Abstract
The spectrum of vascular inflammatory disease ranges from atherosclerosis and hypertension, widespread conditions affecting large proportions of the population, to the vasculitides, rare syndromes leading to fast and irreversible organ failure. Atherosclerosis progresses over decades, inevitably proceeding through multiple phases of disease and causes its major complications when the vessel wall lesion ruptures, giving rise to lumen-occlusive atherothrombosis. Vasculitides of medium and large arteries progress rapidly, causing tissue ischemia through lumen-occlusive intimal hyperplasia. In both disease entities, macrophages play a decisive role in pathogenesis, but function in the context of other immune cells that direct their differentiation and their functional commitments. In atherosclerosis, macrophages are involved in the removal of lipids and tissue debris and make a critical contribution to tissue damage and wall remodeling. In several of the vasculitides, macrophages contribute to granuloma formation, a microstructural platform optimizing macrophage-T cell interactions, antigen containment and inflammatory amplification. By virtue of their versatility and plasticity, macrophages are able to promote a series of pathogenic functions, ranging from the release of cytokines and enzymes, the production of reactive oxygen species, presentation of antigen and secretion of tissue remodeling factors. However, as short-lived cells that lack memory, macrophages are also amendable to reprogramming, making them promising targets for anti-inflammatory interventions.
Keywords: cytokine, efferocytosis, giant cell, macrophage polarization, reactive oxygen species
1. Introduction
Macrophages are resident phagocytic cells, which are critically involved in host defense by regulating protective inflammatory responses as well as tissue repair and healing. More recent evidence places macrophages at the center of steady-state tissue homeostasis, dealing with waste products and tissue regeneration. Macrophages are equipped with pattern recognition receptors (PRRs), which interact with pathogen-associated molecular patterns, enabling them to efficiently phagocytose pathogens and infected cells as well as secrete defense-relevant mediators and inflammatory cytokines (1, 2). Macrophages have further roles as antigen-presenting cells, effectively bridging innate and adaptive immunity. In an elegant feed-forward loop, the activation of antigen-specific T cells results in the amplification of macrophage response (1).
Macrophages are located in all organs to detect, ingest and process debris, dead cells and foreign materials and are numerous in chronically inflamed, nonhealing lesions, such as the atherosclerotic plaque. Macrophages are now recognized as key players in the pathogenesis of atherosclerosis, contributing to all stages of the disease process (3). At the same time, macrophages are critical components in vasculitides, aggressive inflammatory diseases that occur within and around blood vessels and lead to severe vascular damage and tissue ischemia. In several of the vasculitic syndromes, granuloma formation can occur, with highly activated macrophages and surrounding T cells forming complex lymphoid microstructures. How the microenvironment of the vascular wall affects macrophage differentiation and activation is not understood. Similarly, how macrophages shape inflammatory responses that occur in the vasculature is only partially known. Vascular inflammatory disease forms a spectrum, with smoldering, slowly progressive inflammatory wall damage typical for atherosclerosis, which progresses slowly over decades. Vasculitides, which lead to life-threatening complications within days to weeks, represent the other end of the spectrum. Highly inflammatory, tissue-destructive macrophages participate in the entire spectrum of vessel wall inflammatory disease. Vascular disease remains one of the major killers in the Western world, absorbing much of the health care costs spent on chronic disease. Insights gained from one disease condition on the spectrum of vascular inflammatory disease may have major impact on understanding how abnormal immuno-inflammation can be regulated. In this article, we have reviewed the current knowledge on how macrophages can misfunction and miscommunicate to threaten vascular integrity and blood supply to dependent organ systems.
2. Recruitment of macrophage to vascular inflammatory lesions
Macrophages represent a major component of vessel wall infiltrates, where they may form granulomatous arrangements (Table 1). It is well known that macrophages can live in healthy tissues for extended time periods, and multiple subsets of tissue-residing macrophages have been identified, such as microglia, dermal macrophages, and splenic marginal zone and metallophilic macrophages (4). In contrast with such resident macrophages in which primitive yolk-sac derived macrophages can be a precursor, inflammatory conditions recruit circulating monocytes and develop them into macrophages (4, 5). In mice, splenic hematopoietic stem and progenitor cells that originate from bone marrow niches can be an extramedullary myelopoietic source of monocytes, which are then available for recruitment to inflammatory sites, including atherosclerotic lesions (6).
Table 1.
Vascular inflammation associated with macrophage
| Disease | Vessel size | Localization of Macrophages | Granulomatous changes | Reference |
|---|---|---|---|---|
| Atherosclerosis | Intima Adventitia |
Absent | (3, 7, 11, 12, 51, 92, 111, 132, 145) | |
| Hypertension | Adventitia | Absent | (10, 15, 146) | |
| Pulmonary hypertension | Adventitia | Absent | (58, 96) | |
| Abdominal aortic aneurysm | Adventitia | Absent | (10, 59, 63, 75, 83) | |
| Giant cell arteritis | Large and medium | Adventitia Media |
Present | (27, 28, 56, 57, 85, 147) |
| Takayasu arteritis | Large and medium | Adventitia Media |
Present | (65, 148) |
| Polyarteritis nodosa | Medium | Transmural | Absent | (62) |
| Kawasaki Disease | Medium | Coronary artery | Present | (64, 77, 84, 116) |
| ANCA-associated vasculitis | Small | Glomeruli | Present | (62, 108–110) |
| Anti-GBM disease | Small | Glomeruli | Absent | (149) |
| Cryoglobulinemic vasculitis | Small | Glomerular tuft | Absent | (150) |
| Primary angiitis of the central nervous system | Transmural | Present | (147) | |
| Thromboangiitis Obliterans | Intima, Thrombi | Absent | (118, 151) |
ANCA, anti-neutrophil cytoplasmic antibody; GBM, glomerular basement membrane.
The current paradigm holds that macrophages differentiate from monocytes once they transition from the circulation into tissue. The steps that regulate monocyte entry into the specialized tissue site of the arterial wall are independent of the source of the cells and depend on the upregulation of molecules that mediate the arrest of circulating monocytes by the leukocyte adhesion cascade on activated endothelial cells (ECs) (7, 8). As for the recruitment of macrophages into vascular inflammatory sites, two pathways with opposing directions are suspected to be relevant; the “inside-out” pathway, taking inflammatory cells from the main endothelial lumen into the wall in a radial pattern (9), and the “outside-in” pathway, in which inflammatory cells enter through the microvessels at the backside of the vascular wall and penetrate towards the macrolumen (10). An important aspect of this discussion is, of course, the size of the affected blood vessel, which dictates the absence or presence of wall microvessels. Determined by body size, human medium and large vessels (including coronary arteries) have such a diameter that the vessel wall requires a separate microvascular support system to secure oxygen and nutrient supply to the vessel; the vasa vasorum system. In contrast, the radius of mouse blood vessels is so small, and the wall thickness is so low, that oxygen and nutrients can easily diffuse into the wall tissues. This fundamental difference between man and mice provides a considerable challenge in translating pathogenic studies from one species to the other.
a. The “Inside-out” model
In the “inside-out” model, injured ECs express surface adhesion molecules and inflammatory mediators that participate in monocyte homing to the endothelium and eventual transmigration into the media (10). This step includes: (a) monocyte influx from the circulating blood because of the activation of ECs and elevation of chemotactic factors; (b) differentiation and activation of macrophages according to the microenvironment in the inflammatory region; and (c) retention of macrophages and amplification of the inflammation (11). Several chemokine receptors (CCR) as well as adhesion molecules expressed on the surface of monocytes have been implicated in facilitating the accumulation of macrophages (12). In atherosclerosis, three major CCR-chemokine pairs are considered to be involved in monocyte transmigration including CCR2-monocyte chemotactic protein 1 (MCP-1), CX3C-chemokine receptor 1 (CX3CR1)-CX3C-chemokine ligand 1 (CX3CL1), and CCR5-CCL5 (13). Genetic depletion of these three pairs led to 90% reduction of atherosclerosis in ApoE−/− mice (14). In addition, monocyte recruitment in the atherosclerotic plaque is enhanced by modified LDL (7). In experimental hypertension, CCR2-mediated responses are reported to be critical to the process of macrophage recruitment (15). Details of these chemokine systems in macrophages have been described in previous reviews (7, 16). Another notable molecule is sphingosine-1-phosphate (S1P), which is a bioactive lipid. Human macrophages express the relevant receptors, S1PR1-4 (17). Among S1PRs, S1PR2 inhibits macrophage migration, and depletion of S1PR2 enhances macrophage recruitment in vivo (18). Conversely, S1PR3 mediates chemotactic effects and promotes macrophage recruitment in atherosclerosis in ApoE−/− mice (19).
b. The “Outside-in” model
The “outside-in” model suggests that vascular inflammation starts from the adventitia and progresses toward the inner direction (10). It has been reported that the adventitial vasa vasorum undergoes marked expansion in atherosclerosis and vasculitis (20–22). Moreover, increased vasa vasorum neovascularization and macrophage presence in early atherosclerotic lesions before plaque neovascularization have been shown (23–25). Also, hypertension is accompanied by infiltration of the adventitia and perivascular adipose tissue by macrophages and other inflammatory immune cells (26). In giant cell arteritis (GCA), the entry of macrophages is in the vasa vasorum, and the prominent characteristic of GCA is that they migrate into an adventitial-intimal direction through tissue space (27). In this vasculitic syndrome, macrophages positioned in the adventitia produce proinflammatory cytokines, while collagenase-producing macrophages accumulate at the intima-media border of the inflamed vessel (28). It has been proposed that adventitial cells recruit macrophages and lymphocytes by altering the matrix and the redox status expression of adhesion molecules (10). Considerable uncertainty remains as to the specifics of adventitial cells that participate in the establishment and the regulation of inflammatory lesions. Notably, human arteries contain a population of endogenous dendritic cells (DCs), which have been named vascular DCs and which reside at the adventitia-media border (29). In the model system of GCA, such vascular DCs have been implicated in providing initial signals to recruit inflammatory cells, activate T cells, and trap inflammatory responses in the vessel wall (30–32). Remarkably, adventitial DCs in human arteries express a vessel-specific pattern of PRRs, imposing artery-specific recognition abilities on different arterial beds (29).
The retention of macrophages in the tissue niche is an indispensable element for continuation of inflammation. According to in vivo experiment using ApoE−/− mice, the chemokine receptor CCR7 is induced in foam cells during atherosclerosis regression, and lesion size and foam cell content are preserved when CCR7 function is abrogated (33). The signals that guide macrophages to exit inflammatory lesions, either by reverse transmigration through the endothelium to the lumen or by migrating through the media to adventitial lymphatics, remain to be determined (7).
3. Macrophage polarization
When activated with different environmental signals, macrophages make a commitment to polarize into distinct functional subpopulations (34). Such macrophage subsets are broadly classified into classically activated macrophage or M1, and alternatively activated macrophage or M2. The M1 differentiation program is defined by responses to the proinflammatory cytokine interferon (IFN)-γ and by the activation of Toll-like receptors (TLRs), such as TLR4 (35). M2 macrophages are further subdivided into M2a (induced by interleukin (IL)-4 or IL-13), M2b (induced by immune complexes in combination with IL-1β or lipopolysaccharide), and M2c (induced by IL-10, transforming growth factor (TGF)-β, or glucocorticoids) (35). M1 macrophages contribute to Th1 responses, and mediate inflammatory and tissue disruptive reactions (34). M2 macrophages manifest Th2-associated effector functions, and are considered anti-inflammatory or tissue repairing cells; expressing IL-10, scavenger receptors (SRs), and mannose receptors (11, 34).
a. Molecular mechanisms of macrophage polarization
Macrophage polarization is regulated by a broad range of contributors, including signaling molecules and transcription factors (reviewed in detail previously) (36, 37). IFN-γ skews macrophage function toward the M1 program via signal transducer and activator of transcription (STAT)1. TLR4 signaling leads to activation of nuclear factor (NF)-κB and interferon regulatory factor (IRF)-3. Activation of NF-κB results in the production of inflammatory mediators, and production of IFN-β through IRF-3 induces IRF-5 and following transcription of cytokines (IL-12, IL-23, tumor necrosis factor (TNF)-α), which contribute to Th1 and Th17 responses (36, 38). IL-4 and IL-13 skew macrophage function toward the M2a program via STAT6, which in turn activates transcription of genes such as Krüppel-like factor (KLF)4, peroxisome proliferator-activated receptor (PPAR)γ, and PPARδ that are associated with M2 macrophage activation (39–44). Importantly, STAT signaling pathway is strictly controlled by suppressor of cytokine signaling; M2a stimuli induce cytokine signaling 1 which inhibit STAT1 (45). Similarly, NF-κB activation is regulated by the KLF family; KLF2 and KLF4 inhibit its activity whereas KLF6 acts cooperatively (36, 46, 47). Interestingly, NF-κB activation itself induce anti-inflammatory genes, which are involved in the resolution of inflammation (48).
b. Polarized macrophages in vascular inflammation
Polarized macrophages contribute to both, atherosclerotic disease and vasculitides and provide a wide spectrum of disease relevant functions (Table 2). In terms of polarization, atherosclerotic lesions contain both M1 and M2 macrophages (49). The phenotype of macrophages in the inflammatory region is not always consistent, rather, they can polarize into different subtypes according to their microenvironmental changes (7). Khallou-Laschet et al. have evaluated the phenotype of macrophages in ApoE−/− mice (50). In these experiments, early atherosclerotic lesions contain mainly M2 macrophages, while more progressed lesions are dominantly infiltrated by M1 macrophages, indicating that the macrophages are polarized according to surrounding inflammation. Stoger et al. have investigated human atherosclerosis, and have demonstrated a prominent and continued presence of both M1 and M2 macrophages during human atherosclerotic plaque development (51). In the plaque shoulders, which are important predilection sites for plaque rupture, M1 macrophages exist as the major subset, while fibrous cap regions have no significant differences in subsets. The authors also found that adventitial macrophages near atherosclerotic lesions are selectively polarized towards the M2 phenotype. Adventitial M2 macrophages outnumber their M1 counterparts by 2- to 3-fold (51). In the late phases of atherosclerosis, M1 macrophages facilitate the formation of the necrotic core and plaque destabilization, which bring about thrombotic events (52, 53). The role of M2 macrophages in atherosclerosis is still controversial. However, the finding that deletion of the transcription factors NR4A1 and KLF4, both of which promote M2 macrophage polarization and inhibit M1 macrophage polarization, results in acceleration of atherosclerosis suggests that pathways that promote M2 polarization of macrophages are primarily protective (7).
Table 2.
Macrophage polarization and vascular inflammation
|
|
||
|---|---|---|
| M1 | M2 | |
| Atherosclerosis | Atherogenic | Atheroprotective and atherogenic |
| Endothelial activation Chemotaxis of inflammatory cells Weakening of cap Oxidative stress Prothrombotic activity Enlargement of necrotic core ? antigen presentation |
Uptake of oxidized lipoproteins Efferocytosis Production of IL-10 and TGF-β Promotion of neoangiogenesis |
|
|
| ||
| Vasculitis | Vasculitogenic | Vasculitogenic |
| Granuloma formation Giant cell formation Endothelial activation Oxidative stress Digestion of elastic tissues Media thinning Intimal hyperplasia ? antigen presentation |
Granuloma formation Giant cell formation Neoangiogenesis Intimal hyperplasia ? efferocytosis |
|
Inflammatory responses in vasculitis are much more pronounced than those observed in atherosclerosis. The clinical correlate is a strong acute phase response in vasculitis, whereas inflammation-induced acute phase responses in atherosclerotic disease (e.g. elevation of C-reactive protein) are subtle (54, 55).
GCA lesions have features of a Th1 response, and both M1 (inducible nitric oxide synthase (iNOS)-positive) and M2 (CD163-positive) macrophages are present in vasculitic temporal arteries (56, 57). Ciccia et al. have proposed that IL-33 is involved in the M2 polarization, because Th2 cytokines (except for IL-33) are not detected in inflamed temporal arteries (56). In pulmonary hypertension, pulmonary arteries of humans, calves, and rats contain increased numbers of CD163-positive cells, particularly in the adventitia (58). Aortic aneurysmal segments, induced by continuous Angiotensin II infusion of ApoE−/− mice, exhibit accumulation of M2 macrophages in regions of medial disruption, predominantly in the adventitia (59).
Ohlsson et al. have reported that serum from AAV patients with anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) induces a macrophage subtype mainly resembling M2c (60). The relevance of this finding is difficult to assess as insufficient data are available to which extent macrophages in AAV patients are biased towards any of the functional subspecializations. In many other clinical conditions characterized by vascular inflammation, detailed analyses of macrophages in the blood vessel lesions and in the circulation are lacking, deeming any effort to define common macrophage-centric abnormalities premature (Table 1).
4. Pathogenic functions of macrophages in vascular inflammation
Pathogenic roles of macrophages in vascular inflammation range from secretion of soluble factors, such as cytokines, growth factors and enzymes, to the production of reactive oxygen species (ROS) (Table 2). Related to their phagocytic capabilities, macrophages can participate in debris removal and efferocytosis and evidence has been presented that they can mediate cytotoxic functions. Finally, macrophages are key players in regulating T cells, through antigen presentation, expression of costimulatory ligands and the release of mediators that modulate lymphocyte function (Figure 1). Especially in atherosclerosis, macrophages ingest the deposited normal and modified lipoproteins, transforming them into cholesterol-laden foam cells. Foam cells persist in plaques and promote disease progression through several mechanisms (7). Also, oxidized cholesterol esters have numerous proinflammatory effects on macrophages, some of them mediated by TLR4 signaling (61).
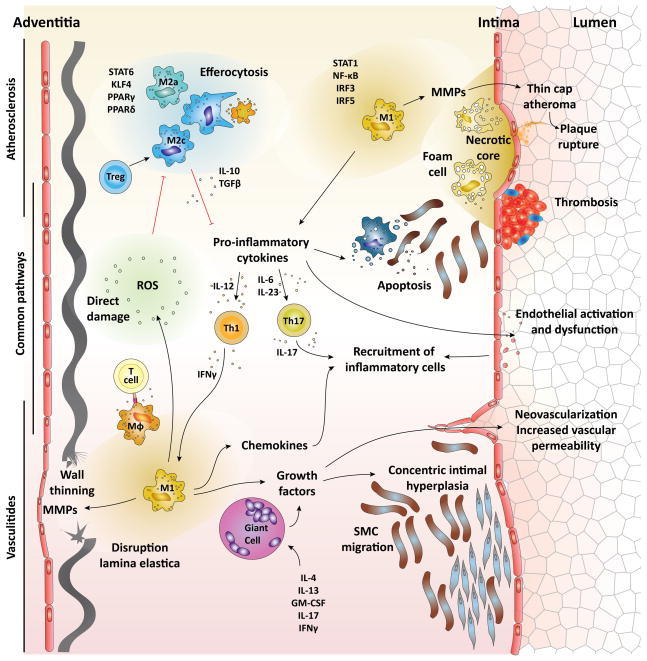
Figure 1.
Pathophysiology of macrophages in vascular inflammation. The major pathophysiology of macrophages in vascular inflammation is outlined. Th1 cytokine IFN-γ and stimulation of TLRs primes macrophages to polarize into M1 macrophages through STAT1, NF-κB, IRF3, and IRF5. Th2 cytokine IL-4 and IL-13 provide differentiation signals for M2a macrophages through STAT6, KLF4, PPARγ and PPARδ. Treg skew macrophages into M2c by IL-10. Proinflammatory cytokines secreted by M1 macrophages induce activation and dysfunction of the endothelium, SMC and macrophage apoptosis, thrombus formation, and differentiation of Th1 and Th17. Recruitment of inflammatory cells into the inflammatory lesions is augmented by the activated endothelium, chemokines, ROS, and IL-17. MMPs solubilize extracellular matrix, resulting in thin cap fibroatheroma and thinning of the wall. M1 macrophages and giant cells produce growth factors. Growth factors induce neovascularization and vascular hyperpermeability, and promote the migration and expansion of SMCs, which results in intimal hyperplasia. ROS directly damages the arterial wall, promotes macrophage recruitment, and impairs efferocytosis. Apoptosis of macrophages and SMCs is a prominent feature of atherosclerosis, and impairment of their clearance results in enlargement of the necrotic core. M2c and efferocytosis produce cytokines such as TGF-β and IL-10, which inhibit inflammatory cell recruitment and suppress the proinflammatory cytokine production, respectively.
4-1. Secreted factors
a. Cytokine
M1 macrophages secrete an armamentarium of proinflammatory cytokines, including IL-1β, IL-6, IL-8, IL-12, IL-23, IL-27, and TNF-α (11). Such M1 macrophage cytokines have been implicated as critical amplifiers of inflammation in the pathogenesis of atherosclerosis, abdominal aortic aneurysms (AAA), GCA, Takayasu arteritis (TAK), Kawasaki disease (KD), and AAV (7, 28, 62–67).
Proinflammatory cytokines manifest their biological effects through a plethora of pathways. First, cytokines, especially TNF-α, restructure the intercellular junctions, which facilitate leukocyte transmigration (66). Cytokines activate ECs and induce endothelial expression of integrin ligands, especially vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1, which lead to the recruitment of more inflammatory cells into the inflammatory lesions (7, 68, 69). In KD, it has been proposed that inflammatory cells recruited by macrophage cytokines damage the ECs and smooth muscle cells (SMCs), initiating complex inflammatory responses underlying vasculitis (64). Such mechanisms may have a role in many of the scenarios presenting as arterial wall inflammation.
M1-derived cytokines bring about endothelial dysfunction by down-regulation of endothelial NOS (eNOS) expression and promotion of oxidative stress through ROS and reactive nitrogen species production (70).
In advanced stages of atherosclerosis, proinflammatory cytokines promote cell apoptosis and matrix degradation, which result in destabilization of atherosclerotic plaques. Particularly IL-1β and TNF-α can induce SMC and macrophage apoptosis and promote Fas-Fas ligand killing (66, 67), inducing tissue injury and accelerating the need for wound healing.
IL-1β and TNF-α increase tissue procoagulant activity and suppress anticoagulant activity mediated by thrombomodulin-protein C (71). Proinflammatory cytokines modify the fibrinolytic properties of EC, by decreasing the production of tissue plasminogen activator and increasing the production of type I plasminogen activator inhibitor (72). Taken together, proinflammatory cytokines have ability to effectuate thrombus formation, which results in acute coronary syndromes, a clinically important complication of atherosclerosis.
Meanwhile, M2-derived cytokines such as TGF-β and IL-10 are considered to have anti-inflammatory effects by inhibiting inflammatory cell recruitment and suppressing the feed-forward loops of proinflammatory cytokine production, respectively (11, 73, 74). Curiously, there is the possibility that M2 macrophages also display proatherogenic functions, as IL-4 induces CD36 expression, which promotes the uptake of oxidized LDL (11).
b. Chemokines
A crucial function of macrophages lies in their ability to secrete chemokines, thus shaping the composition of the inflammatory infiltrate that forms in a tissue site. MCP-1 is highly expressed in atherosclerotic lesions and in the aneurysmal aortic wall, and is involved in both initiation and amplification of monocyte recruitment to the arterial wall layers (75, 76). Macrophage-derived chemokines may represent a major amplification system in vasculitis as well. Sera of patients with a history of KD induce expression of MCP-1, CCR2, and iNOS in THP-1 macrophages in vitro, suggesting feed-forward cycles of macrophage activation (77). In terms of possible signals inducing chemokine production, microRNA-155 has been shown to induce MCP-1 and enhance plaque formation through repressing Bcl6 (78), suggesting abnormalities in cell-internal regulation networks. M2 macrophages are potent producers of CCL18, which can recruit naïve T cells to the inflamed site, giving them a potentially disease-enhancing role (79).
c. Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a major product of macrophages, enabling myeloid cells to actively digest matrix, and their production is also influenced by proinflammatory and anti-inflammatory cytokines (66, 80).
MMPs have been consistently seen in the inflamed arterial wall and have been implicated to contribute to atherosclerosis, AAA, GCA, and KD (66, 80–85). Macrophages are thought to destabilize the atherosclerotic plaque via production and secretion of MMPs, which solubilize extracellular matrix and destroy the fibrous cap (82). The release of MMPs and apoptotic death of SMCs collectively result in the conversion of stable fibroatheromas into vulnerable thin cap fibroatheromas in atherosclerosis and progressive weakness of the aortic wall in AAA (81, 83). Even in GCA, activated macrophages in the intima-media junctions produced MMPs and ROS and played an important role in damaging the medial layer (85). iNOS and MMP9 have been placed at the site of vascular wall inflammation in KD (84).
d. Growth factors
A major pathogenic mechanism in vasculitis is the formation of intimal hyperplasia, occluding the vascular lumen and obstructing blood flow to dependent organs. Neither superficial breakdown of the endothelial layer nor superimposed thrombotic occlusions appear to be relevant in vasculitic tissue ischemia. Growth, migration and secretory activity of SMCs forming the hyperplastic intima depend on appropriate growth factors. Also, the expanding intimal layer needs to be supplied with oxygen and nutrients, necessitating the formation of neomicrovessels. Production of growth factors, such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), has been reported for GCA, TAK and KD (65, 86, 87). VEGF supports increased neovascularization, and PDGF promotes the migration of and expansion of SMCs in GCA and TA. Increased vascular permeability and dilation of coronary arteries, pathognomic events in KD, have been attributed to the excess production of VEGF and PDGF (64).
e. ROS
Oxidative stress is a pathological phenomenon resulting from the imbalance in the production of ROS and the ability of biological systems to detoxify the reactive intermediates. ROS production as a means of attacking pathogens is one of the most important mechanisms through which macrophages protect the host. Excess production of ROS, leading to the damage of membranes, proteins and DNA is believed to play a critical role in vascular disease and convincing evidence indicatess that oxidative stress contributes to atherosclerosis and GCA (85, 88–90). In macrophages, the NADPH oxidase Nox2 is one of the dominant sources of ROS generation and is a signifying product of M1 macrophages (91). Nox2 is by far not the sole source of ROS in macrophages, but Nox4, mitochondria, myeloperoxidase (MPO), xanthine oxidase, lipoxygenase, and the uncoupling of NOS all add to the production of ROS (92). ROS promotes macrophage recruitment and impairs efferocytosis in atherosclerotic plaques (93, 94). Oxidative modifications of LDL particles enhance the proinflammatory functions of lipids (95). In GCA, lipid peroxidation mediated by reactive oxygen intermediates is an important pathway of arterial wall damage (85, 89).
f. Other secreted factors
In pulmonary hypertension, Tian et al. have reported that leukotriene (LT) B4 derived by macrophages acts to injure the endothelium, with inhibition of LTB biosynthesis reversing pulmonary hypertension (96).
4-2. Apoptosis
Apoptosis of macrophages is a noticeable feature of atherosclerosis at all stages; its pathogenic role in vasculitides has not been explored (97–100). It is considered in early atherosclerotic lesions that an increase of macrophage apoptosis causes the attenuation of atherogenesis. On the other hand, impairment of removal for apoptotic macrophages in late stages of atherosclerosis results in the enlargement of the lipid core. Deposition of debris drives proinflammatory responses and furthers apoptotic signals for SMCs, ECs, and leukocytes within the plaques (100). Apoptosis of SMCs in plaque leads to thinning in the fibrous cap, considered an important prerequisite for plaque rupture (101).
Moreover, macrophages within atherosclerotic plaques express Fas-ligand, providing a partner for Fas expressing neighboring cells, and possibly mediating apoptosis of SMCs and ECs (102, 103). Interestingly, the lesional macrophages themselves are considered prone to undergo apoptosis through the Fas pathway (104). NO increases macrophage Fas-ligand and SMC Fas expression, promoting macrophage-induced SMC killing (67, 101). Oxidized lipids can also trigger apoptosis in endoplasmic reticulum-stressed macrophages by a mechanism involving both CD36 and TLR2 (98).
4-3. Phagocytosis and efferocytosis
Phagocytosis protects the arterial tissue from exposure to harmful proinflammatory deposits and immunogenic contents of dying cells, and further limits the overall inflammatory process by restricting lesion growth through postapoptotic necrosis (105). Removal of apoptotic cells, a constant source of proinflammatory signal, is an important tissue protective mechanism, and is called efferocytosis. Insufficient efferocytosis in clearing apoptotic cells from the lesions may function to amplify tissue damage and chronicity of inflammation. In atherosclerosis, M2 macrophages mainly conduct efferocytosis so as to eliminate apoptotic cells and their harmful effects (11, 99). Efferocytosis inhibits macrophages from producing the proinflammatory cytokines through the autocrine/paracrine secretion of TGF-β and IL-10 (11, 106, 107). In advanced experimental atherosclerosis, efferocytosis is typically impaired, and this leads to the assemblage of apoptotic cells which impair the resolution of inflammation (105).
The involvement of efferocytosis of macrophages for the pathogenicity of AAV has been proposed; yet exact mechanisms are still unclear (62). Harper et al. have described that neutrophils from AAV patients show an increased apoptotic rate, and ANCA opsonization of apoptotic neutrophils increases the uptake of neutrophils by macrophages and subsequent secretion of IL-1 and IL-8 (108). Other studies, however, have supported the notion that efferocytosis in AAV is impaired, rather than being hyperactive. van Rossum et al. have suggested a role for pentraxin 3 in delaying macrophage uptake of apoptotic neutrophils in AAV (109). In addition, proteinase 3 (PR3), an autoantigen recognized by ANCA, also seems to impair macrophage efferocytosis when PR3 is externalized during neutrophil apoptosis (110).
Macrophage PRRs, such as the scavenger receptors, CD36, and scavenger receptor-A are intimately involved in the process of apoptotic cell removal (111). Regulation of such PRRs on plaque-residing macrophages may, therefore, represent a critical event in plaque inflammation.
4-4. Giant cells
Fusion of macrophages leads to the formation of multinucleated giant cells, a hallmark of a granulomatous responses (112). Typically, granulomas are formed if the host fails to eliminate antigen. Granulomas display a unique architecture, with highly activated macrophages surrounding a core, that is sometimes necrotic, the most outer layer of the structures are often T cells and granuloma formation is a T cell-dependent mechanism. Giant cells are so typical for GCA that they are part of the disease’s name. In GCA, multinucleated giant cells are often identified along the fragmented internal elastic lamina. They retain secretory activity and are an important source of VEGF (85). The precise mechanism leading to the formation of multinucleated giant cells are still unknown. A multitude of factors, including IL-4 and IL-13, granulocyte-macrophage colony-stimulating factor, IL-17A, IFN-γ and lectins have all been considered capable of promoting the formation of multinucleated giant cells (112).
4-5. Interaction with T cells
M1 and M2 macrophages are often understood as counterparts of Th1 and Th2 cells, respectively. M1 macrophages produce IL-12 and IL-23, which direct the differentiation and expansion of Th1 and Th17 cells (113). Conversely, the Th1 product IFN-γ primes macrophages to differentiate into M1 cells. Also, the Th2 cytokine, IL-4, provides critical differentiation signals for M2 cells. A macrophage-T cell partnership of pathogenic relevance is suspected in atherosclerosis, GCA, TAK, KD, anti-glomerular basement membrane disease, AAV, and thromboangiitis obliterans (TAO) (3, 27, 65, 114–119). In all these conditions, macrophages and T cells colocalize in the disease lesions, supporting the concept that a mutual dependence of both cell types initiates and sustains pathologic inflammation. While there is a growing body of evidence connecting T cells and macrophages, the molecular details and the specific cell populations participating in disease-relevant cross-talk are not understood.
Particularly, IFN-γ exerts various biological effects that are predicted to either promote lesion development or destabilize established lesions in atherosclerosis (3). These effects include stimulation of proinflammatory cytokine and chemokine secretion, and production of ROS and MMPs by macrophages (111). IFN-γ is recognized as a critical factor in GCA, with the vascular lesions having typical features of Th1 lesions (27). IFN-γ–producing T cells are surrounded by highly activated macrophages, and interaction of these two types of cells leads to the formation of the granulomas predominantly in the medial layer of the vascular wall.
As for the participation of Th17 in vascular inflammation, Gan et al. examined experimental murine anti-MPO-induced glomerulonephritis, and found IL-17A secreted by Th17 cells promoted the recruitment of pathogenic macrophages to the inflammatory region in response to MPO as an autoantigen (115). Smith et al. have shown a similar effect of IL-17A on recruitment of macrophages in ApoE−/− mice (114).
In a model of KD using Rag1−/− mice, T cells are required for the development of the arteritis, and the interaction of macrophages and DCs with T cells is necessary for the pathologic manifestations of coronary arteritis (116). Macrophages are common effectors for both CD4 and CD8 T cell-dependent injury in anti-glomerular basement membrane disease (119) and macrophage depletion diminishes the recruitment of T cells to the kidney and provides renal protection (120, 121). Activation of macrophages in the intima in the association with T cells have a key role in thromboangiitis obliterans (117, 118).
5. Macrophages as diagnostic tools
Positron emission tomography (PET) imaging using [18F] fluorodeoxyglucose (FDG) has been applied for the vascular inflammation with the goal to identify high-risk plaques and quantify the disease burden in vasculitides (122, 123). [18F]FDG PET imaging is founded on the excessive demand of glucose in inflammatory cells, particularly macrophages in vascular inflammation. Activated M1 macrophages undergo a switch to glycolysis, which increases the uptake of radiolabeled glucose accumulation (124). PET imaging in combination with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) visualizes FDG uptake in carotid plaques and FDG accumulation is associated with inflammation of atherosclerotic plaques related to macrophage infiltration (125, 126). Because cellular studies in human macrophages have indicated higher metabolic fluorodeoxymannose (FDM) uptake and mannose receptors are upregulated in high-risk plaques in humans, Tahara et al. have utilized radiolabeled mannose, [18F]FDM, to demonstrate superior imaging characteristics for atherosclerosis (122). As for vasculitis, PET may be useful for large vessel vasculitis (123), but CT and MRI based imaging approaches can provide important information on wall structure and blood pooling (54).
However, PET imaging is not a specific procedure to detect macrophages. Ultrasmall superparamagnetic particles of iron oxide (USPIO) that enable visualization of macrophages residing in the plaques using MRI promise a functionally more relevant approach (127, 128). Furthermore, new macrophage-targeted agents have been developed to delineate the disease in terms of biological processes, which are undetectable when using traditional morphological imaging techniques (129). The application of these imaging modalities to vasculitides might lead to further understanding of how macrophages exhibit their pathogenicity.
6. Macrophages as therapeutic targets
Considering the central position of macrophages in the pathogenic events underlying vascular inflammatory disease, macrophages emerge as a promising therapeutic target to selectively suppress damaging immunity in the blood vessel wall (Table 3). Macrophages themselves can be depleted by using pharmacological agents such as mammalian target of rapamycin inhibitors (130). However, depletion of macrophages can have both harmful effects of worsening diseases as well as beneficial effects in decreasing the inflammatory activities in experimental hyperlipidemia (131).
Table 3.
Therapeutic strategies targeting macrophage function
|
|
|
| Depletion of macrophages | Clodronate Nitric oxide donors Mammalian target of rapamycin inhibitors Protein synthesis inhibitors Statins |
|
| |
| Reduction of macrophage recruitment/retention | Prodrug of dexamethasone VCAM-1 inhibitors (e.g. AGI-1067, CAM741) Notch1 TRAF1 TSP-1 |
|
| |
| Suppression of proinflammatory capabilities | Induction of M2 ( IL-4, M-CSF) IL-1β inhibition ( e.g. Canakinumab) Prevention of oxidative damage (e.g. GSH) Resetting efferocytosis ( e.g. IL-10, LXR agonists) Notch inhibitors |
Because the effects of macrophage-depleting reagents are nonspecific, more precise targets need to be identified, with the ultimate goal to eliminate pathogenic macrophages in a highly selective fashion. Macrophage-centric interventions can be divided into two major categories: reducing macrophage recruitment/retention and suppression of proinflammatory capabilities.
6-1. Reducing macrophage recruitment/retention
The adjustment of macrophage recruitment is a fascinating therapeutic approach not only for the treatment itself, but also for the prevention of vascular inflammation (132). In this regard, inhibitors of VCAM-1 synthesis and modulators for the chemokine system, such as the vascular protectant succinobucol (AGI-1067) and a selective inhibitor of VCAM-1 synthesis in ECs (CAM741), have been explored (11). Although such interventions have attenuated atherosclerosis development in animal models, their therapeutic effects on human atherosclerosis are not confirmed yet (133). As a drug, a dexamethasone prodrug can effectively impair macrophage infiltration although its mechanism is not fully understood (134). In addition, Notch1, tumor necrosis factor receptor-associated factor 1, and thrombospondin-1 are reported to be involved in the recruitment of macrophages and might provide elegant options to target macrophage-dependent pathology (63, 135, 136). However, therapeutic strategies targeting macrophage recruitment also need to accommodate their potential harmful side resulting from the disruption of housekeeping functions of macrophages in vascular tissues. Therefore, the timing of intervention seems to be crucial even in regard to inhibiting macrophage recruitment (7).
6-2. Suppression of proinflammatory capabilities
Manipulating factors that inhibit M1 macrophage polarization or promote M2 macrophage polarization has been proposed as a potential therapeutic strategy. Specifically, boosting M2 macrophages could have beneficial effects in accelerating wound healing and stabilizing the vessel wall. A possible approach could be to deliver IL-4 or macrophage colony-stimulating factor to the site-of-interest and facilitate localized induction of M2 macrophages although the resident macrophages, but not recruited macrophages, might be preferentially targeted (7, 137).
The clinical trial, Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), is evaluating the efficacy of IL-1β inhibition in reducing cardiovascular events based on the inflammation hypothesis of atherosclerosis in humans (138). In regard to manipulating ROS production, enhancing an endogenous antioxidant such as glutathione, which prevents oxidative damage, may prove to be more effective in managing human cardiovascular disease (92, 139). Antioxidant strategies in atherosclerosis have proven disappointing in numerous large clinical trials.
Current efforts to reset efferocytotic activity in the atherosclerotic plaque have focused on maintaining levels of efferocytosis using substances such as IL-10 or liver X receptor (LXR) agonists (7). It has been predicted that increasing lipid efflux using LXR agonists or inhibition of microRNA-33 may favorably affect disease-sustaining macrophages (140, 141).
Progress in rectifying macrophage function in vascular inflammation depends on a much better understanding of the factors that control the activities of these cells. An unanswered question is whether the primary abnormalities lay within the pathogenic macrophages themselves or whether the cells are actually normal, but are swayed towards excess inflammatory behavior through microenvironmental cues. A recent study has broadened the view of how the tissue microenvironment can shape the function of immune cells, biasing them towards disease-inducing functional activities. Piggott et al. have reported that disruption of Notch signaling effectively suppressed both T cell and macrophage functions in inflamed human arteries (142). This study suggested that immunostromal communications are relevant in guiding innate and adaptive immune responses in the arterial wall and that such communication pathways are potential therapeutic targets. The uniqueness of the tissue site, being accessible through adventitial vasa vasorum, offers opportunities for developing new molecular approaches in treating inflammatory disease. Bringing together the study of atherosclerosis and vasculitides creates new opportunities to learn from the aggressive inflammatory abnormalities in rare vasculitic conditions and apply new knowledge to the huge patient base that is affected by the inflammatory condition of atherosclerosis. A combination of molecular finesse and technical breakthroughs that permit selective delivery of reagents to the arterial wall will pave the way to test nanoparticles, reconstituted lipoproteins, siRNAs, and small molecule inhibitors to reeducate inflammatory macrophages that have settled in the wall layers of arteries (7, 143, 144).
7. Conclusion
Macrophages are powerful innate immune cells protecting the host from infection and malignancy and are equally sophisticated when it comes to supporting chronic inflammatory lesions. Macrophages are key drivers of vascular inflammation, a spectrum of diseases that ranges from aggressive, life-threatening vasculitis to slowly progressive atherosclerosis. Vasculitides of small blood vessels, e.g AAV, as well as vasculitides of medium and large vessels, such as GCA and TAK, critically depend on pathogenic macrophages. Macrophages occupy the atherosclerotic plaque, at times transforming into the typical lipid-laden foam cells. Macrophages cause tissue damage through a multiplicity of functions, all connected to their inherit ability to rapidly attract other immune cells, release large amounts of tissue-injurious mediators and phagocytose waste and dead cells. Due to their specialization in inflammatory amplification mechanisms, M1 cells are considered the most likely candidates for causing vessel wall inflammation. It is equally possible that a loss of protective macrophage function leaves the host susceptible to nonhealing inflammation and disorganized vessel wall remodeling. To which extent pathogenic macrophages result from faulty microenvironmental signals versus cell indigenous abnormalities is insufficiently understood. Answering this question is critical to develop appropriate therapeutic strategies. The importance of host age, particularly in atherosclerosis, suggests that vascular wall aging is a critical component of disease. Equally important must be determinants imposed by the tissue environment, as all vasculitides and atherosclerosis share the stringency in tissue tropism, meaning that they almost exclusively occur in an anatomically defined part of the vascular tree. Immune cell aging fundamentally changes the functionality of innate and adaptive immune cells. How the tissue aging process affects the propensity to attract and retain inflammatory cells in the vessel wall is unexplored. Exploiting the phagocytic ability of macrophages to load them with specific cargo will provide new avenues for immunomodulatory therapy in restricted tissue sites.
Review criteria.
We searched PubMed for the search terms “ANCA associated vasculitis”, “atherosclerosis”, “giant cell arteritis”, “Kawasaki disease”, “macrophage”, “Takayasu arteritis”, “vascular inflammation”, and “vasculitis”. Publications from the past 10 years were analyzed for pathogenic studies. If appropriate to support scientific concepts, older publications were included. Case reports were not included, unless providing unique insights into pathogenic pathways. The date of the last search was 03 July 2014.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR042547, RO1 HL117913, R01 AI044142, RO1 AI108906 and P01 HL058000 to CMW and R01 AI108891 and R01 AG045779 to JJG). Research studies informing this work received critical support from the Govenar Discovery Fund.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Interest statement No conflict of interest is declared by the authors.
References
- 1.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 9.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 10.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218:1376–1384. doi: 10.1016/j.imbio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 13.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 15.Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, Taylor WR. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Duong CQ, Bared SM, Abu-Khader A, Buechler C, Schmitz A, Schmitz G. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim Biophys Acta. 2004;1682:112–119. doi: 10.1016/j.bbalip.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 20.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis - Quid novi? Thromb Haemost. 2007;97:873–879. [PubMed] [Google Scholar]
- 22.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gossl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia--implications for vulnerable plaque-development. Atherosclerosis. 2007;192:246–252. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 26.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38:20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9:731–740. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weyand CM, Wagner AD, Bjornsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weyand CM, Liao YJ, Goronzy JJ. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J Neuroophthalmol. 2012;32:259–265. doi: 10.1097/WNO.0b013e318268aa9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol. 2005;115:38–46. doi: 10.1016/j.clim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 35.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 36.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 39.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Z, Sun X, Icli B, Wara AK, Feinberg MW. Role of Kruppel-like factors in leukocyte development, function, and disease. Blood. 2010;116:4404–4414. doi: 10.1182/blood-2010-05-285353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 45.Whyte CS, Bishop ET, Ruckerl D, Gaspar-Pereira S, Barker RN, Allen JE, Rees AJ, Wilson HM. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–854. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- 46.Date D, Das R, Narla G, Simon DI, Jain MK, Mahabeleshwar GH. Kruppel-like transcription factor 6 regulates inflammatory macrophage polarization. J Biol Chem. 2014;289:10318–10329. doi: 10.1074/jbc.M113.526749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahabeleshwar GH, Kawanami D, Sharma N, Takami Y, Zhou G, Shi H, Nayak L, Jeyaraj D, Grealy R, White M, et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44. doi: 10.3389/fphys.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371:50–57. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abd TT, Eapen DJ, Bajpai A, Goyal A, Dollar A, Sperling L. The role of C-reactive protein as a risk predictor of coronary atherosclerosis: implications from the JUPITER trial. Curr Atheroscler Rep. 2011;13:154–161. doi: 10.1007/s11883-011-0164-5. [DOI] [PubMed] [Google Scholar]
- 56.Ciccia F, Alessandro R, Rizzo A, Raimondo S, Giardina A, Raiata F, Boiardi L, Cavazza A, Guggino G, De Leo G, et al. IL-33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis. 2013;72:258–264. doi: 10.1136/annrheumdis-2012-201309. [DOI] [PubMed] [Google Scholar]
- 57.Wagner AD, Bjornsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 58.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, et al. Adventitial Fibroblasts Induce a Distinct Proinflammatory/Profibrotic Macrophage Phenotype in Pulmonary Hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged Infusion of Angiotensin II in apoE(−/−) Mice Promotes Macrophage Recruitment with Continued Expansion of Abdominal Aortic Aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohlsson SM, Linge CP, Gullstrand B, Lood C, Johansson A, Ohlsson S, Lundqvist A, Bengtsson AA, Carlsson F, Hellmark T. Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin Immunol. 2014;152:10–19. doi: 10.1016/j.clim.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 61.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol. 2013;8:139–160. doi: 10.1146/annurev-pathol-011811-132453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hans CP, Koenig SN, Huang N, Cheng J, Beceiro S, Guggilam A, Kuivaniemi H, Partida-Sanchez S, Garg V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler Thromb Vasc Biol. 2012;32:3012–3023. doi: 10.1161/ATVBAHA.112.254219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin Exp Immunol. 2011;164(Suppl 1):20–22. doi: 10.1111/j.1365-2249.2011.04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev. 2011;11:61–67. doi: 10.1016/j.autrev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 67.Stoneman VE, Bennett MR. Role of Fas/Fas-L in vascular cell apoptosis. J Cardiovasc Pharmacol. 2009;53:100–108. doi: 10.1097/FJC.0b013e318198fe60. [DOI] [PubMed] [Google Scholar]
- 68.Barks JL, McQuillan JJ, Iademarco MF. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–4538. [PubMed] [Google Scholar]
- 69.Wang SC, Kanner SB, Ledbetter JA, Gupta S, Kumar G, Nel AE. Evidence for LFA-1/ICAM-1 dependent stimulation of protein tyrosine phosphorylation in human B lymphoid cell lines during homotypic adhesion. J Leukoc Biol. 1995;57:343–351. doi: 10.1002/jlb.57.2.343. [DOI] [PubMed] [Google Scholar]
- 70.Simionescu M. Cellular dysfunction in inflammatory-related vascular disorders’ review series. The inflammatory process: a new dimension of a 19 century old story. J Cell Mol Med. 2009;13:4291–4292. doi: 10.1111/j.1582-4934.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114:321–327. doi: 10.1016/j.thromres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 72.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008;100:969–975. [PubMed] [Google Scholar]
- 73.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 75.Colonnello JS, Hance KA, Shames ML, Wyble CW, Ziporin SJ, Leidenfrost JE, Ennis TL, Upchurch GR, Jr, Thompson RW. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J Vasc Surg. 2003;38:138–146. doi: 10.1016/s0741-5214(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 76.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 77.Cheung YF, KO, Tam SC, Siow YL. Induction of MCP1, CCR2, and iNOS expression in THP-1 macrophages by serum of children late after Kawasaki disease. Pediatr Res. 2005;58:1306–1310. doi: 10.1203/01.pdr.0000183360.79872.1c. [DOI] [PubMed] [Google Scholar]
- 78.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adema GJ, Hartgers F, Verstraten R, deVries E, Marland G, Menon S, Foster J, Xu YM, Nooyen P, McClanahan T, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 80.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 81.Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399–411. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 83.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 84.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 85.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 86.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–633. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 88.Manea A, Simionescu M. Nox enzymes and oxidative stress in atherosclerosis. Front Biosci (Schol Ed) 2012;4:651–670. doi: 10.2741/s291. [DOI] [PubMed] [Google Scholar]
- 89.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 90.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–1013. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tavakoli S, Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal. 2012;17:1785–1795. doi: 10.1089/ars.2012.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiao M, Zhao Q, Lee CF, Tannock LR, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, Asmis R. Thiol oxidative stress induced by metabolic disorders amplifies macrophage chemotactic responses and accelerates atherogenesis and kidney injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–1786. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 95.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 96.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5:200ra117. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andres V, Pello OM, Silvestre-Roig C. Macrophage proliferation and apoptosis in atherosclerosis. Curr Opin Lipidol. 2012;23:429–438. doi: 10.1097/MOL.0b013e328357a379. [DOI] [PubMed] [Google Scholar]
- 98.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tabas I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr Drug Targets. 2007;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 100.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 101.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 102.Imanishi T, Han DK, Hofstra L, Hano T, Nishio I, Liles WC, Gown AM, Kiener P, Schwartz SM. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002;161:175–175. doi: 10.1016/s0021-9150(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 103.Seshiah PN, Kereiakes DJ, Vasudevan SS, Lopes N, Su BY, Flavahan NA, Goldschmidt-Clermont PJ. Activated monocytes induce smooth muscle cell death: role of macrophage colony-stimulating factor and cell contact. Circulation. 2002;105:174–180. doi: 10.1161/hc0202.102248. [DOI] [PubMed] [Google Scholar]
- 104.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 105.Tabas I. Macrophage apoptosis in atherosclerosis: consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2333–2339. doi: 10.1089/ars.2009.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Harper L, Cockwell P, Adu D, Savage CO. Neutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Kidney Int. 2001;59:1729–1738. doi: 10.1046/j.1523-1755.2001.0590051729.x. [DOI] [PubMed] [Google Scholar]
- 109.van Rossum AP, Fazzini F, Limburg PC, Manfredi AA, Rovere-Querini P, Mantovani A, Kallenberg CG. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004;50:2667–2674. doi: 10.1002/art.20370. [DOI] [PubMed] [Google Scholar]
- 110.Gabillet J, Millet A, Pederzoli-Ribeil M, Tacnet-Delorme P, Guillevin L, Mouthon L, Frachet P, Witko-Sarsat V. Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. J Immunol. 2012;189:2574–2583. doi: 10.4049/jimmunol.1200600. [DOI] [PubMed] [Google Scholar]
- 111.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 2010;21:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, Wong M, Doherty TM, Lehman T, Crother TR, et al. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J Immunol. 2009;183:5311–5318. doi: 10.4049/jimmunol.0901395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee T, Seo JW, Sumpio BE, Kim SJ. Immunobiologic analysis of arterial tissue in Buerger’s disease. Eur J Vasc Endovasc Surg. 2003;25:451–457. doi: 10.1053/ejvs.2002.1869. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi M, Ito M, Nakagawa A, Nishikimi N, Nimura Y. Immunohistochemical analysis of arterial wall cellular infiltration in Buerger’s disease (endarteritis obliterans) J Vasc Surg. 1999;29:451–458. doi: 10.1016/s0741-5214(99)70273-9. [DOI] [PubMed] [Google Scholar]
- 119.Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D’Souza M, Milton G, Holdsworth SR. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109:134–142. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010;298:F248–254. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, Tahara A, Constantinescu CC, Zhou J, Boersma HH, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 123.Blockmans D. PET in vasculitis. Ann N Y Acad Sci. 2011;1228:64–70. doi: 10.1111/j.1749-6632.2011.06021.x. [DOI] [PubMed] [Google Scholar]
- 124.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 125.Jezovnik MK, Zidar N, Lezaic L, Gersak B, Poredos P. Identification of Inflamed Atherosclerotic Lesions In Vivo Using PET-CT. Inflammation. 2014;37:426–434. doi: 10.1007/s10753-013-9755-3. [DOI] [PubMed] [Google Scholar]
- 126.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 127.Sigovan M, Bessaad A, Alsaid H, Lancelot E, Corot C, Neyran B, Provost N, Majd Z, Breisse M, Canet-Soulas E. Assessment of age modulated vascular inflammation in ApoE−/− mice by USPIO-enhanced magnetic resonance imaging. Invest Radiol. 2010;45:702–707. doi: 10.1097/RLI.0b013e3181f16e5a. [DOI] [PubMed] [Google Scholar]
- 128.Kooi ME, V, Cappendijk C, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 129.Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 2013;6:1327–1341. doi: 10.1016/j.jcmg.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Croons V, Martinet W, De Meyer GR. Selective removal of macrophages in atherosclerotic plaques as a pharmacological approach for plaque stabilization: benefits versus potential complications. Curr Vasc Pharmacol. 2010;8:495–508. doi: 10.2174/157016110791330816. [DOI] [PubMed] [Google Scholar]
- 131.Calin MV, Manduteanu I, Dragomir E, Dragan E, Nicolae M, Gan AM, Simionescu M. Effect of depletion of monocytes/macrophages on early aortic valve lesion in experimental hyperlipidemia. Cell Tissue Res. 2009;336:237–248. doi: 10.1007/s00441-009-0765-2. [DOI] [PubMed] [Google Scholar]
- 132.Schulz C, Massberg S. Atherosclerosis-Multiple Pathways to Lesional Macrophages. Sci Transl Med. 2014;6:239ps232. doi: 10.1126/scitranslmed.3008922. [DOI] [PubMed] [Google Scholar]
- 133.Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L’Allier PL, et al. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:1761–1768. doi: 10.1016/S0140-6736(08)60763-1. [DOI] [PubMed] [Google Scholar]
- 134.Yuan F, Tabor DE, Nelson RK, Yuan H, Zhang Y, Nuxoll J, Bynote KK, Lele SM, Wang D, Gould KA. A dexamethasone prodrug reduces the renal macrophage response and provides enhanced resolution of established murine lupus nephritis. PLoS One. 2013;8:e81483. doi: 10.1371/journal.pone.0081483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Missiou A, Kostlin N, Varo N, Rudolf P, Aichele P, Ernst S, Munkel C, Walter C, Stachon P, Sommer B, et al. Tumor necrosis factor receptor-associated factor 1 (TRAF1) deficiency attenuates atherosclerosis in mice by impairing monocyte recruitment to the vessel wall. Circulation. 2010;121:2033–2044. doi: 10.1161/CIRCULATIONAHA.109.895037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kirsch T, Woywodt A, Klose J, Wyss K, Beese M, Erdbruegger U, Grossheim M, Haller H, Haubitz M. Endothelial-derived thrombospondin-1 promotes macrophage recruitment and apoptotic cell clearance. J Cell Mol Med. 2010;14:1922–1934. doi: 10.1111/j.1582-4934.2009.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 139.Callegari A, Liu Y, White CC, Chait A, Gough P, Raines EW, Cox D, Kavanagh TJ, Rosenfeld ME. Gain and loss of function for glutathione synthesis: impact on advanced atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:2473–2482. doi: 10.1161/ATVBAHA.111.229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piggott K, Deng J, Warrington K, Younge B, Kubo JT, Desai M, Goronzy JJ, Weyand CM. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123:309–318. doi: 10.1161/CIRCULATIONAHA.110.936203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pan H, Myerson JW, Hu L, Marsh JN, Hou K, Scott MJ, Allen JS, Hu G, San Roman S, Lanza GM, et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013;27:255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spears LD, Razani B, Semenkovich CF. Interleukins and atherosclerosis: a dysfunctional family grows. Cell Metab. 2013;18:614–616. doi: 10.1016/j.cmet.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014 doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mihm B, Bergmann M, Bruck W, Probst-Cousin S. The activation pattern of macrophages in giant cell (temporal) arteritis and primary angiitis of the central nervous system. Neuropathology. 2013 doi: 10.1111/neup.12086. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Li L, Wang L, Chang Q, Pu J. Comparison of clinical and pathological characteristics of isolated aortitis and Takayasu arteritis with ascending aorta involvement. J Clin Pathol. 2012;65:362–366. doi: 10.1136/jclinpath-2011-200424. [DOI] [PubMed] [Google Scholar]
- 149.Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab Invest. 2011;91:978–991. doi: 10.1038/labinvest.2011.61. [DOI] [PubMed] [Google Scholar]
- 150.Rastaldi MP, Ferrario F, Crippa A, Dell’Antonio G, Casartelli D, Grillo C, D’Amico G. Glomerular monocyte-macrophage features in ANCA-positive renal vasculitis and cryoglobulinemic nephritis. J Am Soc Nephrol. 2000;11:2036–2043. doi: 10.1681/ASN.V11112036. [DOI] [PubMed] [Google Scholar]
- 151.Ketha SS, Cooper LT. The role of autoimmunity in thromboangiitis obliterans (Buerger’s disease) Ann N Y Acad Sci. 2013;1285:15–25. doi: 10.1111/nyas.12048. [DOI] [PubMed] [Google Scholar]