Abstract
Decubitus ulcers occur in an estimated 2.5 million Americans each year at an annual cost of $11 billion to the U.S. health system. Current screening and prevention techniques for assessing risk for decubitus ulcer formation and repositioning patients every 1–2 hours are labor-intensive and can be subjective. We propose use of a Bluetooth-enabled fabric-based pressure sensor array as a simple tool to objectively assess and continuously monitor decubitus ulcer risk.
I. Introduction
Decubitus ulcers (also known as pressure ulcers or bedsores) occur in an estimated 2.5 million Americans each year, at an annual cost of $11 billion to the U.S. health system [1], [2]. In a hospital environment, they often arise as a secondary ailment while the patient is bedridden and being treated for their primary condition. Unfortunately, these ulcers extend a patient’s hospital stay by 6.5 to as many as 15.6 days and can result in much pain, disfigurement, and occasionally death [3]. Patients particularly susceptible to decubitus ulcers include patients who are immobile, bedridden or have decreased nerve function and cannot sense the formation of an ulcer. Elderly patients are at an increased risk for decubitus ulcers because decreases in tissue elasticity amplifies the effects of shear-induced tissue damage [4]. Surgical patients are also at increased risk for decubitus ulcer development because they may be laying in a fixed position on the operating table for hours, during which constant pressure can instigate tissue damage [5], [6].
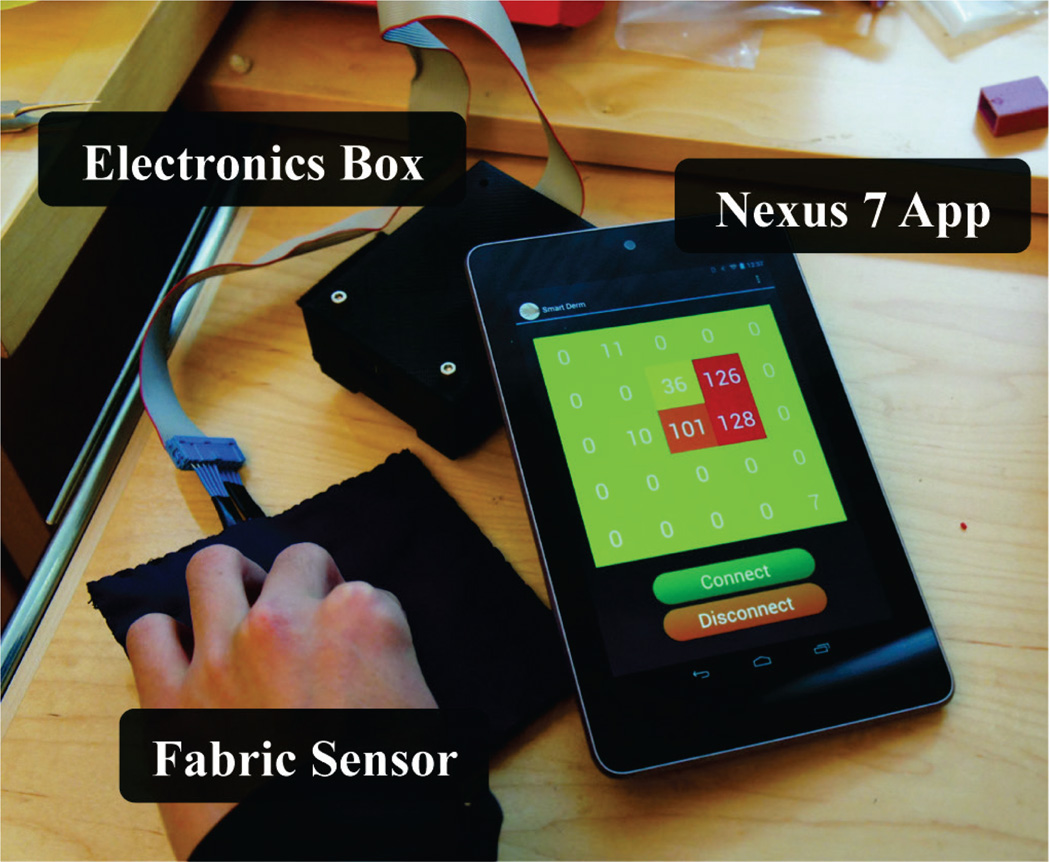
The U.S. Centers for Medicare and Medicaid Services considers decubitus ulcers as a preventable hospital error, and as of 2008, has stopped reimbursing the cost of treatment for stage III and stage IV hospital-acquired ulcers [7]. Because costs incurred from hospital-acquired ulcers must now be absorbed by hospitals, many have instituted standardized procedures, checklists, and ulcer prevention programs. Current screening tools can be subjective and provide risk assessment only at specific snapshots in a patient’s hospital stay [8]. Additionally, prevention techniques such as patient repositioning or use of commercially available pressure redistribution mats are labor intensive and results are often unsatisfactory [2], [9]. We have created a low-cost, wireless, and unobtrusive fabric-based pressure sensor to continuously monitor the tissue status in at-risk areas (Fig. 1).
Figure 1.
Pressure on the fabric sensor is acquired and digitized by the microcontroller in the electronics box, then transmitted over Bluetooth v2.1 to an Anrdoid application running on a Nexus 7 device. The next iteration of this device will involve miniaturization of the electronics box and use of Bluetooth Low Energy technology.
II. Background
A. Pathophysiology
Decubitus ulcers are caused by a combination of pressure, friction, shearing forces, and moisture and most frequently occur over bony prominences such as the back of the head, pelvis, sacrum, greater trochanter of the hip, and heels [6], [10]. Normal tissue capillary blood pressure is typically between 12–32 mmHg [6], [11]. Prolonged pressure can cause occlusion of blood vessels, resulting in local tissue ischemia and eventually tissue death. Friction results from superficial layers of the skin rubbing against another surface (e.g. bed sheets), which leads to skin breakage and the formation of an open wound. Shearing forces involve the sliding of bone and subcutaneous tissue layers when the skin is fixed in place by friction. These forces can pinch off blood vessels and augment tissue damage. Moisture on the skin due to incontinence, perspiration, or excessive wound drainage can cause softening and weakening of the skin, making it easier for breakage of skin [12–14]. When left unattended, these factors cause the formation and progression of decubitus ulcers from Stage I to Stage IV [4], [15].
B. Current Prevention Techniques
Multiple decubitus ulcer risk assessment tools are used worldwide with the most popular being the Braden, Norton, and Waterlow scales [16], [17]. The Braden scale is most popular in the U.S. and involves a 1 to 6 ranking of a patient’s sensory perception, moisture, activity, mobility, nutrition, and friction and shear to generate a composite risk score. While quantitative, scoring can be subjective and studies have shown high variability in scoring between clinicians [8], [16], [17]. Assessment is typically performed during admission, discharge, and changes in the patient’s condition.
Prevention and management of decubitus ulcers involves patient repositioning and pressure redistribution devices. Patient repositioning typically occurs every 1–2 hours to prevent tissue ischemia, though determination of timing is somewhat arbitrary [18], [19]. Patients at high risk for or have already acquired an ulcer are managed with special support surfaces such as foam or gel cushions that relieve or redistribute pressure [9]. Hydrocolloid-or foam-based wound dressings are commonly used upon identification of Stage I pressure ulcers to reduce friction [20]. Special beds and mattresses utilizing air, foam, gel, and water are also used to reduce friction and shear [13], [15].
C. Our Approach
Our approach involves enhancing current clinical workflows by creating a wound dressing with integrated pressure sensor that better quantify clinically relevant factors and monitor decubitus ulcer risk continuously. Evidence in literature shows an inverse relationship between time and pressure in decubitus ulcer formation, meaning that optimal repositioning intervals are different for each patient depending on factors such as body mass index (BMI) and comorbidities [14], [19]. We aim to capture spatial-temporal pressure maps at high-risk locations (e.g. bony prominences) and fuse the data with BMI and co-morbidities. These measurements would be trended over time and eventually combined with predictive analytics to establish an objective tool for determining decubitus ulcer risk. Furthermore, we aim to do this in a low-cost fashion by using a disposable fabric-based transducer that attaches to electronics for data acquisition and wireless transmission.
III. Device
A. Fabric-Based Pressure Sensor Array
Off-the-shelf conductive fabric was used to create a 3-layer variable resistor array that is approximately 4 inches in both length and width (Fig. 2). The top layer consists of alternating thick conductive columns and thin nonconductive columns of Zebra Cloth fabric (Eeontex, Pinole, CA). The middle layer is a single sheet of piezoresistive fabric with 105Ω/sq. range surface resistivity (LR-SL-PA-10E5, Eeontex, Pinole, CA). The bottom layer consists of alternating thick conductive rows and thin nonconductive rows of Zebra Cloth fabric. Each element in the resistive array is defined by the intersection of a column of conductive fabric with a row of conductive fabric. Wires are attached to each row and column of conductive with copper tape on one end and to data acquisition hardware on the other end.
Figure 2.
(A) Each element in a fabric sensor array is connected in series with a 100kΩ resistor to create a variable resistor divider. The microcontroller scans through each element in the m × n array to sample a pressure reading. (B) Top view of fabric sensor array. (C) Side view of fabric sensor array.
B. Electronic Hardware
The sensing hardware comprises of a protoboard with ATMEL 1284P microcontroller (ATMEL, San Jose, CA) and RN-41 Bluetooth v2.1 (Roving Networks, Los Gatos, CA) ICs. Rows in the resistive array are powered to VCC by general purpose IO (GPIO) pins on microcontroller, while columns are connected to the microcontroller’s onboard ADC. Rows and columns are powered such that all conductive paths remain open except for the row and column specifying a single element in the array. This connects the fabric element, which acts as a variable resistor, in series with a 100kΩ resistor to create a variable resistor divider (Fig. 2). The microcontroller firmware scans through all rows and columns to acquire voltage readings, which are proportional to pressure on the fabric element. Data from each element is packaged along with row and column coordinates into a 3-byte packet and wirelessly transmitted to a paired Nexus 7 (Google, Mountain View, CA) tablet over a Bluetooth link.
C. Software
Data is unpackaged and visualized in an Android 4.1 (“Jelly Bean”) application written for the Nexus 7 tablet (Fig. 3). The application comprises of UI buttons to establish or disconnect a wireless link to sensor hardware. Upon establishing a connection, incoming pressure data is visualized on a the screen as a grid of squares, which indicate the numerical pressure value and change the background color of squares proportional to the intensity of pressure. The application is multithreaded to allow for simultaneous data receiving and UI updating.
Figure 3.
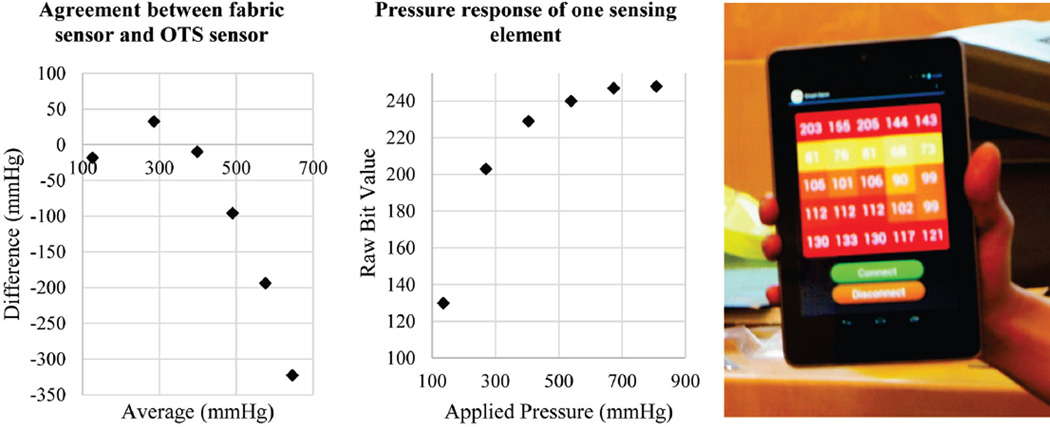
Left: A Bland-Altman plot showing agreement of fabric sensor to a commercial sensor. Middle: Extended pressure response of a single sensing element showing limitations in dynamic range. Raw bit values from measurement range from 0–255. Right: Measurement output when the fabric sensor is placed on a cushioned chair and a human sits on the sensor. Raw bit values on each square represent pressure values from the sensor.
IV. Calibration
Calibration was performed by placing the fabric-based sensor on top of a commercial scale (HBF-510W, Omron, Kyoto, Japan). The fabric-based sensor itself was not heavy enough to register a weight measurement. Sensing elements in the fabric array were individually loaded and applied pressure was computed by using the cross-sectional area of the sensing element and the weight registered on the scale. Pressure values were compared to the output from the sensing element. Fig. 3 shows a Bland-Altman analysis, as well as raw sensor output over an extended range.
V. Discussion
Prior experiments applying external pressure over the greater femoral trochanter in swine have shown muscle damage when 500 mmHg of pressure is applied for 4 hours and skin damage when 800 mmHg is applied for 8 hours [21]. The same experiments also found that skin breakdown did not occur when pressures of 200 mmHg were applied for 15 hours. While our sensor can produce measurements within this range, it becomes non-linear beyond approximately 300 mmHg and thus has reduced sensitivity. Data produced while sitting on the sensor shown in Fig. 3, indicates that our sensor can capture a relevant range of values for monitoring decubitus ulcer risk in human subjects.
Our device enables rapid capturing of pressure data in a simple and inexpensive manner. The fabric sensor is approximately the same size as commonly used wound dressings. Our intent is to eventually integrate this sensor technology into foam or hydrocolloid wound dressings used to reduce friction when a stage I pressure ulcer is identified. The fabric-based transducer is detachable and disposable while the electronic hardware can be reprocessed and reused. Because ulcer formation is typically constrained to specific bony prominences on the patient’s body, a pressure sensor of this size is adequate since the clinician can select high-risk locations depending on the patient’s positioning. The sensor data will not only indicate if there is excessive pressure at a particular location, but can also indicate whether the patient has shifted their weight off of the sensor entirely. Either case may warrant patient repositioning depending on the specific use case.
Operating room usage is particularly compelling because rates for decubitus ulcers acquired in surgery are much higher than those acquired elsewhere in the hospital [3], [5]. During many surgeries, patients are typically immobile for long periods of time, but it is inconvenient for surgeons to reposition the patient. However, if adequate warning of decubitus ulcer formation was feasible, it may be possible for surgeons to schedule patient repositioning during a surgery on an as-needed basis.
Compared to existing devices such as pressure relieving mattresses, our device does not require substantial hospital investment in new infrastructure since the fabric-based sensor would be disposable. Existing pressure sensor pads and sheets typically have higher resolution, but are very expensive ($5000–10000/pad). Furthermore, hospital staffs frequently complain that large mattress-size sensors with an integrated alarm/alert system result in excessive false alarms given the large sensing surface area. In contrast, while our design has limited resolution, it is low-cost (approx. $1/pad), low profile, and highly portable. Because the sensor is always attached to the area of interest, the chance of a false positive alarm can be minimized. The proposed form factor could enable use cross many clinical scenarios and be employed in both operating rooms as well as in patient rooms.
The current device is the first step to realizing our vision for near real-time pressure map monitoring, and subsequent use of the data for prediction and risk assessment of decubitus ulcer formation. However, the current device has several limitations; for example, it is not waterproof. In future designs, we intend to waterproof the sensor by laminating the exposed surface with plastic or synthetic materials. Currently, copper wire is used to interface the fabric sensor to the microcontroller. Compared to fabric, these wires are relatively rigid and could potentially contribute to the development of pressure ulcers. In future design iterations, we will employ conductive thread in place of wires as we examine how to best integrate conductive fabric into a wound dressing. Furthermore, the current electronic hardware is rather large, is not power optimized, and does not yet have data logging capability. In the next iteration, we will employ Bluetooth v4.0 Low Energy to achieve greater power efficiency and size reduction of the electronic hardware. Data logging capability will be added to the software for data collection in an IRB-approved clinical study at our institution. Measurement repeatability and device-to-device variability will also be more thoroughly characterized.
The fabric-based pressure-sensing array may be considered as the physical layer of an overall risk management system, which would also involve fusing pressure measurements with patient-specific factors from their medical record to develop a real-time decubitus ulcer risk index. Such a real-time index could be used to improve decubitus ulcer risk determination and serve as a basis for a more sophisticated alert system for the hospital staff, allowing more individualized treatment while allowing clinicians to focus their energies on treating the patient’s primary ailment.
VI. Conclusion
We have created an inexpensive fabric-based pressure sensor array using off-the-shelf components that can be used to quantify pressure-related risk in decubitus ulcer formation. With the use of pressure and time data for detection along with patient comorbidity data, such a device may enable better and more efficient clinical management of patients who are at risk for decubitus ulcers.
Acknowledgment
The authors would like to thank Dr. Michael Harrison, Dr. Shinjiro Hirose, Dr. Thomas K. Hunt, Dr. Eveline Shue, Dr. Steven Kim, Susan Barbour, and Shelley Diane for their useful discussion and clinical advice.
Research supported by the Mount Zion Health Fund, the NSF (1240380) and the FDA (2P50FD003793).
References
- 1.Russo A, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers among Adults 18 Years and. 2008;(64):1–9. [PubMed] [Google Scholar]
- 2.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA : the journal of the American Medical Association. 2006 Aug.296(8):974–984. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 3.Schouchoff B. Pressure Ulcer Development in the Operating Room. Critical Care Nursing Quarterly. 2002;25(1):76–82. [Google Scholar]
- 4.Dharmarajan TS, Ugalino JT. Pressure Ulcers: Clinical Features and Management. Hospital Physician. 2002 Mar;:64–71. [Google Scholar]
- 5.Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. Journal of wound, ostomy, and continence nursing : official publication of The Wound, Ostomy and Continence Nurses Society / WOCN. 1999 May;26(3):130–136. doi: 10.1016/s1071-5754(99)90030-x. [DOI] [PubMed] [Google Scholar]
- 6.Price MC, Whitney JD, King CA, Doughty D. Development of a risk assessment tool for intraoperative pressure ulcers. Journal of wound, ostomy, and continence nursing : official publication of The Wound, Ostomy and Continence Nurses Society / WOCN. 2005;32(1):19–30. doi: 10.1097/00152192-200501000-00006. quiz 31–2. [DOI] [PubMed] [Google Scholar]
- 7.Mattie AS, Webster BL. Centers for Medicare and Medicaid Services’ ‘never events’: an analysis and recommendations to hospitals. The health care manager. 2008;27(4):338–349. doi: 10.1097/HCM.0b013e31818c8037. [DOI] [PubMed] [Google Scholar]
- 8.Kottner J, Dassen T. Pressure ulcer risk assessment in critical care: interrater reliability and validity studies of the Braden and Waterlow scales and subjective ratings in two intensive care units. International journal of nursing studies. 2010 Jun.47(6):671–677. doi: 10.1016/j.ijnurstu.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Thorne S, Sauvé K, Yacoub C, Guitard P. Evaluating the pressure-reducing capabilities of the gel pad in supine. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2009;63(6):744–750. doi: 10.5014/ajot.63.6.744. [DOI] [PubMed] [Google Scholar]
- 10.Hartley L. Reducing pressure damage in the operating theatre. British journal of perioperative nursing : the journal of the National Association of Theatre Nurses. 2003 Jun.13(6):249–251. 253–254. doi: 10.1177/175045890301300602. [DOI] [PubMed] [Google Scholar]
- 11.Hedrick-Thompson JK. A review of pressure reduction device studies. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing. 1992 Dec.10(4):3–5. [PubMed] [Google Scholar]
- 12.Niezgoda Ja, Mendez-Eastman S. The effective management of pressure ulcers. Advances in skin & wound care. 2006 Feb;19(Suppl 1):3–15. doi: 10.1097/00129334-200601001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DR. Prevention and treatment of pressure ulcers. Journal of the American Medical Directors Association. 2006 Jan.7(1):46–59. doi: 10.1016/j.jamda.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Kanj LF, Wilking SV, Phillips TJ. Pressure ulcers. Journal of the American Academy of Dermatology. 1998 Apr.38(4):517–36. doi: 10.1016/s0190-9622(98)70113-6. quiz 537–8. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DR. Prevention and treatment of pressure ulcers: what works? what doesn’t? Cleveland Clinic journal of medicine. 2001 Aug.68(8):704–707. 710–714, 717–722. doi: 10.3949/ccjm.68.8.704. [DOI] [PubMed] [Google Scholar]
- 16.O’Tuathail C, Taqi R. Evaluation of three commonly used pressure ulcer risk assessment scales. British journal of nursing (Mark Allen Publishing) 2011;20(6):S27–S28. doi: 10.12968/bjon.2011.20.Sup2.S27. S30, S32 Passim. [DOI] [PubMed] [Google Scholar]
- 17.Anthony D, Parboteeah S, Saleh M, Papanikolaou P. Norton, Waterlow and Braden scores: a review of the literature and a comparison between the scores and clinical judgement. Journal of clinical nursing. 2008 Mar.17(5):646–53. doi: 10.1111/j.1365-2702.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- 18.Peterson MJ, Schwab W, van Oostrom JH, Gravenstein N, Caruso LJ. Effects of turning on skin-bed interface pressures in healthy adults. Journal of advanced nursing. 2010 Jul.66(7):1556–1564. doi: 10.1111/j.1365-2648.2010.05292.x. [DOI] [PubMed] [Google Scholar]
- 19.Gefen A. How Much Time Does it Take to Get a Pressure Ulcer ? Integrated Evidence from Human, Animal, and In Vitro Studies. Ostomy Wound Management. 2008;54(10):26–35. [PubMed] [Google Scholar]
- 20.Hollisaz MT, Khedmat H, Yari F. A randomized clinical trial comparing hydrocolloid, phenytoin and simple dressings for the treatment of pressure ulcers [ISRCTN33429693] BMC dermatology. 2004 Jan.4(1):18. doi: 10.1186/1471-5945-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel RK, Priest DL, Wheatley DC. Etiologic factors in pressure sores: an experimental model. Archives of physical medicine and rehabilitation. 1981 Oct.62(10):492–498. [PubMed] [Google Scholar]