Fund for Thyroid Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHDS
- Allan-Herndon-Dudley syndrome
- Dio1
- deiodinase type 1
- Dio2
- deiodinase type 2
- Dio3
- deiodinase type 3
- DITPA
- diiodothyropropionic acid
- MCT8
- monocarboxylate transporter 8
- Mct8KO
- Mct8-deficient mouse
- NS
- not significant
- RER
- respiratory exchange ratio
- TEE
- total energy expenditure
- TH
- thyroid hormone.
References
- 1. Allan W, Herndon CN, F. C. D Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 2. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. [DOI] [PubMed] [Google Scholar]
- 4. Di Cosmo C, Liao XH, Ye H, et al. Mct8 deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum T3 levels. Endocrinology. 2013;154:4885–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. [DOI] [PubMed] [Google Scholar]
- 6. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E. Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther. 1992;263:163–169. [PubMed] [Google Scholar]
- 8. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. [DOI] [PubMed] [Google Scholar]
- 9. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology. 2009;150:4450–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97:4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao XH, Di Cosmo C, Dumitrescu AM, et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology. 2011;152:1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrara AM, Liao XH, Gil-Ibanez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishii H, Inada M, Tanaka K, et al. Induction of outer and inner ring monodeiodinases in human thyroid gland by thyrotropin. J Clin Endocrinol Metab. 1983;57:500–505. [DOI] [PubMed] [Google Scholar]
- 14. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trajkovic-Arsic M, Visser TJ, Darras VM, et al. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology. 2010;151:802–809. [DOI] [PubMed] [Google Scholar]
- 16. Stasilli NR, Kroc RL, Meltzer RI. Antigoitrogenic and calorigenic activities of thyroxine analogues in rats. Endocrinology. 1959;64:62–82. [DOI] [PubMed] [Google Scholar]
- 17. Barker SB, Kiely CE, Jr, Klitgarrd HM, Dirks HB, Jr, Wang SC, Wawzonek S. Metabolic effects of some halogenated acrylic acid analogues of thyroxine. Endocrinology. 1951;48:70–74. [DOI] [PubMed] [Google Scholar]
- 18. Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol. 2004;18:384–401. [DOI] [PubMed] [Google Scholar]
- 19. Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18:205–216. [DOI] [PubMed] [Google Scholar]
- 20. Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003;14:85–90. [DOI] [PubMed] [Google Scholar]
- 21. Ferrara AM, Liao XH, Gil-Ibanez P, et al. Placenta passage of the thyroid hormone analog DITPA to male wild-type and Mct8-deficient mice. Endocrinology. 2014;155:4088–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]


