Abstract
A long history of lead arsenate application in orchards has led to significant accumulation of Pb and As in the topsoil. Besides the threat that such soils represent for the environment, reclamation of old orchards for agricultural purposes implies the exposure of humans to Pb and As. In this study we assessed the influence of vegetable compost addition (as a sustainable agricultural practice) to contaminated acidic orchard soil on Pb and As bioavailability, assessed with two selective non-exhaustive chemical extractions and with an in vivo bioaccumulation test with an isopod (P. scaber). The treatment with compost caused a significant increase in soil pH and total carbon content, resulting in a consistent decrease of Pb bioavailability. In contrast, the bioavailability of As increased, indicating that a complementary treatment should be used for reducing the bioavailability of As in old orchard soils. This is the first report on the use of As accumulation in P. scaber as a tool for the assessment of As bioavailability in contaminated orchard soil.
Keywords: accumulation, arsenic, bioavailability, compost, lead, Porcellio scaber
Introduction
Trace metals, such as lead (Pb) and arsenic (As) in soil are not biodegraded by natural processes, and therefore they persist in soil after their introduction, leading to long-term effects. Agricultural inputs in the past were the major source of As in soils. Inorganic arsenicals have been widely used in agriculture as pesticides or plant defoliants. Among them, lead arsenate (PbAsO4) was the most extensively used of the arsenical insecticides from the 1890’s until the introduction of organic pesticides in the 1940s (Adriano, 2001; Smith et al., 1998), resulting in heavy As and Pb soil contamination. Although lead arsenate is no longer used in orchards, these soils are often converted to other uses such as the production of vegetables. Composts, commonly used in vegetable production, when added to contaminated soil can affect the bioavailability and mobility of trace metals, either increasing or decreasing them, depending on the element (Cao et al., 2003; Kabata-Pendias and Adriano, 1995; Clemente et al., 2010; Baldantoni et al., 2010; Shuman et al., 2001; Zheljazkov and Warman, 2004; Mench et al., 2003). The impact of toxic metals on the environment depends on their chemical forms as they are rarely 100% bioavailable. The term bioavailability refers to the portion of a substance or element in a soil that is available for absorption into living organisms, such as humans, animals, or plants (Hettiarachichi and Pierzynski, 2004). It is usually lower than the potential bioavailability, which is easier to assess and can be thus used as a practical alternative to bioavailability measurements (Wragg et al., 2007). The assessment of the total concentrations of trace metals in soil is not sufficient to assess their environmental impact, since it is known that total concentration is not an accurate predictor of their bioavailability and mobility in soil. Several non-exhaustive selective chemical extractions have been developed for the assessment of trace metal potential bioavailability and mobility. In a comparative study on the efficiency of five non-exhaustive chemical extractions for trace metal extraction, McBride et al. (2009) have concluded that less aggressive tests would be more sensitive to chemical lability of trace metals in soil and thus more suitable for the assessment of their bioaccessibility. For the purpose of this study we have chosen to use the Modified Morgan’s Extraction (MME) as a simple one-step extraction test as this test is widely used for nutrient extraction in several Northeastern states and is used in the Cornell soil heath test for the assessment the availability, toxicity and elemental imbalances of micronutrients (McBride et al, 2009; Schindelbeck et al., 2008; Idowu et al., 2009). More elaborate in vitro digestion models have been developed to better estimate the oral-bioavailability of soil contaminants to humans through accidental soil ingestion (Oomen et al., 2002; Oomen et al., 2003). The two-step Physiologically Based Extraction Test (PBET) was first designed by Ruby et al. (1996) to mimic the conditions in the human digestive tract, where the soil is first digested in a simulated stomach phase at pH 2.5, followed by an intestinal phase at neutral pH. In general, this method is considered a good surrogate for the more expensive in vivo tests with experimental animals like rats, pigs and primates (Ruby et al., 1996; Cave et al., 2002; Rodriguez et al., 1999).
Past studies have shown that chemical soil extractions are not suitable for describing the bioavailable fraction of trace metals, due to the dynamic and complex nature of metal-soil and metal-organism interactions (Cave et al., 2002; Udovic et al., 2009). A simple in vivo test with isopods as indicator organisms was therefore proposed as a supplement to chemical extractions, providing a more complete and relevant picture of the bioavailability of trace metals in soil to organisms. Terrestrial isopods accumulate metals from their environment in proportion to their concentration in the soil (Hopkin et al., 1993), and therefore they appear very suitable as indicators of the metal bioavailable fraction in polluted soil and leaf litter (Gál et al., 2008; Udovic et al., 2009).
The aim of this study was to assess the influence of vegetable compost addition on Pb and As bioavailability in historically contaminated orchard soils. The suitability of two chemical extraction tests and an in vivo accumulation test with isopods (Porcellio scaber) was studied for the assessment of Pb and As bioavailability in these soils. The correlations between the results of chemical extractions and the results of the accumulation test in the isopod Porcellio scaber are discussed with the aim of identifying a simple tool to use in the assessment of the potential environmental hazard represented by soils with a history of pollution with lead arsenate. To the authors’ knowledge, this is the first report on the use of an isopod accumulation test as a tool to assess the bioavailability of As in contaminated soil.
Materials and methods
Soil and soil analysis
Soils (silty clay loam soils, Hudson series) containing different Pb and As concentrations were sampled at three 4-month old experimental garden plots established in 2010 at Dilmun Hill Student Organic Farm in Ithaca, NY (USA). Soils without compost (A, B and C; hereinafter: non-amended soils) and soils with 10% (w/w) added compost (A+10%, B+10% and C+10%; hereinafter: amended soils) were sampled at each experimental plot. Soil from a nearby managed field at Caldwell Field (Ithaca, NY) was sampled as control, uncontaminated site. The soils were air-dried, homogenized and sieved as required by the method. Soil pH was measured in a 1/2 (w/v) 0.01M CaCl2 suspension. Soil samples were analyzed for total carbon content (C %) by the modified Walkley-Black titration (ISO 14235, 1998).
Potential bioavailability of Pb and As
The potential bioavailability of Pb and As was assessed with the single-step Modified Morgan’s Extraction (MME) and with a two-step Physiologically Based Extraction Test (PBET).
The Modified Morgan’s Extraction was initially developed for the assessment of the biological availability of macronutrients in soil , but has been shown to be useful in evaluating trace element availability (McBride et al., 2009). It was performed by extracting 10g of sieved (2 mm) air-dried soil for 15 minutes on an orbital shaker (180 rpm) in 50 mL of extraction solution containing 0.65 M NH4OH and 1.25 M CH3COOH buffered to pH 4.8 ± 0.05. The suspension was paper-filtered (Whatman No. 2) and As and Pb concentrations determined in the filtrate by ICP-AES (described below). All the extractions were conducted in triplicate. The method is described in more detail in Recommended Soil Testing Procedures for the Northeastern United States, Second Edition, 1995, University of Delaware Agricultural Experiment Station Bulletin No. 493.
The PBET used is based on the method of Ruby et al. (1996), designed to approximate the gastrointestinal tract parameters of 2-3 years old children and is used to assess the potential bioavailability of trace metals in soil to humans (Turner and Ip, 2007). Pediatric physiological characteristics were chosen for the method, since children ingest more soil and dust particles than adults, mainly due to their mouthing behavior, and are thus more exposed to soil pollutants (Davis and Mirick, 2006). The PBET involves a first step extraction at pH 2.5, which simulates soil digestion in the stomach (stomach phase), and a second step extraction at pH 7.0, which simulates the small intestine phase. For the stomach phase, 0.5 g of sieved soil sample (< 250 mm) was extracted in a 250 mL Erlenmeyer flask in an incubator orbital shaker at 180 rpm for 2 h at constant temperature (37ºC) in simulated gastric fluid (50 mL). The gastric fluid was prepared to contain 1.25 g of pepsin (porcine, Sigma), 0.50 g of citrate, 0.50 g of malate, 420 mL of lactic acid and 500 mL of acetic acid per liter, adjusted to pH 2.50 ± 0.05. The pH of the reaction mixture was checked every 30 min and adjusted with 1M HCl as necessary to keep it at a value of 2.50 ± 0.05. After 2 h, the reaction mixture was titrated to pH 7 with saturated NaHCO3 solution, followed by addition of 175 mg of bile salts (porcine, Sigma) and 50 mg of pancreatin (porcine, Sigma) were added, thus simulating small intestine conditions. After 2 h, the reaction solutions were centrifuged at 1600 g for 10 min. The liquid fraction was decanted and analyzed for As and Pb by ICP-AES (see below) as the small intestine fraction.
The PBET was conducted in triplicate. Only As and Pb concentrations in the intestinal fraction were measured, since the metals are absorbed into the blood system from the intestine (Turner and Ip, 2007).
As and Pb accumulation in Porcellio scaber
Adult specimens of a terrestrial isopod Porcellio scaber were purchased from Carolina Science (Burlington, NC), kept in laboratory at constant temperature (24 °C) and fed with hay pellets (Hertz, USA). For the experiment, 6 adult specimens of 30 to 110 mg fresh weight were exposed for 14 days (Bibič et al., 1997; Udovic et al., 2009) to 200 g of air dried experimental soil (control soil, non-amended and amended soils) in plastic vessels (16.5 cm x 16.5 cm, 379 mL) with perforated plastic lids. Four replicate vessels were used for each soil treatment . The experimental soils in plastic vessels were moistened daily with deionized water in one corner of the experimental vessels, in order to achieve a soil moisture gradient. No other food than soil was presented to the animals. After exposure, the animals were removed from the vessels and fed with hay pellets for 24 h to remove metals from their digestive systems. They were then frozen and lyophilized (Udovic et al., 2009). For the digestion, the six animals in each of the four experimental vessels were pooled; there were therefore 4 replicates for each soil treatment. We assumed that by pooling the animals, we would average variability among individuals, thus simplifying the test. The animals were then completely digested in a nitric/perchloric acid mixture (volume ratio 7:1) and total Pb and As. concentrations in whole animals were determined by ICP AES at the Robert W. Holley Center for Agriculture and Health (Dr. M. Rutzke, analyst). Bioaccumulation factors (BAFs) were used to express Pb and As accumulation in animals. They were calculated as the ratio of total Pb and As in the animals to their pseudototal concentration measured in soil (see below).
Trace metal analysis
Most contaminant metals in soils are not structural in minerals; therefore a “pseudototal” analysis using strong acid digest without the necessity of dissolution of silicates by hydrofluoric acids is sufficient to estimate total metals (Ure, 1996). Air-dried samples of non-amended, amended and control soils (3 g) were ground in an agate mill, sieved (<150 μm), digested in 18 mL of nitric/hydrochloric acid mixture (volume ratio 1:7) and diluted with deionized water up to 100 mL. Pseudototal Pb and As concentrations were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES) (SPECTRO CIROS CCD– ICP Spectrophotometer) at the Cornell Nutrient Analysis Laboratory. Pb and As in PBET and Modified Morgan’s extracts were determined by ICP-AES directly. Standard reference material from the National Institute for Standards and Technology (NIST SRM 2702 and SRM 2709) were used in the digestion and analyses as part of the QA/QC protocol. The recovery percentages were 73 and 84% for Pb and As, respectively. Reagent blanks were used to ensure accuracy of the analysis.
Data analysis
The significance of measured treatment differences in soil characteristics (pH, total organic carbon), Pb, As, S and P pseudototal concentrations, Pb and As potential bioavailability and Pb and As accumulation in P. scaber in non-amended and amended soils were determined by t-test at 95% confidence level (p<0.05). Linear regression analysis was performed to assess correlations between Pb and As concentrations in animals, Pb and As in soil and their potentially bioavailable concentration in soil. Statgraphics software (Statgraphics Plus for Windows 4.0, Statistical Graphics, Herndon, VA, USA) and Microsoft Office Excel 2007 were used.
Results and discussion
Soil amendments
Soil properties assessed for control, non-amended and amended soils are presented in Table 1. The non-amended soils used in the study were weakly to strongly acidic, with pH values ranging from 4.66 ± 0.1 to 6.38 ± 0.1. After the 10% (w/w) addition of compost, the pH significantly increased (p<0.05) by 1.5, 1.3 and 1.2 units in soils A, B and C, respectively. Pb and As pseudototal concentrations in the experimental control and non-amended soils formed a gradient ranging from 18 to 820 mg kg−1 for Pb and from 5.3 to 172 mg kg−1 for As (Table 1). The small, but significant (p<0.05) differences in Pb and As pseudototal concentrations between non-amended and amended soils can be attributed to soil dilution with compost or to the spatial variability of Pb and As in soil at this site (Arai et al., 2006). By adding compost, the pseudototal concentration of phosphorus and sulfur and the total organic carbon content in soils significantly (p<0.05) increased by factors up to 3, 2.9 and 4.1, respectively (Table 1).
Table 1.
Selected soil properties, Pb and As pseudototal concentration (assessed with aqua regia digestion) and their potential bioavailability assessed with the Modified Morgan's Exctraction and with the Physiologically Based Extraction Test (PBET, only the concentrations in the intestinal phase are shown) in control soil (CO) and in low, medium and highly polluted soil, with and without the addition of 10 % (w/w) compost (A, A + 10%, B, B + 10%, C, C + 10%, respectively). Results are presented as means of three replicates ± SD. LOQ, below the limit of quantification.
| CO | A | A + 10% | B | B + 10% | C | C+10% | |
|---|---|---|---|---|---|---|---|
| pH | 5.23 ± 0.1 | 4.66 ± 0.1 | *7.03 ± 0.1 | 5.88 ± 0.1 | *7.38 ± 0.1 | 6.38 ± 0.1 | *7.72 ± 0.1 |
| Total C (%) | 0.22 ± 0.04 | 5.1 ± 0.2 | *20.4 ± 0.3 | 5.1 ± 0.2 | *15.4 ± 3.2 | 3.3 ± 0.4 | *13.6 ±2.5 |
|
Pseudototal concentration
|
|||||||
| Pb (mg kg−1) | 18.4 ± 0.3 | 64.9 ± 0.9 | *35.9 ± 15.5 | 317.5 ± 2.8 | *296.8 ± 3.6 | 647.5 ± 17.5 | *820.4 ± 9.5 |
| As (mg kg−1) | 5.3 ± 0.1 | 31.7 ± 0.5 | *11.3 ± 0.1 | 84.1 ± 0.6 | *76.2 ± 0.6 | 171.7 ± 4.3 | 171.4 ± 1.9 |
| P (mg kg−1) | 647 ± 13 | 522 ± 8.5 | *1417 ± 12 | 827 ± 6.7 | *2450 ± 48 | 1142 ± 15 | *2690 ± 115 |
| S (mg kg−1) | 171 ± 3 | 280 ± 6.4 | *576 ± 8.5 | 300 ± 7.7 | *878 ± 29 | 396 ± 2.4 | *928 ± 68 |
|
Modified Morgan's Extraction
|
|||||||
| Pb (mg kg−1) | 0.4 ± 0.02 | 10.9 ± 0.1 | *0.89 ± 0.06 | 35.2 ± 2 | *14.1 ± 0.3 | 79.2 ± 3.2 | *46.2 ± 3.3 |
| As (mg kg−1) | 0.13 ± 0.01 | 0.31 ± 0.02 | *0.37 ± 0.04 | 1.03 ± 0.03 | 3.18 ± 0.07 | 6.08 ± 0.08 | *12.61 ± 0.13 |
|
PBET (intestinal phase) |
|||||||
| Pb (mg kg−1) | LOQ | 11.2 ± 1.7 | LOQ | 54.8 ± 2.5 | *20.2 ± 3.7 | 119.3 ± 9.2 | *89.2 ± 4 |
| As (mg kg−1) | LOQ | 5.8 ± 0.3 | *1.8 ± 1.6 | 18.8 ± 1 | 20.7 ± 1.6 | 65.7 ± 1.2 | *71.5 ± 1.3 |
significant difference (LSD,p<0.05) between soil without and soil with the addition of 10 % (w/w) compost.
Pb and As potential bioavailability
The results of the selective chemical extraction methods used in this study showed that the easily accessible compost is an effective stabilizing agent for the limitation of Pb potential bioavailability in polluted soil (Table 1). The MME showed a 12.3, 2.5 and 1.7-fold significant decrease (p<0.05) of Pb potential bioavailability in A, B and C soils, respectively. The very large reduction in extractable Pb from soil A is probably the result of the initially very low pH of this soil ( 4. 66) and the fact that compost addition raised the pH to 7. 03) . The compost amendment in soil A decreased the potential Pb bioavailability assessed with PBET below the limit of detection, while in soils B and C it decreased by factors of 2.7 and 1.3, respectively. Conversely, As potential bioavailability increased by factors up to 3.1 and 2.2 after compost addition, as assessed with MME and PBET, respectively. The soil pH is likely to be the most important factor controlling the conversions of trace metal chemical forms in soil (Smith, 2009; Zheljazkov and Warman, 2004; Jordaõ et al., 2006). The solubility of Pb is known to be inversely proportional to the soil pH, phosphate compounds content and humic materials. Humic materials are introduced into the soil by adding compost and seem to be the main sites of Pb sorption in soil by strong complxation, while Pb-phosphate compounds are likely to precipitate (Song and Greenway, 2004; Smith, 2009; Grimes at al., 1999; Adriano, 2001). At higher soil pH values, Pb species in soil are converted from the more labile chemical forms into less labile ones. On the other hand, As tends to become more soluble and mobile at higher soil pH values as chemisorption on minerals is less favorable at higher pH. In addition, humic acids, to which As may be adsorbed, tend to dissolve at higher soil pH values, resulting in As mobilization (Guisquiani et al., 1998; Kabata-Pendias and Pendias, 1992; Li and Thornton, 2001; Newton et al., 2006; Arai et al., 2006; Carbonell-Barrachina et al., 1999; Smedley and Kinniburgh, 2002; Gàl et al., 2007; Adriano, 2001; Yu and Klarup, 1994; McBride, 1994). Arsenic in soil can be mobilized also by competitive displacement by phosphate from anion adsorption sites (Cao et al., 2003; Woolson et al., 1973; Peryea, 1991; Hartley et al., 2009; 2010). The high phosphate in the compost used in this study may have contributed to As mobilization (Table 1). In the present study, the potential bioavailability of As assessed with MME was significantly positively correlated (p<0.01) with pseudototal phosphorus (R2 = 0.4818).
A statistical comparison of the results of MME and PBET showed, that in the case of Pb the results of both methods are consistent (R2 = 0.9568, p = 0.0000). The intercept of the linear regression function between MME and PBET is statistically not different than 0 and the slope is not different than 1 (p<0.001). It indicates that the simpler MME could be used instead of the more complex PBET to assess Pb potential oral bioavailability in soil.
Pb and As accumulation in P. scaber
The majority of isopods (95 %) survived the 14-day long exposure to experimental soil. The duration of exposure was determined on the basis of preliminary studies, where the authors concluded that 14 days of exposure under suboptimal condition does not severely affect animals. On the other hand, this is long enough for them to accumulate substantial amounts of bioavailable metals (Bibič et al. 1997; Udovic et al., 2009). Differently from a previous study, where the animals were exposed to highly polluted soil with Pb concentrations ranging from 1200 to 4300 m kg−1 (Udovic et al., 2009), Pb concentrations here were lower. However, the animals accumulated measurable Pb and As amounts in their bodies, without experiencing considerable stress, as their body weights did not significantly (p<0.05) change during the exposure time (data not shown). To the authors’ knowledge this is the first report on As accumulation in P. scaber exposed to contaminated agricultural soil without additional food in a short-term accumulation test.
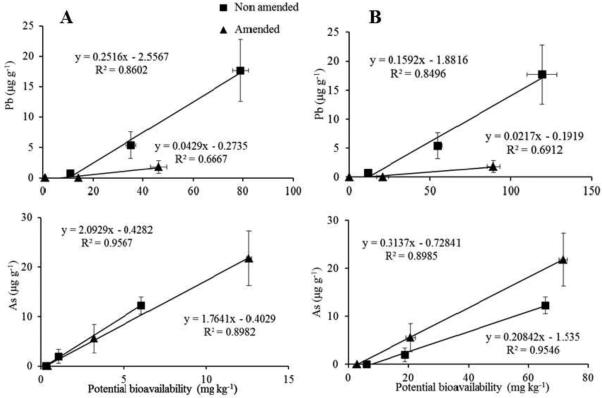
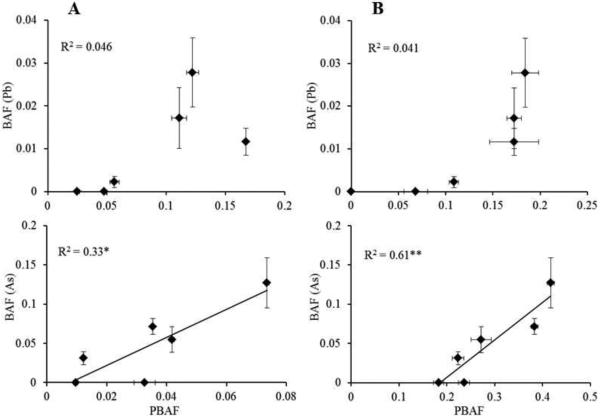
The increasing concentrations of Pb and As in soil were reflected in the accumulated Pb and As in animals (Table 2). As expected, the lowest Pb and As accumulation was measured in animals exposed to soil A and the highest in animals exposed to soil C. The results are in concordance with the reports of trace metals accumulated in isopods exposed to remediated soils (Udovic et al., 2009), in isopods collected in differently polluted areas (Blanuša et al., 2002; Gàl et al., 2008; Heikens et al., 2001; Hussein et al., 2006), or exposed to food spiked with increasing concentrations of trace metals (Gräff et al., 1997; Odendaal and Reinecke, 2004; Witzel, 1998). The opposite effects of compost addition on Pb and As bioavailability in soil already predicted with the chemical extraction tests were reflected also in P. scaber accumulation. The BAFs for Pb decreased below the limit of quantification in soils A and B and by a factor of 12.5 in soil C, while for As the BAFs increased by factors up to 1.8 (Table 2). The clear difference between the BAFs for non-amended and amended soils indicates that compost application is a very effective remediation approach for polluted soils for limiting the bioavailability of Pb, but not for As. The difference is evident also in Figure 1, where the correlations between the potential bioavailability of Pb and As assessed with MME and PBET and the accumulated amount of Pb and As in animals are presented separately for non-amended and amended soils. The high correlations between the MME and PBET values and the accumulated Pb and As in animals suggest that the fractions of soil Pb and As dissolved using the chemical extraction tests in this study correspond to the fractions of Pb and As available to soil organisms. To test this hypothesis, we expressed the potential bioavailability of Pb and As in soils with potential bioavailability factors (PBAFs), i.e. as the ratio between the Pb and As extractable with either MME or PBET and their pseudototal concentration in soil, and compared them with respective BAFs. The correlations are graphically presented in Figure 2. The Pb PBAFs and BAFs were not correlated, while for As the correlations for both MME and PBET with the BAFs were significant (p<0.05 and p<0.01, respectively). The slope of PBAF (from the MME test) vs. the BAF was different than one, but the intercept was equal to zero. Conversely, the slope of PBET vs. the BAF was equal to one, but the intercept was different from zero, indicating that the PBAFs are not equal to the BAFs. The PBET tends to overestimate the bioavailability of As for organisms (Figure 2). However, published studies comparing the PBET results to animal models give contrasting conclusions. Ruby et al. (1996) compared the results of the PBET with a New Zealand White rabbit model and the Cynomologus primate model, finding the PBET to be overpredictive. Conversely, Rodriguez and Basta (1999) reported that the PBET underestimates the bioavailability of As in calcine materials, compared to the in vivo immature swine model. This indicates the need to include bioassays in similar studies in order to gain more complete and meaningful information on the bioavailability of trace metals in polluted soils.
Table 2.
Pb and As concentrations and respective bioaccumulation factors (BAFs) for Porcellio scaber (whole bodies) after 14-days exposure to control soil (CO), three non-amended soils (A, B and C) and respective soils amended with 10 % (w/w) compost (A + 10%, B + 10% and C + 10%). Results are presented as average of 4 samples, each consisting in 6 pooled animals, with respective standard deviations.
| Accumulation | BAF | |||
|---|---|---|---|---|
| Pb (μg g−1) | As (μg g−1) | Pb | As | |
|
|
||||
| CO | LOQ | LOQ | LOQ | LOQ |
| A | 0.75 ± 0.2 | LOQ | 0.012 ± 0.003 | LOQ |
| A + 10% | LOQ | LOQ | *LOQ | LOQ |
| B | 5.4 ± 2.2 | 2.6 ± 0.7 | 0.017 ± 0.007 | 0.031 ± 0.008 |
| B + 10% | *LOQ | 5.5 ± 2.9 | *LOQ | *0.055 ± 0.017 |
| C | 17.7 ± 5.1 | 12.3 ± 1.8 | 0.028 ± 0.008 | 0.071 ± 0.01 |
| C + 10% | *1.8 ± 1.1 | *21.8 ± 5.5 | *0.002 ± 0.001 | *0.127 ± 0.032 |
significant diference between animals exposed to non-amended and amended soil (t-test, p<0.05).
LOQ, below the limit of quantification.
Figure 1.
Linear relationships between accumulated Pb and As in Porcellio scaber exposed to non-amended soils and soils amended with 10 % (w/w) compost and Pb and As potential bioavailability in respective soils, as assessed by the Modified Morgan’s extraction test (A) and by a Physiologically Based Extraction Test (PBET) (B). Data are presented as averages ± STDEV.
Figure 2.
Comparisons of Pb and As potential bioavailability factors (PBAF) and their bioaccumulatiooonnn factors (BAF). PBAFs are calculated as the ratio between the potential bioavailability of Pb and As, as assessed by Modified Morgan’s Extraction (A) and the Physiological Based Extraction Test (B), and their respective pseudototal concentrations in soils. BAFs are calculated as the ratio between the accumulated Pb and As and their respective pseudototal concentrations in soils. Results are presented as average ± STDEV. Asterisks denote statistical significance (*p>0.05; **p>0.01). Only significant linear correlations are graphically presented.
Conclusions
Using only chemical extraction methods for the assessment of trace metal bioavailability in soil, the complex soil-organism-pollutant interactions may not be adequately evaluated. Thus , we may lose important information on the risk such polluted soils pose to the environment and to humans, as well as information on the effect of remediation actions applied to the polluted soils. The results of this study indicate the efficacy of using common compost amendments in reducing the bioavailability of Pb in agricultural soils, but also that a complete assessment of trace metals in soil should be performed in order to detect and counteract side effects such as increased As bioavailability when both Pb and As are present in the soil. A complementary stabilization method focused on As should be therefore used in remediation of soil contaminated by long-term application of lead arsenate.
P. scaber accumulated Pb and As in a concentration-dependent manner and in proportion to Pb and As bioavailability, not to their total soil concentrations. The simple accumulation test used in this study showed potential for use as an in vivo test for the assessment of Pb and As bioavailability, in addition to the commonly used chemical extraction tests. To our knowledge, this is the first report on As accumulation in P. scaber exposed to agricultural soil historically contaminated with lead arsenate and remediated by compost application. The results of similar studies provide the base information for the future development of mathematical models for the prediction of trace metal bioavailability. In this study we showed that the simpler MME could replace the more complex PBET in the assessment of the potential bioavailability of Pb. In the case of As, both MME and PBET showed to be correlated to As accumulation in P. scaber; they could be therefore used for the assessment of As bioavailability, with some correction factors applied.
References
- Adriano DC. Trace Elements in Terrestrial Environments. 2nd Springer-Verlag; New York: 2001. pp. 219–261. [Google Scholar]
- Almendras ML, Carballa M, Diels L, Vanbroekhoven K, Chamy R. Prediction of heavy metal mobility and bioavailability in contaminated soil using sequential extraction and biosensors. Journal of Environmental Engineering. 2009;135:839–844. [Google Scholar]
- Arai Y, Lanzirotti A, Sutton SR, Newville M, Dyer J, Sparks DL. Spatial and temporal variability of arsenic solid-state speciation in historically lead arsenate contaminated soils. Environmental Science and Technology. 2006;40:673–679. doi: 10.1021/es051266e. [DOI] [PubMed] [Google Scholar]
- Baldantoni D, Leone A, Iovieno P, Morra L, Zaccardelli M, Alfani A. Total and available soil trace element concentrations in two Mediterranean agricultural systems treated with municipal waste compost or conventional mineral fertilizers. Chemosphere. 2010;80:1006–1013. doi: 10.1016/j.chemosphere.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Bibič A, Drobne D, Štrus J, Byrne AJ. Assimilation of zinc by Porcellio scaber (Isopoda, Crustacea) exposed to zinc. Bulletin of Environmental Contamination and Toxicology. 1997;58:814–821. doi: 10.1007/s001289900407. [DOI] [PubMed] [Google Scholar]
- Blanuša M, Mrković-Milić R, Durbešić P. Lead and Cadmium in soil and isopoda woodlice in Croatia. Ecotoxicol Environ Saf. 2002;52:198–202. doi: 10.1006/eesa.2002.2173. [DOI] [PubMed] [Google Scholar]
- Cao X, Ma LQ, Shiralipour A. Effects of cmpost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris vittata L. Environmental Pollution. 2003;126:157–167. doi: 10.1016/s0269-7491(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Jugsujinda A, Burlo F, De Laune RD, Patrick WH. Arsenic chemistry in municipal sewage sludge as affected by redox potential and pH. Water Research. 1999;34:216–224. [Google Scholar]
- Cave MR, Wragg J, Palumbo B, Klinck BA. R&D Technical Report P5-062/TR02. Environmental Agency; Bristol UK: 2002. Measurement of the bioaccessibility of arsenic in UK soils. [Google Scholar]
- Clemente R, Hartley W, Riby P, Dickinson NM, Nepp NW. Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ. Pollut. 2010;158:1644–1651. doi: 10.1016/j.envpol.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Gàl J, Hursthouse A, Cuthbert S. Bioavailability of arsenic and antimony in soils from an abandoned mining area, Glendinning (SW Scotland) Journal of Environmental Science and Health Part A. 207;42:1263–1274. doi: 10.1080/10934520701435585. [DOI] [PubMed] [Google Scholar]
- Gál J, Markiewicz-Patkowska J, Hursthouse A, Tatner P. Metal uptake by woodlice in urban soils. Ecotoxicol. Environ. Safety. 2008;69:139–149. doi: 10.1016/j.ecoenv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Gál J, Markiewicz-Patkowska J, Hursthouse A, Tatner P. Metal uptake by woodlice in urban soils. Ecotoxicology and Environmental Safety. 2008;69:139–149. doi: 10.1016/j.ecoenv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Gräff S, Berkus M, Alberti G, Köhler HR. Metal accumulation strategies in saprophagous and phytophagous soil invertebrates: a quantitative comparison. BioMetals. 1997;10:45–53. [Google Scholar]
- Grimes SM, Taylor GH, Cooper J. The availability and binding of heavy metals in compost derived from household waste. J Chem Technol Biotechnol. 1999;74:1125–1130. [Google Scholar]
- Guisquiani PL, Concezzi L, Businelli M, Machioni A. Fate of pig sludge liquid fraction in calcareous soil: agricultural and environmental implications. Journal of Environmental Quality. 1998;27:364–371. [Google Scholar]
- Guo G, Zhou Q, Ma LQ. Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: A review. Environmental Monitoring and Assessment. 2006;16:513–528. doi: 10.1007/s10661-006-7668-4. [DOI] [PubMed] [Google Scholar]
- Hartley W, Dickinson NM, Clemente R, French C, Piearce TG, Sparke S, Lepp NW. Arsenic stability and mobilization in soil at an amenity grassland overlying chemical waste (St. Helens, UK) Environ. Pollut. 2009;157:847–856. doi: 10.1016/j.envpol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Hartley W, Dickinson NM, Riby P, Leese E, Morton J, Lepp NW. Arsenic mobility and speciation in a contaminated urban soil are affected by different methods of green waste compost application. Environmental Pollution. 2010;158:3560–3570. doi: 10.1016/j.envpol.2010.08.015. [DOI] [PubMed] [Google Scholar]
- He ZL, Yang XE, Stoffella PJ. Trace elements in agrosystems and impacts on the environment. Journal of Trace Elements in Medicine and Biology. 2005;19:125–140. doi: 10.1016/j.jtemb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Heikens A, Peijnenburg WJGM, Hendriks AJ. Bioaccumulation of metals in terrestrial invertebrates. Environmental Pollution. 2001;113:385–393. doi: 10.1016/s0269-7491(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Hettiarachichi GM, Pierzynski GM. Soil lead bioavailability and in situ remediation of lead-contaminated soils: a review. Environmental Progress. 2004;23:78–93. [Google Scholar]
- Hopkin SP, Jones DT, Dietrich D. The isopod Porcellio scaber as a monitor of the bioavailability of metals in terrestrial ecosystems: towards a global ‘woodlouse watch’ scheme. The Science of the Total Environment. 1993;134(Supplement 1):357–365. [Google Scholar]
- Hussein MA, Obuid-Allah AH, Mohammad AH, Scott-Fordsmand JJ, Abd El-Wakeil KF. Seasonal variation in heavy metal accumulation in subtropical population of the terrestrial isopod, Porcellio laevis. Ecotoxicology and Environmental Safety. 2006;63:168–174. doi: 10.1016/j.ecoenv.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Idowu OJ, van Es HM, Abawi GS, Wolfe DW, Schindelbeck R, Moebius-Clune BN, Gugino BL. Use of an integrative soil heath test for evaluation of soil management impacts. Renewable Agriculture and Soil Systems. 2009;24:214–224. [Google Scholar]
- ISO 14235 . Soil Quality – Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization; Geneve, Switzerland: 1998. [Google Scholar]
- Jordaõ CP, Nascentes CC, Cecon PR, Fontes RLF, Pereira JL. Heavy metal availability in soil amended with composted urban solids. Environ Monit Asses. 2006;112:309–326. doi: 10.1007/s10661-006-1072-y. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A, Adriano DC. Trace metals. In: Rechcigl JE, editor. Soil Amendments and Environmental Quality. CRC Press, Inc.; Boca Raton, Florida: 1995. pp. 139–167. [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace Elements in Soils and Plants. CRC Press; Boca Raton: 1992. [Google Scholar]
- Langdon CJ, Piearce TG, Meharg AA, Semple KT. Interactions between earthworms and arsenic in soil environment: a review. Environmental Pollution. 2003;124:361–373. doi: 10.1016/s0269-7491(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Magdoff F, van Es H. Building Soils for Better Crops. 3rd Sustainable Agriculture Publications; Waldorf, MD: 2009. [Google Scholar]
- Malandrino M, Abollino O, Buoso S, Giacomino A, LA Gioia C, Mentasti E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere. 2011;82:169–178. doi: 10.1016/j.chemosphere.2010.10.028. [DOI] [PubMed] [Google Scholar]
- McBride M. Environmental Chemistry of Soils. Oxford University Press; New York: 1994. [Google Scholar]
- McBride MB, Pitiranggon M, Kim B. A comparison of tests for extractable copper and zinc in metal-spiked and field-contaminated soil. Soil Sci. 2009;174:439–444. [Google Scholar]
- Mench M, Bussière S, Boisson J, Castaing E, Vangronsveld J, Ruttens A, Da Koe T, Bleeker P, Assunção A, MAnceau A. Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments. Plant and Soil. 2003;249:187–202. [Google Scholar]
- Newton K, Amarasiriwardena D, Xing B. Distribution of soil arsenic species, lead and arsenic bound to humic acid molar mass fractions in a contaminated apple orchard. Environmental Pollution. 2006;143:197–205. doi: 10.1016/j.envpol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Nriagu JO. Arsenic in the environment. Part 2: Human health and ecosystem effects. Advances in Environmental Science and Technology. 194;27:1–293. [Google Scholar]
- Odendaal JP, Reinecke AJ. The sublethal effects and accumulation of cadmium in the terrestrial isopod Porcellio laevis Latr. (Crustacea, Isopoda) Archives of Environmental Contamination and Toxicology. 1999;36:64–69. doi: 10.1007/s002449900443. [DOI] [PubMed] [Google Scholar]
- Oomen AG, Hack A, Minekus M, Zeijdner A, Cornelis C, Schoeters W, van der Wiele T, Wragg J, Rompelberg CJM, Sips AJAM, van Wijnen JH. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environmental Science and Technology. 2002;36:3326–3334. doi: 10.1021/es010204v. [DOI] [PubMed] [Google Scholar]
- Oomen AG, Rompelberg CJM, Bruil MA, Dobbe CJG, Pereboom DPKH, Sips AJAM. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Archives of Environmental Contamination and Toxicology. 2003;44:281–287. doi: 10.1007/s00244-002-1278-0. [DOI] [PubMed] [Google Scholar]
- Peryea FJ. Phosphate induced release of arsenic from soils contaminated with lead arsenate. Soil Sci. Soc. Am. J. 1991;55:1301–1306. [Google Scholar]
- Rodriguez RR, Basta NT. An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environmental Science and Technology. 1999;33:642–649. [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiological based extraction test. Environmental Science and Technology. 1996;30:422–430. [Google Scholar]
- Schindelback RR, van Es HM, Abawi GS, Wolfe DW, Whitlow TW, Gugino BK, Idowu OJ, Moebius-Clune BN. Comprehensive assessment of soil quality for landscape and urban management. Landscape and Urban Planning. 2008;88:73–80. [Google Scholar]
- Shuman LM, Dudka S, Das K. Zinc forms and plant availability in a compost amended soil. Water Air and Soil Pollution. 2001;128:1–11. [Google Scholar]
- Smedley PL, Kiniburgh DG. A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry. 2002;17:517–568. [Google Scholar]
- Smith SR. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste compost compared to sewage sludge. Environment International. 2009;35:142–156. doi: 10.1016/j.envint.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Smith E, Naidu R, Alston AM. Arsenic in the soil environment: A review. Advances in Agronomy. 1998;64:149–195. [Google Scholar]
- Song QJ, Greenway GM. A study of the elemental leachability and retention capability of compost. J Environ MOnit. 2004;6:31–37. doi: 10.1039/b310840f. [DOI] [PubMed] [Google Scholar]
- Turner A, Ip KH. Bioaccessibility of metals in dust from the indoor environment: application of a Physiologically Based Extraction Test. Environ. Sci. Technol. 2007;41:7851–7856. doi: 10.1021/es071194m. [DOI] [PubMed] [Google Scholar]
- Udovic M, Drobne D, Lestan D. Bioaccumulation in Porcellio scaber (Crustacea, Isopoda) as a measure of the EDTA remediation efficiency of metal-polluted soil. Environ. Pollut. 2009;157:2822–2829. doi: 10.1016/j.envpol.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Vermeulen F, Van der Brink NW, D’Havé H, Mubiana VK, Blust R, Bervoest L, De Coen W. Habitat type-based bioaccumulation and risk assessment of metal and As contamination in earthworms, beetles and woodlice. Environmental Pollution. 2009;157:3098–3105. doi: 10.1016/j.envpol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Witzel B. Uptake, storage and loose of cadmium and lead in the woodlouse Porcellio scaber (Crustacea, Isopoda) Water Air and Soil Pollution. 1998;108:51–68. [Google Scholar]
- Woolson EA, Axley JH, Kearney PC. Chemistry and phytotoxicity of arsenic in soils. 2. Effects of time and phosphorous. Soil Science Society of America Journal. 1973;37:254–259. [Google Scholar]
- Wragg J, Cave M, Nathanail P. A study of the relationship between arsenic bioaccessibility and its solid-phase distribution in soils from Wellingborough, UK. Journal of Environmental Science and Health Part A. 2007;42:1303–1315. doi: 10.1080/10934520701436062. [DOI] [PubMed] [Google Scholar]
- Wragg J, Cave MR. R&D Technical Report P5-062/TR/01. Environmantal Agency; Bristol: 2002. In-vitro Methods for the Measurement of the Oral Bioaccessibility of Selected Metals and Metalloids in Soils: a Critical Review. [Google Scholar]
- Yu J, Klarus D. Extraction kinetics of copper, zinc, iron and manganese from contaminated sediment using disodium ethylenediaminetetraacetate. Water Air and Soil Pollution. 1994;75:205–225. [Google Scholar]
- Zheljazkov VD, Warman PR. Phytoavailability and fractionation of copper, manganese, and zinc in soil following application of two composts to four crops. Environmental Pollution. 2004;131:187–195. doi: 10.1016/j.envpol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Zheljezakov VD, Warman PR. Source-separated municipal solid waste compost application to Swiss chard and basil. Journal of Environmental Quality. 2004;33:542–552. doi: 10.2134/jeq2004.5420. [DOI] [PubMed] [Google Scholar]