Abstract
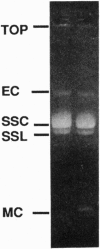
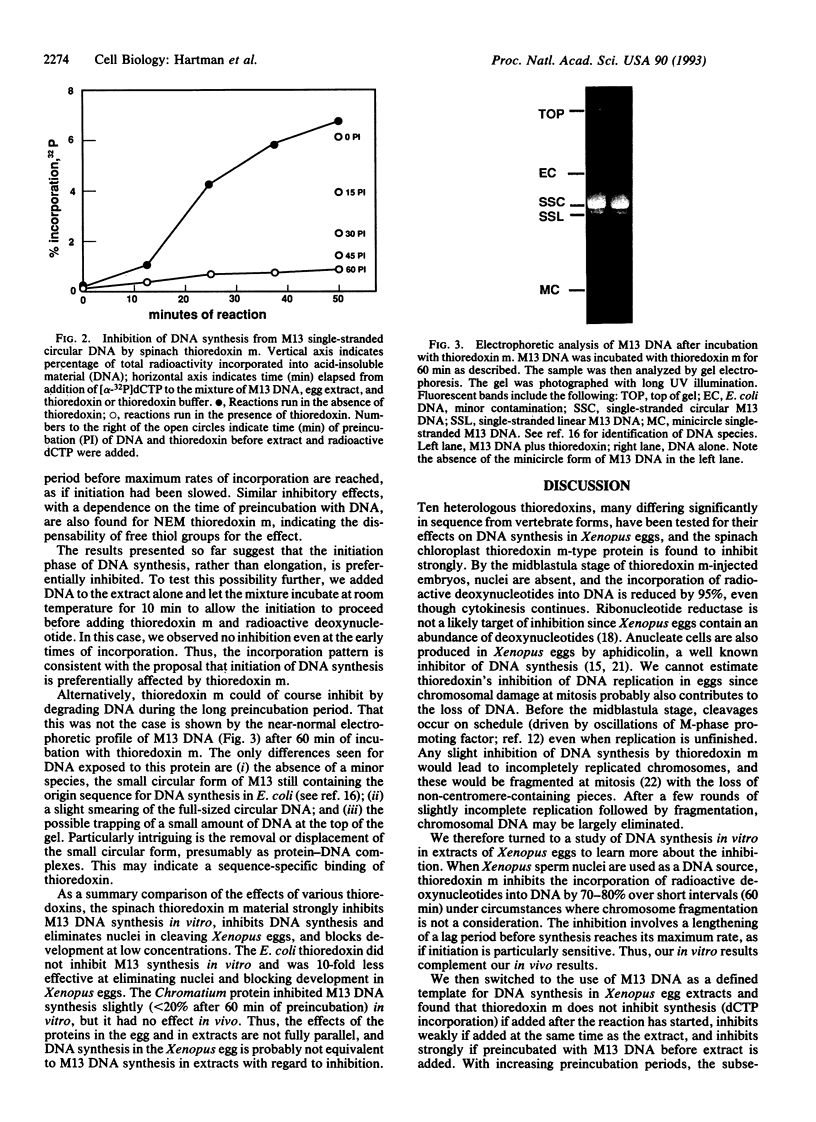
A role for thioredoxin in metazoan DNA synthesis has been assessed by injecting rapidly dividing Xenopus eggs with purified heterologous thioredoxins, which might act as inhibitors if they were to replace resident thioredoxins in some but not all reaction steps. Of 10 tested proteins, spinach chloroplast thioredoxin m is the most potent inhibitor. Eggs cleave and produce cells lacking nuclei. DNA synthesis is severely reduced. Development arrests before gastrulation. In egg extracts, thioredoxin m inhibits incorporation of radioactive dCTP into DNA of sperm nuclei and M13 phage. Inhibition exceeds 90% when thioredoxin m and M13 DNA are preincubated together. The data support the interpretation that thioredoxins normally participate in initiation of metazoan DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brachet J., Hubert E., Lievens A. The effects of -amanitin and rifampicins on amphibian egg development. Rev Suisse Zool. 1972;(Suppl):47–63. [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Dasso M., Newport J. W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990 Jun 1;61(5):811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Di Berardino M. A., Hoffner N. Origin of chromosomal abnormalities in nuclear transplants--a reevaluation of nuclear differentiation and nuclear equivalence in amphibians. Dev Biol. 1970 Oct;23(2):185–209. doi: 10.1016/0012-1606(70)90094-1. [DOI] [PubMed] [Google Scholar]
- Gan Z. R. Yeast thioredoxin genes. J Biol Chem. 1991 Jan 25;266(3):1692–1696. [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman H., Syvanen M., Buchanan B. B. Contrasting evolutionary histories of chloroplast thioredoxins f and m. Mol Biol Evol. 1990 May;7(3):247–254. doi: 10.1093/oxfordjournals.molbev.a040602. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Huber H. E., Russel M., Model P., Richardson C. C. Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem. 1986 Nov 15;261(32):15006–15012. [PubMed] [Google Scholar]
- Landström U., Løvtrup-Rein H., Løvtrup S. Control of cell division and cell differentiation by deoxynucleotides in the early embryo of Xenopus laevis. Cell Differ. 1975 Dec;4(5):313–325. doi: 10.1016/0045-6039(75)90016-0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991 May 15;266(14):9194–9202. [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin genes in Saccharomyces cerevisiae: map positions of TRX1 and TRX2. Yeast. 1992 Feb;8(2):117–120. doi: 10.1002/yea.320080206. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Newport J., Dasso M. On the coupling between DNA replication and mitosis. J Cell Sci Suppl. 1989;12:149–160. doi: 10.1242/jcs.1989.supplement_12.13. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. The role of thioredoxin in filamentous phage assembly. Construction, isolation, and characterization of mutant thioredoxins. J Biol Chem. 1986 Nov 15;261(32):14997–15005. [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland H. R., Pestell R. Q. Determination of the nucleoside triphosphate contents of eggs and oocytes of Xenopus laevis. Biochem J. 1972 Apr;127(3):597–605. doi: 10.1042/bj1270597. [DOI] [PMC free article] [PubMed] [Google Scholar]