INTRODUCTION
The most effective management of postoperative crises is prevention. This starts with preoperative preparation and patient screening. There are many factors that can be controlled and improved by the patient, including but not limited to, smoking cessation, cardiopulmonary rehab and teaching/setting peri-operative expectations. Equally as important is patient selection, which is influenced by pulmonary function tests, cardiopulmonary reserve, and pre-existing comorbidities. After the operation, the care team can also greatly improve outcomes with aggressive cardiopulmonary therapies, ambulation, vigilant monitoring and frequent assessments of the patient. Even when all of these guidelines are followed not all early complications can be avoided.
A recent review of the National Cancer Database by Rosen et al found a 2.6% mortality from lobectomy [1]. Morbidity associated with lobectomy is 10% to 50%, and increases in the elderly [2]. The population being treated by thoracic surgeons continues to age and the complexity of the operations is increasing as neoadjuvant therapies become more prevalent. Early postoperative crises after lobectomy will occur, necessitating prompt and effective management strategies. The most common complications after pulmonary resection are listed below in Table 1, in this chapter we will focus on the early complications after lobectomy [3].
Table 1.
Post-lobectomy Morbidity and Mortality
| ACOSOG Z0030 Trial | |
|---|---|
| Patients | 1,023 |
| Mortality | 1.4 |
| Reoperation for Bleeding | 1.5 |
| Prolonged Air Leak | 7.6 |
| Empyema | 1.1 |
| Pneumonia | 2.5 |
| Bronchopleural Fistula | 0.5 |
| Atelectasis | 6.4 |
| ARDS | 0.7 |
| Atrial Fibrillation | 14.4 |
For the purposes of this chapter we will define early post-operative crisis as those that develop within 48 hours of the operation.
AIR LEAK
Prolonged air leak is the most common complication after pulmonary resection, with a reported incidence of 15–18% [4]. Prolonged air leak is defined as a leak lasted more than 7 days after pulmonary resection. Cerfolio et al reported an incidence of 25% on postoperative day 1 and 20% on postoperative day 2 [5,6]. There are many techniques available to reduce parenchymal air leaks post-operatively and a variety of factors that influence any given patients propensity for developing an air leak. Patient factors that increase risk of developing an air leak include emphysematous lungs, larger parenchymal resection and therefore less parenchymal apposition to chest wall, and inadequate drainage of air by thoracostomy tube(s).
PREVENTION STRATEGIES
Bronchial stump buttressing with a muscle flap. In patients with an infected pleural space or who are on chronic immunosuppression, coverage of the stump with vascularized muscle may prevent bronchial stump breakdown [7]. The intercostal muscle is an excellent coverage option and is harvested from the intercostal space upon entry into the chest at the level of the thoracotomy. It is important to plan ahead, as this muscle needs to be harvested prior to placing the rib spreader in an open operation to prevent crushing the vascular supply. Dissection is begun on the inferior rib and the muscle is mobilized using a periosteal elevator posteriorly to the paraspinous muscle. Attention is then turned to the superior rib taking great care to maintain the neurovascular bundle. The muscle is divided as far anteriorly as possible before encountering or endangering the internal mammary artery.
Pleural tent [8]. A pleural tent is developed by releasing the pleural from the chest wall over the top half of the hemithorax and allowing it to drape over the remaining lung. The space above the pleura fills with blood and fluid negating it as potential space and creating tissue apposition between the lung surface and the parietal pleura. The pleural tent is developed by incising the parietal pleural with electrocautery at the level of the access incision if the lobectomy was done thoracoscopically or the thoracotomy incision if the lobectomy was done in an open manner. It is released bluntly from the chest wall, taking care to keep it intact. It is continued circumferentially in the chest until there is enough laxity that the pleura is in contact with the remaining inflated lung. The use of a pleural tent can be especially helpful in patients with emphysema, who are at a high risk of air leak, and those who will be left with a large potential pleural space after resection.
Suture closure of identified leaks [9]. Assessing lung parenchyma for air leak is possible by filling the thoracic cavity with sterile water and having the anesthesia team inflate the remaining lung. Once a site of leak is identified simple figure of eight suture closure of the visceral pleura can decrease post-operative air leak.
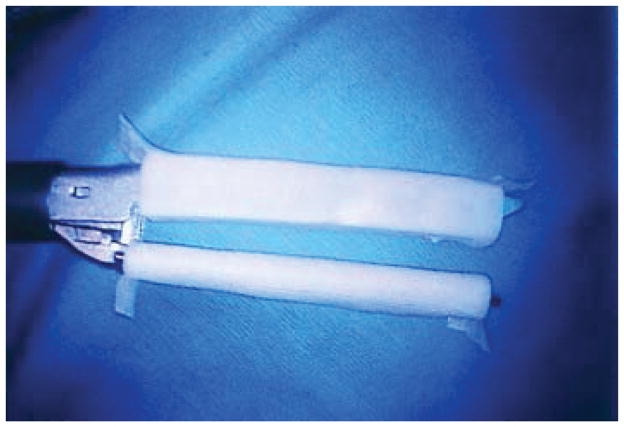
Buttressed staple line [10,11,12] There are a variety of products that can be used to buttress the staple line. They are designed to work with major stapling devices. The most commonly used are bovine pericardium, polytetrafluoroethylene PTFE or a collagen matrix (Peri Strips Dry). They are applied to the stapler prior to firing as shown and function as full length pledgets along the entirety of the staple line, see Figure 1 [13].
Fissureless operation [14]. The pulmonary artery is frequently accessed via the fissure by dissecting free the parenchymal tissue with electrocautery or sharp dissection. This can lead to air leaks from the divided parenchyma. In a fissureless lobectomy the pulmonary artery is exposed via the posterior hilum. As branches and the ongoing PA are identified the parenchyma is stapled sequentially. This allows all parenchyma to be stapled and divided thus avoiding division with cautery or sharply [15]
Sealants [16,17]. A Cochrane database review included 16 randomized trials with 1642 patients found that surgical sealants reduced postoperative air leaks and time to chest tube removal, but did not report a reduction in length of postoperative hospital stay [18]. A variety of sealants are available, these include: fibrin glue, cyanoacrylate, gelatin-resorcinol cross-linked with formaldehyde or glutaraldehyde, collagen, gelatin-based tissue adhesives, and polyurethane-based adhesives. They are activated by a variety of techniques and then applied to the divided parenchymal surface with an applicator in a stream or spray.
Expedited Liberation from Mechanical Ventilation. The vast majority of patients are extubated in the operating room after a lobectomy. Reducing the time on positive pressure ventilation and minimizing peak airway pressure decreases incidence of postoperative alveolar air leak.
Figure 1.
Butressed Stapler
TREATMENT STRATEGIES
The timing and severity of the air leak will affect treatment strategies.
Non-Operative Management. If the air leak is small and expiratory chest tube management is the first step in management. Cerfolio et al [19] published a randomized study in which chest tubes were continued on suction or placed to water seal on postoperative day 2. By postoperative day 3 the leak had resolved in 67% of those on water seal, and only 7% on suction. It is advised that air leaks are best treated with water seal of the chest tube as long as a pneumothorax does not develop or increase and respiratory function is not compromised. Air leaks that do not resolve should be placed to one-way valve for discharge with the goal of tube removal in clinic at follow-up.
Operative Management. If the air leak develops suddenly or increases to a continuous leak, a high index of suspicion is necessary for a bronchopleural fistula (BPF). The risk is highest after a pneumonectomy, right greater than left (8.6% vs 2.3% respectively) [20]. BPF development after lobectomy in the first 48 hours is very rare. Factors that increase the risk of BPF development include immunocompromise, neoadjuvant radiation, infection (uncommon as an early postoperative issue), steroid use, diabetes [21], skeletonizing the bronchial stump with dissection, and leaving a long bronchial stump [22]. Prevention of BPF is paramount due to high mortality rates if it occurs [23]. Particularly with pneumonectomy the bronchial stump should be covered with autologous tissue. Tissue options include pericardium, mediastinal fat pad, intercostal muscle flap, parietal pleura and azygous vein [22].
Development of a BPF as an early postoperative complication after lobectomy is a surgical problem that is solved by a return to the operating room. Diagnosis of a BPF can be clinical, but is confirmed bronchoscopically. If the bronchoscopy is negative and the index of suspicion remains high a ventilation perfusion scan can be performed. This will demonstrate the tagged gas exiting the pleural space through the chest tube.
In the operating room the chest is re-entered through the incisions made during the primary operation. Depending on the initial approach an intercostal muscle flap can be harvested as shown above upon re-exploration. If the intercostal muscle is not viable from prior retraction or injury attention can be turned to a vascularized mediastinal fat pad or the latissimus or serratus muscles. The pleural space is cleared of fluid and debris and the bronchial stump is identified and debrided. If the stump is healthy, primary repair can be attempted, of greater importance is well vascularized autologous tissue coverage. The covered stump needs to be tested for any remaining leak before chest closure.
PNEUMONIA/MUCOUS PLUGGING
Pneumonia is a significant concern for thoracic surgeons, with an incidence is reported up to 6% in some studies [24]. Patients are at increased risk after chest surgery for poor pulmonary hygiene, which can lead to the development of atelectasis and progression to pneumonia and/or mucous plugging. Atelectasis causes a ventilation/perfusion mismatch that results in hypoxemia and respiratory decline. Factors that increase the risk of atelectasis include poor cough, poor pain control, impaired pulmonary function at baseline and anatomic factors such as chest wall or diaphragm dysfunction.
PREVENTION STRATEGIES
Chest physiotherapy. Chest physiotherapy (CPT) including coughing and percussion, early ambulation, and incentive spirometry is the standard approach for postoperative prophylaxis against atelectasis and sputum retention.
Aggressive Strategies. Nasotracheal suctioning, intermittent positive pressure ventilation, inhaled mucolytics, and bedside may become necessary in patients with recalcitrant mucous plugging and atelectasis to encourage and produce an effective cough.
Pain Control. Poor postoperative pain control decreases a patients’ ability to participate in the standard CPT activities, which impairs their ability to clear their secretions. The use of narcotic pain medication can improve pain control, but may lead to altered mental status and impaired ability to clear secretions, and the cycle continues. Debate exists over optimal pain control strategy. There is good evidence that video-assisted thoracoscopic surgery causes less postoperative pain than open thoracotomies [25]. Epidural analgesia, intercostal nerve blocks and patient controlled intravascular analgesia (PCA) are the most common methods used. Each method has advantages and disadvantages. Epidural analgesia provides excellent pain relief, but carries the risk of bradycardia, hypotension and urinary retention. Intercostal nerve blocks are short acting and may not be effective for the duration of patient recovery. PCA’s are effective, but narcotics can lead to altered mental status and respiratory depression as described above [26]. Effective pain control is the goal and surgeon preference the deciding factor.
Smoking Cessation. Preoperative smoking cessation is a necessity to reduce sputum production and retention [27]. The risk of pulmonary complications decreased the longer the interval between smoking cessation. The optimal timing of smoking cessation is not defined. Nakagawa et al found that a minimum of 4 weeks of smoking abstinence was needed to see a reduction in pulmonary complications [28]. Other studies of cardiac patients concluded that 8 weeks of abstinence was best, but the cohort of patients is not entirely transferable [29]. Thoracic surgeons need to weigh the risk of smoking activity with delaying a cancer operation.
TREATMENT STRATEGIES
-
Antibiotic Treatment. The patient needs to be treated for nosocomial bacteria with broad spectrum antibiotics. The choice of antibiotic depends on the center. Prior to initiation of antibiotic therapy blood and sputum cultures should be sent. The most effective way to obtain an airway specimen is by bronchoalveolar lavage (BAL) from the airway with abnormality on CXR or the airway with copious secretions on bronchoscopy.
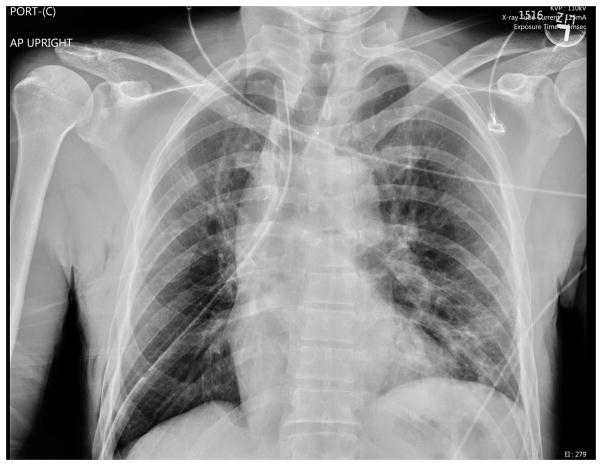
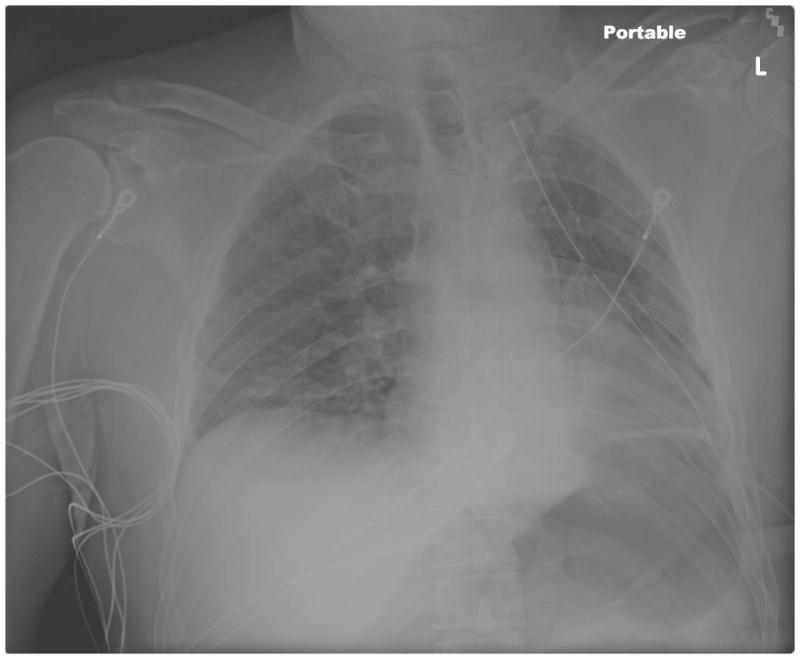
2. Aggressive Chest Physiotherapy. Continue standard post-operative CPT as noted in the prevention strategies remains paramount. The addition of an expectorant and/or chest percussion can improve atelectasis. Therapeutic bronchoscopy to physically remove the mucous plug may also prove necessary. Figures 2, 3, 4 demonstrate the progression of atelectasis to mucous plugging and improvement after therapeutic bronchoscopy. The left lung does not return to its immediate post-operative expansion, but there is significant improvement after bronchoscopy.
Ventilatory Support. If the infection or effect on breathing mechanics is severe enough the patient may require respiratory support with the ventilator as the lung recovers. The goal of ventilator support is to allow effective gas exchange. There are multiple modes of ventilation, the details of which are too broad for this topic.
Figure 2.
Immediate Postoperative Chest X-Ray
Figure 3. Postoperative Mucous Plugging Chest X-Ray.
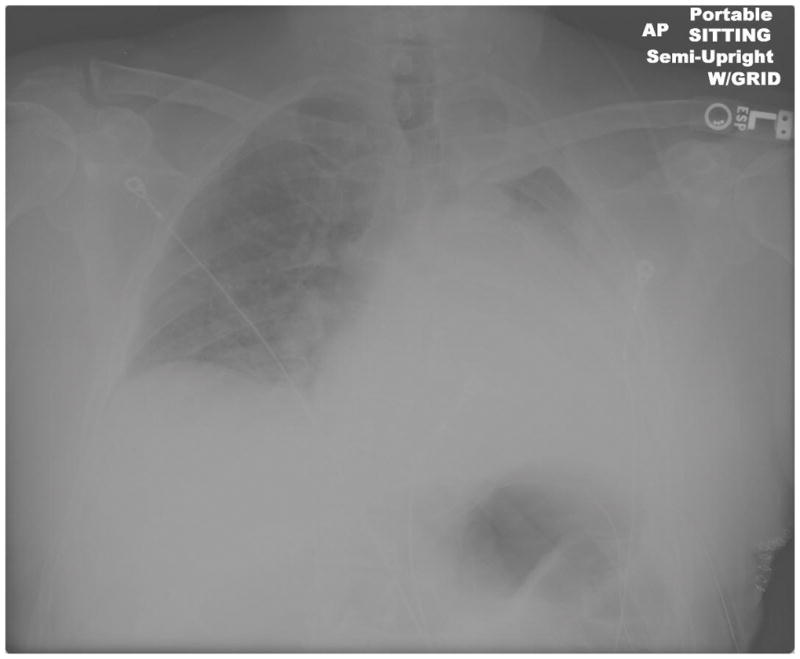
Arrows denote mediastinal shift to the side of the collapse
Figure 4.
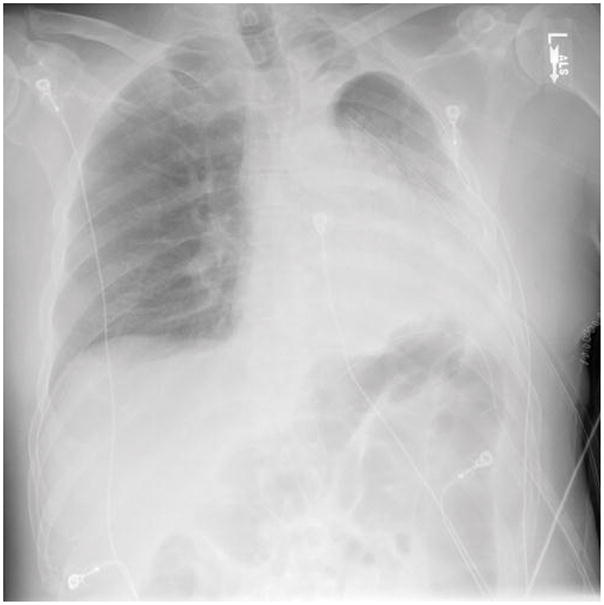
Post-bronchoscopy Chest X-Ray
ATRIAL FIBRILLATION
Atrial fibrillation is the most common arrhythmia following pulmonary resection with an incidence of 10–40% for all pulmonary resections, and 33% for lobectomy [30]. It most commonly occurs on postoperative day 2 or 3, but can occur at any time during recovery [31]. The mechanism of atrial fibrillation after non-cardiac thoracic surgery is unknown, but there are multiple factors associated with developing post-operative atrial fibrillation. Risk factors include: age greater than 70 years old, amount of lung resected (right pneumonectomy carries the greatest risk), incision (clamshell), previous episode of congestive heart failure, male gender, prior arrhythmia and blood transfusions [32].
PREVENTION STRATEGIES
There are strategies to treat atrial fibrillation once it has occurred, but much like air leaks, the best treatment is prevention. Management of fluid balance and electrolyte levels can decrease the incidence of atrial fibrillation. Multiple medications including β-blockers, calcium-channel blockers, amiodarone and digoxin have been used as prophylaxis against the development of atrial fibrillation [33]. Riber et al completed a meta-analysis and found amiodarone and magnesium sulfate to be the most effective and safest drugs as prophylaxis against postoperative atrial fibrillation [34]. Amiodarone had a relative risk of 0.32 and a number needed to treat of 4.8. Overall Riber found the risk of postoperative atrial fibrillation reduced from 25.1% to 13.4 with the use of drug prophylaxis.
The STS published a practice guideline in 2011 which included recommendations for atrial fibrillation prophylaxis [35]. Patients taking β-blockers preoperatively should continue postoperative administration, and titrated as blood pressure tolerates. In patients not taking β-blockers preoperatively, diltiazem and amiodarone should be considered for prophylaxis after a lobectomy. Flecainide and digoxin are not recommended as prophylaxis against postoperative atrial fibrillation [35].
TREATMENT STRATEGIES
If a patient develops atrial fibrillation postoperatively treatment is guided by their hemodynamic stability. Unstable patients need to be transferred to the ICU and electrically cardioverted. The STS practice guidelines further state that hemodynamically stable patients should be chemically cardioverted with rate controlling medication for at least 24 hours. If chemical cardioversion is unsuccessful, electrical cardioversion can be used after 24 hours. The recommended agent of choice for rate control is a β1-selective blocker in the absence of moderate-severe COPD and diltiazem if moderate-severe COPD is present. For rhythm control IV amiodarone or oral flecainide is recommended. Amiodarone should be avoided in ventilated patients, those who have had a pneumonectomy or significant underlying lung disease due to pulmonary toxicity. Flecainide should be avoided with any underlying structural heart condition [35]. Nearly 50% of patients will convert to NSR within 12 hours of rate control [36] with β-blockade or calcium-channel blockers, see Figure 5 for algorithm. If atrial fibrillation is recalcitrant to cardioversion or recurs for >48 hours, anticoagulation should be considered. In patients with two or more risk factors for a stroke (age >75 years, hypertension, impaired left ventricular function, prior stroke or transient ischemic attack), anticoagulation with warfarin is reasonable. For patients with fewer than two risk factors for stroke and patients considered not suitable for warfarin, aspirin, 325 mg daily, is reasonable.
Figure 5. Management of post-operative atrial Fibrillation.
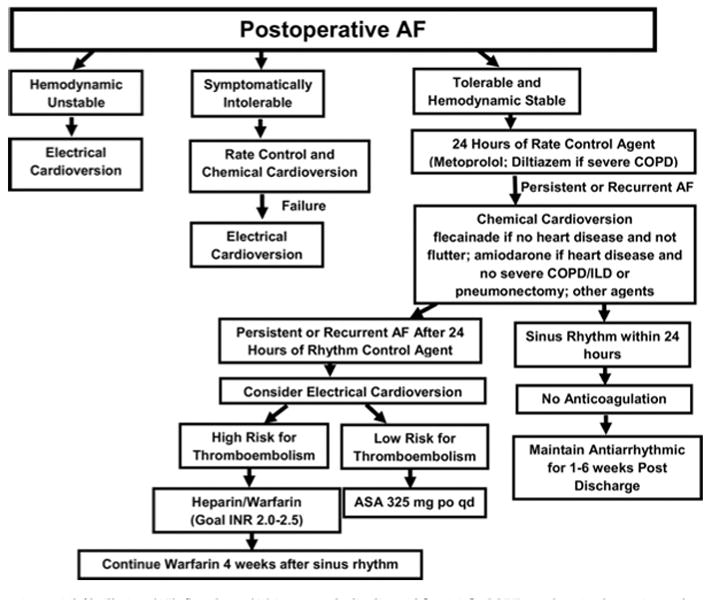
[35]
ASA: acetylsalicylic acid [aspirin]; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; INR: international normalized ratio
RIGHT MIDDLE LOBE TORSION
Postoperative lobar torsion is a rare, but life-threatening complication after pulmonary resection with a prevalence of 0.09–0.4% [37]. Torsion of the right middle lobe (RML) accounts for 70% of cases in the literature. Twisting of the lobe on its pedicle can lead to ischemia, pulmonary infarction and gangrene unless treated promptly. Factors that increase the risk of torsion include a complete fissure, lack of adhesions and complete mobilization of remaining lung [38].
PREVENTION STRATEGIES
Torsion can be prevented with simple tacking of the RML to the remaining right lower or upper lobes [39]. Tacking can be performed with a simple figure-of-eight suture through the visceral pleural to maintain lobar orientation or with an endoscopic stapling device (pneumopexy)[40]. Neither of these techniques is required and visualization of lobar expansion at the conclusion of the operation is adequate. Signs and symptoms of torsion include sudden and unexplained dyspnea and tachypnea refractory to oxygen supplementation, early high fever, and copious secretions (hemorrhagic or clear) [41]. Chest x-ray may demonstrate sudden opacification of previously expanded lobe, see Figures 6 and 7.
Figure 6.
Immediate Post-operative Chest X-Ray
Figure 7. Post-operative Day 1.
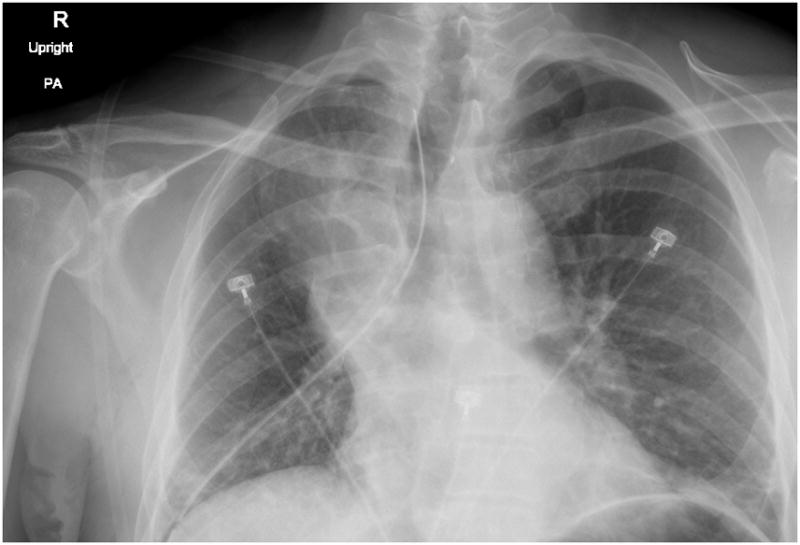
(arrow indicates RML opacification)
Diagnosis is confirmed with bronchoscopy, with visualization of a compressed lobar bronchus with a classic “fish mouth” appearance [42]. CT scan with contrast will demonstrate loss of blood flow and bronchial cut-off. Transesphageal echo may show right heart strain and turbulent flow and possibly thrombus in the pulmonary vein. A high index of suspicion is important and can improve patient outcomes if reoperation is undertaken before ischemia or gangrene occur.
TREATMENT STRATEGIES
Treatment of lobar torsion necessitates a completion lobectomy if ischemic necrosis or gangrene is present. It is important to evaluate for venous thrombosis before untwisting the lobe to avoid a potentially fatal stroke [43]. This can be accomplished with CTA preoperatively and/or intraoperative transesophageal echo. Re-expansion pulmonary edema can also occur and may require prolonged mechanical ventilation and frequent therapeutic bronchoscopy [44]. If the torsion is incomplete it may be possible to preserve the twisted lobe. Pexy of the lobe to the adjacent lobe should be performed with a stapler or suture.
HEMORRHAGE
The incidence of postoperative bleeding after lobectomy requiring at least 4 units of packed red blood cells was found to be 2.9% [45]. If the patient drains greater than 1L in 1 hour or 200mL/hr for 4 hours coagulation labs need to be checked, and, if normal, reexploration is indicated. Meticulous inspection of all dissection fields and incision sites prior to closing and ensuring hemostasis can help avoid postoperative hemorrhage that requires reexploration. There are multiple sources for postoperative bleeding. Sirbu et al reported the possible sources as mediastinal or bronchial vessel (23%), intercostal vessel (17%), pulmonary vessel (17%), and unidentifiable (41%) [46]. A systematic approach is best to inspect all areas of the thorax when reexploration is undertaken for hemothorax including the lymph node beds, the inferior pulmonary ligament and all staple lines. A dental mirror can be useful to get a full view of all aspects of incisions and chest tube sites.
CHYLOTHORAX
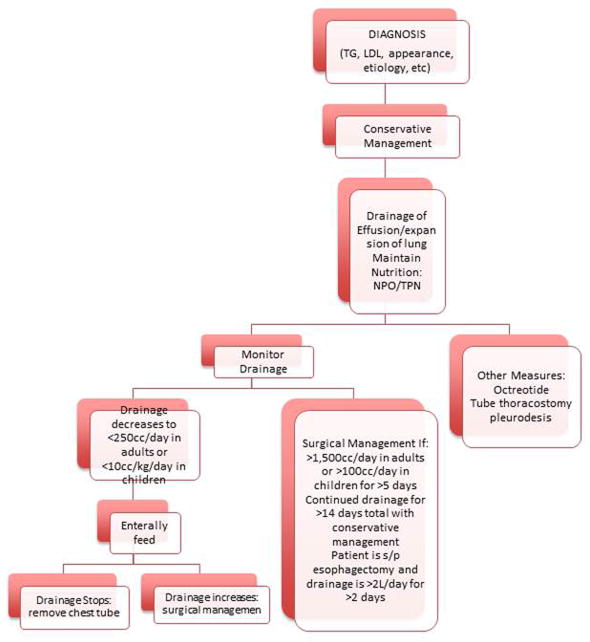
Chylothorax occurs due to injury of the thoracic duct. The incidence of chylothorax with pulmonary resection is 0.7–2% [48]. Aggressive mediastinal lymph node dissection and incomplete ligation of lymph node channels can lead to development of chylothorax. Diagnosis and treatment algorithm is described in Figure 8. Triglyceride level >110mg/dL and lymphocyte count >90% are diagnostic. Attempts at conservative management for 5–7 days is accepted as long as the chest tube drainage decreases and the patient’s nutritional balance can be maintained. Indications for surgical intervention include: drainage of >1,500cc/day in adults or >100cc/day in children for >5 days, continued drainage for >14 days total despite maximal medical management, or if the patient is s/p esophagectomy and drainage is >2L/day for >2 days [47]. Surgical management involves accessing the right chest and ligating the thoracic duct as low as possible. Direct repair is very challenging due to difficulty in locating the injury directly.
Figure 8. Management Algorithm for Chylothorax.
TG=triglyceride, NPO=nothing per os, TPN=total parenteral nutrition, s/p=status post [47] Ziarnik E, Nesbitt J.
NERVE INJURY
Phrenic Nerve
A variety of thoracic surgical procedures can lead to phrenic nerve injury. When focusing on lobectomy there is a higher risk of injury in the setting of adhesions to the pericardium and at the time of mediastinal lymph node dissection. Symptoms include dyspnea on exertion and diminished activity tolerance. Chest X-ray would demonstrate an elevated hemidiaphragam ipsilateral to the lobectomy. Diagnosis can be confirmed with a fluoroscopic sniff test, which would demonstrate paradoxical movement of the diaphragam on the side of injury. Depending on the degree of respiratory impairment, expectant management may be tolerated. If the patient is unable to maintain quality of life due to breathlessness then diaphragm plication is indicated after one year with no return of function.
Recurrent Laryngeal Nerve
Injury to the recurrent laryngeal nerve (RLN) can be detected very early postoperatively and should be suspected in patients with weak voices, hoarseness and weak cough. As the patient is transitioned to a normal oral diet during recovery they may also experience aspiration after taking liquids. The symptoms are related to glottic incompetence and culminate in diminished airway protection and increased risk of pulmonary complications [49]. Diagnosis is made with direct fiberoptic laryngoscopy which will demonstrate lack of movement of the effected cord with phonation. Once vocal cord dysfunction is confirmed the next step is to determine if it is temporary or permanent. If the nerve was knowingly or purposefully injured during the conduct of the lobectomy, there is no need for further investigation. Treatment options include vocal cord injection or laryngeal framework surgery to medialize the paralyzed cord [50]. Early intervention most often involves collagen injection to medialize the cord and re-establish a competent glottis. Timing of intervention should be a joint decision between the thoracic surgeon and otolaryngologist. Early medicalization can reduce pulmonary complications by reducing aspiration, improving cough and pulmonary toilet [51].
SUMMARY
Immediate post operative complications are common after lobectomy. Prevention strategies in the operating room and in the early post operative setting can minimize these risks. However, when they occur, a proactive approach may minimize the long term sequelae.
Acknowledgments
Support: Dr. Grogan is a recipient of the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service Career Development Award (10-024). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Contributor Information
Elena Ziarnik, Resident in Thoracic Surgery, Department of Thoracic Surgery, Vanderbilt University Medical Center
Eric L. Grogan, Tennessee Valley Healthcare System, Nashville Campus, Assistant Professor, Thoracic Surgery, Vanderbilt University Medical Center
References
- 1.Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the national cancer database. Ann of Thorac Surg. 2014 doi: 10.1016/j.athoracsur.2014.07.007. Article in Press. [DOI] [PubMed] [Google Scholar]
- 2.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–9. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Annals of Thoracic Surgery. 2006;81(3):1013–9. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Rice TW, Kirby TJ. Prolonged air leak. Chest Surg Clin North Am. 1992;2:802–811. [Google Scholar]
- 5.Cerfolio RJ, Tummula RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66:1726–173. doi: 10.1016/s0003-4975(98)00958-8. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bass CS, Katholi C. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001;71:1613–1617. doi: 10.1016/s0003-4975(01)02474-2. [DOI] [PubMed] [Google Scholar]
- 7.Babu AN, Mitchell JD. Technique of muscle flap harvest for intrathoracic use. Operative Tech in Thorac and Cardiovasc Surg; A Comparative Atlas. 2010;15:41–52. [Google Scholar]
- 8.Uzzaman MM, Daniel RJ, Mhandu PC, et al. A meta-analysis assessing the benefits of concomitant pleural tent procedure after upper lobectomy. Ann Thorac Surg. 2014;97:365–72. doi: 10.1016/j.athoracsur.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Fell SC, DeCamp MM. Technical aspects of lobectomy: General Thoracic Surgery. 2009;28:425–427. [Google Scholar]
- 10.Hazelrigg S, Boley TM, Naunheim KS, et al. Effect of bovine pericardial strips on air leak after stapled pulmonary resection. Ann Thorac Surg. 1997;63:1573–1575. doi: 10.1016/s0003-4975(97)00126-4. [DOI] [PubMed] [Google Scholar]
- 11.Stammberger UZ, Klepetko W, et al. Buttressing the staple line in lung volume reduction surgery; a randomized three-center study. Ann Thorac Surg. 2000;70:1820–5. doi: 10.1016/s0003-4975(00)01903-2. [DOI] [PubMed] [Google Scholar]
- 12.Itoh E, Matsuda S, Yaauchi K, et al. Synthetic absorbable film for prevention of air leaks after stapled pulmonary resection. J Biomed Mater Res. 2000;53(6):640–5. doi: 10.1002/1097-4636(2000)53:6<640::aid-jbm4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Fischel RJ, McKenna RJ. Bovine pericardium versus bovine collagen to buttress staples for lung reduction operations. Ann Thorac Surg. 1998;65:217–219. doi: 10.1016/s0003-4975(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Caro A, Roca-Calvo MJ, Lanzas JT, et al. The approach of the fused fissure with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg. 2007;31:203–8. doi: 10.1016/j.ejcts.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Temes RT, Willms CD, Santiago AE, et al. Fissureless lobectomy. Ann Thorac Surg. 1998;65:282–284. doi: 10.1016/s0003-4975(97)01268-x. [DOI] [PubMed] [Google Scholar]
- 16.Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection; meta-analysis. Ann Thorac Surg. 2010;90:1779–85. doi: 10.1016/j.athoracsur.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Tansley P, Al-Mulhim F, Lim E, et al. A prospective, randomized, controlled trial of the effectiveness of Bioglue in treating alveolar air leaks. J Thorac Cardiovasc Surg. 2006;132:105–112. doi: 10.1016/j.jtcvs.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Belda-Sanchis J, Serra-Mitjan M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane database Syst Rec. 2010;1:CD003051. doi: 10.1002/14651858.CD003051.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001;71:1613–1617. doi: 10.1016/s0003-4975(01)02474-2. [DOI] [PubMed] [Google Scholar]
- 20.Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. J Thorac Cardiovasc Surg. 1992;104:1456–1464. [PubMed] [Google Scholar]
- 21.Deschamps C, Bernard A, Nichols FC, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg. 2001;72:243–248. doi: 10.1016/s0003-4975(01)02681-9. [DOI] [PubMed] [Google Scholar]
- 22.Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg. 2001;72:1662–17667. doi: 10.1016/s0003-4975(01)03096-x. [DOI] [PubMed] [Google Scholar]
- 23.Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg. 1992;104:1456–1464. [PubMed] [Google Scholar]
- 24.Deslauriers J, Ginsberg RJ, Piantadosi S, et al. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106:329S–330S. doi: 10.1378/chest.106.6_supplement.329s. [DOI] [PubMed] [Google Scholar]
- 25.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 26.Luketich JD, Land SR, Sullivan EA. Thoracic epidural versus intercostal catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg. 2005;79:1845–1850. doi: 10.1016/j.athoracsur.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 27.Bonde P, McManus K, McAnespie M, et al. Lung surgery: identifying the subgroup at risk for sputum retention. Eur J Cardiothorac Surg. 2002;22:18–22. doi: 10.1016/s1010-7940(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa M, Tanaka H, Tsukuma H, et al. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001:705–710. doi: 10.1378/chest.120.3.705. [DOI] [PubMed] [Google Scholar]
- 29.Warner MA, Divertie MB, Tinker JH. Preoperative cessation of smoking and pulmonary complications in coronary artery bypass patients. Anesthesiology. 1984;60:380–383. doi: 10.1097/00000542-198404000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Roselli EE, Murthy SC, Rice TW, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg. 2005;130:438–444. doi: 10.1016/j.jtcvs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Curtis JJ, Parker BM, McKenney CA, et al. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann Thorac Surg. 1998;66:1766–1771. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 32.Asamura H, Naruke T, Tsuchiya R, et al. What are the risk factors for arrhythmias after thoracic operations? A retrospective multivariate analysis of 267 consecutive thoracic operations. J Thorac Cardiovasc Surg. 1993;106:1104–1110. [PubMed] [Google Scholar]
- 33.Sedrakyan A, Treasure T, Browne J, et al. Pharmacologic prophylaxis for postoperative atrial tachyarrhythmia in general thoracic surgery: evidence from randomized clinical trials. J Thorac Cardiovasc Surg. 2005;129:997–1005. doi: 10.1016/j.jtcvs.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Riber LP, Larsen TB, Christensen TD, et al. Postoperative atrial fibrillation prophylaxis after lung surgery: systematic review and meta-analysis. Ann Thorac Surg. doi: 10.1016/j.athoracsur.2014.06.069. In press. [DOI] [PubMed] [Google Scholar]
- 35.Fernando HC, Jaklitsch MT, Walsh GL, et al. The STS practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: an executive summary. Ann Thorac Surg. 2011;92:1144–52. doi: 10.1016/j.athoracsur.2011.06.104. [DOI] [PubMed] [Google Scholar]
- 36.Amar D. Postoperative atrial fibrillation. Heart Dis. 2002;4:117–123. doi: 10.1097/00132580-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Apostolakis E, Koletsis EN, Panagopoulos N, et al. Fatal stroke after completion pneumonectomy for torsion of left upper lobe following left lower lobectomy. J Cardiothorac Surg. 2006;1:25. doi: 10.1186/1749-8090-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones JM, Paxton LD, Graham AN. Acute postoperative lobar torsion associated with pulmonary arterial rupture. J Thorac Cardiovasc Surg. 2003;126(1):303. doi: 10.1016/s0022-5223(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 39.Kutlu CA, Olgac G. Pleural flap to prevent lobar torsion: a novel technique. Eur J Cardiothorac Surg. 2006;30:943–944. doi: 10.1016/j.ejcts.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Fell SC, DeCamp MM., Jr Chapter 28:Technical aspects of lobectomy; General thoracic surgery. 2005;7:425–427. [Google Scholar]
- 41.Ponn R. Complications of pulmonary resection. General Thoracic Surgery. 2005;9:566–67. [Google Scholar]
- 42.Schamaun M. Postoperative pulmonary torsion: report of a case and survey of the literature including spontaneous and posttraumatic torsion. Thorac Cardiovasc Surg. 1994;42:116–21. doi: 10.1055/s-2007-1016469. [DOI] [PubMed] [Google Scholar]
- 43.Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg. 2006;47:609–612. [PubMed] [Google Scholar]
- 44.Sakai M, Kurimori K, Seaki Y, et al. Video-assisted thoracoscopic conservative repair of postoperative lobar torsion. Ann Thorac Surg. 2014;98:e119–e121. doi: 10.1016/j.athoracsur.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 45.Harpole DH, Jr, DeCamp MM, Jr, Daley J, et al. Prognostics models of thirty-day mortalityand morbidity after major pulmonary resection. J Thorac Cardiovasc Surg. 1999;117:969–979. doi: 10.1016/S0022-5223(99)70378-8. [DOI] [PubMed] [Google Scholar]
- 46.Sirbu H, Busch T, Aleksic I, et al. Chest reexploration for complications after lung surgery. Thoracic Cardiovasc Surg. 1999;47:73–76. doi: 10.1055/s-2007-1013114. [DOI] [PubMed] [Google Scholar]
- 47.Ziarnik E, Nesbitt J. Chylothorax after esophageal surgery. Gastrointestinal, Hepatobiliary, and Pancreatic Surgery: A Procedure Based Guide for Complex Peri-operative Situations and Complications. In Press. [Google Scholar]
- 48.Kutlu CA, Sayar A, Olgac G, et al. Chylothorax: a complication following lung resection in patients with NSCLC: chylothorax following lung resection. Thorac Cardiovasc Surg. 2003;51:342–345. doi: 10.1055/s-2003-45423. [DOI] [PubMed] [Google Scholar]
- 49.Murty GE, Kelly PJ, Bradley PJ. Tussometry: an objective assessment of vocal cord function. Ann Otolarygolofy, Rhinology and Laryngology. 1993;102:743–7. doi: 10.1177/000348949310201001. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharyya N, Batirel H, Swanson SJ. Improved outcomes with early vocal fold medicalization for vocal fold paralysis after thoracic surgery. Auris Nasus Larynx. 2003;30:71–75. doi: 10.1016/s0385-8146(02)00114-1. [DOI] [PubMed] [Google Scholar]
- 51.Mom T, Filaire M, Advenioer D. Concomitant type 1 thyroplasty and thoracic operations for lung cancer: preventing respiratory complications assosciated with vagus or recurrent laryngeal nerve injury. J Thorac Cardiovasc Surg. 2001;121:642–8. doi: 10.1067/mtc.2001.112533. [DOI] [PubMed] [Google Scholar]