Abstract
Acrolein (Acr) is a ubiquitous environmental contaminant; it also can be generated endogenously by lipid peroxidation. Acr contains a carbonyl group and an olefinic double bond; it can react with many cellular molecules including amino acids, proteins and nucleic acids. In this review article we focus on updating information regarding: 1) Acr induced DNA damage and methods of detection, 2) repair of Acr-DNA damage, 3) mutagenicity of Acr-DNA adducts, 4) sequence specificity and methylation effect on Acr-DNA adduct formation, and 5) the role of Acr in human cancer. We have found that Acr can inhibit DNA repair and induces mutagenic Acr-dG adducts and that the binding spectrum of Acr in the p53 gene in normal human bronchial epithelial cells is similar to the p53 mutational spectrum in lung cancer. Since Acr-DNA adduct has been identified in human lung tissue, and that Acr causes bladder cancer in human and rat models, we conclude that Acr is a major lung and bladder carcinogen, and its carcinogenicity arises via induction of DNA damage and inhibition of DNA repair.
Keywords: Acrolein, DNA Damage, Lung Cancer, Mutation, Repair
Introduction
Acrolein (Acr) is a ubiquitous environmental contaminant. It comes from different sources, including industrial, food, cigarette smoke, incomplete combustion, and cooking [1]. Acr also can be generated endogenously, it is a by-product of lipid peroxidation [2]. Although the levels of Acr in normal cells and in cells under oxidative stress have yet to be established, the interactions of Acr with many cellular molecules including amino acids, proteins and nucleic acids have been extensively studied [3–5]. The most common reaction between Acr and cellular molecules is the Michael addition with and without subsequent cyclization [3–6]. It is conceivable that Acr interactions might profoundly affect the functions of these modified molecules. The chemistry and biology of Acr and Acr-DNA adducts have been thoroughly examined in many excellent articles [1–7] This review focuses on updating information regarding: 1) Acr induced DNA damage and methods of detection, 2) repair of Acr-DNA damage, 3) mutagenicity of Acr-DNA adducts, 4) the role of sequence context and cytosine methylation on Acr-DNA adduct formation, and 5) the role of Acr in human cancer. Most of the information was obtained from experimental results that were accomplished by treating cells with exogenous Acr, since little is known about the consequences of elevated endogenous Acr.
Acrolein distribution in human cells
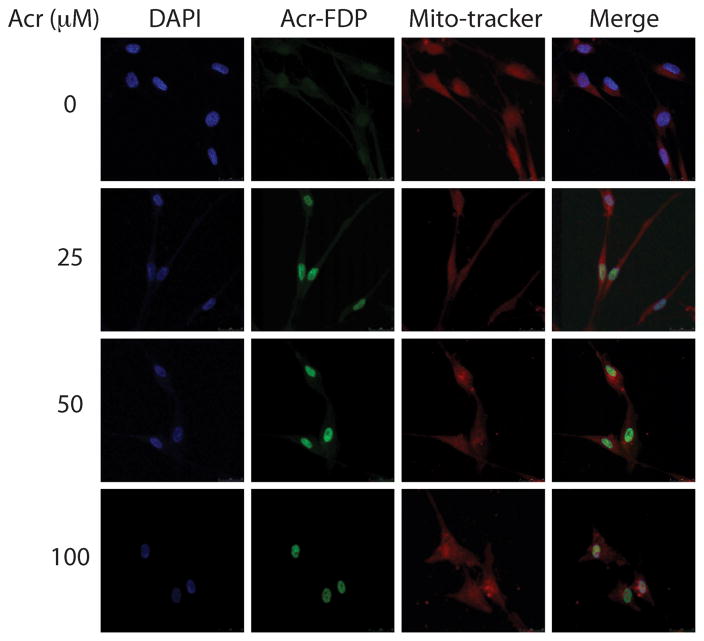
Acr can cross membrane barriers and readily enters human cells [7]. Using anti-hemocyanin-acrolein antibodies Lou et al. have reported that Acr is mainly located in the cytoplasm region of myocytes [8]. Using an anti-Acr-FDP-lysine (Nε-(3-formyl-3,4-dehydropiperidino)-lysine) antibody specifically for Acr-lysine we found that Acr-lysine is preferentially trapped in the nucleus compared with other regions of the cytoplasm in cultured normal human lung fibroblasts (Fig. 1). If we assume that only the conjugated Acr is recognized by the Acr-lysine antibody then those Acr molecules are not “free form”. Whether bonded Acr can further react with nuclear components such as nuclear proteins and DNA, and whether Acr can remain in free form in cytoplasm are unclear.
Figure 1.
Acrolein distribution in normal human lung fibroblasts (CCL202). Cultured CCL202 cells were treated with Acr (0–100 μM, 1 h), fixed, stained with anit-Acr-FDP-lysine (Nε-(3- formyl-3,4-dehydropiperidino)-lysine) antibody followed by goat anti-mouse FITC-conjugated secondary antibody, and then examined by microscopy. The method is the same as previously described [8]. Note: DAPI stained DNA, Acr-FDP stained Acr-lysine adducts and Mito-tracker stained mitochondria.
Acr-DNA interactions in vitro
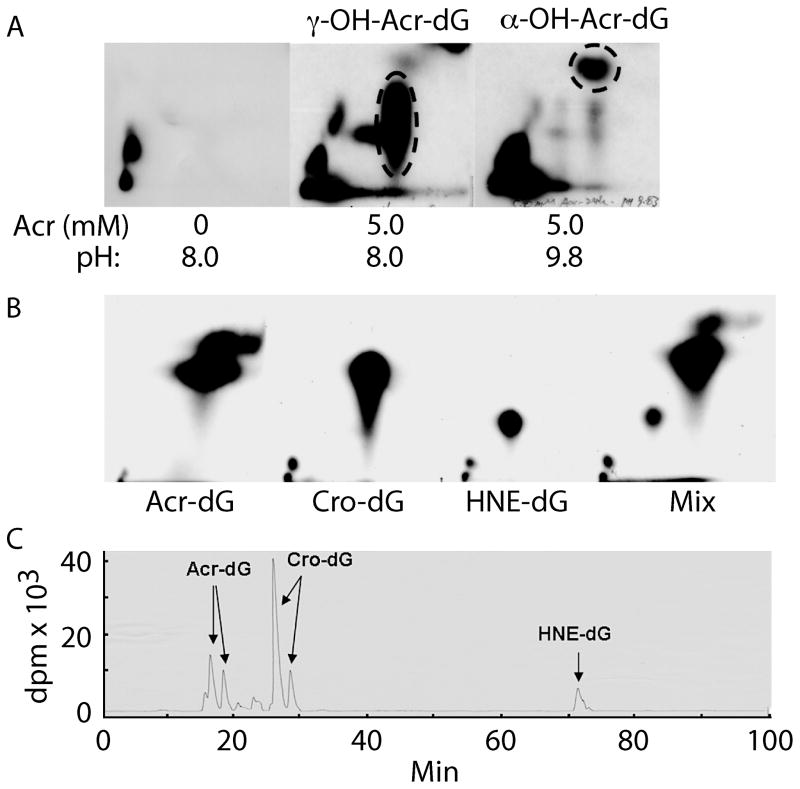
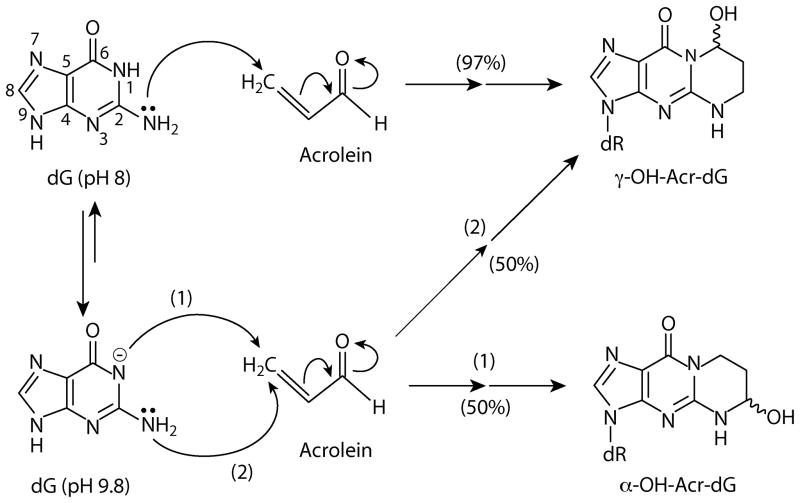
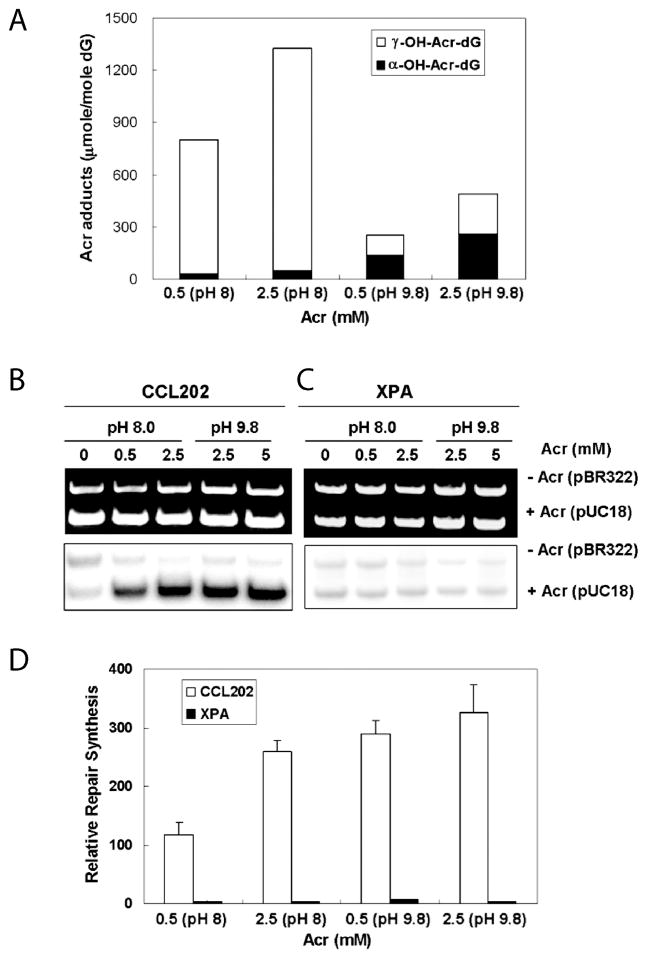
It has been well established that excyclic amine of guanine residues is a strong Michael addition donor for the olefinic double bond of Acr. At neutral to mild basic pH Acr –DNA interactions generate mainly γ-hydroxy-1,N2-propano-2′-deoxyguanosine (γ-OH-Acr-dG) adducts [6]. However, we found that at high pH both α- and γ-OH-Acr-dG adducts are formed (Fig. 2A). It appears that at neutral and mild pH the N1 of the guanine residue has a higher nucleophilicity than the excyclic amine group. Therefore, it attacks the carbonyl group of the Acr to form primarily γ-OH-Acr-dG adducts. However, at high pH the nucleophilicity of N1 and excyclic amine at guanine residue are reduced and equalized, hence these two moieties have equal probability of attacking the olefinic double bond, as well as the carbonyl group of acrolein, and consequently form both α- and γ-OH-Acr-dG adducts (Fig. 3).
Figure 2.
Analysis of acrolein induced DNA adduct under pH 8 and pH 9.8 reaction conditions by 32P post-labeling, 2 dimensional thin layer chromatography (2D-TLC) (A) followed by high performance liquid chromatography (HPLC). The DNA adducts in the indicated spots that contain Acr-dG were extracted and further separated by HPLC. Acr-dG, and Cro-dG co-migrate in 2D-TLC (B). Acr-dG, Cro-dG and HNE-dG can be well separated by HPLC (C). The methods for Acr-DNA modification and adduct analysis are the same as previously described [9, 11].
Figure 3.
Hypothetical acrolein – deoxyguanosine reactions under pH 8 and pH 9.8 conditions that lead to formation of α-OH-Acr-dG and γ-OH-Acr-dG adducts.
Detection of Acr-dG adducts by the 32P post-labeling-two-dimensional thin layer chromatography (2D-TLC) and high performance liquid chromatography (HPLC)
Two methods have been well established to quantify Acr-DNA adducts: 1) 32P post-labeling label followed by two-dimensional thin layer chromatography (2D-TLC) and high performance liquid chromatography (HPLC) [9], and 2) liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) [10]. The initial step for both methods is to digest DNA to single 3′-mono-phosphate nucleotides by phosphodiesterase. The adducted mono-phosphate nucleotides are separated from normal nucleotide phosphate, first by solid phase extraction and then followed by LC-ESI-MS/MS. Accurate mass can be identified in the eluent, therefore this method renders a very reliable result [10]. However, this method requires a relatively large amount of genomic DNA. Thus, it is quite a challenging task to apply this method to clinical samples.
For 32P post-labeling analysis, the digested mono-phosphate nucleotides are 5′-end phosphorylated with 32P followed by nuclease P1 (NP1) digestion; adducted nucleotides are relatively resistant to NP1, and the resulting 5′,3′-bisphosphate adducted nucleotides can be separated from normal 5′-32P-mono-phosphate normal nucleotides by 2D-TLC [9]. Although α-OH-Acr-dG from γ-OH-Acr-dG can be well separated in our 2D-TLC system, these two types of adducts co-migrate with crotonaldehyde-adducted deoxyguanosine (Cro-dG) and other unknown DNA adducts, therefore further separation of α- and γ-OH-Acr-dG from Cro-dG and others is necessary (Fig. 2B). We found that the HPLC step can separate Acr-dG, Cro-dG and 4-hydroxy-2-nonenal (HNE)-dG (Fig. 2C). We found that this method is highly reproducible with high recovery (80%). Furthermore, only 5 μg of DNA is needed for adduct analysis using 32P post-labeling 2D-TLC-HPLC method, and this method can simultaneously analyze Acr-dG, Cro-dG, and HNE-dG adducts. Although the LC-ESI-MS/MS method can also have the advantage of separating Acr-dG from others, it requires 50 μg DNA for the same analysis, in addition to requiring the availability of expensive LC-ESI-MS/MS equipment.
Analysis of Acr-dG formation at the DNA sequence level
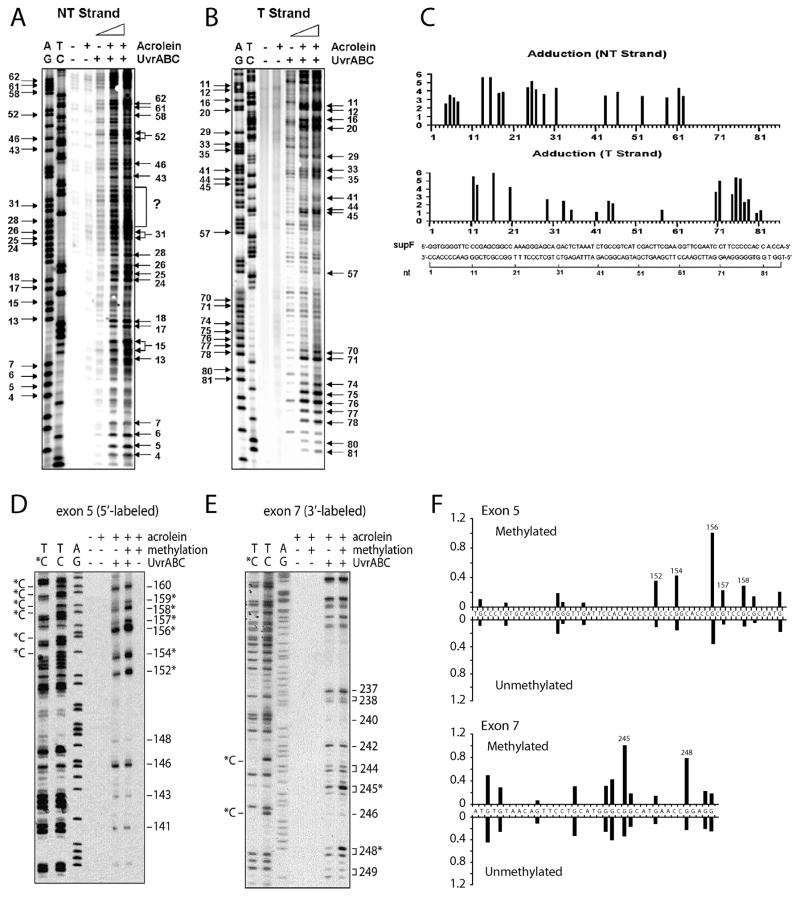
We have found that Escherichia coli nucleotide excision repair enzymes, UvrA, UvrB and UvrC working in concert (collectively, known as UvrABC nuclease), can incise Acr-dG specifically and quantitatively [9, 11]. Using UvrABC incision with and without ligation-mediated PCR, we have developed methods to map the distribution of Acr-dG in the p53 gene and the supF gene at the nucleotide level in the defined DNA fragments and in genomic DNA isolated from human cells [9, 11]. We have found that 1) Acr-DNA adducts form exclusively at guanine residues in DNA, 2) sequences of a run of guanine residues, -GA- and –AG- are the preferential sites for Acr-DNA adduct formation, and 3) the C5 cytosine methylation at –CpG- sequence greatly enhances Acr-dG adduction at this site (Fig. 4). Our results indicate that Acr-dG formation is affected by DNA sequence context and cytosine methylation [9, 11].
Figure 4.
Sequence specificity and cytosine methylation effect on Acr-dG adduct formation. A) and B) are 32P 5′-single end labeled supF DNA fragments. D) and E) are 32P 5′-single end labeled exon 5 of the p53 gene and 32P 3′-single end labeled exon 7 of the p53 gene fragments, respectively (with and without methylated at –CpG sequences by CpG methylase). Labeled DNA were modified with Acr and then reacted with UvrABC. The resultant DNAs were separated by denaturing DNA sequencing gel electrophoresis. C) and F) are quantitations. AG and TC represent Maxam and Gilbert sequencing reaction products; the positions of the methylated cytosines, which are resistant to Maxam Gilbert reactions, are indicated by C* [9, 11].
Acrolein-induced cytotoxicity and mutations in mammalian cells
Acr has been shown to be both cytotoxic and mutagenic to Chinese hamster ovary (CHO) cells [12–15]. However, its genotoxic and cytotoxic effects in human cells are less straightforward [16, 17]. It has also been demonstrated that Acr induces a concentration-dependent cell killing and mutation increase in nucleotide excision repair (NER) deficient xeroderma pigmentosum complement group A (XPA) cells [18]. These results indicate that Acr-induced DNA damage is not only mutagenic but also cytotoxic. However, although Acr induces a concentration-dependent cell killing in NER proficient human fibroblasts and the D37 for these cells is 4–5 times higher than for XPA cells, intriguingly, Acr does not induce a significant number of mutations in NER proficient human cells even at the level that it induces 90% of cell killing [18]. Kim et al. [19] also failed to detect Acr-induced mutation in the cII transgene in transgenic mouse fibroblasts treated with the Acr (<100 μM) at the concentrations that causes a substantial amount of cell killing and DNA damage detected by an extremely sensitive terminal transferase-dependent PCR method. Together, these results suggest that either Acr-induced DNA damage is efficiently repaired in normal human fibroblasts and mouse ES cells or that the path that Acr induces cell death in normal human and mouse ES cells is different from the path for XPA cells.
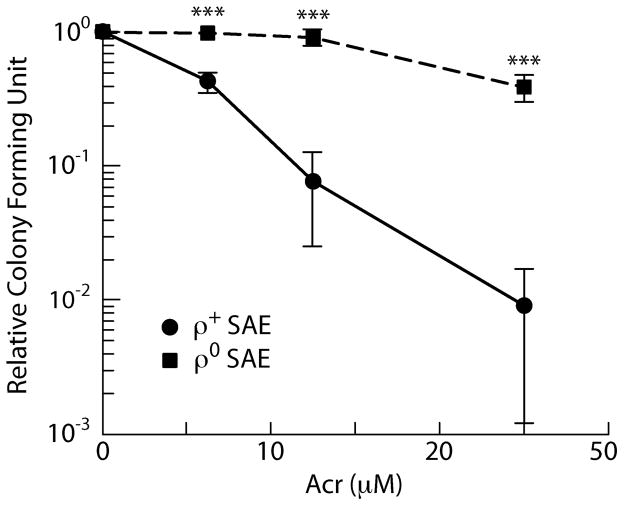
Since Acr can readily interact with many proteins and numerous cellular components it is conceivable that multiple pathways can lead to cell death by Acr and that DNA damage is one of them. For example, it has been found that Acr can induce apoptosis through interaction with mitochondria [20–26]. To further test this possibility, we determined the Acr-induced cytotoxicity and DNA damage in mitochondria depleted rho zero cells and their parental human lung epithelial cells. We have found that rho zero cells are much more resistant to Acr-induced cell killing [27] (Fig. 5). These results indicate that mitochondria indeed mediate Acr-induced cell death. These results also indicate that unlike many DNA damaging agents that induce cytotoxicity through their genotoxicity, the relationship between Acr-induced cytotoxicity and genotoxicity is not equal and is cell type dependent.
Figure 5.
Acrolein-induced cell killing in human small airway epithelial (SAE) cells and their mitochondria depleted derivatives (ρ∘). Exponentially growing SAE and cells ρ∘ cells were treated with different concentrations of acrolein for 1 h and the cell survival was determined by colony forming ability. The method for generating ρ∘ cells is the same as described by Huang [27].
Mutagenicity of DNA adducts: α-OH-Acr-dG versus γ-OH-Acr-dG
While the relationship between Acr-induced cytotoxicity and genotoxicity is not straightforward, the mutagenicity of Acr-induced DNA adducts is no less confusing. It is well established that modifications of deoxyguanosine mono-phosphate result in generating two stereoisomeric α-OH-Acr-dG adducts and two stereoisomeric γ-OH-Acr-dG Acr adducts (Fig. 3). Although the γ-OH-Acr-dG adduct is the major DNA adduct detected in cultured human cells that have been treated with Acr, both α-OH-Acr-dG and γ-OH-Acr-dG are detected in human lung tissues [10]. Therefore, it is essential to assess the mutagenicity of these two types of DNA adducts in order to understand the carcinogenicity of Acr. While the mutagenicity of Acr-induced DNA adducts in CHO and human cells has been firmly established a question remains: which isomeric Acr-dG is mutagenic? Using a construct that contains a site-specific α-OH-Acr-dG, it has been shown that both G to T and G to A mutations are induced at the adducted guanine position in both mammalian and Escherichia coli cells [28]. Hence, it is generally believed that α-OH-Acr-dG adducts are mutagenic. The mutagenicity of γ-OH-Acr-dG is controversial. Using a construct containing a site-specific γ-OH-Acr-dG, both Moriya’s and Marnett’s laboratories have shown that no significant mutations were observed at the adducted guanine site [29, 30]. On the other hand, using a supF containing shuttle vector randomly modified with Acr that generates mainly γ-OH-Acr-dG adducts (97%), both Yagi and Tang’s laboratories have found that γ-OH-Acr-dG adducts are mutagenic and induce G to T and G to A mutations at the adducted guanines [11, 31]. Using a site-specific construct containing a γ-OH-Acr-dG adduct Lloyd’s laboratory has also found G to T and G to A mutations at the adducted guanine position in Cos cells [32–34]. These results indicate that both sequence context and host cells plays a role in determining the Acr-dG adduct mutagenicity. Caution must be exercised in generalizing results, particularly those results obtained from a site-directed Acr-dG containing constructs, because site specific Acr-DNA adduct induced mutations represent only a “snapshot” of Acr-DNA adduct mutagenicity at that particular sequence context and may not represent the mutagenicity of the same adduct at other sequences.
Since we have found that Acr-DNA modifications under various pH conditions produce different ratios of Acr-dG stereoisomers (Fig. 2), we modified supF containing shuttle vectors under pH 8 and 9.8 conditions to produce plasmid DNA with either mainly γ-OH-Acr-dG (97%) or plasmid DNA with 50% γ-OH-Acr-dG adducts and 50% α-OH-Acr-dG adducts. We have found that the latter adduct ratio induced significantly less mutations/DNA adducts than the former in both normal human bronchial epithelial (NHBE) cells and in normal human lung fibroblasts (NHLF) (CCL202) (Table 1). Intriguingly, we also found that the γ-OH-Acr-dG adduct is more mutagenic in NHBE cells than in NHLF. In contrast, the α-OH-Acr-dG adduct is more mutagenic in NHLF than in NHBE cells. These results suggest that α-OH-Acr-dG adduct could be either repaired efficiently and/or are less mutagenic than the γ-OH-Acr-dG adduct.
Table 1.
Mutations in supF gene induced by Acr-modifications under pH 8 and 9.8 conditions manifested in normal human bronchial epithelial cells (NHBE) and normal human lung fibroblasts (CCL202)*.
| Cell lines | pH | 8 | 8 | 8 | 9.8 | 9.8 |
|---|---|---|---|---|---|---|
| Acr (mM) | 0 | 0.5 | 2.5 | 2.5 | 5 | |
| NHBE | No. mutants/Total colonies | 3/25120 | 128/9940 | 31/1483 | 2/15624 | 23/56448 |
| Mutation frequency (×104) | 1.2 | 128.8 | 209.0 | 1.3 | 4.1 | |
| CCL202 | No. mutants/Total colonies | 11/101440 | 78/51216 | 53/9920 | 45/74400 | 114/75360 |
| Mutation frequency (×104) | 1.1 | 15.2 | 53.4 | 6.0 | 15.1 |
Acr-DNA modifications and supF mutation detection are the same as previously described (9,11)
Acr-DNA and Acr-protein-DNA crosslinks
It has been found that γ-OH-Acr-dG adduct can undergo further chemical reactions forming interstrand or intrastrand DNA cross-links at 5′-CpG-3′ sequences. Acr can also cause DNA-protein conjugates. The chemistry and biological processing of these cross-links were extensive reviewed by Minko et al. and Stone et al. [5, 35]. Interestingly, Minko et al. [36] have found that while mutations are rarely generated during processing of these interstrand or intrastrand DNA cross-links, the reduced γ-OH-Acr-dG-peptide conjugates can be more mutagenic than the corresponding mono-adduct and its potential to cause mutations is dependent on the peptide attachment. Current data suggest that γOH-Acr-dG-peptide conjugates are formed as intermediates in the process of repairing γ-OH-Acr-dG [37]. These results suggest that the mutagenicity of γ-OH-Acr-dG can be derived from adduct itself and its peptide conjugates.
Repairability of α-OH-Acr-dG adducts versus γ-OH-Acr-dG adducts
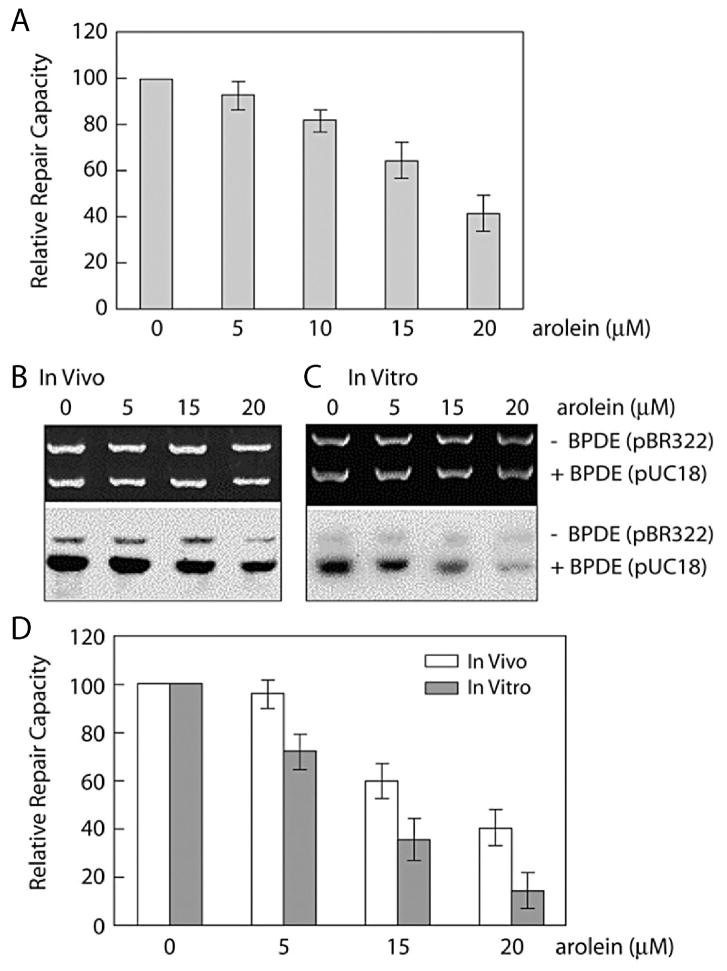
To determine the repairability of the two stereoisomeric Acr-dG adducts, supercoiled plasmid DNA was modified with Acr under pH 8 and pH 9.8 conditions that produce different percentages of α-OH-Acr-dG adducts versus γOH-Acr-dG adducts; these DNAs were then used as substrates for in vitro DNA damage dependent repair synthesis which was carried out using cell lysates isolated from human lung fibroblasts (CCL202) and XPA cells (Fig. 6). We have found that 1) DNA modified with Acr under pH 9.8 conditions produces lower total Acr-dG adducts but significantly higher α-OH-Acr-dG/γ-OH-Acr-dG ratios than DNA modified with Acr under pH 8.0 conditions, and 2) DNA modified with Acr under pH 9.8 conditions induces more repair synthesis than DNA modified with Acr under pH 8.0 conditions. These results indicate that α-OH-Acr-dG adducts induce more repair synthesis than γ-OH-Acr-dG adducts suggesting that α-OH-Acr-dG adducts are better substrates for excision repair. We also found that lysates from XPA cells are deficient in carrying out repair synthesis for both substrates, indicating that NER is the major mechanism for Acr-dG adduct repair (Fig. 6).
Figure 6.
α- and γ-OH-Acr-dG induced DNA repair synthesis carried out by cell lysates isolated from normal human lung fibroblasts (CCL202) and xeroderma pigmentosum group A (XPA) cells. Supercoiled plasmid DNA pUC18 was modified with Acr under pH 8 and pH 9.8 conditions which produce different ratios of α-OH-Acr-dG : γ-OH-Acr-dG adducts (3 : 97 for pH 8 and 50 : 50 for pH 9.8). These Acr-modified pUC18 and pBR322 were used as substrates for in vitro repair synthesis in the presence of 32P-dATP, the same manner as previously described [9]. (A) Acr-dG adduct analysis and (B–D) in vitro repair synthesis carried out by cell lysates of CCL202 and XPA cells. Note: DNA modified with Acr under pH 9.8 conditions produces lower total Acr-dG adducts but higher α-OH-Acr-dG/γ-OH-Acr-dG ratios than DNA modified with Acr under pH 8.0 conditions, and DNA modified with Acr under pH 9.8 conditions induces more repair synthesis than DNA modified with Acr under pH 8.0 conditions. These results indicate that α-OH-Acr-dG adducts are better substrates for repair synthesis.
Acrolein effect on DNA repair
Because Acr contains a carbonyl group and an olefinic double bond, once it enters cells Acr can interact with numerous cellular components such as proteins and DNA through Michael additions. Two systems have been used to measure Acr effect on DNA repair: host cell reactivation (HCR) of UV-modified vector containing luciferase gene and in vitro DNA damage dependent repair synthesis. In the former, luciferase will not be expressed until the UV damage in the target gene is repaired. In the latter, the level of repair synthesis, which can be measured by incorporation of 32P labeled nucleotides, is directly proportional to the DNA repair capacity in the cell lysates [9]. We have found that cells treated with Acr have lower HCR for UV-irradiated luciferase, indicating that the Acr treatment causes an inhibitory effect on DNA repair (Fig. 7A) [9]. This interpretation was further confirmed by results showing that cell lysates isolated from Acr-treated cells have lower capacity to carry out repair synthesis using UV-damaged templates (Fig. 7B & D) [9]. The reduction of repair capacity in cells treated with Acr apparently comes from Acr-repair protein interactions based on results which show that the addition of Acr directly to the cell free cell lysates can also effectively inhibit cell lysates from carrying out repair synthesis (Fig. 7C & D) [9].
Figure 7.
Inhibitory effect on DNA repair capacity by Acr. (A) Host cell reactivation (HCR) assay. UV-irradiated luciferase reporter plasmids and unmodified β-galactosidase plasmids were transfected into normal human lung fibroblasts (CCL202) (NHLF) with Acr treatments, and the luciferase and β-galactosidase activities were measured post-transfection. (B–D) In vitro DNA repair synthesis assay. UV-irradiated pUCC18 and unmodified pBR322 plasmids were used as DNA substrates for in vitro DNA repair synthesis assay. In (B) NHLF were treated with Acr first and the cell extracts were used for repair assay. In (C) Acr was added directly into cell extracts prepared from untreated NHLF. In the upper panel are ethidium bromide-stained gels, and the lower panel are autoradiographs of the same gels. In (D) the relative repair capacity was calculated from (B) and (C) [9].
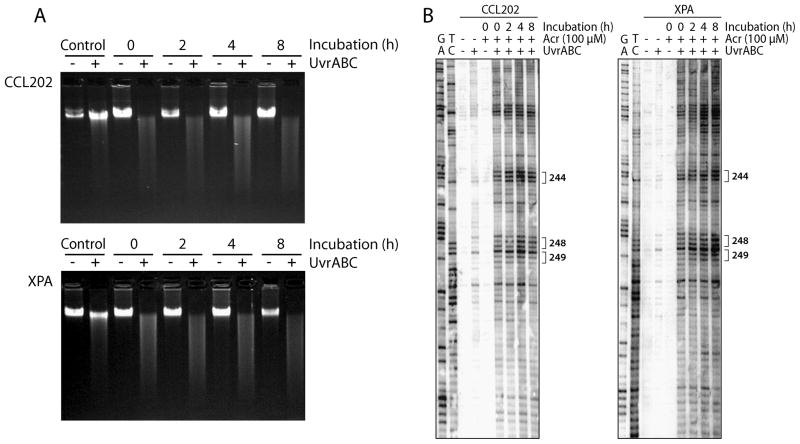
In the process of mapping Acr-DNA adduct repair kinetics in the p53 gene in cells treated with Acr, we found that Acr-dG adducts are not significantly repaired in the p53 gene, as well as in genome overall, after 8 h incubation in NHLF; similar results were found in XPA cells (Fig. 8A & B). Since we have found that Acr-dG adducts are substrates for NER and that Acr cause an inhibitory effect on DNA repair for UV-induced DNA damage, together, these results suggest that Acr treatment causes two detrimental effects: DNA damage and reduction of repair capacity for Acr-induced DNA adducts.
Figure 8.
Lack of repair of Acr-induced DNA damage in the p53 gene (A) as well as in genome overall (B) in normal human NER proficient NHLF (CCL-202) and NER deficient XPA cells. Exponentially growing cells at 70% confluency were treated with Acr (100 μM) for 6 h, then incubated in fresh medium without Acr for 0, 2, 4, and 8 h. The genomic DNAs were isolated and reacted with UvrABC which can cut Acr-dG adducts [9, 11]. Acr-DNA adducts formed in exon 7 of the p53 gene were mapped at the nucleotide level by the UvrABC-LMPCR method as previously described [9]. Note: Acr-DNA adducts were not repaired at the genome level and at the codons 244, 248 and 249 of the p53 gene.
Conclusions
Acr is not only a ubiquitous environmental contaminant, but also can be generated endogenously under oxidative stress. Ample evidence indicates that Acr interacts with many cellular components, resulting in deleterious effects. We have shown that through the interaction with DNA Acr induces two major stereoisomeric DNA adducts: α–OH-Acr-dG and γ–OH-Acr-dG. These two types of DNA adducts are also found in human lung tissue and furthermore, the amount of Acr-dG is significantly higher than benzo(a)pyrene diol epoxide (BPDE)-dG adducts [38, 39] The mechanisms that lead to the production of these two types of adducts is unclear. Nonetheless, both types of Acr-dG are mutagenic in different assay systems [11, 31–33]. Acr also has also been found to cause an inhibitory effect on DNA repair [9]. It seems that this effect is through its direct interaction with DNA repair proteins [9].
Intraperitoneal injection of Acr causes bladder cancer in rat models [3, 40, 41]. Metabolically produced Acr, by antitumor drugs such as cyclphosphamide and ifosfamide, can cause bladder cancer in humans [3, 40, 41]. We have found that the distribution of the Acr-DNA adduct in the p53 gene coincides with the p53 mutational pattern in cigarette smoke-related lung cancer [9]. Furthermore, the Acr-dG formed in the p53 gene, as well as in genome overall, is poorly repaired (Fig. 8). Therefore, based on the findings that: Acr-induces DNA damage that is mutagenic, Acr causes an inhibitory effect on DNA repair, and Acr causes bladder carcinogenesis, we conclude that Acr is a carcinogen for human lung and bladder cancers. This conclusion raises an intriguing question: If Acr has such detrimental effects then why to date there is no evidence to imply Acr in cancers other than lung and bladder cancers in human? The answer probably will be found by understanding how Acr is metabolized in human body. Except for occupational and/or accidental exposure, which can occur anywhere, in the body, inhalation is the major entry route for exogenous Acr to the body. It is conceivable that bronchial epithelial cells are the first targets to be exposed to inhaled Acr. Once entering the blood stream, Acr is most likely to interact with amino acids and serum proteins through Schiff base formation. Those protein or amino acid-associated Acr is no longer “active” in causing detrimental effects until they reach the renal system, which under normal physiological conditions will free Acr from proteins and amino acids. The freed Acr is excreted in urine, accumulated in the bladder, and consequently causes detrimental effects to urothelial cells, including initiating bladder carcinogenesis.
In summary, Acr is a ubiquitous environmental contaminant; it is abundant in cigarette smoke and in cooking fumes. Acr is very reactive with various cellular components including amino acids, proteins and nucleic acids; it causes an inhibitory effect on DNA repair and induces mutagenic Acr-dG adducts. Acr causes bladder cancer in human and rat models. The binding spectrum of Acr in the p53 gene in normal human bronchial epithelial cells is similar to the p53 mutational spectrum in lung cancer. Therefore, we conclude that Acr is a major lung and bladder carcinogen, and its carcinogenicity arises via induction of DNA damage and inhibition of DNA repair.
Acknowledgments
This work was supported by National Institutes of Health Grants (CA114541, ES014641, CA99007, and ES00260).
Abbreviations
- Acr
acrolein
- 2D-TLC
two dimensional thin layer chromatography
- HPLC
high performance liquid chromatography
- α- and γ-OH-Acr-dG
α- and γ-hydroxy-1,N2-propano-2′-deoxyguanosine
- NP1
nuclease P1
- Cro-dG
crotonaldehyde adducted propano-dG
- HNE
4-hydroxy-2-nonenal
- HNE-dG
HNE adducted propano-dG
- CHO
Chinese hamster ovary
- NER
nucleotide excision repair
- XPA
xeroderma pigmentosum complement group A
- D37
dose yielding 37% survival
- NHBE
normal human bronchial epithelial
- NHLF
normal human lung fibroblasts
- HCR
host cell reactivation
- BPDE
benzo(a)pyrene diol epoxide
- LC-ESI-MS/MS
liquid chromatography electrospray ionization tandem mass spectrometry
Footnotes
All authors declare there is no conflict of interest.
References
- 1.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 3.Comes RMM, Eggleton M. 2002 [Google Scholar]
- 4.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, et al. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chemical research in toxicology. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 7.Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 8.Luo J, Hill BG, Gu Y, Cai J, et al. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am J Physiol Heart Circ Physiol. 2007;293:H3673–3684. doi: 10.1152/ajpheart.00284.2007. [DOI] [PubMed] [Google Scholar]
- 9.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chemical research in toxicology. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HT, Zhang S, Hu Y, Tang MS. Mutagenicity and sequence specificity of acrolein-DNA adducts. Chemical research in toxicology. 2009;22:511–517. doi: 10.1021/tx800369y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Wu MH, Ludeman SM, Grdina DJ, Dolan ME. Role of O6-alkylguanine-DNA alkyltransferase in protecting against cyclophosphamide-induced toxicity and mutagenicity. Cancer research. 1999;59:3059–3063. [PubMed] [Google Scholar]
- 13.Cai Y, Wu MH, Xu-Welliver M, Pegg AE, et al. Effect of O6-benzylguanine on alkylating agent-induced toxicity and mutagenicity. In Chinese hamster ovary cells expressing wild-type and mutant O6-alkylguanine-DNA alkyltransferases. Cancer research. 2000;60:5464–5469. [PubMed] [Google Scholar]
- 14.Hansen RJ, Ludeman SM, Paikoff SJ, Pegg AE, Dolan ME. Role of MGMT in protecting against cyclophosphamide-induced toxicity in cells and animals. DNA Repair (Amst) 2007;6:1145–1154. doi: 10.1016/j.dnarep.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanel A, Averill-Bates DA. Inhibition of acrolein-induced apoptosis by the antioxidant N-acetylcysteine. J Pharmacol Exp Ther. 2007;321:73–83. doi: 10.1124/jpet.106.114678. [DOI] [PubMed] [Google Scholar]
- 16.Irwin RD. NTP Technical Report on the comparative toxicity studies of allyl acetate (CAS No. 591-87-7), allyl alcohol (CAS No. 107-18-6) and acrolein (CAS No. 107-02-8) administered by gavage to F344/N rats and B6C3F1 mice. Toxic Rep Ser. 2006;1–73:A71–H10. [PubMed] [Google Scholar]
- 17.Jia L, Zhang Z, Zhai L, Bai Y. Protective effect of lipoic acid against acrolein-induced cytotoxicity in IMR-90 human fibroblasts. J Nutr Sci Vitaminol (Tokyo) 2009;55:126–130. doi: 10.3177/jnsv.55.126. [DOI] [PubMed] [Google Scholar]
- 18.Curren RD, Yang LL, Conklin PM, Grafstrom RC, Harris CC. Mutagenesis of xeroderma pigmentosum fibroblasts by acrolein. Mutat Res. 1988;209:17–22. doi: 10.1016/0165-7992(88)90104-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim SI, Pfeifer GP, Besaratinia A. Lack of mutagenicity of acrolein-induced DNA adducts in mouse and human cells. Cancer Res. 2007;67:11640–11647. doi: 10.1158/0008-5472.CAN-07-2528. [DOI] [PubMed] [Google Scholar]
- 20.Roy J, Pallepati P, Bettaieb A, Averill-Bates DA. Acrolein induces apoptosis through the death receptor pathway in A549 lung cells: role of p53. Canadian journal of physiology and pharmacology. 88:353–368. doi: 10.1139/Y09-134. [DOI] [PubMed] [Google Scholar]
- 21.Kern JC, Kehrer JP. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chemico-biological interactions. 2002;139:79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochemistry international. 2005;47:449–457. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Tanel A, Averill-Bates DA. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochimica et biophysica acta. 2005;1743:255–267. doi: 10.1016/j.bbamcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Luo C, Long J, Wei D, Liu J. Acrolein is a mitochondrial toxin: effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion. 2006;6:136–142. doi: 10.1016/j.mito.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Liu Z, Luo C, Jia H, et al. Lipoamide protects retinal pigment epithelial cells from oxidative stress and mitochondrial dysfunction. Free radical biology & medicine. 2008;44:1465–1474. doi: 10.1016/j.freeradbiomed.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy J, Pallepati P, Bettaieb A, Tanel A, Averill-Bates DA. Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chemico–zbiological interactions. 2009;181:154–167. doi: 10.1016/j.cbi.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang XS. The Roles of Mitochondria and Reactive Intermediate Species in Asbestos-Induced Mutagenesis. Columbia University; 2009. [Google Scholar]
- 28.Yang IY, Chan G, Miller H, Huang Y, et al. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 29.VanderVeen LA, Hashim MF, Nechev LV, Harris TM, et al. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J Biol Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 30.Yang IY, Hossain M, Miller H, Khullar S, et al. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J Biol Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 31.Kawanishi M, Matsuda T, Nakayama A, Takebe H, et al. Molecular analysis of mutations induced by acrolein in human fibroblast cells using supF shuttle vector plasmids. Mutat Res. 1998;417:65–73. doi: 10.1016/s1383-5718(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 32.Kanuri M, Minko IG, Nechev LV, Harris TM, et al. Error prone translesion synthesis past gamma-hydroxypropano deoxyguanosine, the primary acrolein-derived adduct in mammalian cells. J Biol Chem. 2002;277:18257–18265. doi: 10.1074/jbc.M112419200. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez AM, Minko IG, Kurtz AJ, Kanuri M, et al. Comparative evaluation of the bioreactivity and mutagenic spectra of acrolein-derived alpha-HOPdG and gamma-HOPdG regioisomeric deoxyguanosine adducts. Chemical research in toxicology. 2003;16:1019–1028. doi: 10.1021/tx034066u. [DOI] [PubMed] [Google Scholar]
- 34.Minko IG, Washington MT, Kanuri M, Prakash L, et al. Translesion synthesis past acrolein-derived DNA adduct, gamma-hydroxypropanodeoxyguanosine, by yeast and human DNA polymerase eta. J Biol Chem. 2003;278:784–790. doi: 10.1074/jbc.M207774200. [DOI] [PubMed] [Google Scholar]
- 35.Stone MP, Cho YJ, Huang H, Kim HY, et al. Interstrand DNA cross-links induced by alpha,beta-unsaturated aldehydes derived from lipid peroxidation and environmental sources. Acc Chem Res. 2008;41:793–804. doi: 10.1021/ar700246x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minko IG, Kozekov ID, Kozekova A, Harris TM, et al. Mutagenic potential of DNA-peptide crosslinks mediated by acrolein-derived DNA adducts. Mutat Res. 2008;637:161–172. doi: 10.1016/j.mrfmmm.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Kozekov ID, Kozekova A, Rizzo CJ, et al. Minor groove orientation of the KWKK peptide tethered via the N-terminal amine to the acrolein-derived 1,N2-gamma-hydroxypropanodeoxyguanosine lesion with a trimethylene linkage. Biochemistry. 2010;49:6155–6164. doi: 10.1021/bi100364f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexandrov K, Cascorbi I, Rojas M, Bouvier G, et al. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers’ lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–1977. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- 39.Gyorffy E, Anna L, Gyori Z, Segesdi J, et al. DNA adducts in tumour, normal peripheral lung and bronchus, and peripheral blood lymphocytes from smoking and non-smoking lung cancer patients: correlations between tissues and detection by 32P-postlabelling and immunoassay. Carcinogenesis. 2004;25:1201–1209. doi: 10.1093/carcin/bgh131. [DOI] [PubMed] [Google Scholar]
- 40.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 41.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]