Abstract
Rhopalurus junceus scorpion venom has been identified as a natural extract with anticancer potential. Interestingly, this scorpion venom does not cause adverse symptoms in humans. However, there is scarce information about its composition and enzymatic activity. In this work, we determined the electrophoretic profile of the venom, the gelatinase and caseinolytic activity, and the phospholipase A2 (PLA2) and hemolytic activity. The effect of different venom doses (6.25, 12.5 and 25 mg/kg) on gastrocnemius muscle was also measured as CK and LDH activity in serum. The presence of hyaluronidase was determined by turbidimetric assay. The effect of different fractions obtained by gel filtration chromatography were evaluated at different concentrations (0.05, 0.1, 0.2, 0.4, 0.6mg/ml) against lung cancer cell A549 and lung normal cell MRC-5 using MTT assay. The electrophoretic profile demonstrated the presence of proteins bands around 67kDa, 43kDa, 18.4kDa and a majority band below 14.3kDa. The venom did not showed caseinolytic, gelatinase, PLA2 and hemolytic activity even at highest venom concentration used in the study. Scorpion venom only showed a significant toxic effect on gastrocnemius muscles identified by CK and LDH release after subcutaneous injection of 12.5 and 25mg/kg. Low molecular weight fractions (<4kDa) induced a significant cytotoxicity in A549 cells while high molecular weight proteins (45–60kDa) were responsible for hyaluronidase activity and toxic effect against MRC-5. Experiments indicate that Rhopalurus junceus scorpion venom has low enzymatic activity, which could contribute to the low toxic potential of this scorpion venom.
Keywords: Rhopalurus junceus, scorpion venom, hyaluronidase, high molecular weight proteins, cancer cells
INTRODUCTION
Scorpion venom is a mixture of various active substances which includes a variety of biologically active components: enzymes, peptides, nucleotides and other unknown substances (Zlotkin et al, 1978; Possani et al, 1999; Possani et al, 2000). The biological activities of scorpion venom enzymes include proteolysis, PLA2, hyaluronidases, phosphatases and acetylcholinesterases (Tan and Ponnudurai, 1992; Almeida et al, 2002; Khodadadi et al, 2012). These enzymes contribute to various pathological changes in skin, blood cells, cardiovascular and central nervous systems (Seyedian et al, 2010) associated to local necrosis, intravascular hemolysis and acute renal failure/damage (Heidarpour et al, 2012). Particularly, PLA2 is present in some scorpion species and have shown to be toxic to mammals and cause edema and/or hemolysis of mammalian erythrocytes (Zamudio et al, 1997; Conde et al, 1999; Valdez-Cruz et al, 2004). Hyaluronidases usually have an indirect effect increasing the absorption of active peptides and permit that toxic components rapidly spread and induce systemic reactions (Pessini et al, 2001; Morey et al, 2006).
Rhopalurus junceus (R. junceus) is endemic specie from Cuba. It belongs to genera Rhopalurus included in the Bhutidae family. This family includes the most dangerous species related to human scorpionism. However, in Cuba there is no report of fatal sting from this or another species in the country. Because of this the scorpions are not consider medically important species. Interestingly, the R. junceus venom has become a very popular treatment in traditional medicine in Cuba for pain, inflammatory diseases and cancer. Some scientific evidences suggest that this scorpion has low toxic effect (García-Gómez et al, 2011; Díaz-García et al, 2013). However, the presence of some enzymes can be related with some toxic effect (Rodríguez-Ravelo et al, 2013) and this topic has been rarely investigated in this species. In this study, we analyzed the enzymatic activity present in R. junceus venom and correlated it with the biological activity.
MATERIALS AND METHODS
Reagents
Dulbecco’s modified Eagle’s medium was purchased from GIBCO/BRL (Caithershurg, MD). Fetal bovine serum (FBS) was purchased from Hyclone. Gelatin from porcine skin, hyaluronic acid sodium salt from rooster comb, α-casein from bovine milk, bovine serum albumin Fraction V (BSA), calcium chloride, tris-base and molecular weight size markers for electrophoresis were obtained from Promega (Promega, USA). All other reagents were of analytical grade and provided from commercial sources.
Venom source
Rhopalurus junceus scorpions were maintained in individual plastic cage in the laboratories belonging to the Laboratories of Biofarmaceuticals and Chemistries Productions (LABIOFAM). Scorpions kept alive in the laboratory were milked for venom extraction, once a month, by electrical stimulation. Venom was dissolved in distilled water and centrifuged at 15000xg for 15min. The supernatant was filtered by 0.2µm syringe filter, freeze and stored at -20°C until used. The protein content was calculated by the method of Lowry modified (Herrera et al, 1999).
Animals
Balb/c male mice with an average weight of 20±2gm were obtained from the National Center for Laboratory Animal Breeding (CENPALAB, Havana, Cuba). Mice were housed in controlled temperature and humidity rooms and food and water were administered ad libitum. The experimental procedures using animals were approved by the Institutional Committee for the Care and Use of Laboratory Animals (Protocol 2013/3) and performed in accordance with the International Guiding Principles for Biomedical Research Involving Animals.
SDS-PAGE
The protein components in venom (50μg) were analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS- PAGE), under non-reducing conditions in 16% (w/v) polyacrylamide gels (Laemmli, 1970). Electrophoresis was carried out at 15mA by approximately 2hr and then gels were stained with Coomassie Blue G-250 for identification of protein bands. Molecular mass markers were included in all runs.
Zymography assays
For zymography assays SDS-PAGE (16%, w/v) was prepared and co-polymerized with either gelatin or casein (0.2%). Scorpion venom (90μg) was run at above conditions. The gels were washed in Triton X-100 for 30min to remove SDS. Additionally, the gels were washed three times with 0.1M Tris-HCl, pH 8 and were incubated overnight at 37°C in reaction buffer (50mM Tris-HCl, pH 7.4, 200mM NaCl, 10mM CaCl2) for gelatin and casein zymography. Afterward, the gels were stained for 30min with Coomassie blue G-250 dye, followed by destaining for 30min in 45% (v/v) methanol, 45% H2O (v/v) and 10% (v/v) acetic acid. The clear zone of substrate indicated the presence of either gelatin or casein degrading activities. Trypsin (3µg/well) and gelatinase A (collagenase type IV, 10ng/well) were used as positive control of experiments.
Phospholipase A2 activity
The phospholipase A2 activity in venom was determined by using the egg yolk-agarose method of Habermann y Hardt (Habermann and Hardt, 1972). Three different scorpion venom solutions were prepared at 1, 5 and 10mg/ml. Each concentration was applied with unique volume of 10µl in triplicate in each well. The extract of sea anemone Condylactis gigantea (1mg/ml) was applied at10ml and was used as positive control. A clear zone around the application well indicates enzymatic activity.
Direct hemolysis determination
Mouse erythrocytes were separated from freshly collected blood, washed and suspended in 1.5ml tubes containing a citrate buffer solution (66mmol/L Na3C6H5O7, 44mmol/L C6H8O7, 9:1, v/v) to avoid coagulation. Varying concentrations of venom (0.12, 0.25, 0.5, 1, 2mg/ml) were incubated with the red blood cells at 37°C during 2hr. The tubes were centrifuged at 14000xg for 3min using a centrifuge (Eppendorf centrifuge 5410 model, Germany). Finally, 100µl from each supernatant solution was placed in a well of a 96-well plate and the percentage of hemolysis was determined by measuring the amount of hemoglobin released from the erythrocyte suspension at 540nm using a microplate reader (ELISA MRX Revelation Dynex Technologies). The percentage of hemolysis of each sample was calculated using the formula: % hemolysis = A540nm of treated cells/A540nm of positive control × 100%. For negative and positive controls, the human erythrocytes were suspended either in respective buffered saline solution (0% lysis) or 1% (v/v) Triton X-100 (100% lysis). The experiment used three replicate and was repeated 2 times.
Toxicity in mice gastrocnemius muscle by evaluation of CK and LDH activities from serum samples
Animals were separated in five groups. The first group (PC) served as positive control, and was injected in the muscle with 50ml of the Condylactis gigantea sea anemone extract which has previously demonstrated to affect gastrocnemius muscle (Romero et al, 2010). The second group (NC) received an injection of 100ml of physiological saline solution (0.9%, w/v, NaCl). The remaining three groups received an injection of 100ml of scorpion venom doses 6.25, 12.5 and 25mg/kg, respectively. After 2hr of venom injection, the blood was collected from the orbital sinus from each mouse and kept for 1hr at 37oC. Serum was obtained by centrifugation at 10,000xg for 5min and kept at -20oC until use.
The creatine kinase (CK) and lactate dehydrogenase (LDH) were assayed in sera using a commercial kit (Sigma, EUA) and absorbance was measured at 340 nm. The enzyme activities values were expressed in international units (U/l). Each experiment used five replicate and was repeated 2 times.
Separation of venom component by flow pressure liquid chromatography (FPLC)
The crude venom (3mg) was dissolved in ammonium acetate buffer (50mM, pH 7.2) and centrifuged at 10 000xg at 4°C for 15min. Supernatant was injected and fractioned on a superdex 75 HR 10/30 column implemented on a FPLC system. This column has a separation range from 2kDa to 60kDa proteins. Fractions were eluted with the same buffer at flow rate of 0.5ml/min and the absorbance was monitored at 280nm.
Molecular weight determination of R. junceus venom fractions
The molecular weight of venom fractions was determined by the gel filtration chromatography method. The superdex 75 HR 10/30 column was calibrated with standard molecular weight markers (Amersham Pharmacia). The elution pattern of each standard molecular weight marker was monitored at 280nm and flow rate at 0.5ml/min. The elution volumes of the standard proteins of known molecular weights and the fractioned venom proteins were determined under similar conditions. A calibration curve was prepared by plotting Ve/Vo (Ve = elution volume and Vo = void volume) vs logarithmic molecular weights (logM) of marker proteins. The void volume of the column was determined using bromophenol blue.
Hyaluronidase assay
The activity of hyaluronidase enzyme was determined by the turbidimetric method described by Pearce in 1953 (Pearce, 1953). Briefly, the hyaluronic acid (BDH, England) was dissolved in buffer solution (NaAc 0.1M; NaCl 0.15M; pH 6) at final concentration of 200µg/ml. The scorpion venom, the bovine testicle hyaluronidase (Sigma, EUA) (positive control) and the fractions obtained by gel filtration chromatography were evaluated at 200µg/ml, 0.2µg/ml and 5µg/ml, respectively. The final reaction volume for each sample was 1ml. The mixtures of hyaluronic acid with the scorpion venom, the five fractions and bovine testicle hyaluronidase were incubated individually during 10min at 37°C. After this period, the samples were transferred to a water bath kept at 70°C for 30min, in order to inactivate the enzyme. Finally, the samples were mixed with 2ml of acetate buffer (0.1% bovine albumin Fraction V; 0.1M NaAc; pH 3.75) and incubated at room temperature. Five minutes after mixing, the absorbance was read in a spectrophotometer (MRC, Spain) at 540nm. A sample containing hyaluronic acid without scorpion venom was used as negative control of experiments. The absorbance decrease at 540nm in samples is proportional to hyaluronidase activity. All analyses were made in triplicate. The experiments were repeated three times.
Cell lines and culture
The human cancer cell lines A549 (lung carcinoma ATCC CCL-185™) and MRC-5 (normal human lung fibroblast ATCC CCL-171™) used in the experiments were obtained from ATCC culture collection. The A549 cell line was maintained in Dulbecco’s modified Eagle’s medium supplemented with heat inactivated fetal bovine serum (FBS), 10% (v/v), penicillin (100U/ml), and streptomycin (100μg/ml). The MRC-5 cell line was maintained in RPMI-1640 supplemented with 10% (v/v) FBS, penicillin (100U/ml) and streptomycin (100μg/ml).
In vitro cell viability assay (MTT assay)
The effect of scorpion venom fraction from FPLC, on cell viability, was determined by the MTT assay ( Mosmann, 1983). A549 and MRC-5 cell lines (1x104/well) were plated in 50μl of medium/well in 96-well culture plates (Costar Corning, Rochester, NY) and incubated overnight to recovery and cell adhesion in a humidified atmosphere of 5% CO2 (v/v) at 37°C. After incubation, 50μl of different concentrations (0.05, 0.1, 0.2, 0.4, 0.6mg/ml) of each fraction, dissolved in medium were added individually. Cells with culture medium and without venom fractions were used as untreated growth control. Five wells were included in each concentration. After treatment for 72hr, 10μl of 5mg/ml of sterile MTT (pH 4.7) was added per well and cultivated for another 3hr. The supernatant was carefully removed, 150μl DMSO was added per well and shaken for 15min at 37oC. The absorbance was measured with a microplate reader (ELISA MRX Revelation Dynex Technologies) at 560nm with 630nm as reference. Absorbance from untreated cells was considered as 100% of growth and used for viability calculation. The effect of scorpion venom on the viability for human cell lines panel was expressed as the % viability, using the formula: %viability= A560nm of treated cells/A560nm of control cells x100%. The IC50 values (concentration that causes 50% reduction of cell) for both types of human cell lines were determined. The experiments were performed three times.
Statistical analysis
Quantitative data were expressed as mean ±SD. Data from positive control, scorpion venom and/or fraction samples were compared against control without treatment using Mann-Whitney U test. CK and LDH values from scorpion venom doses-treated mice were compare against non-treated mice using Mann-Whitney U test. The IC50 values were determined by interpolation of tendency line from linear regression curve. For all analysis we used the GraphPad Prism version 5.01 for Windows, (GraphPad Software, San Diego California, USA). Significant differences were considered for p<0.05.
RESULTS
SDS-PAGE and enzymatic activity of R. junceus venom
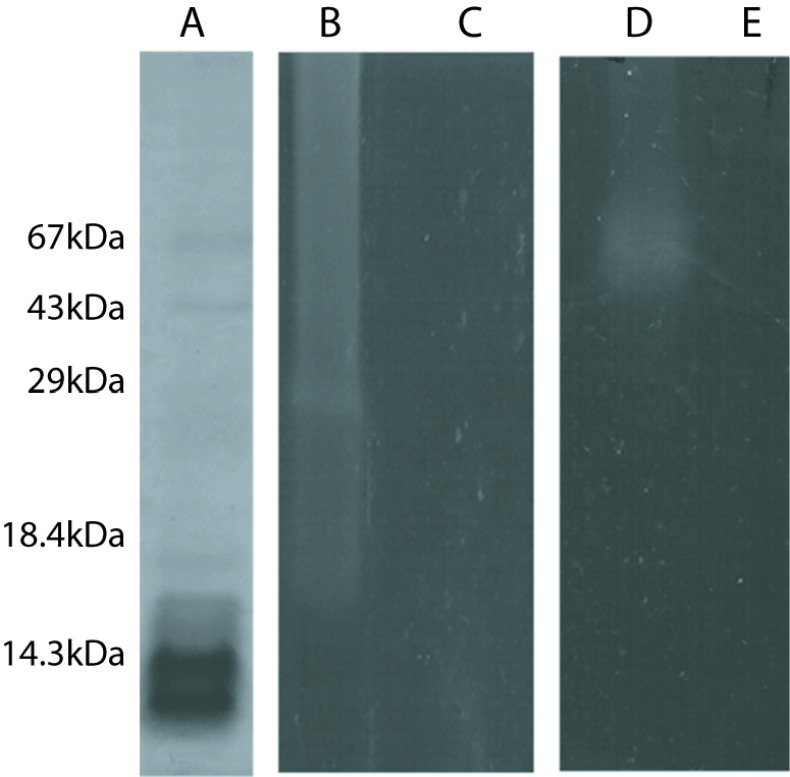
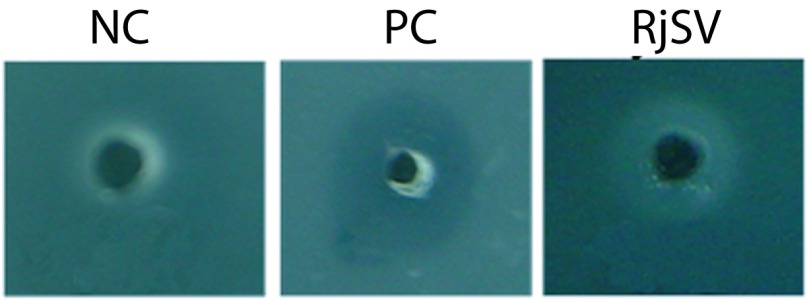
In SDS-PAGE technique the electrophoretic profile of R. junceus scorpion venom was obtained. The protein bands were observed at 67kDa, 43kDa, 18.4kDa and below 14.3kDa (Figure 1A). The band observed below 14.3kDa was prominent among the rest. Zymography technique with casein and gelatin are observed in Figure 1. Clear band was observed with the positive control used for casein (Figure 1B) and gelatin (Figure 1D). R. junceus venom revealed the absence of proteolytic activity with both enzymes substrates (Figure 1C, 1E). Additionally, by the egg-yolk method there was no evidence of phospholipase A2 activity from R. junceus venom, even at the highest concentration used in the study (Figure 2).
Figure 1.
Protein components and proteolytic activity of R. junceus venom. (A) protein components of R. junceus venom were separated by SDS-PAGE and stained with Comassie blue dye. Zymographies were performed for caseinolytic activity. (B): positive control; (C): scorpion venom, gelatinase activity (D): positive control; (E): scorpion venom.
Figure 2.
Phospholipase A2 activity of R. junceus venom. The enzyme activity was determined by using the egg yolk-agarose method. NC: negative control; PC: positive control (Condylactis gigantea sea anemone extract); RjSV: R. junceus scorpion venom (1, 5, 10mg/ml).
Effect of venom on murine erythrocytes cells
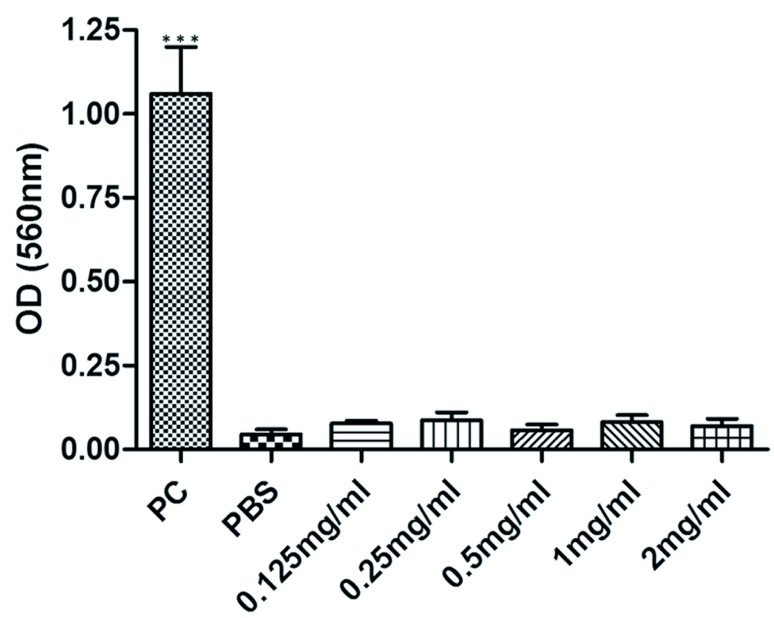
The hemolytic activity was evaluated on murine erythrocytes cells. In the experiment, positive control produced a red color showing that it causes total lysis in red blood cells (Figure 3). In contrast, hemolytic effect was not observed in murine erythrocytes at venom concentration ranging from 0.125mg/ml-2mg/ml (Figure 3).
Figure 3.
Effect of R. junceus concentrations (0.12–2mg/ml) on hemolysis of murine erythrocytes. The percentage of hemolysis was determined by measuring the amount of hemoglobin released from the erythrocyte suspension at 540nm. Values represent the means ±SD from two independent experiments. 1% (v/v) Triton X-100 was used as positive control. The p values were obtained comparing the control group versus every group of treatment by Mann-Whitney U test. Significant differences ***p<0.001.
Effect of venom on gastrocnemius muscles
To determine the effect on gastrocnemius muscles, different scorpion venom doses were inoculated in gastrocnemius muscles. The enzyme assays showed a significant increment (p<0.01) in the level of CK (287.2±47U/l) and LDH (451.02±67.5U/l) activities in the serum from positive control group compared to negative controls (CK: 90.9± 28.3U/l; LDH: 185.7±26.3U/l) (Figure 4). Scorpion venom doses increased CK and LDH levels in a dose-dependent manner. At dose of 6.25mg/kg there were no differences with negative control group (Figure 4). Meanwhile, the levels of these enzymes were significantly increased at 12.5mg/kg (p<0.05) (CK: 157.2±28.4U/l; LDH: 340.9±52.3U/l) and for 25mg/kg (p<0.001) (CK: 275.7±53.1U/l; LDH: 388.8±54.9U/l) (Figure 4).
Figure 4.
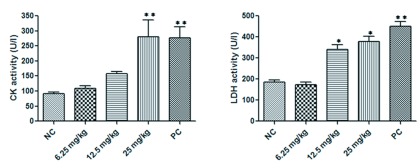
Effect of R. junceus venom doses (6.25, 12.5, 25mg/kg) on CK and LDH activity in serum. The CK and LDH activities were measured at 340 nm. The enzyme activities values were expressed in international units (U/l). Values represent the means ±SD from two independent experiments. The p values were obtained comparing the negative control (NC) versus every group of treatment by Mann-Whitney U test. Significant differences *p<0.05; **p<0.01. PC: positive control (Condylactis gigantea sea anemone extract).
Separation of venom component by flow pressure liquid chromatography (FPLC)
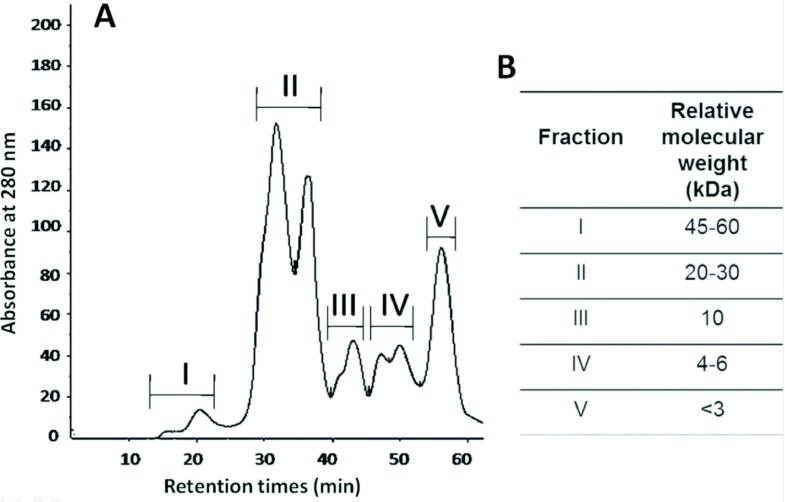
The crude venom was separated into five selected fractions (I-V) by gel filtration chromatography on a superdex 75 column (Figure 5A). The relative molecular weight for each selected fraction was obtained from calibration curve (Figure 5B).
Figure 5.
Separation of R. junceus venom. A: chromatographic profile using a superdex 75 molecular exclusion column on FPLC system. Fractions were eluted at flow rate of 0.5ml/min and the absorbance was monitored at 280nm. B: Relative molecular weight from the five selected fraction.
Determination of hyaluronidase activity
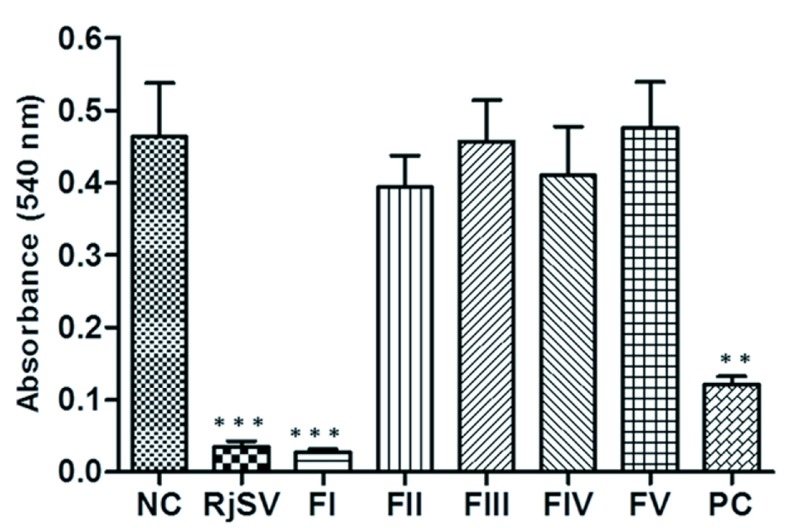
The presence of hyaluronidase activity was evaluated in crude venom and the five fractions obtained by gel filtration chromatography. Bovine testicle hyaluronidase (positive control) showed a significant decrease of absorbance (0.12±0.01) compare to negative control (0.44±0.02) (p<0.05) (Figure 6). Similarly, crude venom showed a significant decrease of absorbance (0.03±0.01) against control (p<0.01) (Figure 6). Among all fractions obtained from gel filtration chromatography, only FI induced a significant difference in absorbance (0.03±0.01) compare to negative control (p<0.01). The remaining fractions had no differences against control (Figure 6).
Figure 6.
Effect of R. junceus venom and fractions on hyaluronic acid. The activity of hyaluronidase enzyme was determined by the turbidimetric method at 540nm. Values represent the means ± SD from three independent experiments. Bovine testicle hyaluronidase (0.2µg/ml) was used as positive control. The p values were obtained comparing the negative control (NC) versus every group of treatment by Mann-Whitney U test. Significant differences ***p<0.001, **p<0.01. RjSV: R. junceus scorpion venom; PC: positive control.
Biological activity
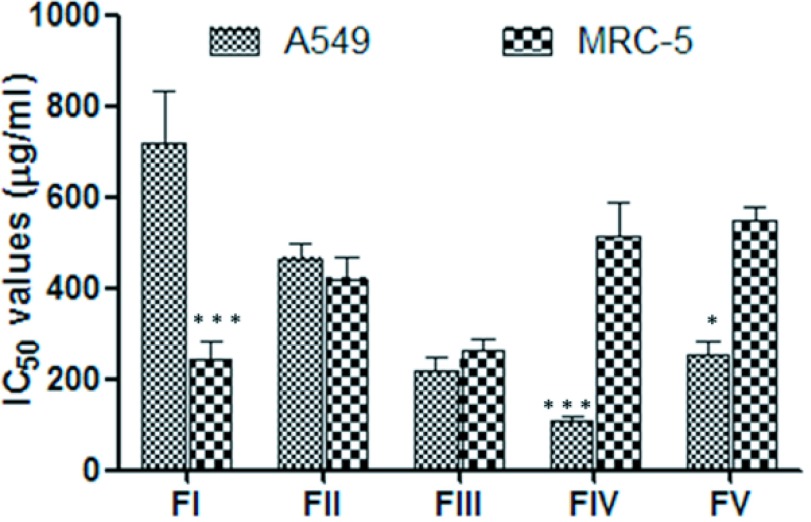
To identify the biological effect of different scorpion venom fractions, on cell viability, increased concentration were applied to A549 and MRC-5 cell lines. The FI fraction induced a decrease in cell viability in MRC-5 with IC50 values of 244.5±65µg/ml significant lower (p<0.001) than IC50 in A549 (717.65±110µg/ml) (Figure 7). This fraction showed the lowest IC50 values against MRC-5 cells, among all fractions. The FII and FIII did not show differences between IC50 values from cancer and normal cells (Figure 7). The IC50 values obtained for FIV (107.4±36µg/ml) in A549 were significant lower (p<0.05) than the IC50 values from MRC-5 cells (514.5±98µg/ml) (Figure 7). Similarly, FV showed IC50 values in A549 (251.6±58µg/ml) significantlylower (p<0.01) than the observed for MRC-5 cells (546.5±42µg/ml) (Figure 7). The FIV fraction showed in A549, the lowest IC50 values among all fractions evaluated.
Figure 7.
Graphics of IC50 values from venom fraction in A549 and MRC-5 cells. IC50 values were obtained after R. junceus venom fractions treatment (0.05, 0.1, 0.2, 0.4, 0.6mg/ml) for 72hr in A549 and MRC-5 cells using the MTT assay. Values represent the means ± SD from three independent experiments. The p values were obtained comparing the IC50 values from MRC-5 against IC50 values from A549 in each fraction by Mann-Whitney U test. Significant differences ***p<0.001 **p<0.01 and *p<0.05.
DISCUSSION
In this work we studied the enzymatic activity of venom from the Cuban endemic scorpion Rhopalurus junceus. First we fractionated the venom components using SDS-PAGE. The electrophoretic profile showed to be similar to most scorpion venom species reported previously, where a high content of low molecular proteins below 14kDa has been described (Keskin and Koç, 2006; Borges et al, 2010; de Roodt et al, 2010; Khoobdel et al, 2013; Xu et al, 2014). Low molecular weight proteins (toxins) under 14kDa are responsible for main toxicological effects observed in human envenomation ( Possani et al, 2000; Escobar et al, 2003;Velásquez and Escobar, 2004) and besides recently become interesting active components with a variety of pharmacological effects (Ding et al, 2014).
Using zymography techniques we did not detected the presence of caseinolytic or gelatinolytic activity in R. junceus venom, suggesting the lack of proteolytic enzymes. There are some reports which identify a variety of proteolytic activities in scorpion venoms (Almeida et al, 2002; Seyedian et al, 2010; Brazón et al, 2014; Ortiz et al, 2014). The highly toxic venom from Hemiscorpius lepturus scorpion have shown some enzymatic activities like gelatinolytic and caseinolytic identified through zymography techniques (Heidarpour et al, 2012). This scorpion venom exhibits various pathological changes in skin, blood cells, cardiovascular and central nervous systems (Seyedian et al, 2010) associated to local necrosis, intravascular hemolysis, and acute renal failure/damage where enzymes are directly implicated (Heidarpour et al, 2012). Besides, some metalloproteinases have been identified in Tytius sp (Venancio et al, 2013; Brazón et al, 2014). In T. discrepans, metalloproteases and serine proteases are responsible for fibrinogenolytic activity as part of envenomation syndrome (Brazón et al, 2014). While in T. serrulatus has been described the presence of gelatinolytic activity trough zymography (Almeida et al, 2002). Some of these proteinases are implicated in hypotension and bradycardia induction related to scorpion stings (Venancio et al, 2013) and/or is suggested that can undergo post-translational modifications, activating toxin precursors (Almeida et al, 2002). In our study, the PLA2 activity was not detected in R. junceus venom even at highest venom concentration used in the study (10mg/ml) and there was no hemolytic effect in red blood cells (2mg/ml). The PLA2 activity is not present in all scorpion venom (Almeida et al, 2012; Venancio et al, 2013). However, the presence of this enzyme is usually related to tissue necrosis, hemorrhages and clinical signs provoked by the stings of venomous animals (Valdez-Cruz et al, 2004; Valdez-Cruz et al, 2007). IpTxI and Phospholipin are two examples of PLA2 isolated from the venom of the African scorpion Pandinus imperator (Zamudio et al, 1997; Conde et al, 1999). IpTxI catalyzes the hydrolysis of phospholipids and the reaction yields free fatty acids and lysophospholipids (Zamudio et al, 1997). Authors suggested that this free fatty acids bind to ryanodine receptors on sarcoplasmic reticulum or to a closely associated protein controlling the intracellular Ca2+ mobilization (Zamudio et al, 1997). Phaiodactylipin a PLA2 isolated from the scorpion Anuroctonus phaiodactylus has shown direct hemolytic effect on erythrocytes in vitro and inflammation on rat muscle in vivo (Valdez-Cruz et al, 2004) probably as part of the envenomation process. Recently, has been isolated and purified a PLA₂ from the Egyptian scorpion Scorpio maurus palmatus (Louati et al, 2013). This PLA₂exhibits hemolytic activity toward human, rabbit or rat erythrocytes. Authors demonstrated that this hemolytic activity is related to its ability to interact with phospholipids monolayer similar to those of phospholipases isolated from other venoms (Louati et al, 2013).
Other high-molecular-weight enzymes like hyaluronidases have been identified in scorpion venom (Morey et al, 2006; Batista et al, 2007). Hyaluronidases in venoms act as spreading factors because they facilitate toxin diffusion in the tissue of the prey by catalyzing the hydrolysis of the glycosaminoglycans in their connective tissues, thus causing systemic envenomation (Morey et al, 2006). The molecular weight of the enzymes identified are around 52kDa for Palamneus gravimanus (Morey et al, 2006), 51kDa for Tytius serrulatus (Pessini et al, 2001) and 44.8kDa for hyaluronidase from the Brazilian scorpion Tityus stigmurus (Batista et al, 2007). Previous studies with R. junceus venom have reported the presence of hyaluronidase activity through zymography techniques at 45kDa levels (Rodríguez-Ravelo et al, 2013). In our study, the hyaluronidase activity was observed in the fraction FI (from gel filtration chromatography) corresponding to molecular weight around 45–60kDa this range comprise the previously reported hyaluronidases. Intramuscular injection of R. junceus venom in gastrocnemius muscle assay induced a significant increase of LDH and CK enzymes in serum, but only at highest doses (12.5mg/kg and 25mg/kg), to best of our knowledge, not previously tested for other scorpion species, suggesting a low toxic potential of this scorpion venom. Besides, FI fraction was responsible for the great toxicity to normal cells probably due to the presence of hyaluronidase. However, additional experiments are needed to confirm this suggestion and to establish the relation between hyaluronidase activity and muscles damage.
The low molecular weight fractions (FIV and FV) from R. junceus venom showed a significant and selective cytotoxicity against the lung cancer cell line A549. Recent studies have investigated the biological effect of low molecular weight polypeptides (PESV), from Buthus martensii karsh (BMK), which represent the major biologically active components in the BMK venom (Zhang et al, 2009; Li et al, 2014). These polypeptides of 50–60 amino acids extracted from crude venom of BMK contain also anti-tumor activity against tumor growth of S180 sarcoma and H22 hepatocellular carcinoma in mice (Li et al, 2014). These observations increase the pharmacological relevance of low molecular weight proteins from scorpion venoms.
In conclusion, R. junceus scorpion venom does not contains proteolytic and PLA2 activities and this is a very important feature because add elements that contribute to understanding the low toxic potential of this specie. Hyaluronidase activity could be, at least partially, responsible for toxicity observed in R. junceus venom through in vivo and in vitro experiments. The selective effect against cancer cells is located mainly in the low molecular weight fraction which could represent the initial step for identifying the pure active components present in this scorpion venom.
ACKNOWLEDGMENTS
The authors are grateful to colleagues from Technical and Scientific support Department of Tropical Medicine Institute “Pedro Kourí” in their support to maintain cell culture.
COMPETING INTEREST
None declared.
REFERENCES
- Almeida D, Scortecci K, Kobashi L, et al. Profiling the resting venom gland of the scorpion Tityus stigmurus through a transcriptomic survey. BMC Genomics. 2012;13:362. doi: 10.1186/1471-2164-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F, Pimenta A, De Figueiredo S, et al. Enzymes with gelatinolytic activity can be found in Tityus bahiensis and Tityus serrulatus venoms. Toxicon. 2002;40:1041–1045. doi: 10.1016/s0041-0101(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Batista C, Román-González S, Salas-Castillo S, et al. Proteomic analysis of the venom from the scorpion Tityus stigmurus: biochemical and physiological comparison with other Tityus species. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:147–157. doi: 10.1016/j.cbpc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Borges A, Rojas-Runjaic F, Diez N, et al. Envenomation by the scorpion Tityus breweri in the Guayana Shield Venezuela: report of a case efficacy and reactivity of antivenom and proposal for a toxinological partitioning of the Venezuelan scorpion fauna. Wilderness Environ Med. 2010;21:282–289. doi: 10.1016/j.wem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Brazón J, Guerrero B, D’Suze G, et al. Fibrin(ogen)olytic enzymes in scorpion (Tityus discrepans) venom. Comp Biochem Physiol B Biochem Mol Biol. 2014;168:62–69. doi: 10.1016/j.cbpb.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Conde R, Zamudio F, Becerril B, et al. Phospholipin a novel heterodimeric phospholipase A2 from Pandinus imperator scorpion venom. FEBS Lett. 1999;460:447–450. doi: 10.1016/s0014-5793(99)01392-7. [DOI] [PubMed] [Google Scholar]
- de Roodt A, Coronas F, Lago N, et al. General biochemical and immunological characterization of the venom from the scorpion Tityus trivittatus of Argentina. Toxicon. 2010;55:307–319. doi: 10.1016/j.toxicon.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Díaz-García A, Morier-Díaz L, Y F-H, et al. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5–12. [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chua P, Bay B, et al. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med (Maywood) 2014;239:387–393. doi: 10.1177/1535370213513991. [DOI] [PubMed] [Google Scholar]
- Escobar E, Rivera C, Tincopa L. Acción de la toxina Hl3 sobre el musculo esquelético. Rev Peru Biol. 2003;10:88–93. [Google Scholar]
- García-Gómez B, Coronas F, Restano-Cassulini R, et al. Biochemical and molecular characterization of the venom from the Cuban scorpion Rhopalurus junceus. Toxicon. 2011;58:18–27. doi: 10.1016/j.toxicon.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Habermann E, Hardt K. A sensitive and specific plate test for the quantitation of phospholipase. Anal Biochem. 1972;50:163–173. doi: 10.1016/0003-2697(72)90495-2. [DOI] [PubMed] [Google Scholar]
- Heidarpour M, Ennaifer E, Ahari H, et al. Histopathological changes induced by Hemiscorpius lepturus scorpion venom in mice. Toxicon. 2012;59:373–378. doi: 10.1016/j.toxicon.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Herrera Y, Heras N, Cardoso D. Adaptación a microplacas y validación de la técnica de Lowry. VacciMonitor. 1999;3:7–11. [Google Scholar]
- Keskin N, Koç H. A study on venom proteins of Iurus dufoureius asiaticus Birula 1903 (Scorpiones: Iuridae) Turkiye Parazitol Derg. 2006;30:59–61. [PubMed] [Google Scholar]
- Khodadadi A, Pipelzadeh M, Vazirianzadeh B, et al. An in vitro comparative study upon the toxic properties of the venoms from Hemiscorpius lepturusAndroctonus crassicauda and Mesobuthus eupeus scorpions. Toxicon. 2012;60:385–390. doi: 10.1016/j.toxicon.2012.04.348. [DOI] [PubMed] [Google Scholar]
- Khoobdel M, Zahraei-Salehi T, Nayeri-Fasaei B, et al. Purification of the Immunogenic Fractions and Determination of Toxicity in Mesobuthus eupeus (Scorpionida: Buthidae) Venom. J Arthropod Borne Dis. 2013;7:139–146. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li W, Xin Y, Chen Y, et al. The anti-proliferative effects and mechanisms of low molecular weight scorpion BmK venom peptides on human hepatoma and cervical carcinoma cells in vitro. Oncol Lett. 2014;8:1581–1584. doi: 10.3892/ol.2014.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louati H, Krayem N, Fendri A, et al. A thermoactive secreted phospholipase A2 purified from the venom glands of Scorpio maurus: relation between the kinetic properties and the hemolytic activity. Toxicon. 2013;72:133–142. doi: 10.1016/j.toxicon.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Morey S, Kiran K, Gadag J. Purification and properties of hyaluronidase from Palamneus gravimanus (Indian black scorpion) venom. Toxicon. 2006;47((2)):188–195. doi: 10.1016/j.toxicon.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular grow and survival: application to proliferation and citotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ortiz E, Rendón-Anaya M, Rego S, et al. Antarease-like Zn-metalloproteases are ubiquitous in the venom of different scorpion genera. Biochim Biophys Acta. 2014;1840:1738–1746. doi: 10.1016/j.bbagen.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Pearce R. The turbidimetric estimation of hyaluronate. Biochem J. 1953;55:467–472. doi: 10.1042/bj0550472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessini A, Takao T, Cavalheiro E, et al. A hyaluronidase from Tityus serrulatus scorpion venom: isolation characterization and inhibition by flavonoids. Toxicon. 2001;39:1495–1504. doi: 10.1016/s0041-0101(01)00122-2. [DOI] [PubMed] [Google Scholar]
- Possani L, Becerril B, Delepierre M, et al. Scorpion toxins specific for Na+ channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Possani L, Merino E, Corona M, et al. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochiemie. 2000;82:861–868. doi: 10.1016/s0300-9084(00)01167-6. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ravelo R, Coronas F, Zamudio F, et al. The Cuban scorpion Rhopalurus junceus (Scorpiones Buthidae): component variations in venom samples collected in different geographical areas. J Venom Anim Toxins Incl Trop Dis. 2013;19 doi: 10.1186/1678-9199-19-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L, Marcussi S, Marchi-Salvador D, et al. Enzymatic and structural characterization of a basic phospholipase A2 from the sea anemone Condylactis gigantea. Biochimie. 2010;92:1063–1071. doi: 10.1016/j.biochi.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Seyedian R, Pipelzadeh M, Jalali A, et al. Enzymatic analysis of Hemiscorpius lepturus scorpion venom using zymography and venom-specific antivenin. Toxicon. 2010;56:521–525. doi: 10.1016/j.toxicon.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Tan N, Ponnudurai G. Comparative study of the enzymatic hemorrhagic procoagulant and anticoagulant activities of some animal venoms. Comp Biochem Physiol C Comp Pharmacol. 1992;103:299–302. [PubMed] [Google Scholar]
- Valdez-Cruz N, Batista C, Possani L. Phaiodactylipin a glycosylated heterodimeric phospholipase A from the venom of the scorpion Anuroctonus phaiodactylus. Eur J Biochem. 2004;271:1453–1465. doi: 10.1111/j.1432-1033.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- Valdez-Cruz N, Segovia L, Corona M, et al. Sequence analysis and phylogenetic relationship of genes encoding heterodimeric phospholipases A2 from the venom of the scorpion Anuroctonus phaiodactylus. Gene. 2007;396:149–158. doi: 10.1016/j.gene.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Velásquez L, Escobar E. Purificación y caracterización parcial de una toxina (Hm3) del veneno de Hadruroides mauryi (Francke y Soleglad 1980) (Scorpiones Iuridae) Rev Peru Biol. 2004;11:13–18. [Google Scholar]
- Venancio E, Portaro F, Kuniyoshi A, et al. Enzymatic properties of venoms from Brazilian scorpions of Tityus genus and the neutralisation potential of therapeutical antivenoms. Toxicon. 2013;69:180–190. doi: 10.1016/j.toxicon.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Xu X, Duan Z, Di Z, et al. Proteomic analysis of the venom from the scorpion Mesobuthus martensii. J Proteomics. 2014;106:162–180. doi: 10.1016/j.jprot.2014.04.032. [DOI] [PubMed] [Google Scholar]
- Zamudio F, Conde R, Arévalo C, et al. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I a heterodimeric protein from the scorpion Pandinus imperator. J Biol Chem. 1997;172:11886–11894. doi: 10.1074/jbc.272.18.11886. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu L, Wang Z, et al. Anti-proliferation Effect of Polypeptide Extracted from Scorpion Venom on Human Prostate Cancer Cells in vitro. J Clin Med Res. 2009;1:24–31. doi: 10.4021/jocmr2009.01.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotkin E, Miranda F, Rochat H. Chemistry and pharmacology of Buthidae scorpion venoms. In: Bettini, editor. Handbook of Experimental Pharmacology. Springer Verlag Berlin; 1978. pp. 317–369. [Google Scholar]