Abstract
Background
Recurrent aortic arch obstruction following the Norwood procedure (NP) continues to be a source of morbidity. We sought to determine if a modified interdigitating technique for aortic arch reconstruction during the NP decreased recurrent arch obstruction.
Methods
142 consecutive infants undergoing the NP were divided into groups based upon surgical technique: Group 1 (n=79, 1/1999 to 5/2003) underwent arch reconstruction with complete coarctectomy followed by anastomosis of the descending aorta to the transverse arch. Group 2 (n=63, 6/2003 to 9/2006) underwent complete coarctectomy plus a modified interdigitating technique. Catheterization prior to Stage 2 palliation was reviewed for hemodynamics, angiographic arch dimensions, and a coarctation index (CI) was calculated.
Results
Reintervention for recurrent coarctation occurred in 28% (22/79) of Group 1 patients compared with 2% (1/63) of Group 2 patients (p = 0.001). Aortic pressures, gradients, dimensions, and CI were consistently more favorable for Group 2.
Conclusion
Coarctectomy plus an interdigitating arch anastomosis was superior to coarctectomy alone and resulted in a dramatically decreased incidence of recurrent arch obstruction.
Introduction
The documented incidence of recurrent coarctation of the aorta following the Norwood procedure (NP) ranges from 5 to 40 % (1–5). Factors contributing to recoarctation include surgical technique and contraction of residual ductal tissue not resected at the time of the NP. Several modifications have been made to the original arch reconstruction technique described by Norwood (6), yet the incidence of recoarctation and its resultant morbidity remain significant. The increased afterload to the single ventricle caused by recoarctation may result in ventricular dysfunction and atrioventricular valve regurgitation, increasing the risk of morbidity and mortality. The purpose of this paper is to describe the incidence of recoarctation following the NP in a recent cohort of single ventricle patients at the Children’s Hospital of Wisconsin and to identify factors contributing to long-term arch patency after aortic arch reconstruction in children with significant systemic outflow obstruction.
Methods
The clinical records of 142 consecutive infants undergoing the NP at the Children’s Hospital of Wisconsin between January 1999 and September of 2006 were reviewed after Institutional Review Board approval. The focus of this retrospective analysis was to determine the difference in the incidence of recurrent obstruction using two different techniques of arch reconstruction. In all 142 patients a coarctectomy was performed as part of the arch reconstruction. Prior to June of 2003 the posterior edge of the descending thoracic aorta was sutured to the posterior edge of the distal arch without interdigitation. Beginning in June of 2003 the interdigitating arch reconstruction technique was added. This surgical procedure has been previously described. (1,7). Briefly, our interdigitating technique is as follows: The hypoplastic aorta, brachiocephalic vessels, ductus arteriosus, and pulmonary arteries are fully mobilized. A Gore-Tex graft is anastomosed to the innominate artery that is utilized for arterial cannulation for cardiopulmonary bypass and subsequent cerebral perfusion during arch reconstruction and becomes the systemic-to-pulmonary artery shunt in patients undergoing a classic Norwood. Mobilization of the proximal descending aorta is assisted by division of the first 2 to 3 sets of intercostals arteries. After cooling to a bladder temperature of 18°C, a brief period of circulatory arrest averaging 10 minutes occurs to give cardioplegia and perform an atrial septectomy. Regional cerebral perfusion is then initiated via the innominate artery shunt. The aortic isthmus is divided. The pulmonary artery side of the ductus arteriosus is ligated and all ductal tissue is resected from the descending aorta. Two indicators are used to ensure that all ductal tissue is excised. First, the arterial wall of the ductus is thicker and the tissue is more friable than that of native descending aortic tissue and excision is continued until thin tissue characteristic of the descending aorta is reached. Second, the ductal tissue excision is continued to within 1–2 mm of the first set of intercostals arteries since the intercostal arteries arise from the descending aorta and not the ductus arteriosus, this indicates complete ductal excision. The undersurface of the aortic arch is then incised from the divided isthmus proximally down the hypoplastic ascending aorta and a cutback in the pulmonary root is made at a precise site allowing for creation of a nonstenotic anastomosis between the aortic and pulmonary root. A cutback is made in the posterior left lateral descending thoracic aorta. The open distal arch is then sutured within the cutback in the descending thoracic aorta in an interdigitating fashion creating a large native tissue-to-tissue connection. Pulmonary homograft tissue is utilized to complete the construction of the neoaorta and the systemic to pulmonary artery shunt, whether a modified BT shunt or a RV to PA conduit, is constructed during rewarming. The total cohort of patients for this study was divided into two chronological groups, those undergoing the Norwood procedure between January 1999 and May of 2003 without an interdigitating arch reconstruction (Group 1, n=79) and those undergoing the Norwood procedure between June 2003 to September of 2006 with an interdigitating arch reconstruction (Group 2, n=63).
Demographics and primary diagnosis of the two groups at the Norwood procedure as defined by echocardiography are detailed in tables 1 and 2. Clinical records were reviewed for any reinterventions following the Norwood procedure (surgical or balloon dilation/stenting) to address aortic arch or brachiocephalic vessel anatomic or physiologic abnormalities. Timing of these reinterventions in relation to the Norwood procedure was recorded.
Table 1.
Patient demographics
| Group 1 NP 1/1999–5/2003 |
Group 2 NP 6/2003–9/2006 |
P value | |
|---|---|---|---|
| Number of patients | 79 | 63 | |
| Male (%) | 48 (61) | 39 (62) | .9 |
| Age at Norwood (days) | 6.1 ± 4.1 | 7.6 ± 7.7 | .13 |
| Weight at Norwood (Kg) | 3.1 ± 0.5 | 3.3 ± 0.6 | .16 |
| Height at Norwood (cm) | 49.9 ± 3.0 | 50.2 ± 6.7 | .76 |
Table 2.
Patient diagnostic groups
| Diagnostic Group | Group 1 | Group 2 | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Hypoplastic left heart syndrome | 65 | 82 | 52 | 83 |
| Double inlet left ventricle with coarctation | 3 | 4 | 2 | 3 |
| Right ventricular hypoplasia with TGA | 6 | 8 | 3 | 5 |
| Right dominant unbalanced atrioventricular septal defect | 1 | 1 | 2 | 3 |
| Double outlet right ventricle with left ventricular hypoplasia | 4 | 5 | 3 | 5 |
| Interrupted aortic arch with left ventricular hypoplasia | 1 | 2 | ||
Angiography from cardiac catheterization prior to stage 2 palliation was reviewed. Neoaortic dimensions were recorded in the following positions: neoaortic valve, neoaortic sinuses, point of maximal dimension of the ascending neoaorta in both the anterior-posterior and lateral views, transverse neoaortic arch, distal neoaortic arch anastomosis, proximal descending aorta and aorta at the diaphragm. The coarctation index (CI) was calculated as the ratio of the narrowest arch segment divided by the diameter of the aorta at the level of the diaphragm (8). Blood pressure measured directly in the ascending and descending aorta and pulmonary and systemic flow measurements were recorded from catheterization reports.
Statistical analysis
Results are expressed as mean ± standard deviation or median with ranges as appropriate. Data was analyzed using SPSS 13.0 statistical software (SPSS Inc. Chicago, IL). A descriptive analysis of the sample was performed. Continuous variables were compared using student t-tests (mean ± standard deviation) and Mann-Whitney test (median and range). Categorical variables were compared using chi-square technique.
Results
Hypoplastic left heart syndrome made up the majority of patients in Group 1 (82%) and Group 2 (83%) (Table 2). The frequency of aortic and mitral atresia was similar for the two groups (42% vs. 36%, p=0.5) as was the size of the ascending aortic dimensions measured by preoperative echocardiogram (3.5 mm ± 1.7 vs. 3.6 ± 1.7, p=0.8). Thirty-three percent of Group 1 and 36% of Group 2 patients had ascending aortas measuring 2 mm or less (p=0.7). Patient age, weight, height and gender distribution at the time of the NP was similar for the two groups. All patients in Group 1 had Blalock-Taussig (BT) shunts; 49% (31/63) of Group 2 patients had right ventricle to pulmonary artery (RV-PA) conduits.
Pre-stage 2 palliation catheterizations were performed at a younger age in Group 2 patients (3.3 ± 1.0 vs. 3.8 ± 1.4 months, p= 0.03); however, height, weight and body surface area were similar for the two groups. Group 1 patients had increased systolic and mean blood pressure in the ascending aorta and increased systolic and mean blood pressure gradients from the ascending to descending aorta (Table 3). Diastolic blood pressures were similar for the two groups in the ascending and descending aorta. All patients were able to maintain mean pressures in the descending aorta greater than 40 mmHg regardless of the angiographic or hemodynamic severity of recoarctation. For all patients undergoing reintervention (16%, n=23/142) the average systolic blood pressure gradient was 22.6 ± 17.6 mmHg compared to those who did not undergo reintervention 2.1 ± 5.9 mmHg (p< 0.001).
Table 3.
Catheterization hemodynamics
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Ascending aorta systolic blood pressure (mmHg) | 93.0 ± 16.3 | 77.9 ± 13.9 | .001 |
| Ascending aorta diastolic blood pressure (mmHg) | 34.1 ± 6.6 | 32.9 ± 4.9 | .25 |
| Ascending aorta mean blood pressure (mmHg) | 57.7 ± 9.4 | 50.6 ± 7.6 | .001 |
| Descending aorta systolic blood pressure (mmHg) | 84.8 ±13.7 | 75.6 ± 16.2 | .001 |
| Descending aorta diastolic blood pressure (mmHg) | 33.9 ± 6.9 | 32.9 ± 4.4 | .37 |
| Descending aorta mean blood pressure (mmHg) | 54.3 ± 8.9 | 49.2 ± 7.5 | .001 |
| Systolic blood pressure gradient | 8.3 ± 14.3 | 2.5 ± 7.5 | .005 |
| Diastolic blood pressure gradient | 0.46 ± 2.5 | 0.3 ± 2.6 | .62 |
| Mean blood pressure gradient | 3.5 ± 4.0 | 1.4 ± 2.5 | .001 |
Angiographic measurements of the aortic arch are detailed in Table 4. Group 1 patients had increased dimensions at the neoaortic sinus, proximal descending aorta, and aorta at the diaphragm and decreased transverse arch and distal neoaortic arch anastomosis dimensions. The coarctation index was greater in Group 2 patients.
Table 4.
Angiographic dimensions
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Neoaortic valve (mm) | 12.2 ± 2.3 | 11.7 ± 2.2 | .23 |
| Neoaortic sinus (mm) | 17.7 ± 3.5 | 16.2 ± 2.1 | .004 |
| Ascending neoaorta maximal dimension anterior-posterior (mm) | 17.4 ± 3.5 | 17.2 ± 3.5 | .76 |
| Ascending neoaorta maximal dimension lateral (mm) | 17.0 ± 4.2 | 16.5 ± 2.8 | .53 |
| Transverse arch (mm) | 10.3 ± 2.5 | 11.6 ± 2.4 | .009 |
| Distal neoaortic arch anastomosis (mm) | 6.2 ± 1.9 | 6.8 ± 1.4 | .04 |
| Proximal descending aorta (mm) | 7.8 ± 1.7 | 6.3 ± 1.0 | .001 |
| Aorta at diaphragm (mm) | 8.1 ± 1.3 | 6.8 ± 0.9 | .001 |
| Coarctation Index | 0.8 (0.3–1.7) | 1.0 (0.4–1.7) | .001 |
| Recoarctation intervention, n (%) | 22(28) | 1(2) | .001 |
Reintervention for recoarctation was performed in 28% (22/79) of Group 1 patients at a median age of 116 days (range 43–1091). Three were effectively palliated with balloon dilation, four had balloon dilation followed by surgical arch reconstruction and fifteen had surgical repair at the time of stage 2 palliation The incidence of reintervention for recoarctation in Group 2 patients was 2% (1/63). This patient underwent successful balloon dilation of the recoarctation at 4 months of age. No additional arch surgeries were necessary in Group 2 patients. Hypoplastic left heart syndrome was the primary diagnosis in 91% (21/23) of patients with recoarctation. Survival through stage 2 palliation were similar for the two groups as is current survival for Group 1 (82%) and Group 2 (84%) patients.
Discussion
Obstruction to systemic blood flow is poorly tolerated in infants with single ventricle physiology who have undergone the Norwood procedure. In these patients the single ventricle is responsible for pumping blood to both the pulmonary and systemic circulation and any obstruction to flow worsens an already inefficient system of parallel circulation. Recoarctation increases systemic resistance resulting in excessive pulmonary blood flow with increased volume load and increased wall stress to a single ventricle with limited reserve. Even mild arch obstruction can lead to ventricular dysfunction, increased atrioventricular valve regurgitation, and diminished systemic perfusion. A study by Fraisse et al (9) also showed that residual arch obstruction significantly increases the risk for mortality. Most publications document the frequency of recoarctation to be in the range of 10–40% (1–5).
Recurrent arch obstruction appears to be affected both by surgical technique and failure to completely resect ductal tissue from the aorta prior to completion of the arch reconstruction (10). This series compares two groups in which complete coarctectomy was performed. With each technique care was taken to completely resect all ductal tissue prior to completing the arch reconstruction. In addition to coarctectomy, our current technique uses an interdigitating technique in which the open distal transverse arch is sutured into a cutback in the posterior left lateral descending thoracic aorta. This results in a large native tissue-to-tissue connection similar to an extended end-to-end coarctation repair. With this technique the incidence of recurrent aortic arch obstruction has dramatically decreased to 2% in patients undergoing the Norwood procedure since 6/2003 compared to a nearly 30% incidence in patients operated upon prior to utilization of the interdigitating technique.
Burkhart et al has reported similar results with the interdigitating technique (1). In that study three surgical techniques utilized in different eras were compared. The initial group of patients underwent a classic Norwood with incision across the coarctation followed by patch reconstruction with homograft tissue. The incidence of recoarctation with this technique was 46%. The second group of patients underwent arch reconstruction with an autologous technique described initially by Fraser and Mee (11). With this technique all ductal tissue is resected and the arch is reconstructed by anastomosing the distal aorta, aortic arch and the main pulmonary artery together. This group of patients had a recoarctation rate of 15%. Finally, the most recent 33 patients underwent an interdigitating and the incidence of recoarctation was 0%.
Limitations
This was a retrospective study comparing of non-contemporary groups with all the limitations of that study design. A learning curve and greater surgeon experience may have contributed to the improved results in the later group of patients.
Conclusion
The avoidance of recurrent arch obstruction is an important goal in the treatment of patients requiring the Norwood procedure. Coarctectomy plus an interdigitating arch anastomosis was superior to coarctectomy alone resulting in a dramatically decreased incidence of recurrent arch obstruction and is the technique of choice for the Norwood procedure.
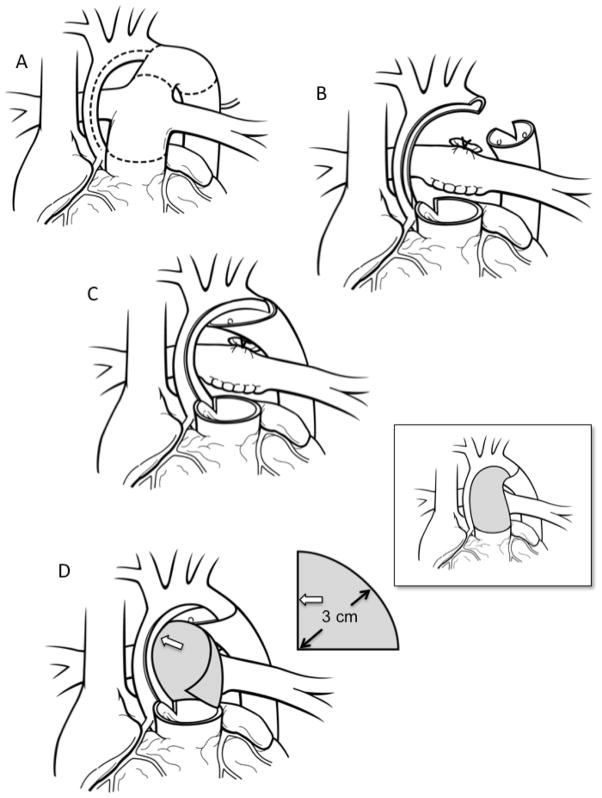
Figure 1.
Coarctectomy combined with an interdigitating arch reconstruction. A) Preoperative anatomy. The dotted lines indicate the areas to be incised. B) The aortic isthmus is divided and all ductal tissue is excised. The undersurface of the arch is incised and this incision is carried down the medial aspect of the ascending aorta. Cutbacks are performed in the posterior left lateral aspect of the proximal descending aorta as well as the pulmonary root leftward of the commissure that is adjacent to the ascending aorta. C) A large interdigitating tissue-to-tissue connection is created between the open distal arch and the descending aorta. The descending aorta can be brought as far proximally as the distal ascending aorta. Finally, the adjacent points of the ascending aorta and pulmonary root cutback are sutured together. D) A patch of homograft material is used to complete the arch reconstruction and neoascending aorta. A flat piece of homograft material is used that is in the shape of a quarter of a circle with a radius of 3cm. The straight edge of the graft (open arrow) is sutured to the inner curvature and the curved outer edge of the patch is sutured to the outer curvature. The patch is tailored as the suture-line transitions to the pulmonary root. Although a large portion of the original patch may be trimmed away the 3cm radius ensures that the neoascending aorta will be without obstruction as this corresponds to the circumference of the typical pulmonary root of the typical patient undergoing the Norwood procedure. The final reconstruction is shown in the inset.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burkhart H, Ashburn D, Konstantinov I, De Oliviera N, Benson L, Williams W, Van Arsdell G. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:61–5. doi: 10.1016/j.jtcvs.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 2.Ishino K, Stumper O, De Giovanni J, Silove E, Wright J, Sethia B, Brawn W. The modified Norwood procedure for hypoplastic left heart syndrome: early to intermediate results of 120 patients with particular reference to aortic arch repair. J Thorac Cardiovasc Surg. 1999;117:920–30. doi: 10.1016/s0022-5223(99)70373-9. [DOI] [PubMed] [Google Scholar]
- 3.Chessa M, Dindar A, Vettukattil J, Stumper O, Wroght J, Silove E, De Giovanni J. Balloon angioplasty in infants with arch obstruction after the modified stage I Norwood procedure. Am Heart J. 2000;140:227–31. doi: 10.1067/mhj.2000.108238. [DOI] [PubMed] [Google Scholar]
- 4.Tworetzky W, McElhinney D, Burch G, Teitel D, Moore P. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Cathet Cardiovasc Intervent. 2000;50:54–8. doi: 10.1002/(sici)1522-726x(200005)50:1<54::aid-ccd11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Zellers T. Balloon angioplasty for recurrent coarctation of the aorta in patients following staged palliation for hypoplastic left heart syndrome. Am J Cardiol. 1999;84:231–2. doi: 10.1016/s0002-9149(99)00242-8. [DOI] [PubMed] [Google Scholar]
- 6.Norwood W, Lang P, Hansen D. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;306:23–6. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 7.Tweddell J. The Norwood procedure with an innominate artery-to-pulmonary artery shunt. Operative Techniques in Thoracic and Cardiovascular Surgery. 2005;7:123–40. [Google Scholar]
- 8.Lemler M, Zellers T, Harris K, Ramaciotti C. Coarctation index: identification of recurrent coarctation in infants with hypoplastic left heart syndrome after the Norwood procedure. Am J Cardiol. 2000;86:697–99. doi: 10.1016/s0002-9149(00)01058-4. [DOI] [PubMed] [Google Scholar]
- 9.Fraisse A, Colan S, Jonas R, Gauvreau K, Geva T. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J. 1998;135:230–6. doi: 10.1016/s0002-8703(98)70086-9. [DOI] [PubMed] [Google Scholar]
- 10.Machii M, Becker A. Nature of coarctation in hypoplastic left heart syndrome. Ann Thorac Surg. 1995;59:1491–4. doi: 10.1016/0003-4975(95)00154-d. [DOI] [PubMed] [Google Scholar]
- 11.Fraser C, Mee R. Modified Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg. 1995;60(suppl):S546–9. doi: 10.1016/0003-4975(95)00848-9. [DOI] [PubMed] [Google Scholar]