Abstract
Controlled immune responses to infection and injury involve complex molecular signaling networks with coordinated and often opposing actions. Eicosanoids and related bioactive lipid mediators derived from polyunsaturated fatty acids constitute a major bioactive lipid network, which is among the most complex and challenging pathways to map in a physiological context. Eicosanoid signaling, similar to cytokine signaling and inflammasome formation, has been viewed as primarily a pro-inflammatory component of the innate immune response; however, recent advances in lipidomics have helped to elucidate unique eicosanoids and related docosanoids with anti-inflammatory and pro-resolution functions. This has advanced our overall understanding of the inflammatory response and its therapeutic implications. The induction of a pro- and anti-inflammatory eicosanoid storm through the activation of inflammatory receptors by infectious agents is reviewed.
Eicosanoids are locally acting bioactive signaling lipids derived from arachidonic acid and related polyunsaturated fatty acids (PUFAs) that regulate a diverse set of homeostatic and inflammatory processes 1,2 linked to numerous diseases. Inhibiting the formation or receptor-mediated actions of classical eicosanoids (that is prostaglandins and leukotrienes) by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), by the leukotriene inhibitor zileuton, and by leukotriene receptor antagonists during inflammation remains a prevailing strategy to alleviate pain, swelling, fever and asthmatic conditions. However, pleiotropic effects are becoming increasingly appreciated for most eicosanoids and their related docosanoids.
Hundreds of structurally and stereochemically distinct eicosanoid species can be made from arachidonic acid and other ω6-derived PUFAs such as dihomo-γ-linolenic acid (DGLA) whose origin is the 18-carbon essential fatty acid linoleic acid as well as ω3-derived PUFAs from α-linolenic acid (ALA) including eicosapentaenoic acid (EPA) which can be further elongated to docosapentaenoic acid (DPA) and further desaturated to docosahexaenoic acid (DHA). Although the physiological roles of only a few of the eicosanoid and related docosanoid species are well understood, some of the agonists and receptors that activate inflammasome formation and the cytokine storm that accompanies infection3,4 appear to also initiate the release of arachidonic acid and related PUFAs, resulting in an eicosanoid storm5.
Mass spectrometric-based lipidomic profiling6 is now being used to identify, monitor and quantify hundreds of distinct eicosanoid and related PUFA species7 that appear to be involved in infection and inflammation, as well as in its resolution8. These lipidomics approaches, sometimes referred to as “eicosadomics”, facilitate the identification of the ‘eicosadome’ and can be used to accurately determine global changes in cellular lipids during specific physiological processes. Lipidomics is currently being used to more effectively screen for potential disease biomarkers9, and to provide scientists with a mechanistic understanding of eicosanoid biosynthesis and signaling at the cellular and multicellular tissue level. Integrating the latest genomics (transcriptomics), proteomics and lipidomics of pro-inflammatory and pro-resolution eicosanoid and related PUFA production should lead to new insights on infection and inflammation.
In this review, we will provide and discuss our current understanding of cellular eicosanoid metabolism2,10–12 and signaling, as well as their physiological consequences, including homeostatic, pro-inflammatory and resolving functions. We will also consider the importance of subcellular enzyme compartmentalization and stimulatory contexts involved in the regulation of eicosanoid metabolism5,13–16. Although the production of cytokines is considered a hallmark of infectious disease, we will highlight the importance of the accompanying temporal production of both pro-inflammatory and anti-inflammatory (pro-resolution) eicosanoids and related docosanoids during the initiation and resolution of infection. The recent progress in lipidomic monitoring of arachidonic acid and related PUFA metabolism provides us with the comprehensive perspective needed to tackle the challenges of therapeutic targeting of eicosanoid pathways, which range from traditional enzyme inhibition17,18 with NSAIDs and prostanoid mimetics, to “natural” fish oil ω3 fatty acid supplementation19,20.
Eicosanoid biosynthesis and function
Eicosanoids arise from the oxidation of arachidonic acid and related PUFAs by cyclooxygenase21 (COX), lipoxygenase22 (LOX), and cytochrome P450 (CYP) enzymes, or via non-enzymatic free radical mechanisms (Figure 1; Supplementary information (Figures 1, 2, 3). Although eicosanoids are most frequently associated with inflammation, they also have homeostatic functions (Box 1).
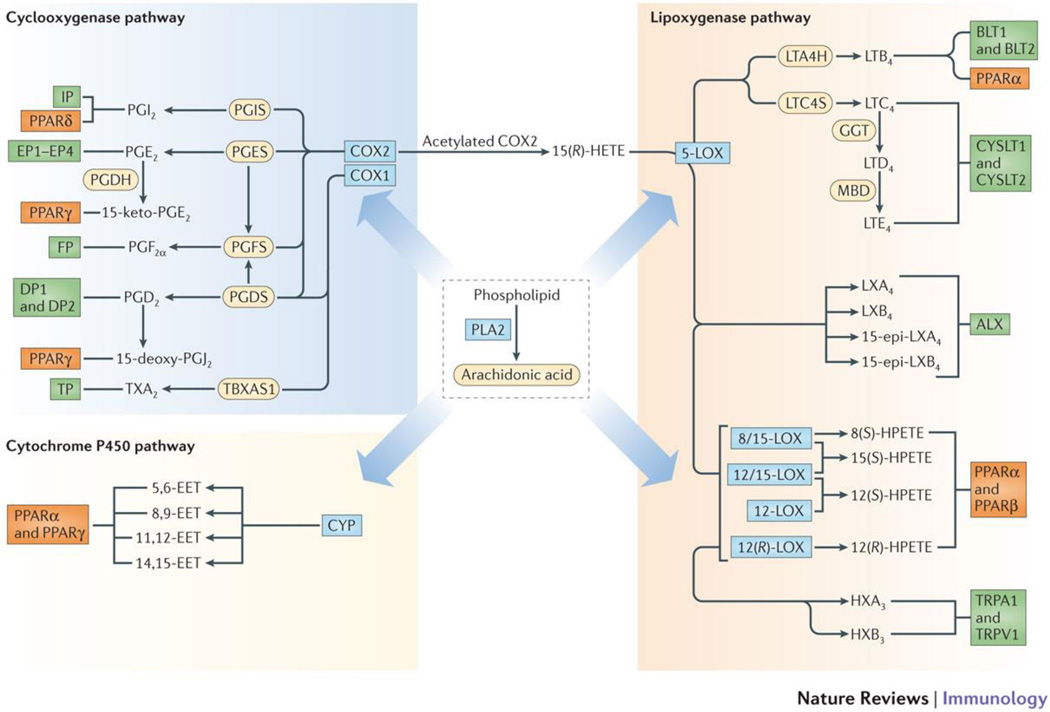
Figure 1. Eicosanoid biosynthesis and receptor signaling.
Lipidomic view of phospholipase A2 (PLA2), cyclooxygenase-1 and -2 (COX1/2), 5-lipoxygenase (5-LOX), 8-, 12-, and 15-lipoxygenases (8/12/15-LOX) and cytochrome P450 epoxyhydrolase (CYP) pathways of eicosanoid biosynthesis derived from arachidonic acid. Downstream enzymes are shown as yellow ovals followed by eicosanoid species and their receptors as green boxes. The peroxisome-proliferator activating receptors (PPARs), indicated as orange boxes, that are potentially activated by the eicosanoids are also shown.. The CYP ω-hydroxylase pathway and lipid species derived from other fatty acids are not shown. Abbreviations: COX, cyclooxygenase; CYP, cytochrome P450; EET, epoxyeicosatrienoic acid; GGT, gamma-glutamyl transferase; HETE, hydroxyeicosatetraenoic acid; HX, hepoxilin; LOX, lipoxygenase; LT, leukotriene; LTAH, LTA4 hydrolase; LTCS, LTC4 synthase; LX, lipoxin; MBD, membrane bound dipeptidase; PLA2, phospholipase A2; PPAR, peroxisome proliferator-activated receptor; TRPA1, transient receptor potential ankyrin 1; TRPV1, transient receptor potential vanilloid 1; and TX, thromboxane.
Box 1. Homeostatic Function of Eicosanoids.
Classic eicosanoids play important homeostatic roles ranging from regulating vascular leakage and barrier formation, to protecting mucosal integrity in the stomach and regulating platelet aggregation. For example, the COX1-derived metabolite TxA2 is produced by platelets and some other cell types and plays a homeostatic role in platelet aggregation, as well as a role in the platelet response to injury. By contrast, COX2-derived PGI2 produced by endothelial cells inhibits platelet aggregation and promotes vasodilation.
Unique therapeutic side effects have been attributed to blocking COX1 or COX2 isoforms: COX1-specific inhibitors can cause stomach toxicity and delays in blood clotting, while COX2-specific inhibitors (coxibs) largely avoid stomach toxicity and promote platelet aggregation. Although coxibs potently inhibit COX2, which is upregulated during inflammation, the resulting vascular imbalance of PGI2 and TxA2 has been associated with an increase in myocardial infarction and stroke events in coxibs-treated patients. The most potent and specific COX-2 inhibitor, rofecoxib, was removed from world markets a decade ago because of cardiovascular risk.
The significance and complexity of eicosanoid signaling in critical physiological processes has recently been evaluated in detail73,74. As a result, low doses of aspirin are now commonly prescribed as cardioprotection agents, and limit thromboxane formation by COX1 in platelets without inhibiting COX2-mediated PGI2 formation by endothelial cells. One approach that could potentially circumvent this is to inhibit the major inflammatory PGE2 synthase (mPGES1), which is coupled to (and upregulated with) COX2 in many tissues during inflammation . However, this approach is questionable in light of the several anti-inflammatory effects that have now been attributed to PGE2.
Fish oil-derived ω3 fatty acid supplementation is also commonly prescribed for the treatment of various inflammatory ailments and for cardioprotection. The clearest evidence for this reasoning is the ability of ω3 EPA and DHA to inhibit arachidonic acid metabolism by COX-1 (but less so by COX2)20, similar to low dose aspirin. However, the cardioprotective effects of ω3 fatty acids may be obscured by the widespread use of statins, which appear to have anti-inflammatory properties.
The vast collection of lipids produced by these eicosanoid and related biosynthetic pathways is the starting point for in vivo eicosanoid signaling networks in cells through their cognate receptors (Figure 1; Table 1). Cells are highly selective as to the specific eicosanoids they make but the amounts are altered by the activation state and the physiological conditions of the specific tissues in which they reside. For example, macrophages produce an array of arachidonic acid-derived prostaglandins in response to Toll-like receptor (TLR) stimuli but the specific prostaglandins and their quantities produced after stimulation differ dramatically between macrophages of different tissue origin and isolation method — for example, thioglycollate-elicited peritoneal macrophages and bone marrow-derived macrophages, as well as between primary macrophages and a transformed macrophage cell line23. Furthermore, the expression of receptors for specific eicosanoids is also cell and tissue specific.
Table 1.
Eicosanoid pathways, mediators, receptors, and their physiological roles.
| Major Pathway | Mediator | Receptor | Physiological responses and biochemical effects | Reference |
|---|---|---|---|---|
| COX | PGE2 | EP1, EP2, EP3 and EP4 |
Vasodilation and vascular leakage; hyperalgesia; fever; IL-10 ↑ TNF-α ↓; PMN eicosanoid class switching |
25,44,79–86 |
| 15-keto- PGE2 |
PPARγ | Adipogenesis ↑; mucus retention ↓; bicarbonate secretion ↑ |
51,67 | |
| PGD2 | DP1 | Mast cell maturation, vasodilation, neuroprotection |
87–89 | |
| DP2 | Eosinophil recruitment and allergic response ↑ | 90,91 | ||
| 15d PGJ2 | PPAR-γ | Adipogenesis ↑ | 92,93 | |
| PGF2α | FP | Uterine, vascular and respiratory smooth muscle contraction |
94 | |
| Intraocular pressure ↓ | 95 | |||
| PGI2 | IP | Platelet aggregation ↓; hyperalgesia; vasodilation; IL-10 ↑ TNF-α ↓ |
25,83,96–98 | |
| PPAR-δ | Embryo implantation | 99,100 | ||
| TxA2 | TP | Platelet aggregation ↑; vasoconstriction; T-cell activation ↓ |
101,102,103,104 | |
| 5-LOX | LTB4 | BLT1 | Neutrophil recruitment and vascular leakage | 26,27,80 |
| BLT2 | Enhanced epithelial barrier function | 105 | ||
| PPAR-α | Negative feedback of LTB4 biosynthesis | 106,107 | ||
| LTC4, LTD4, LTE4 |
CysLT1, CysLT2 |
Bronchoconstriction; vascular leakage; neutrophil extravasation |
26,108 | |
| 8-,12-,15-LOX | HpETEs, HETEs and diHETEs |
TRPV1 | Hyperalgesia | 109 |
| PPAR-α,γ | Fatty acid translocase/CD36 ↑ | 28,29 | ||
| CYP | EETs | PPAR-α,γ | Vasodilation; antihyperalgesia; COX-2 expression ↓ | 110–112 |
| (LOX-LOX; COX-LOX) | HXA3 HXB3 |
TRPA1 | Hyperalgesia | 113 |
| TRPV1 | ||||
| ? | Mucosal epithelial-directed neutrophil recruitment | 114–116 | ||
| LXA4, 15- epi-LXA4, LXB4,15- epi-LXB4 |
ALX | Neutrophil recruitment ↓; Efferocytosis ↑ | 8 | |
| Membrane-esterified eicosanoids |
PL-HETE | ? | Modulation of signal transduction; lipoxin precursor storage |
117,118 |
| PL-PG | ? | TLR response ↓ | 119 | |
| Non-enzymatic | IsoP | ? | Function not established | |
| AA-NO2 | COX-1 inhibition | 120 |
Studies of eicosanoids in inflammation have mainly focused on the signaling pathways activated by lipids produced by the COX enzymes, since they collectively elicit the cardinal signs of inflammation, including heat, swelling, redness, pain, and loss of function24. Understanding the physiological roles of these lipids is complicated by the differing roles they play in different tissues; for example, the binding of prostaglandin E2 (PGE2) to its cognate GPCR receptors, the EP receptor family, in neurons causes pain associated with inflammation, whereas autocrine EP signaling by PGE2 in macrophages (and possibly other leukocytes) can downregulate TNF and up-regulate IL-10 production25, leading to a net reduction in inflammatory signals.
While eicosanoids of the COX pathway control a wide spectrum of processes, the 5-LOX pathway (Figure 1) is more specifically operative during inflammation to promote bronchoconstriction26 and leukocyte recruitment to sites of tissue damage27. While the functions of 5-LOX-initiated leukotrienes in asthma and allergy are well understood, definitive biological functions for the intermediate metabolites 8-hydroperoxyeicosatetraenoic acid (8-HPETE), 12-HPETE and 15-HPETE, as well as their hydroxyeicosatetraenoic acid (HETE) products have not been identified, but some may be ligands for peroxisome proliferator-activating receptor-α (PPARα)28 and PPARγ29, which induce anti-inflammatory effects and modulate liver × receptor (LXR; which regulates cholesterol homeostasis). Thus, metabolites of specific LOXs may be anti-inflammatory, but the interconnections between LOXs and COXs during inflammation confound simple strategies to inhibit individual pathways for therapeutic gain. Despite a direct role for many of the LOX-derived mono-hydroxylated fatty acids produced by a single enzyme, in combination, numerous families of more complex di- and tri-hydroxylated fatty acids (specialized pro-resolving mediators; SPMS) are produced by LOX and COX enzymes that possess remarkably potent and specific binding to GPCRs that accelerate bacterial clearance, neutrophil clearance, and turn on anti-inflammatory cytokine programs.
The CYP pathway (Figure 1) comprises a large number of enzymes that contain a heme iron, and many CYPs are expressed in the liver, but also in other tissues where they inactivate and eliminate toxins and metabolites. The most upstream CYPs in the eicosanoid pathway convert arachidonic acid to epoxyeicosatrienoic acids (EETs) or ω-HETEs2, which are thought to be anti-inflammatory, whereas the downstream diHETEs (which can be formed by soluble epoxide hydrolase (sEH)) are thought to be pro-inflammatory or inactive. Bioactive functions for the ω-HETEs are not well understood, as no cognate receptor or second messenger has been identified to date2,30. It is also important to clarify that epoxidation is essential for the formation of all SPMs and thus, in addition to LOX enzymes, some CYPs and sEH may be partially responsible for the synthesis of SPMs. Clearly, additional work is needed to identify the physiological roles of all the eicosanoids and related PUFAs as well their receptors, and in particular their pro-inflammatory and anti-inflammatory functions.Systematic characterization of prostaglandin and leukotriene structures, biosynthetic pathways, natural receptors and biological functions have resulted in the production of new drug classes in the form of analogs, as well as receptor and enzyme inhibitors. These therapies that target eicosanoids have afforded substantial improvements for treating inflammatory symptoms including swelling and pain, although more chronic diseases, like arthritis and atherosclerosis, and more life-threatening pathogenic diseases are largely unaffected by the inhibition of eicosanoids. As many additional mediators derived from PUFAs during infection and inflammation have continued to be identified and characterized, there is now a greater focus on determining which of these lipid mediators enhance specific aspects of host protection and which mediate a return to homeostasis. Along with regulation of anti-inflammatory responses, specific eicosanoid mediators have been associated with enhancing pathogen clearance, neutrophil clearance, and antibody-mediated immune responses.
Prostaglandins and leukotrienes are produced rapidly after inflammatory initiation and promote the early induction of oedema from postcapillary venules. While this occurs naturally to mediate complement and leukocyte recruitment to an acute site of injury, the eicosanoid-mediated response can be overwhelming and life threatening to the host during septic or toxic shock. For example, prostaglandins produced via COX1 during inflammasome activation have recently been shown to contribute to excessive vascular leakage and lethality in mice and constitute part of a major pro-inflammatory eicosanoid response to infection31. Also, dual inhibition of COX2 and 5-LOX with flavocoxid has been shown to improve survival in mice after caecal ligation and puncture32, with a reduction in the levels of circulating PGE2 and LTB4, an increase in LXA4 expression, and a reduction in lung and liver myeloperoxidase (MPO) levels.
Cellular control of eicosanoid biosynthesis
Phospholipase A2
The majority of eicosanoid metabolism requires free arachidonic acid, but it is primarily stored in an esterified form. Phospholipase A2 (PLA2) enzymes are critical for increasing the levels of free arachidonic acid for metabolism and eicosanoid biosynthesis under most physiological conditions but particularly following inflammatory cell activation. Three members of the PLA2 superfamily have been most implicated in cellular eicosanoid production: cytosolic calcium-dependent PLA2 (cPLA2), cytosolic calcium-independent PLA2 (iPLA2), and secreted PLA2 (sPLA2). iPLA2 is thought to be the primary PLA2 involved in most daily cellular functions, particularly membrane homeostasis and remodeling16,33 and constitutively generating a low level of free fatty acids with relatively minimal specificity for the particular esterified fatty acid, but may include release of arachidonic acid. cPLA2 is largely inactive during homeostatic conditions; but when activated by TLR, purinergic (a Ca2+ mobilizing agonist), and other receptors initiated during an infection or inflammatory response , it translocates to the perinuclear and ER membranes, where it hydrolyses arachidonic acid-containing phospholipids, leading to pro-inflammatory eicosanoids as well as the related ω3 PUFAs EPA, DPA and DHA that are precursors for anti-inflammatory resolvins20. sPLA2 is an inducible enzyme that augments cPLA2 function to control the magnitude and duration of elevated free fatty acid levels including arachidonic acid34.
As the most upstream regulators of eicosanoid biosynthesis, PLA2s regulate the eicosanoid response during different phases of an inflammatory response, and likely do so through various receptor-mediated cues (see below). Indeed, the activation of cPLA2 was shown to be responsible for the storm of pro-inflammatory eicosanoids that accompanies inflammasome formation induced by systemic flagellin or anthrax lethal toxin31. This study highlights the fact that a specific branch of the eicosanoid pathway, namely prostaglandins through COX-1, is life threatening when produced systemically rather than acutely. The specialized delivery of autocoids to an infectious site can therefore breakdown during severe infections and supports the use of COX-1 NSAIDs during severe traumas and infections, while chronic diseases require more refined therapies.
Functional enzyme coupling
Since free fatty acids and oxygenated eicosanoids and related PUFAs can rapidly diffuse out of the cell1,35 or become reincorporated within membrane lipids, and since activated PLA2s are concentrated at specific organelle interfaces, free arachidonic acid and other fatty acid concentrations exist as gradients that restrict most downstream eicosanoid metabolism only to sites in very close proximity to PLA2 activity. For this reason, the co-localization of downstream enzymes in relation to the immediate substrate pool is critical for effective bioactivity and resultant signaling by eicosanoids. Similarly, the close proximity of different cell types to one another is required to create intercellular communication pathways for distinct metabolons and eicosanoid profiles at the site of inflammatory episodes.
The COX pathway has received the most attention regarding the tight co-localization of PLA2 enzymes with downstream enzymes within a cell, defined as functional coupling14 (Figure 2). Since cPLA2 predominantly translocates to the perinuclear membrane and ER, downstream enzymes that are also expressed at, or that can migrate to, these sites can preferentially participate in the metabolism of arachidonic acid. Functional coupling of thromboxane A synthase (TXAS) and PGD synthase (PGDS) with COX1, which all partially co-localize to the ER, has been shown to preferentially produce the eicosanoids thromboxane A2 (TxA2) and PGD2 during short-term stimulation of rat peritoneal macrophages36. Furthermore, microsomal PGE synthase 1 (mPGES1) and PGIS are coupled with COX2 at the perinuclear membrane, and preferentially produce PGE2 and PGI2 during the later phase of the LPS-induced response (3–24 hours of stimulation)36,37.
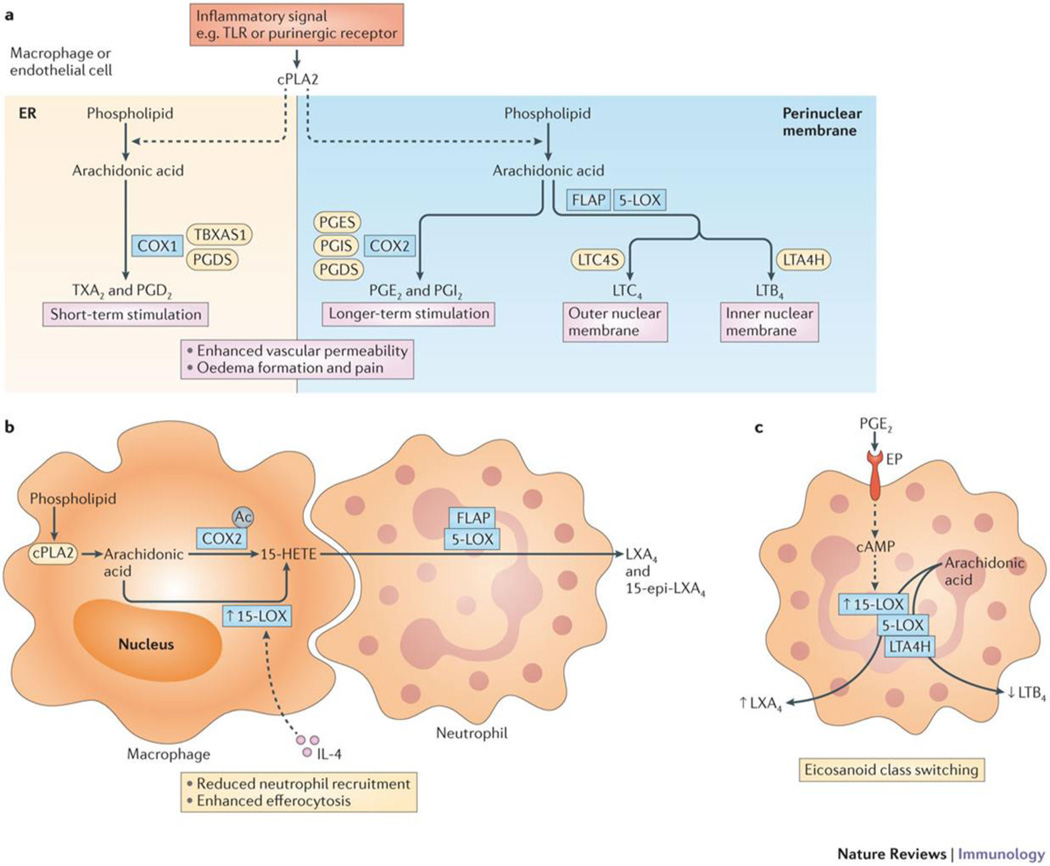
Figure 2. Enzyme functional coupling, transcellular biosynthesis, and eicosanoid class switching.
(a) Functional coupling of cPLA2 with metabolons comprised of COX-1 or COX-2 and different prostaglandin or thromboxane synthases; 5-LOX coupled to LTAH or LTCS; PGES represents microsomal PGES-1 and PGDS represents hematopoietic PGDS, while other isoforms are not shown; coupling schemes are not completely insulated thus products may derive from alternate routes depending on cell type and degree of cell activation. Functions of these products collectively describe the cardinal signs of inflammation. (b) Transcellular biosynthesis of lipoxins from arachidonic acid (AA) involving a cell expressing COX-2 acetylated by aspirin and/or 15-LOX that is upregulated by IL-4, both of which produce the same intermediate 15-HETE, which diffuses or is transported to an adjacent cell where it becomes incorporated in and subsequently released from membrane phospholipids by cPLA2 (not shown). This 15-HETE is converted by 5-LOX (with its functionally coupled FLAP) to LXA4/15-epi-LXA4, thereby initiating eicosanoid class switching. Coordinate macrophage-neutrophil lipoxin biosynthesis and related SPM production (as depicted) provides putative mechanisms to enhance efferocytosis and redirection of neutrophils to the vasculature. (c) Eicosanoid class-switching involves receptor mediated reprogramming of biosynthetic enzyme expression or activation. A neutrophil is depicted being activated by PGE2 binding of a cyclic adenosine monophosphate (cAMP) increasing EP receptor that increases 15-LOX expression, which shunts arachidonic acid (AA) synthesis from LTB4 production by 5-LOX/LTAH to LXA4 by 15-LOX/5-LOX.
Another example is the 5-LOX pathway, where 5-lipoxygenase-activating protein (FLAP; which appears to be constitutively present and facilitates transfer of arachidonic acid substrate to the 5-LOX active site) is responsible for coupling of cPLA2 to 5-LOX at the perinuclear membrane. Note, both cPLA2 and 5-LOX are Ca2+-dependent. Furthermore, the activation-dependent formation of leukotriene C4 (LTC4) and LTB4 is dependent on distinct complexes of LTC4 synthase (LTCS) with 5-LOX at the outer nuclear membrane and LTA4 hydrolase (LTAH) with 5-LOX at the inner nuclear membrane, respectively38, giving rise to two potent but distinct inflammatory mediators.
Understanding these coupling issues underlie the challenge of modulating eicosanoid biosynthesis and function during inflammation because of the dynamic changes in eicosanoid enzyme expression and their subcellular trafficking. A number of these dynamic changes have been recently documented by combining experimental and computational approaches39: in this study essentially complete lipidomics monitoring of eicosanoids was used with kinetic modeling to accurately predict the fluxes and temporal changes in the quantities and composition of cyclooxygenase and lipoxygenase metabolites (based on enzyme transcript expression and functional coupling) in macrophages during stimulation of the TLR-4 and/or the P2×7 purinergic receptor, as well as in the presence of COX1- and COX2-specific inhibitors39. This study demonstrated differential coupling of COX1 and COX2 with downstream synthases and proves that fluxomics modeling can predict inflammatory mediator levels in macrophages as a function of time, even with synergistic activation by TLR-4 (priming) followed by a P2×7 agonist at a later time. Thus, the systems view of eicosanoids provided by lipidomics is improving our ability to predict outcomes of specific enzyme inhibition at the cellular level, and potentially at the physiological level as a computational tool to predict outcomes of drug candidates.
Transcellular eicosanoid metabolism
The biosynthesis of eicosanoids and related lipid species is dramatically increased when cells are exposed to inflammatory stimuli that activate PLA2 and certain downstream enzymes. Often pathway enzymes are robustly upregulated to enhance fatty acid metabolism and eicosanoid biosynthesis; the clearest examples are COX2 and mPGES1. Most cell types produce negligible amounts of eicosanoids in the steady state, and when activated produce only a small number of distinct eicosanoids, which is dictated by the specific enzymes that are expressed in that cell type23. This physiological compartmentalization of the expression of specific eicosanoid biosynthetic enzymes to specific cell types (e.g., macrophages) greatly limits the production of eicosanoids and related lipids. During an immune response, this compartmentalization of specific enzymes can be overcome by the convergence of different cell types at an inflammatory site. Such conditions allow for the synthesis of more complex molecules by cell–cell transfer of intermediate eicosanoid metabolites and/or the metabolism of intermediate substrates, a process known as transcellular biosynthesis40. Transcellular biosynthesis has a role in the synthesis of several COX and 5-LOX metabolites and is particularly important in the formation of ‘complex’ eicosanoids formed by multiple LOX activities.
In isolation, a number of cells express primarily one or two eicosanoid-producing enzymes (e.g. 12-LOX in platelets, 5-LOX in neutrophils, and 15-LOX/COX-2 in epithelial/endothelial cells); however combinations of these cells cooperatively produce lipoxins that are relevant in inflammatory contexts41, while other SPMS are produced through the same or similar mechanisms. The production of epimers of lipoxin A4 (LXA4) and LXB4 (aspirin-triggered lipoxins) that are less prone to degradation by prostaglandin dehydrogenase (PGDH) was first demonstrated using co-cultures of neutrophils with endothelial cells, in which the upstream metabolite 5(S)-HpETE produced by 5-LOX in neutrophils was converted to lipoxins by acetylated COX2 that upregulated 15(R)-HpETE, 15-epi-LXA4 and 15(R)-LXB4 production in the endothelial cells by treating them with aspirin42. The conversion occurs because acetylation of the active site of COX-2 fills its pocket to inhibit the normal COX activity producing prostaglandins thereby promoting 15(R)-HpETE synthesis rather than 15(S)-HpETE; thus aspirin provides a novel activity not possible with other NSAIDs (which bind but do not acetylate COX). Lipoxins promote the resolution of inflammation in humans, which is increased by low-dose aspirin43, and in mouse models of arthritis through the upregulation of 15-LOX activity, as well as by PGE2, both of which are derived from COX244,45.
Recently, it was found that activation of two or more receptors, such as TLR and purinergic receptors, which are known to induce inflammasome formation, also triggers lipoxin synthesis in an immune cell5. In addition, cPLA2 regulates eicosanoid class switching — that is, switch the class of eicosanoids produced by a cell from pro-inflammatory prostaglandins to anti-inflammatory lipoxins — in a parallel process to inflammasome formation and caspase activation 5 (Figure 3). These data suggest that the same receptor-mediated events that lead to inflammasome formation might also trigger an eicosanoid storm that in addition to pro-inflammatory mediators can include mediators that trigger the initiation of resolution.
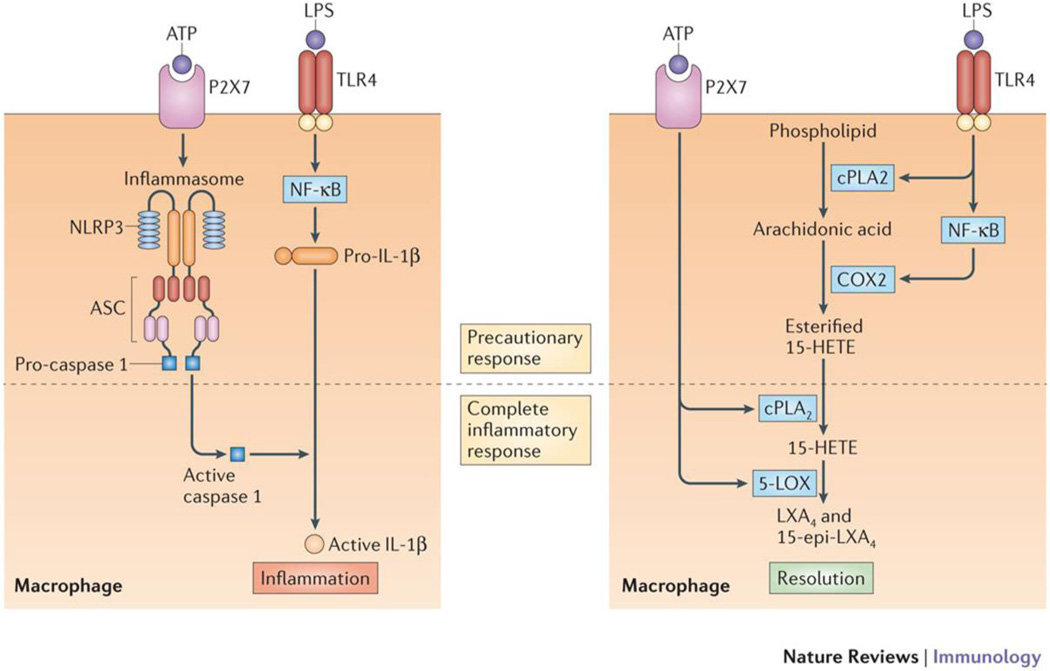
Figure 3. Inflammasome formation and caspase activation parallels lipoxin formation for a complete inflammatory response.
TLR4-mediated priming of macrophages by LPS induces pro-IL-1β production via activation of NF-κB. Subsequent purinergic P2X7 receptor engagement by ATP initiates the process of caspase-1 activation through inflammasome initiation, which converts pro-IL-1β to IL-1β, but a parallel pathway in macrophages exists to initiate the resolution of inflammation. TLR4-mediated priming also activates cPLA2 and induces COX2 production via NF-κB; this results in the release of arachidonic acid and conversion to 15-HETE, which becomes esterified into phospholipids. Subsequent purinergic P2X7 receptor engagement activates cPLA2 and 5-LOX in Ca2+-dependent processes, leading to the release of 15-HETE from membrane phospholipids and conversion of the 15-HETE to lipoxin. The first step in this process is enhanced by aspirin which causes complete eicosanoid class switching from pro-inflammatory prostaglandins to anti-inflammatory lipoxins.
This receptor-mediated single cell formation of lipoxins likely provides a means for cells including macrophages to efficiently enhance production without the need for multiple cell types via transcellular routes, largely through co-localization of numerous enzymes, including COX2, on particular membranes such as the endoplasmic reticulum and nuclear envelope5. Thus, aspirin not only enhances formation of the lipoxin precursor (15(R)-HETE), but also enables concentrated positioning of 15(R)-HETE in close proximity to activated 5-LOX for maximal conversion in a receptor-activated cell.
Further exploration of the specific association of these enzymes will likely provide answers to other related and important questions, for instance, how precursors of SPMs are efficiently transferred via microparticles to other cells for processing. Microparticles shed from neutrophils have been shown to inhibit pro-inflammatory actions of macrophages activated by zymosan or LPS46. More recently, these microparticles were found to contain the DHA derived SPM precursors 14-hydroxydocosahexaenoic acid (14-HDHA) and 17-HDHA and could enhance efferocytosis of apoptotic neutrophils47. If SPM production involves PLA2-mediated mechanisms, for example a role for cPLA2 in releasing arachidonic acid for lipoxin synthesis during inflammasome activation or releasing DHA for SPM synthesis, the effectiveness of nanomedicines that mimic microparticles could be enhanced. Of note, a “resolving sPLA2” has been identified as having a role in the production of SPMs and the resolution of skin inflammation48 and provides a potential candidate for action on microparticles for efficient delivery of SPM precursors to final lipoxygenation pathways.
Lipidomic perspective on the inflammatory process
The cell-specific compartmentalization of eicosanoid pathway enzymes in macrophages, neutrophils and other immune cells can be exploited using lipidomic strategies to assess the homeostatic and inflammatory environment. An elegant example of this approach comes from a study that demonstrates how 12/15-LOX in resident peritoneal macrophages regulates the phenotype of leukocytes entering and exiting the peritoneal cavity during sterile inflammation. Here, the disappearance and reappearance of resident macrophages, as well as the appearance and clearance of infiltrating macrophages, corresponded to the steady fall and eventual rise in the levels of 12-HETE and other LOX products49. Thus, by using temporal lipidomics or fluxomics39 monitoring of specific eicosanoids, one can follow the progression of leukocyte trafficking as the infection progresses.
While the presence of specific leukocytes is commonly used as an indicator of disease severity, lipidomic profiles can potentially be used in addition to cell-specific protein markers as a more complete readout of phenotype and disease severity, but with the added benefit of assessing dietary, metabolic and biosynthetic factors (the quantities of eicosanoids and related molecules derived from EPA, DHA, and other fatty acids). A recent study using a macrophage-specific knockout of NCoR, which is a co-repressor associated with NF-κB-specific genes, showed decreased inflammatory markers and insulin sensitization in obese mice compared with control mice50. Using quantitative profiling, this study showed that the expression of omega-3 fatty acids, which are the precursors of many anti-inflammatory and pro-resolution mediators, were increased, a phenotype that protects against high fat diets. This illustrates the use of quantitative lipidomics to monitor disease phenotypes and severity.
The complexity of eicosanoid biosynthesis is layered in the dynamic expression and intracellular compartmentalization of the biosynthetic machinery that is time-, condition-, and cell type-dependent. Generally, the upregulation of COX2 and mPGES1 begins early during inflammatory programs in cells such as macrophages, endothelial cells and dendritic cells to form PGE2, whereas IL-4- and PGE2-mediated increases in 12/15-LOX begin at later stages in the activation of macrophages, neutrophils and several other cell types that result in increased formation of SPMs that stimulate resolution. The “inactivation” of prostaglandins by downstream enzymes may also function to produce anti-inflammatory PPAR agonists51, and similar downstream mechanisms may also exist for other lipid mediators that are less well understood. This model of progression is tightly regulated and will require further dissection in specific disease states to identify specific intervention strategies.
Eicosanoids in infection and inflammation
A recurring theme in the case of Mycobacterium tuberculosis infections is that PGE2 inhibits necrotic cell death in macrophages resulting in the promotion of pathogen resistance and host protection, while a specific level of TNFα dictated by a delicate balance between LTB4 and LXA4 is critical for maximal control of infection52,53. Although the protective effect of PGE2 in M. tuberculosis infection has been linked to the inhibition of the type I IFN response54, PGE2 has been shown to compromise immunity to influenza A virus by inhibiting macrophage antigen presentation and T cell immunity55. While the roles of different eicosanoid species are emerging as enhancers of various aspects of inflammation and innate immunity in general, skewing the profile of these mediators to effectively treat specific pathogens is likely and will require multiple strategies in line with personalized medicine.
Genetic variability also plays a role in how the host is affected by pathogenic exposure. For example, DBA mice are resistant to developing arthritis associated with Lyme disease following Borrelia borgderferi infection while C3H mice exhibit prolonged inflammation and delayed resolution despite a similar control of bacterial loads; these differences have been profiled with lipidomics56. Resistant DBA mice expressed high levels of protectin 1 (PD1), resolvin D1 (RvD1), hepoxilin A3 (HXA3), PGE2, and 15-keto PGE2 levels in ankle joints while C3H mice expressed high LTE4 levels. This eicosanoid expression pattern was largely sustained for the duration of the disease course, suggesting a defect in eicosanoid class-switching and in anti-inflammatory and pro-resolving mediator synthesis in Lyme disease-susceptible C3H mice. These results are in line with previous studies demonstrating that inflammation resulting from B. borgderferi fails to resolve with 5-LOX57 or COX258 ablation; loss of 5-LOX in particular led to decreased macrophage-mediated efferocytosis and phagocytosis of spirochetes by macrophages and neutrophils.
An example of the importance of eicosanoids and their metabolism in the sequelae of disease-associated inflammation comes from transcriptomic analysis of a cystic fibrosis disease phenotype, where subsequent lipidomic analysis of eicosanoids helped to identify the PGE2 metabolite 15-keto-PGE2 as an endogenous PPARγ agonist and a potential pharmacological therapeutic51 that suggests an important anti-inflammatory action of prostaglandin inactivation. Interestingly, another reported PPARγ agonist, 15-deoxy-PGJ2, has recently been shown to inhibit peritoneal NLRP3-induced IL-1β secretion and leukocyte recruitment59, although the direct mechanism has yet to be identified. The resolvins RvD1 and RvD5 have recently been shown to reduce antibiotic requirements for microbial clearance in mice60 through their ability to synergize with protectins and increase bacterial phagocytosis in human macrophages (Box 2); these data suggest that therapies derived from natural pro-resolving responses can be applied to counteract antibiotic resistance, suggesting that eicosanoid related signaling has bactericidal implications for infection. Eicosanoids like lipoxins, along with these DHA-derived SPMs, appear to be enhancers of microbial clearance that arise after microbicidal components including complement and neutrophils have been deployed partly through the actions of prostaglandins and leukotrienes.
Box 2. Resolution Functions of Eicosanoids (SPMs).
Inflammatory exudates initially serve to neutralize pathogens and local injury; however, this results in an increased presence of leukocytes and pro-inflammatory mediators that can potentially propagate tissue damage and inflammation if not resolved in a timely manner. Lipoxins comprise a family of tri-hydroxy eicosanoids that include LXA4 and LXB4 (as well as aspirin-triggered 15-epi-LXA4 and 15-epi-LXB4)42,75, which inhibit neutrophil recruitment via activation of the LXA4 receptor (ALX–FPR2). Subsequently, this family of specialized pro-resolving mediators (SPMs)8 has grown to include di- and tri-hydroxylated fatty acids derived from the omega-3 fish oils EPA and DHA that have been isolated from inflammatory exudates and leukocytes. SPMs now include the EPA-derived resolvins (RvE1, RvE2, and RvE3) and DHA-derived resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6) as well as protectin D1 (PD1) and macrophage mediators in resolving inflammation (maresins) (MaR1 and MaR2), also derived from DHA. PD1 has also been termed neuroprotectin D1 (NPD1) since it was isolated from and protects retinal pigment epithelial cells from oxidative stress-induced apoptosis76 and displays neuroprotective properties in brain ischemia-reperfusion77. In addition to ALX–FPR2, these mediators activate BLT1, ChemR23 and G protein-coupled receptor 32 (GPR32), which appear to recognize both the stereochemically specific triene/tetraene and di-/tri-hydroxy motifs within the fatty acid backbone. Collectively, SPMs have been shown to enhance bacterial clearance60, down-regulate pro-inflammatory cytokines, and enhance clearance of apoptotic neutrophils (a process termed efferocytosis). Most recently, a sulfido-conjugate family of mediators derived from DHA was identified in mouse inflammatory exudates (maresin conjugates in tissue regeneration; MCTR that promote tissue regeneration in planaria and possess the classic actions of SPMs78.
Recent studies of influenza virus infections in mice have integrated transcriptomics, proteomics of cytokine formation, and lipidomics with disease progression to isolate promising therapeutics61 and biomarkers62 such as protectin D1, one of the many pro-resolution mediators derived from omega-3 fatty acids in fish oil (Box 2). Samples from human patients with high clinical scores and increased levels of cytokines and chemokines also contained significantly higher percentages of eicosanoid metabolites from the LOX and CYP pathways, including those derived from EPA and DHA, compared with healthy individuals. The amounts of individual lipid mediators, including PGE2, LTE4, and 17-hydroxy-DHA (17-HDHA; a 15-LOX derived product of DHA which is a stable form of 17-HpDHA, the precursor to all D-series resolvins and to PD1), were increased in nasopharyngeal lavages from those patients with high cytokine levels and severe symptoms of influenza infection compared to patients with low and medium cytokine production and symptom levels62. More recently, 17-HDHA has been shown to increase the levels of antigen-specific antibody levels when administered to mice in combination with H1N1 influenza virus-derived hemagglutinin, or with ovalbumin, which resulted in increased resistance to live H1N1 infection63. These findings suggest that specific lipid-derived components of potential or existing adjuvants, such as in the oil of Freund’s adjuvant, could be further refined for therapeutics. While some eicosanoids and related species are established mediators that progressively promote the neutralization and clearance of pathogens and trafficking/clearance of leukocytes, understanding which of these molecules and how they are biosynthesized and signal to link innate and adaptive immunity remains unclear, but the studies just mentioned highlight some potential pathways that may be utilized for signaling during a deluge of lipid mediators.
Therapeutic Interventions
Drugs that target eicosanoid pathways (Figure 4) have been used for over a century; aspirin is the oldest of the numerous effective NSAIDS that have been marketed. The discovery that NSAIDs inhibited prostaglandin synthesis through the inhibition of COX17 have led to the development of COX1- and COX2-specific NSAIDs and these molecules have helped to determine the homeostatic importance of this enzyme family (Box 1). In addition, the 5-LOX pathway is a major drug target for treatment of allergic and asthmatic conditions (Figure 4) and the 5-LOX inhibitor zileuton can inhibit all downstream metabolism. Furthermore, leukotriene receptor antagonists (LTRAs), such as montelukast, inhibit the actions of cysteinyl leukotriene receptor 1 (cysLT1), including bronchoconstriction during inflammatory pulmonary events64. The inhibition of COX enzymes by NSAIDs results in a switch to arachidonic acid oxidation by 5-LOX and increased signaling by cysLTs, and therefore combining 5-LOX inhibitors and/or LTRAs with NSAIDs may be more effective. Other LOX pathways as well as CYP pathways, including soluble epoxide hydrolase (sEH) (Figure 4), are also being pursued as drug targets for various inflammatory diseases.
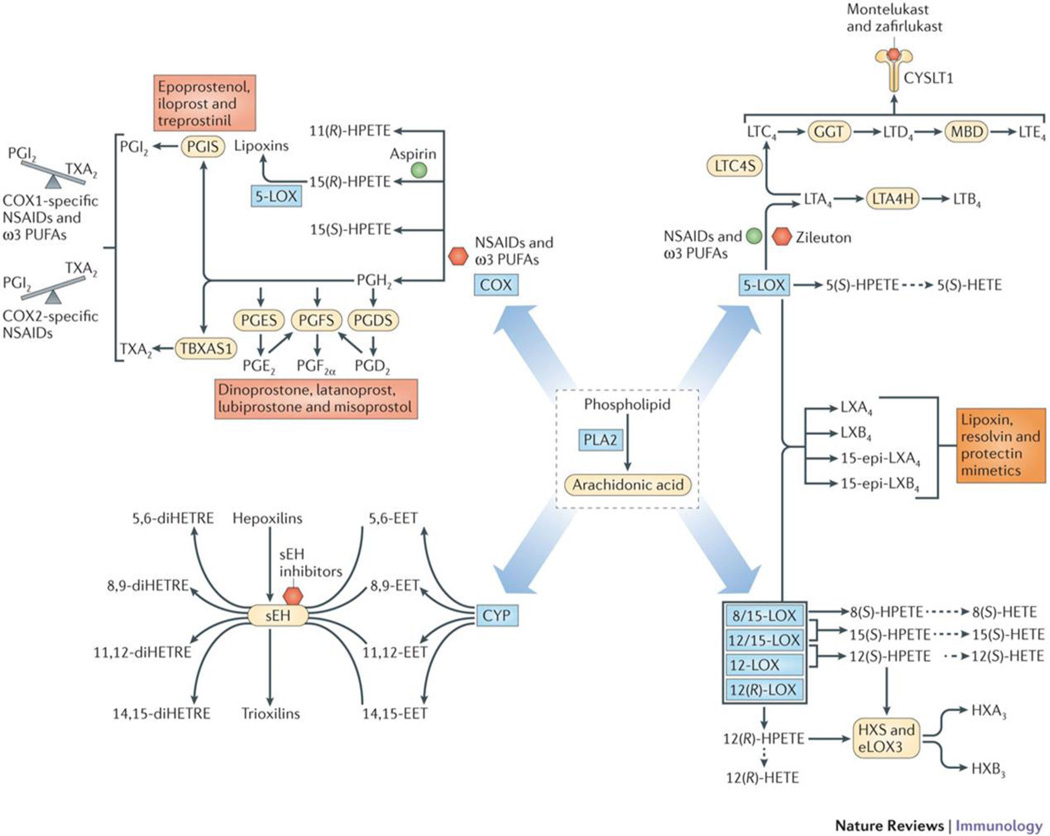
Figure 4. Therapeutics targeting eicosanoid pathways.
Enzymes in the cyclooxygenase pathway generate prostaglandins, thromboxanes, and lipoxins; lipoxygenase pathway enzymes generate leukotrienes, HETEs, and hepoxilins; and cytochrome P450 epoxyhydrolase pathway enzymes generate epoxides and dihydroxy polyunsaturated fatty acids (PUFA). All of the pathways have pharmacological intervention points including enzyme inhibitors and receptor antagonists (red hexagon), product enhancers (green circle) and mimetics (red box). NSAIDs and ω3 PUFAs inhibit COX formation of each product derived from AA (red hexagon), with the exception of one NSAID, aspirin, which enhances COX-2 formation of 15(R)-HETE (green circle) that can be converted to lipoxins by 5-LOX. COX-1-specific NSAIDS and ω3 PUFAs shift the vascular balance to higher PGI2, whereas COX-2 specific NSAIDs shift the vascular balance to higher TxA2 (gray), which are in opposite directions due to coupling of platelet COX-1 with TXAS and endothelial COX-2 with PGIS; analogs of prostaglandins are used clinically to mimic endogenous bioactivity (red box). Montelukast and zafirlukast specifically inhibit activation of cysLT1 (red hexagon). Zileuton inhibits 5-LOX conversion of arachidonic acid (red hexagon), but NSAIDs and ω3 PUFAs can increase conversion (green circle) via shunting of AA from inhibited COX-1 and COX-2. Inhibitors of sEH reduce inactivation of EETs as well as hepoxilins (red hexagon). Lipoxin, resolvin, and protectin mimetics (orange box) are being developed for treatment of ocular, periodontal, and cardiovascular diseases.
The most upstream target for eicosanoid mediators would be PLA2, but clinical trials for cardiovascular indications using a sPLA2 inhibitor [clinicaltrials.gov/, identifier: NCT00743925 and NCT01130246] or a lipoprotein-associated PLA2 inhibitor [clinicaltrials.gov/, identifier: NCT00799903 and NCT01000727] were unsuccessful, and a clinical trial for a cPLA2 inhibitor [clinicaltrials.gov/, identifier: NCT00396955] was stopped because it exhibited the same side effect profile as non-selective NSAIDs16. Future studies with plasma lipidomics monitoring should be able to identify “problematic” side products at an earlier stage in the drug discovery process, especially given the challenges of drug discovery when there is a complex network of enzymes involved. The use of mass spectrometry to help define the global outcomes of lipid profiles in relation to different inflammatory responses is likely to refine the specific eicosanoid targets and reveal which to avoid. For example, while inflammatory bowel disease is an inflammatory condition and standard treatments include the long-term use of salicylate derivatives, aspirin and other traditional NSAIDs are contraindicated.
Though drugs that target COX and 5-LOX pathways comprise arguably the most widely consumed drug class, they are not actually able to stop or resolve innate immune responses alone. This is understandable because cytokines, chemokines, and other bioactive mediators are also critical players in immune signaling. Furthermore, while this review has focused on eicosanoids produced from arachidonic acid, many of the enzymes discussed are also capable of acting on the essential fatty acids containing 18 carbons (linoleic and linolenic acid) as well as on more unsaturated and longer fatty acids (docosanoids) including fish oil-derived ω3 fatty acids (EPA, DPA, and DHA). Recent UPLC/MS-MS technology65 can accurately quantify over 150 eicosanoid and related metabolites (if present) in a 5 minute metabolomics analysis from less than 50 microliters of human plasma and this number is increasing as new standards become available. These new methodologies have replaced older methodologies, such as ELISA and traditional mass spectrometry, where specificity and quantitation can be compromised and these new methodologies are generally carried out on urine or blood plasma.
Novel bioactive oxygenated lipids can also be produced by the traditional “pro-inflammatory” enzymes including COX, LOX, and CYP, and some of these can promote resolution8. Also, several fatty acids, nitrated fatty acids, eicosanoids and related DPA- or DHA-derived oxygenated lipids appear to be agonists of the anti-inflammatory nuclear receptors PPARα, PPARδ, and PPARγ28,29,66–69. None of these PPAR-activating eicosanoids and related species display the same very low nanomolar potencies possessed by prostaglandins, leukotrienes and lipoxins for their natural receptors, questioning the biological relevance of their proposed anti-inflammatory actions1. These criticisms have been voiced again more recently70 and also in reference to the reported in vivo levels of transcellularly formed lipoxins and other SPMs8; more work is clearly needed to resolve these issues.
However, the number of identified PPAR agonists and SPMs continue to mount, multiplying the potential for their in vivo relevance. Thus, the ability to measure nearly all eicosanoids and related species should become increasingly focused on their collective bioactivity rather than as distinct species working in isolation. However, current analytical strategies cannot determine the precise concentration of specific eicosanoids at the receptor level or at confined inflammatory sites in real time, where eicosanoids are rapidly formed and inactivated. Hopefully, future developments in lipidomics such as secondary ion mass spectrometry (SIMS-C60), which allows for profiling of lipids at the single-cell level71, may provide a useful platform to eventually solve the current debate over the presence of and biological relevancies of specific eicosanoids in specific cells during infection and inflammation. Recent studies on the spatial organization of lipids in the human retina and optic nerve by matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry72 identified individual phospholipids, some containing DHA, in different layers of the human retina, suggesting that with future technological advances local pools of released fatty acids and eicosanoid products may be detectable in relevant human tissues under inflammatory conditions.
Conclusion
The overall impacts of the eicosanoid and cytokine storms on host survival are likely context dependent and will require greater focus on age, diet, and genetic variation in addition to the specific pathogenic assault and severity of trauma. Refined application of lipidomics methodologies and similar approaches will be critical to advance the understanding of eicosanoids in health and disease. With the ability that lipidomics brings to now assess changes in the majority of eicosanoid species simultaneously, determining both the pro-inflammatory and anti-inflammatory contributions of eicosanoids in specific diseases will likely accelerate.
The expanded view of eicosanoid signaling since the introduction of lipidomics is remarkably more complex, but the potential for improving therapeutic design is promising. Advancing treatment beyond NSAIDs may need to be more focused on isolating and correcting deficiencies in bioactive eicosanoids, rather than blunting entire pathways. The ability to now analyze hundreds of eicosanoids and related lipid species, alongside the handful of well-characterized prostaglandins and leukotrienes, provides a wealth of possibilities to understand and develop novel treatments for inflammatory and metabolic conditions. A better understanding of the cytokine storm and its integration with the eicosanoid storm that accompanies classic inflammation and its resolution should provide new insights leading to novel strategies for the understanding and treatment of infection and inflammation.
Supplementary Material
Acknowledgements
E.A.D. thanks the US National Institutes of Health (NIH) for National Institute of General Medical Sciences (NIGMS) grants RO1 GM20501-39 and the LIPID MAPS “Glue” grant U54 GM069338 for support of research and scholarly activity and T32 GM007752 for support of graduate training for P.C.N.
GLOSSARY
- Eicosanoids
Bioactive oxygenated polyunsaturated fatty acids containing twenty carbons.
- Non-steroidal anti-inflammatory drugs (NSAIDs)
Non-steroidal anti-inflammatory drugs, such as aspirin and naproxen, are used to ablate the inflammatory response. They work by inhibiting cyclooxygenase-1 and −2 (COX1 and COX2) blocking the biosynthesis of prostaglandins including thromboxane.
- Docosanoids
Bioactive oxygenated polyunsaturated fatty acids containing twenty-two carbons.
- Inflammasome
A molecular complex of several proteins that upon assembly cleaves pro-IL-1 and pro-IL-18, thereby producing active forms for these pro-inflammatory cytokines.
- Toll-like receptor (TLR)
A family of pattern-recognition receptors that recognize conserved molecules from pathogens, such as lipopolysaccharide, that initiate innate immune responses.
- Peroxisome proliferator-activated receptors (PPARs)
Nuclear receptors that participate in the regulation of cellular metabolism and differentiation. PPARs have anti-inflammatory properties by limiting the availability of limited cofactors or blocking promoters of pro-inflammatory genes.
- Specialized pro-resolving mediators (SPMs)
Eicosanoids and docosanoids that promote efferocytosis, the process by which dying cells are engulfed and removed by macrophages in an immunologically inert manner, and also inhibit neutrophil diapedesis and pro-inflammatory cytokine expression. SPMs include lipoxins, resolvins, protectins, maresins, and the newly discovered MCTR sulfido-conjugate series.
- Purinergic receptors
Family of plasma-membrane molecules that are involved in several known cellular functions, such as vascular reactivity, apoptosis and cytokine secretion.
- Resolvins
Lipid mediators that are induced in the resolution phase following acute inflammation. They are synthesized from the essential omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
- Metabolons
Complex of multiple enzymes (bound or in close proximity) that coordinately synthesize eicosanoids and docosanoids which is often dependent on receptor activation and cell-cell transfer of intermediates.
- Eicosanoid class switching
A process by which pro-inflammatory eicosanoid synthesis changes to anti-inflammatory or pro-resolution eicosanoid and docosanoid synthesis.
- Fluxomics
The study of the flux or the change in the concentration of products/metabolites in a biosynthetic pathway as a function of time in a cell.
- Lipoxins
A class of eicosanoids, composed of LXA4 and LXB4, that are produced by lipoxygenase-mediated metabolism of arachidonic acid. They are trihydroxytetraene-containing structures with potent biological activities in the resolution of inflammation.
- Caecal ligation and puncture
An experimental model of peritonitis in rodents, in which the caecum is ligated and then punctured, thereby forming a small hole. This leads to leakage of intestinal bacteria into the peritoneal cavity and subsequent peritoneal infection.
- Myeloperoxidase
A peroxidase enzyme that is most highly expressed by neutrophils. It is a lysosomal protein that is stored in azurophilic granules of neutrophils. Myeloperoxidase produces hypochlorous acid from hydrogen peroxide and chloride ions during the respiratory burst in neutrophils.
- Necrotic cell death
A form of cell death that frequently results from toxic injury, hypoxia or stress. Necrosis involves the loss of cell integrity and release of cell contents into the interstitium. This form of cell death usually occurs together with inflammation. Depending on the context, the self antigens that are released by necrosis could become immunogenic.
- Lyme disease
A disease caused by the bacterium Borrelia burgdorferi or other Borrelia spp. that is transmitted to humans via the bites of infected blacklegged ticks. Symptoms can include skin rash, fever, fatigue, headache, muscle pain, stiff neck and swelling of the knee and other large joints. Most cases can be successfully treated with antibiotics.
- Efferocytosis
The phagocytic clearance of apoptotic cells (from the Latin ‘effero’, meaning to take to the grave or bury) before they undergo secondary necrosis. The process usually triggers an anti-inflammatory response.
- UPLC/MS-MS technology
Ultra-high performance liquid chromatography combined with tandem mass spectrometry for chemical separation and quantitative analysis
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics An integrated omics analysis of eicosanoid biology. Journal of lipid research. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunological reviews. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 4.Latz E, Xiao TS, Stutz A. Activation regulation of the inflammasomes. Nature reviews. Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harkewicz R, Dennis EA. Applications of mass spectrometry to lipids and membranes. Annual review of biochemistry. 2011;80:301–325. doi: 10.1146/annurev-biochem-060409-092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumlao DS, Buczynski MW, Norris PC, Harkewicz R, Dennis EA. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochimica et biophysica acta. 2011;1811:724–736. doi: 10.1016/j.bbalip.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quehenberger O, Dennis EA. The human plasma lipidome. The New England journal of medicine. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada M, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. The Journal of biological chemistry. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 11.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways, environmental carcinogenesis Nature reviews. Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 12.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. The Journal of biological chemistry. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 13.Sala A, Folco G, Murphy RC. Transcellular biosynthesis of eicosanoids. Pharmacological reports : PR. 2010;62:503–510. doi: 10.1016/s1734-1140(10)70306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno N, Takegoshi Y, Kamei D, Kudo I, Murakami M. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochemical and biophysical research communications. 2005;338:70–76. doi: 10.1016/j.bbrc.2005.08.152. [DOI] [PubMed] [Google Scholar]
- 15.Newcomer ME, Gilbert NC. Location, location, location: compartmentalization of early events in leukotriene biosynthesis. The Journal of biological chemistry. 2010;285:25109–25114. doi: 10.1074/jbc.R110.125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical reviews. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vane JR. Biomedicine Back to an aspirin a day? Science. 2002;296:474–475. doi: 10.1126/science.1071702. [DOI] [PubMed] [Google Scholar]
- 18.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Molecular interventions. 2006;6:199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 19.Chen C. COX-2’s new role in inflammation. Nature chemical biology. 2010;6:401–402. doi: 10.1038/nchembio.375. [DOI] [PubMed] [Google Scholar]
- 20.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Progress in lipid research. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Norris PC, Reichart D, Dumlao DS, Glass CK, Dennis EA. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. Journal of leukocyte biology. 2011;90:563–574. doi: 10.1189/jlb.0311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators insights into the resolution of inflammation. Nature reviews. Immunology. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 25.Shinomiya S, et al. Regulation of TNFalpha and interleukin-10 production by prostaglandins I(2) and E(2): studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochemical pharmacology. 2001;61:1153–1160. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 26.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 27.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami K, Ide T, Suzuki M, Mochizuki T, Kadowaki T. Evidence for direct binding of fatty acids and eicosanoids to human peroxisome proliferators-activated receptor alpha. Biochemical and biophysical research communications. 1999;260:609–613. doi: 10.1006/bbrc.1999.0951. [DOI] [PubMed] [Google Scholar]
- 29.Huang JT, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 30.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological reviews. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 31.von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bitto A, et al. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care. 2012;16:R32. doi: 10.1186/1364-8535-16-R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Balsinde J, Balboa MA, Dennis EA. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. The Journal of biological chemistry. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick FA, Soberman R. Regulated formation of eicosanoids. The Journal of clinical investigation. 2001;107:1347–1351. doi: 10.1172/JCI13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buczynski MW, et al. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. The Journal of biological chemistry. 2007;282:22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 36.Naraba H, et al. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- 37.Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. The Journal of biological chemistry. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 38.Mandal AK, et al. The nuclear membrane organization of leukotriene synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kihara Y, et al. Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases. Biophysical journal. 2014;106:966–975. doi: 10.1016/j.bpj.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacological reviews. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 41.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins, leukotrienes, and essential fatty acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 44.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 45.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 47.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miki Y, et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. The Journal of experimental medicine. 2013;210:1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dioszeghy V, et al. 12/15-Lipoxygenase regulates the inflammatory response to bacterial products in vivo. J Immunol. 2008;181:6514–6524. doi: 10.4049/jimmunol.181.9.6514. [DOI] [PubMed] [Google Scholar]
- 50.Li P, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harmon GS, et al. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nature medicine. 2010;16:313–318. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer-Barber KD, Sher A. Cytokine and lipid mediator networks in tuberculosis. Immunological reviews. 2015;264:264–275. doi: 10.1111/imr.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer-Barber KD, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. The Journal of biological chemistry. 2009;284:21599–21612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blaho VA, Zhang Y, Hughes-Hanks JM, Brown CR. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J Immunol. 2011;186:3076–3084. doi: 10.4049/jimmunol.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaho VA, Mitchell WJ, Brown CR. Arthritis develops but fails to resolve during inhibition of cyclooxygenase 2 in a murine model of Lyme disease. Arthritis and rheumatism. 2008;58:1485–1495. doi: 10.1002/art.23371. [DOI] [PubMed] [Google Scholar]
- 59.Maier NK, Leppla SH, Moayeri M. The Cyclopentenone Prostaglandin 15d–PGJ2 Inhibits the NLRP1 and NLRP3 Inflammasomes. J Immunol. 2015;194:2776–2785. doi: 10.4049/jimmunol.1401611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiang N, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morita M, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 62.Tam VC, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–227. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramon S, et al. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 2014;193:6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aharony D. Pharmacology of leukotriene receptor antagonists. American journal of respiratory and critical care medicine. 1998;157:S214–S218. discussion S218-219, S247-218. [PubMed] [Google Scholar]
- 65.Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. Journal of chromatography. A. 2014;1359:60–69. doi: 10.1016/j.chroma.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chou WL, et al. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. The Journal of biological chemistry. 2007;282:18162–18172. doi: 10.1074/jbc.M702289200. [DOI] [PubMed] [Google Scholar]
- 68.Baker PR, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. The Journal of biological chemistry. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groeger AL, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature chemical biology. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passarelli MK, Ewing AG, Winograd N. Single-cell lipidomics: characterizing and imaging lipids on the surface of individual Aplysia californica neurons with cluster secondary ion mass spectrometry. Analytical chemistry. 2013;85:2231–2238. doi: 10.1021/ac303038j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zemski Berry KA, Gordon WC, Murphy RC, Bazan NG. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. Journal of lipid research. 2014;55:504–515. doi: 10.1194/jlr.M044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fitzgerald DJ, Fitzgerald GA. Historical lessons in translational medicine: cyclooxygenase inhibition and P2Y12 antagonism. Circulation research. 2013;112:174–194. doi: 10.1161/CIRCRESAHA.111.300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patrono C, Baigent C. Nonsteroidal anti-inflammatory drugs and the heart. Circulation. 2014;129:907–916. doi: 10.1161/CIRCULATIONAHA.113.004480. [DOI] [PubMed] [Google Scholar]
- 75.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcheselli VL, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. The Journal of biological chemistry. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 78.Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4753–E4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morimoto K, et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol. 2014;192:1130–1137. doi: 10.4049/jimmunol.1300290. [DOI] [PubMed] [Google Scholar]
- 80.Bray MA, Cunningham FM, Ford-Hutchinson AW, Smith MJ. Leukotriene B4: a mediator of vascular permeability. British journal of pharmacology. 1981;72:483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minami T, et al. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. British journal of pharmacology. 2001;133:438–444. doi: 10.1038/sj.bjp.0704092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin CR, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. The Journal of pharmacology and experimental therapeutics. 2006;319:1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 83.Moriyama T, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nature neuroscience. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 85.Treffkorn L, Scheibe R, Maruyama T, Dieter P. PGE2 exerts its effect on the LPS-induced release of TNF-alpha, ET-1, IL-1alpha, IL-6 and IL-10 via the EP2 and EP4 receptor in rat liver macrophages. Prostaglandins & other lipid mediators. 2004;74:113–123. doi: 10.1016/j.prostaglandins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng K, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. Journal of neurochemistry. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- 89.Taketomi Y, et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nature immunology. 2013;14:554–563. doi: 10.1038/ni.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spik I, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 91.Schratl P, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 92.Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 93.Forman BM, et al. 15-Deoxy-delta ,p14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 94.Basu S. Novel cyclooxygenase-catalyzed bioactive prostaglandin F2alpha from physiology to new principles in inflammation. Medicinal research reviews. 2007;27:435–468. doi: 10.1002/med.20098. [DOI] [PubMed] [Google Scholar]
- 95.Woodward DF, et al. Prostaglandin F2 alpha effects on intraocular pressure negatively correlate with FP-receptor stimulation. Investigative ophthalmology & visual science. 1989;30:1838–1842. [PubMed] [Google Scholar]
- 96.Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall An explanation for the anti-thrombotic properties of vascular endothelium. Thrombosis research. 1977;11:323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- 97.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 98.Weiss HJ, Turitto VT. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979;53:244–250. [PubMed] [Google Scholar]
- 99.Lim H, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes & development. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta RA, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartwig JH, et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 102.Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- 103.Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circulation research. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 104.Kabashima K, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nature immunology. 2003;4:694–701. doi: 10.1038/ni943. [DOI] [PubMed] [Google Scholar]
- 105.Iizuka Y, et al. Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory colitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:4678–4690. doi: 10.1096/fj.10-165050. [DOI] [PubMed] [Google Scholar]
- 106.Narala VR, et al. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. The Journal of biological chemistry. 2010;285:22067–22074. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devchand PR, et al. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 108.Moos MP, et al. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:4352–4362. doi: 10.1096/fj.08-113274. [DOI] [PubMed] [Google Scholar]
- 109.Hwang SW, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ng VY, et al. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins & other lipid mediators. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gregus AM, et al. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6721–6726. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mrsny RJ, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 116.Nigam S, Zafiriou MP, Deva R, Ciccoli R, Roux-Van der Merwe R. Structure, biochemistry and biology of hepoxilins: an update. The FEBS journal. 2007;274:3503–3512. doi: 10.1111/j.1742-4658.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 117.Hammond VJ, O’Donnell VB. Esterified eicosanoids: generation, characterization and function. Biochimica et biophysica acta. 2012;1818:2403–2412. doi: 10.1016/j.bbamem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rouzer CA, Marnett LJ. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. The Journal of biological chemistry. 2008;283:8065–8069. doi: 10.1074/jbc.R800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trostchansky A, et al. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. The Journal of biological chemistry. 2011;286:12891–12900. doi: 10.1074/jbc.M110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.