Abstract
Rho kinase mediates the effects of inflammatory permeability factors by increasing actomyosin-generated traction forces on endothelial adherens junctions, resulting in disassembly of intercellular junctions and increased vascular leakage. In vitro, this is accompanied by the Rho kinase-driven formation of prominent radial F-actin fibers, but the in vivo relevance of those F-actin fibers has been debated, suggesting other Rho kinase-mediated events to occur in vascular leak. Here, we delineated the contributions of the highly homologous isoforms of Rho kinase (ROCK1 and ROCK2) to vascular hyperpermeability responses. We show that ROCK2, rather than ROCK1 is the critical Rho kinase for regulation of thrombin receptor-mediated vascular permeability. Novel traction force mapping in endothelial monolayers, however, shows that ROCK2 is not required for the thrombin-induced force enhancements. Rather, ROCK2 is pivotal to baseline junctional tension as a novel mechanism by which Rho kinase primes the endothelium for hyperpermeability responses, independent from subsequent ROCK1-mediated contractile stress-fiber formation during the late phase of the permeability response.
Keywords: Contraction, HUVEC, Endothelial, Permeability, Rho kinase
1. Introduction
Numerous pathological conditions including life-threatening sepsis and acute respiratory distress syndrome (ARDS) are characterized by endothelial barrier failure [1]. The sequelae include uncontrolled fluid extravasation and edema [1,2]. Under acute inflammatory conditions, the post-capillary venules in particular become leaky, whereas in ARDS the alveolar–capillary barrier is the major site of leakage. Despite increasing incidence of sepsis [3] and high mortality rates in ARDS, no treatment is currently available to combat endothelial barrier disruption in these diseases [1].
Endothelial barrier function is controlled principally by cytoskeletal elements that –in addition to local signaling events that weaken the junctions– orchestrate intercellular junctional complexes, and facilitate cell–matrix adhesion [2]. Dysfunction of the endothelial barrier can be elicited through activation of specific receptors by vasoactive agents such as thrombin and vascular endothelial growth factor (VEGF), as well as by interaction of the endothelium with leukocytes. Thrombin-induced signaling via its receptor PAR1 –which is short for protease-activated receptor 1– involves several signaling mechanisms that are simultaneously activated, including the influx of calcium ions, the activation of small Rho GTPases, the activation of various kinases, and the phosphorylation of (junctional) proteins. Among the small GTPases, RhoA is mainly involved in inducing endothelial hyperpermeability, whereas Rac1, Cdc42 and Rap1 contribute to enforcement of an intact barrier function [4].
A key effector molecule of RhoA in regulating vascular permeability is Rho kinase. It has been well-appreciated that enhanced Rho kinase activity upon stimulation by inflammatory mediators such as thrombin has a strong barrier-disruptive effect [5,6]. However, basal Rho kinase activity is also involved in the maintenance of barrier integrity [7,8]. Initial studies in endothelial cells (ECs) showed that transduction of a dominant-negative Rho kinase markedly reduced thrombin-induced formation of F-actin fibers through inhibition of the myosin phosphatase, serving as a paradigm for Rho kinase-mediated vascular hyperpermeability [9]. Yet, their in vivo relevance remained uncertain and has been debated for the microcirculation [10,11]. In cell models, Huveneers et al. showed that the agonist-induced radial F-actin fibers transmit tension to the endothelial junctions [12]. Indeed, pharmacological inhibition of Rho kinase reduces tension to the junctions, and ablates thrombin-induced hyperpermeability of endothelial monolayers by about 50% [6]. The remaining Rho kinase-independent part of the in vitro endothelial hyperpermeability involves regulation by protein tyrosine kinases and protein kinase C zeta [13,14]. In animal models, inhibitors of Rho kinase reduced vascular hyperpermeability induced by vaso-active agents such as VEGF, endotoxin (LPS) and thrombin even more effectively than in vitro [15,16]. But to the contrary, recent reports indicated that treatment of rats with the Rho kinase inhibitor fasudil, while effective in reducing LPS-induced permeability, improving survival in sepsis and preventing ARDS [17], by itself promoted vascular leakage of macromolecules [18]. Taken together, these data suggest the presence of distinct and sometimes even opposing Rho kinase activities determining endothelial morphology and function.
In an attempt to resolve this discrepancy we turned to two distinct isoforms of Rho kinase, ROCK1 and ROCK2, encoded by two different genes [19]. These isoforms are highly homologous except for their PH domains. Their human forms share 64% sequence identity with 89% identity in the catalytic domain [19]. Given their high sequence similarity it is not surprising that available inhibitors do not distinguish between the two ROCK isoforms, except for the ROCK2 inhibitor SLx-2119, also known as KD025, showing 200-fold higher selectivity toward ROCK2 (IC50 105 nmol/L) compared to ROCK1 (IC50 24 µmol/L) [27].
An analysis of Rho kinase knockout-mice suggests that there is no compensation for the loss of either isoform by the other: ROCK2−/− mice show a high fetal death rate [20] while ROCK1−/− die early after birth [21], but ROCK1 null animals that survive develop normally. The developmental effects associated with ROCK1 and ROCK2 deficiencies have limited the use of these animals to evaluate the functions of these molecules in physiology and disease in an isoform-specific manner. Also, most pharmacological inhibitors lack isoform specificity.
Rho kinase is involved in many basic vascular activities such as cellular migration, angiogenesis, and development of tone. Evidence has accumulated over the past decade that enhanced activity of Rho kinase plays an important role in many vascular pathologies including (pulmonary) hypertension, atherosclerosis, diabetes, and vascular leak [22,23]. ROCK isoform-specific regulation has been indicated for some of them [24,25].
Given their discrete form and function, we hypothesized that specifically targeting a single Rho kinase isoform in anti-vascular leak therapy would be an effective and novel strategy. Accordingly, using a combination of in-vitro and in-vivo experiments, we investigated the individual contributions of ROCK1 and ROCK2 to the regulation of endothelial barrier permeability.
2. Results
2.1. Efficacy of ROCK1 and ROCK2 depletion by siRNA treatment in vitro and in vivo
In vitro experiments were performed throughout this study with human umbilical vein endothelial cells (HUVECs). Key findings were verified in primary human pulmonary microvascular endothelial cells (HPMVECs) and presented in the online supplement.
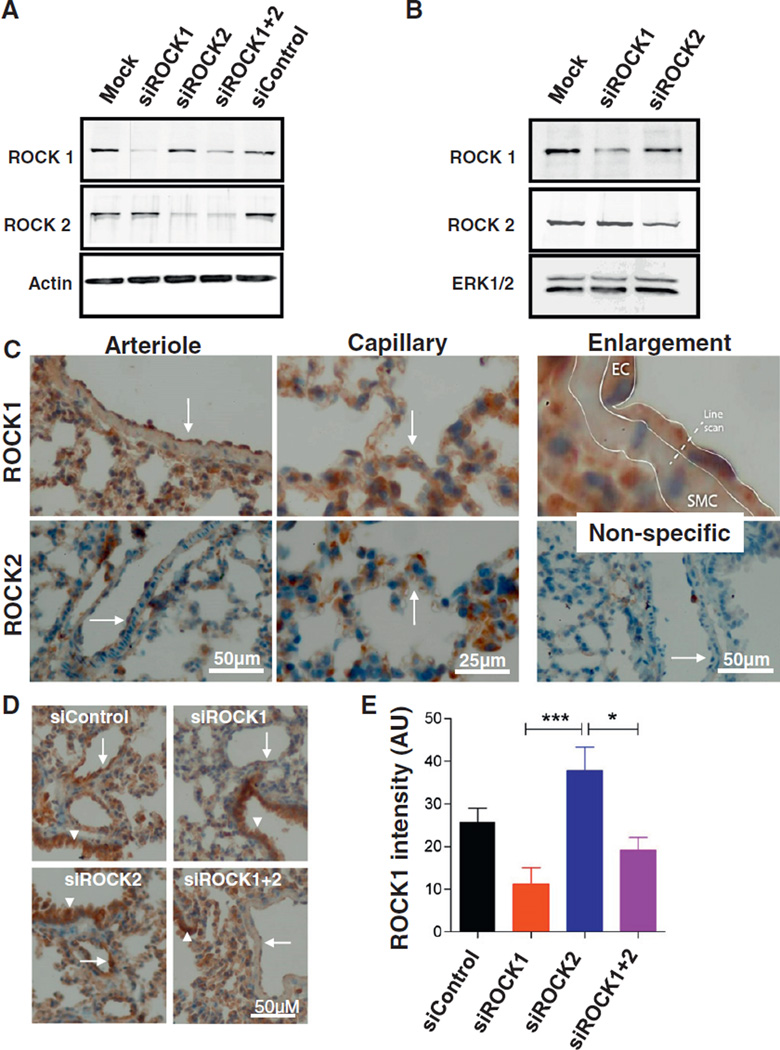
The efficacy of siRNAs to downregulate ROCK protein expression in HUVECs was monitored by Western blotting of cell lysates obtained at 48 h after transfection. A net decrease in ROCK protein expression in each experiment of at least 90% was observed in ROCK1-silenced ECs and of 75% in ROCK2-silenced ECs (see Fig. 1A for representative Western blot).
Fig. 1.
Efficacy of ROCK1 and ROCK2 depletion by siRNA treatment in vitro and in vivo. A: Effect of ROCK1 and/or ROCK2 siRNAs on ROCK protein expression in cultured HUVECs. Representative Western blot showing changes in the expression of ROCK1 and ROCK2 48 h after transfection of HUVECs with ROCK1 or ROCK2 siRNA(s). Blot was probed with ROCK1 (upper panel) or ROCK2 (middle panel) antibodies to verify reduced ROCK expression upon siRNA transfection. Blot was reprobed for actin as a loading control (lower panel). Similar results were obtained in 8 independent experiments. B: Effect of ROCK1 and/or ROCK2 siRNAs on ROCK protein expression in cultured mouse NIH3T3 cells. Representative Western blot showing changes in the expression of ROCK1 and ROCK2 48 h after transfection with ROCK1 or ROCK2 siRNA(s). Blot was probed with ROCK1 (upper panel) or ROCK2 (middle panel) antibodies to verify reduced ROCK expression upon siRNA transfection. Blot was reprobed for ERK1/2 as a loading control (lower panel). Similar results were obtained in 3 independent experiments. C: Representative immune-histochemical staining of ROCK1 and ROCK2 demonstrating their expression in the mouse pulmonary vasculature. ROCK1 and ROCK2 are present in various cell types including endothelial cells. Arrows point to the endothelium at the luminal site of the arteriolar vessel wall (left panels) and to capillaries (middle panels). The upper right panel highlights the endothelial expression of ROCK1. For quantification, line scans were drawn over the vessel wall from the lumen to the cytoplasm of underlying smooth muscle cells. The lower right panel shows non-specific control staining. Left panels: bar = 50 µm; middle panels: bar = 25 µm. D: Effect of ROCK1 and/or ROCK2 siRNAs on ROCK1 protein expression of pulmonary vascular endothelium. The lungs were harvested 48 h after injection of liposomes conjugated with indicated siRNA, as described in the Materials & methods section. Immuno-histochemical staining for ROCK1 was reduced in the endothelium of arterioles (arrows) of ROCK1, but not ROCK2 transduced mice, whereas bronchiolar ROCK1 expression was not affected (arrow heads). Bar = 50 µm. E: Intensity of ROCK1 staining, quantified according to the description in the Materials & methods section. Values represent differences in intensity of staining between endothelial cells and vascular smooth muscle cells. Mean ± SEM of 4–6 vessels from 2 mice per treatment group. *p < 0.05, ***p < 0.001.
To test whether the siRNAs directed at human ROCK sequences were also effective in downregulating mouse ROCK1 and ROCK2, mouse NIH3T3 cells were used as a model cell system. The siRNAs directed to human ROCK1 and ROCK2 were less effective in downregulating mouse ROCK protein expression (data not shown). Therefore, new siRNAs were designed directed to mouse ROCK sequences (see the Materials & methods section), resulting in a >80% downregulation of mouse ROCK1 and ROCK2 protein expressions in vitro (see Fig. 1B for a representative Western blot).
Immuno-histochemical staining of both ROCK1 and ROCK2 revealed their abundant expression in endothelial cells of large pulmonary arterioles as well as in the small capillaries of the mouse lung (Fig. 1C). Their expression was selectively downregulated 48 h after retro-orbital injection of the corresponding siRNAs as evidenced by semi-quantitative immuno-histochemical analysis of arteriolar ROCK1 expression (Fig. 1D, E).
2.2. Silencing of ROCK1 and ROCK2 reveals differential involvement in endothelial permeability
To evaluate the contributions of ROCK1 and ROCK2 to permeability changes of human endothelial monolayers, HUVECs were grown in vitro on porous filters, and permeability for the tracer HRP was measured. The thrombin-induced endothelial hyperpermeability of these monolayers is for about 40–60% dependent on Rho kinase, representing the in vivo PAR1-mediated vascular permeability, whereas the remainder is independent of Rho kinase, involving other signaling pathways as outlined in the Introduction [2,6].
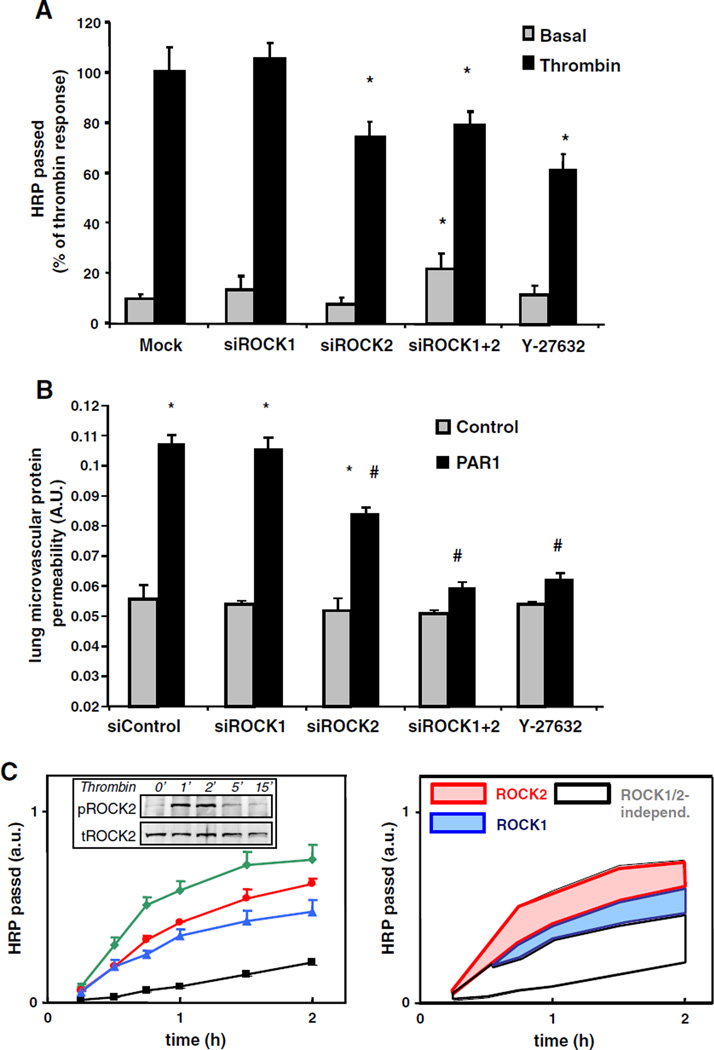
Thrombin elicited a 7.2 ± 0.7-fold increase in the passage of the tracer molecule HRP (Fig. 2A). It is known that PAR1-activating peptides recapitulate the pro-inflammatory effects of thrombin in endothelial cells [26], which we confirmed in vitro where PAR1-peptide and thrombin evoked a comparable transient decrease in endothelial barrier function (Supplementary Fig. S1). Silencing of ROCK1 did not affect thrombin-induced permeability, whereas silencing of ROCK2 significantly reduced thrombin-induced permeability (Fig. 2A). Simultaneous silencing of ROCK1 and ROCK2 further reduced the absolute or net thrombin-induced increase in permeability, and showed an almost similar effect as pharmacological inhibition by Y-27632.
Fig. 2.
ROCK1 and ROCK2 differentially regulate PAR1-mediated endothelial permeability in vitro and in vivo. A: Effects of ROCK silencing on the passage of HRP across HUVEC monolayers under basal conditions and 30 min after exposure to 1 U/mL thrombin. 48 h after transfection with the indicated siRNAs, cells were preincubated for 1 h in medium-199 + 1% HSA and cumulative HRP passage over the 30 min period in the presence or absence of thrombin was measured subsequently as described in the Materials & methods section. For comparison, the effect of the pan-Rho kinase inhibitor Y-27632 is shown (30 min pre-incubation). Values are the mean + SD (4 independent experiments performed in triplicate. Each experiment was performed with HUVECs isolated from a different donor). * indicates significance from appropriate control group (p < 0.05). B: ROCK2 is critically involved in PAR1-mediated mouse lung microvascular permeability. PAR1-peptide (TFLLRN) 1 mg/kg or control peptide (FTLLRN) was injected together with Evans blue albumin retroorbitally into C57/Bl6 mice, 48 h after injection of liposomes conjugated with indicated siRNA. After 30 min, Evans blue-albumin extravasation from the lungs was determined as described in the Materials & methods to quantify lung microvascular protein permeability. Y-27632 (10 mg/kg body weight) was administered intravenously. Data represent mean ± SD of n = 4 mice per group. * indicates significance between PAR1 treatment and appropriate control peptide group within the same group (p < 0.05); # indicates significance from mice injected with control siRNA after PAR1 challenge (p < 0.05). C: Time-dependent effects of Rho kinase inhibitors on the passage of HRP across control and thrombin-stimulated human umbilical vein. Left panel: Confluent monolayers were preincubated for 1 h in medium 199 + 1% HSA, pre-treated with 10 µM Y-27632 (blue), 10 µM SLx-2119 (red) or sham-treated (green) for 30 min. Subsequently, the cumulative HRP passage across the monolayers was measured in the presence of 1 U/mL thrombin, as described in the Materials & methods section. The baseline permeability of monolayers that were not treated with thrombin is shown for comparison (black). The right panel is color-shaded to better visualize the individual contributions of ROCK1 and ROCK2 to the thrombin-enhanced permeability. Values are the mean + SD (experiment performed in triplicate). Inset: ROCK2 is transiently activated in HUVECs upon thrombin-stimulation. Representative Western blot showing changes in the phosphorylation of ROCK2 upon stimulation with 1 U/mL thrombin for the indicated time points. Blot was probed with a phospho-specific ROCK2 antibody (upper panel) and reprobed with a total ROCK2 antibody (lower panel) as a loading control. Similar results were obtained in 3 independent experiments.
To evaluate the contribution of ROCK1 and ROCK2 to PAR1-mediated vascular hyperpermeability in vivo, ROCK1 or ROCK2 siRNA-transduced mice were stimulated with PAR1-peptide for 30 min. The pulmonary microvascular permeability following PAR1-stimulation was determined by measuring Evans blue conjugated albumin (EBA) extravasation (Fig. 2B). None of the siRNA treatments affected baseline permeability. PAR1-peptide evoked a 2-fold increase in EBA extravasation. Silencing of ROCK1 had no effect on PAR1-mediated EBA leakage. Silencing of ROCK2 decreased the PAR1 permeability response by about 50%, while silencing of both ROCK1 and ROCK2 almost completely prevented the increase in EBA extravasation upon treatment with a PAR1-peptide. The potent pharmacological pan-Rho kinase inhibitor Y-27632 was equally effective as silencing of both ROCK1 and ROCK2. These findings were supported by comparable changes in lung wet–dry weight ratios (data not shown).
Together, these data indicate that ROCK2 primarily regulates the thrombin receptor-mediated vascular hyperpermeability response both in vitro and in vivo. ROCK1 is dispensable for this hyperpermeability response, but silencing of ROCK1 enforced the attenuating effect of silencing of ROCK2. These data point to distinct roles of ROCK1 and ROCK2 in the regulation of vascular hyperpermeability.
2.3. Specific pharmacological inhibition of ROCK2 attenuates endothelial hyper-permeability
The role of ROCK2 in regulating the endothelial barrier function was further explored by using the ROCK2-selective inhibitor SLx-2119 [27, 28]. The effects of SLx-2119 were compared to the effects of the pan-Rho kinase inhibitor Y-27632. Probing ROCK2 Ser1366 phosphorylation as a surrogate marker for ROCK2 activation status [29] showed a rapid and transient activation of ROCK2 upon stimulation with thrombin (Fig. 2C inset). ROCK2 activation was entirely prevented by SLx-2119 as well as by downregulation of ROCK2 supporting the specificity of the inhibitor as well as the siRNA sequences, whereas silencing of ROCK1 had no effect on ROCK2 activation (Supplementary Fig. S2).
Pretreatment with SLx-2119 reduced thrombin-induced HRP passage to 74 ± 12% of thrombin-induced HRP flux in the absence of inhibitors (t = 30 min after thrombin stimulation, 12 filters from 4 different donors; p < 0.05), to a similar extent as silencing of ROCK2 (77± 15%). Y-27632 tended to slightly further inhibit thrombin-induced HRP passage (to 62 ± 6%). Detailed analysis (Fig. 2C left panel) of the time-dependency of the ROCK2 effects indicated that ROCK2 mainly was involved in the early phase of the thrombin-induced endothelial permeability changes, whereas only in the later phase ROCK1 additionally contributed to barrier dysfunction (Fig. 2C right panel).
Finally, these findings were verified in human pulmonary microvascular endothelial cells (Supplementary Fig. S3). In these primary cells, the role of Rho kinase in thrombin-induced permeability was even more prominent during the early phase of the permeability response to thrombin: SLx-2119 dose-dependently attenuated thrombin-induced HRP passage, to an almost similar extent as the pan-Rho kinase inhibitor Y-27632.
Thus, specific pharmacological inhibition of ROCK2 attenuates the disruption of human endothelial barrier integrity, in particular during the early phase of disruption. The differences in the time profiles for the involvement of ROCK1 and ROCK2 in endothelial hyper-permeability confirm distinct mechanistic contributions to the regulation of endothelial barrier function.
2.4. ROCK1, but not ROCK2 mediates the formation of thrombin-induced F-actin cytoskeletal stress fibers
The Rho kinase-dependent formation of contractile F-actin stress fibers is a remarkable feature of thrombin-stimulated ECs in vitro, but their presence in intact microvessels is debated [30]. Therefore, it remains to be determined if and how F-actin stress fibers play a role in endothelial permeability.
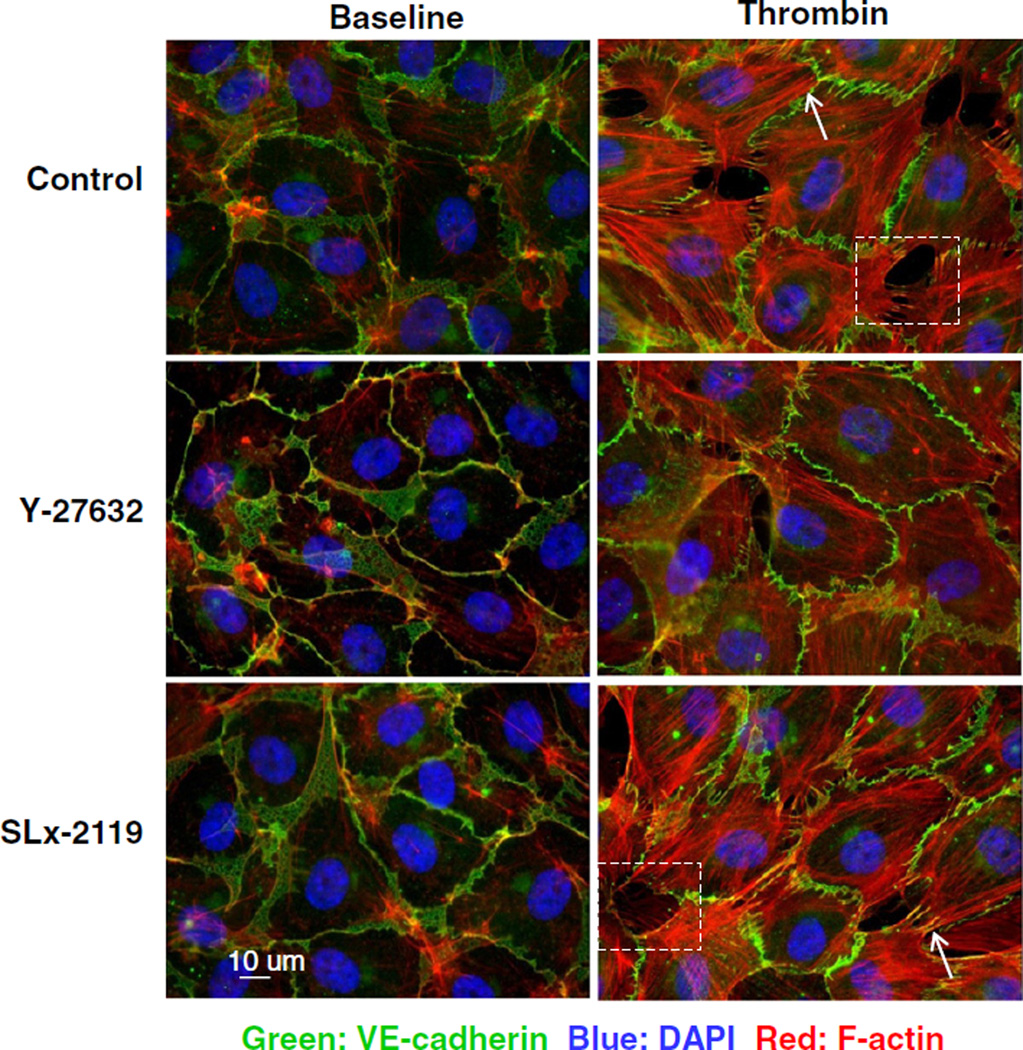
To evaluate whether ROCK2 mediates the thrombin-induced permeability changes through the formation of contractile F-actin stress fibers, endothelial monolayers were stained for F-actin with rhodamine–phalloidin and co-stained for VE-cadherin. HUVECs in a continuous monolayer of closely attached cells were characterized by a cortical F-actin band (Fig. 3) and the presence of some cytoplasmic F-actin filaments. Stimulation with thrombin for 30 min induced a loss of the peripheral F-actin rim, but an overall increase in F-actin staining, especially of F-actin stress fibers (Fig. 3 arrows). Both changes in the F-actin cytoskeleton were attenuated by 30 min pretreatment with Y-27632. Surprisingly, pretreatment with SLx-2119 had no obvious effect on the thrombin-induced F-actin reorganization, indicating that ROCK2 is dispensable for the formation of contractile F-actin stress fibers. Similarly, depletion of ROCK2 by siRNA treatment did not prevent stress fiber formation, whereas depletion of ROCK1 almost completely prevented their appearance (Supplementary Fig. S4).
Fig. 3.
ROCK2 is not essential for thrombin-induced formation of radial F-actin fibers. Y-27632, but not SLx-2119 prevented thrombin-induced cytoskeletal reorganization. F-actin (red) and VE-cadherin (green) staining of HUVECs grown on glass cover slips; cells were counter-stained with DAPI (nuclear staining). HUVECs were preincubated for 1 h in medium 199 + 1% HSA in the absence or the presence of 10 µmol/L of the Rho kinase inhibitors SLx-2119, and Y-27632 where indicated and stimulated for 30 min with 1 U/mL thrombin or sham-treated. Arrows point to radial F-actin fibers. Enlargements of boxed areas are shown is Supplementary Fig. S5A. Similar results were obtained in 4 independent experiments. Bar = 10 µm.
The distribution of VE-cadherin was not altered by ROCK2 inhibition, neither was the connection of thrombin-induced radial F-actin fibers to VE-cadherin affected in the focal adherens junctions (Fig. 3) [12]. Importantly, inter-endothelial gaps did form in ROCK2-inhibited HUVECs, indicating that the radial F-actin fibers were functionally intact, but HUVECs showed a more pronounced protrusive activity filling the gaps at their basal site (compare boxes in Fig. 3; these boxes are enlarged in Supplementary Fig. S5A, B).
Thus, ROCK2, but not ROCK1, is dispensable for the formation of thrombin-induced F-actin stress fibers.
2.5. ROCK2 is a pivotal determinant of baseline intercellular forces
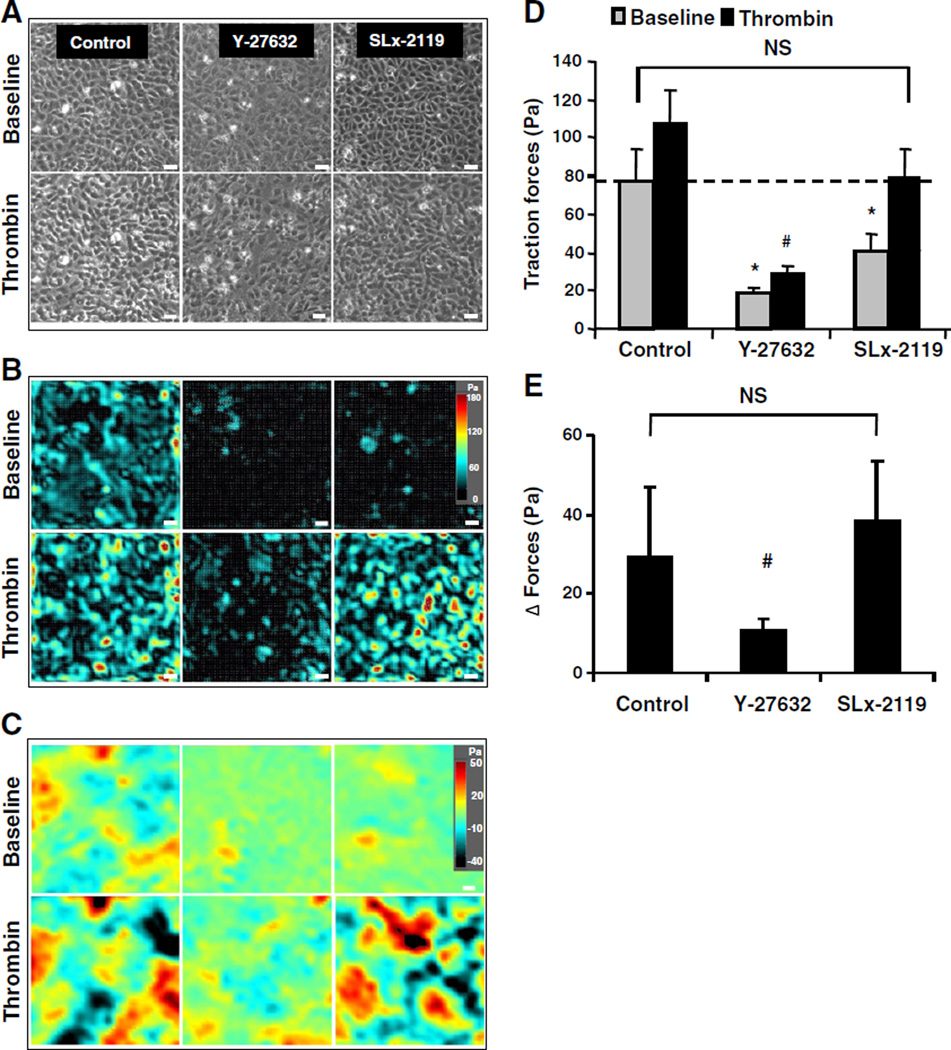
Since F-actin staining does not provide information on whether or not the formed F-actin stress fibers are functional, we sought to directly measure the thrombin-induced contractile forces. We applied a recently reported technique called monolayer traction microscopy to endothelial monolayers [31]. Traction forces exerted by endothelial cells on their extracellular matrix are counter-balanced at the level of the cell–cell junctions. The magnitude of these tractions is proportionally related to the cell–cell forces as a constant fraction (~0.5) [32,36]. The magnitude of these tractions is also proportionally related to the basal cellular tone or pre-stress [33]. HUVECs grown to confluent monolayers were characterized by baseline traction forces of a high magnitude and spatial heterogeneity (Fig. 4A, B). The baseline traction forces were dramatically ablated by Y-27632, and to a lesser extent by SLx-2119 (Fig. 4B). To directly demonstrate that the measured traction forces reflect intercellular forces, intercellular stress maps were generated by a novel technique called monolayer stress microscopy [34]. Both ROCK inhibitors largely reduced baseline intercellular stresses (Fig. 4C).
Fig. 4.
Inhibition of ROCK2 reduces basal traction forces but does not affect thrombin-induced contractile stress enhancements. A, B, C: Representative phase contrast images (A), cell–substrate traction stress maps (B) and intercellular stress maps (C) at baseline and 10 min post-thrombin treatment from each group. HUVEC monolayers were pretreated with the indicated Rho kinase inhibitors for 30 min and subsequently stimulated with thrombin (1 U/mL) for 10 min or sham-treated. Traction stress maps presented in (B) belong to the monolayers presented in (A). Bar = 50 µm. D: Quantification of the effects of different Rho kinase inhibitors on traction forces across all groups, presented as RMS traction stresses. Values are the mean ± SD of 4 independent determinations in duplicate. *p < 0.05 basal RMS traction stresses of monolayers pretreated with Rho kinase inhibitors vs control monolayers. #p < 0.05 thrombin-induced RMS traction stresses of monolayers pretreated with Rho kinase inhibitors vs control monolayers. E: Bar graph showing the effect of the different Rho kinase inhibitors on the net thrombin-induced change in RMS traction stresses calculated from the data presented in (B) (i.e. RMS traction stresses at t = 10 min after thrombin treatment minus RMS traction stresses at baseline). # indicates p < 0.05 thrombin-treated monolayers pretreated with vs without Rho kinase inhibitors.
In sham-treated control cells, the baseline traction forces were significantly enhanced in response to thrombin. These thrombin-induced force enhancements were significantly ablated by pre-treatment with Y-27632 (Fig. 4D, E). In contrast, pretreatment with the ROCK2-selective SLx-2119 did not affect the net thrombin-induced forces (Fig. 4D, E). Similarly, simultaneous silencing of ROCK1 and ROCK2 largely inhibited the thrombin-enhanced contractile force enhancements, whereas silencing of ROCK2 alone did not affect thrombin-induced forces (Supplementary Fig. S6).
Thus, ROCK2 activity is an important determinant of baseline monolayer intercellular forces, but is not involved in thrombin-induced contractile force enhancements.
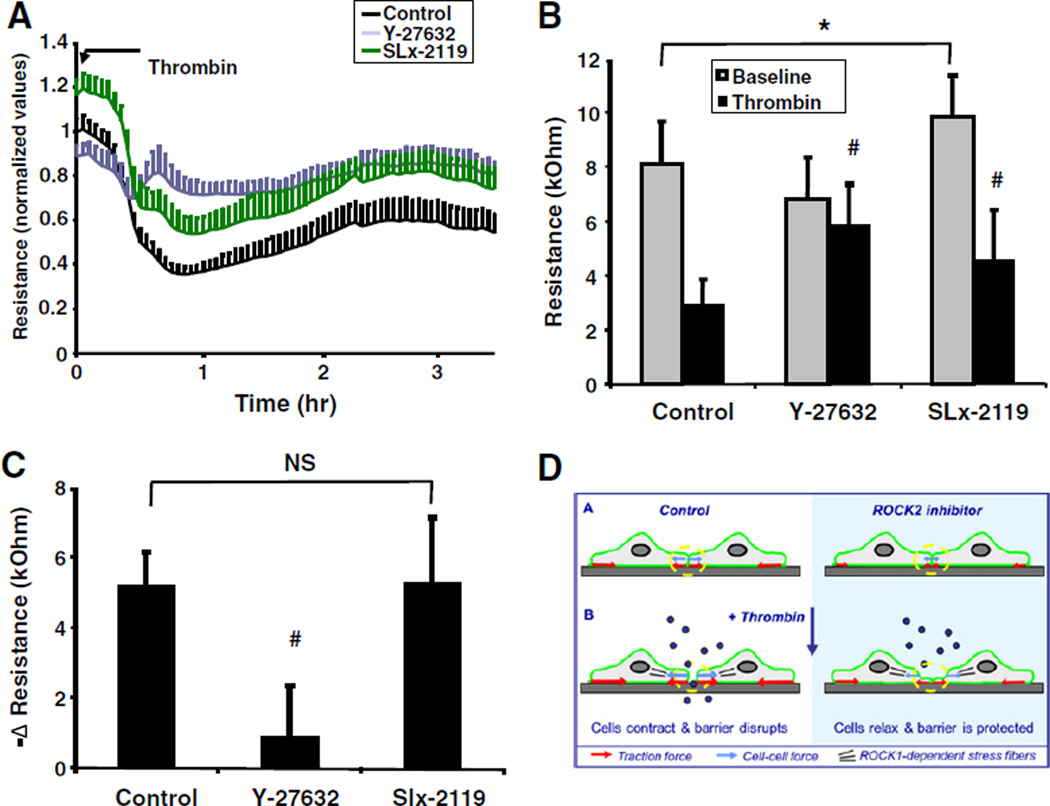
2.6. ROCK2 mediates baseline, but not thrombin-mediated changes in trans-endothelial electrical resistance (TEER)
We next aimed to investigate whether inhibition of ROCK2 is barrier-protective through enforcement of the baseline characteristics of the endothelial barrier.
Silencing of ROCK2 slightly reduced the baseline flux of HRP across endothelial monolayers (0.7 ± 0.2-fold, see also Fig. 2A), whereas silencing of ROCK1 tended to increase basal permeability (1.3 ± 0.6-fold). These trends did not reach statistical significance, but suggested that ROCK1 and ROCK2 might have distinct roles in regulating basal endothelial permeability. Simultaneous silencing of ROCK1 and ROCK2 significantly doubled the HRP flux (2.0 ± 0.6 fold), in line with the previous finding [8,35] that prolonged inhibition of Rho kinase (10 µM Y-27632 for 48 h) induced a similar disruption of baseline barrier integrity.
Next, we evaluated the effect of Rho kinase inhibitors on endothelial barrier integrity by measurement of trans-endothelial electrical resistance or TEER (Fig. 5A and B). We utilized an electrical cell impedance sensing (ECIS) assay, as ECIS allows online monitoring of barrier function, with higher precision than tracer permeability measurements in the transwell assays do. However, it is important to realize that ECIS measurements do not replace tracer permeability measurements as ECIS measurements provide an indirect reflection of barrier function: changes in TEER are not necessarily accompanied by alterations in barrier properties toward larger molecules, i.e. they do not necessarily reflect actual changes in passage of tracers across the endothelium.
Fig. 5.
Inhibition of ROCK2 affects baseline, but not thrombin-induced alterations in trans-endothelial electrical resistance. A: Graph representing the time course of the effect of Rho kinase inhibitors on trans-endothelial electrical resistance of HUVEC monolayers as measured by the ECIS method, see the Materials & methods section. Data are presented as normalized values for resistances of each individual well measured at t = 0 min. Values are the mean ± SD of 16 cultures in 4 independent experiments. B: Bar graph showing that the effect of different Rho kinase inhibitors trans-endothelial electrical resistance at baseline and after stimulation with thrombin (t = 30 min). Data are presented as the absolute values of the same experiments shown in (A). *p < 0.05 basal resistance of monolayers pretreated with Rho kinase inhibitors vs control monolayers. #p < 0.05 resistance of thrombin-stimulated monolayers pretreated with Rho kinase inhibitors vs non-pretreated monolayers that were stimulated with thrombin. C: Bar graph showing the effect of the different Rho kinase inhibitors on the net thrombin-induced change in TEER calculated from the data presented in (B) (i.e. resistance at t = 30 min after thrombin treatment minus resistance at baseline). # indicates p < 0.05 thrombin-treated monolayers pretreated with vs without Rho kinase inhibitors. D: Model depicting that inhibition of ROCK2 promotes vascular barrier stability through lowering of the force balance in endothelial junctions. See the Discussion section for a description of the details on how ROCK2 activity is pivotal to a critical junctional tension for opening of the endothelial barrier in response to inflammatory mediators. In addition, thrombin induces other changes such as a simultaneous decrease in Rac1 activity and changes in phosphorylation of intercellular adhesion proteins that are not shown for the sake of simplicity.
An analysis of the effect of the Rho kinase inhibitors on basal barrier integrity showed that inhibition of ROCK2 with SLx-2119 significantly elevated baseline electrical resistance (Fig. 5A, B), whereas electrical resistance upon treatment with the pan-Rho kinase inhibitor Y-27632 was not elevated. Thus, inhibition of ROCK2 improves endothelial basal barrier integrity, whereas simultaneous inhibition of ROCK1 abolished this effect.
Next, we analyzed the effects of the Rho kinase inhibitors on the thrombin-induced changes in TEER. Thrombin induced a transient decrease in TEER, with a minimal TEER between 45 and 60 min showing a reduction of 64.1 ± 11.8%, which was followed by recovery of the monolayer (Fig. 5A). Pretreatment with the pan-Rho kinase inhibitor Y-27632 largely attenuated the thrombin-induced decrease in TEER (Fig. 5A). In contrast, pretreatment with SLx-2119 resulted in an upward shift of the entire curve, indicating that SLx-2119 increased the initial baseline TEER, without affecting the net thrombin-induced changes. This was further evidenced by the analysis of the maximal thrombin-induced decrease in TEER (Fig. 5C), which was almost entirely ablated by pre-treatment with Y-27632, but not affected by pretreatment with SLx-2119. Similarly, SLx-2119 improved basal barrier integrity in human pulmonary microvascular endothelial monolayers, but did not affect the thrombin-induced drop in TEER (data not shown).
Taken together, these data indicate that ROCK2 activity is critical for the development of a baseline isometric tone and enforcement of baseline barrier characteristics, but does not contribute to the thrombin-induced contractile force enhancements.
3. Discussion
The main finding of the present study is that the Rho kinase isoform ROCK2 mediates passive junctional tension in the endothelium as an entirely novel concept of regulating vascular hyperpermeability by Rho kinase, independent from the well-known role of Rho kinase in F-actin stress fiber formation. Inhibition of ROCK2 attenuates endothelial barrier dysfunction and pulmonary edema. Thus, selective pharmacological inhibition of basal traction forces might provide a novel approach to stabilize the endothelial barrier, and limit vascular leak during derailed inflammatory episodes. These findings shed new light on a longstanding controversy between physiologists and cell biologists whether or not Rho kinase-mediated F-actin stress fibers contribute to vascular hyperpermeability responses (Fig. 5D).
In endothelial monolayers forces are exerted at cell–matrix interactions known as traction forces (indicated by the red arrows in Fig. 5D) and at cell–cell interactions known as cell–cell or intercellular forces (indicated by the blue arrows). The intercellular forces account for nearly one-half of the overall forces in the monolayer and determine junctional tension born by the adherens junctions (upper left) [32,36]. Evidence indicates that classical cadherins, including VE-cadherin are under tension [37–40]. Recently, Rac1 has been shown as a modulator of Rho kinase-mediated junctional tension [53]. The present study shows that baseline forces are substantially reduced with ROCK2 inhibition (upper right), indicating that ROCK2 is a key molecule in the regulation of junctional tension at baseline. Upon stimulation with thrombin, overall forces increase, such that part of the junctions no longer can bear those forces. Cells start to contract and junctional complexes fall apart, resulting in opening of the barrier (lower left). The net increase in thrombin-enhanced contractile forces is not affected by ROCK2 inhibition. However, as these thrombin-induced contractile force enhancements in ROCK2-inhibited cells are now superimposed on a lower level of baseline junctional forces, the net result is a lower level of overall forces. These forces no longer reach a critical threshold necessary for disrupting junctional integrity and opening of the barrier for macromolecules (lower right). Such a critical threshold might be necessary for opening the vascular barrier under inflammatory conditions. At a later stage, F-actin stress fibers are formed in a ROCK1-dependent manner contributing to prolongation of the hyperpermeability response. Thus, ROCK2 activity is pivotal to a critical junctional tension for opening of the endothelial barrier when the interaction of junctional proteins is weakened in response to inflammatory mediators.
Both silencing as well as pharmacological inhibition of ROCK2 reduced thrombin-induced endothelial hyperpermeability. Similarly, in epithelial cells, ROCK2, but not ROCK1 mediated disassembly of the junctions [41]. However, another report indicated that ROCK1 mediated the early, but not late effects of TNFalpha on endothelial permeability, a finding hard to reconcile with the observation in the same report that ROCK2 was the primary Rho kinase activated in the lungs of LPS-treated mice [42,43]. Unfortunately, the authors did not evaluate vascular permeability in these mice.
In the present study we observed that ROCK1 was dispensible for thrombin-induced endothelial hyperpermeability as long as ROCK2 is present in the ECs, but not vice versa. This points to a special form of compensation known as quantitative redundancy. In complete redundancy, the absence or malfunction of either protein alone has no effect, but disruption of both proteins affects cellular behaviour [44,45]., In contrast, two proteins are quantitatively redundant when malfunction of the one protein has no effect, as long as the compensating protein is functioning normally, but the effect of the absence or malfunction of the other is exacerbated by the absence of the former. Quenching of shared (inhibitory) factors by ROCK1 and ROCK2 provides an alternative explanation of the additive effects of silencing of ROCK1 in ROCK2-silenced ECs. In such a case the effects of ROCK1 are secondary and do not require direct involvement of ROCK1 in regulation of endothelial barrier integrity. Of interest, a minimal increase in ROCK1 activity has been reported during sepsis, but its functional role remains to be investigated [42]. The future development of ROCK1-specific inhibitors would provide a valuable tool to further delineate the role of ROCK1.
Another interesting aspect of the current study is the almost complete prevention of PAR1-induced vascular permeability in mice transduced with ROCK1 and ROCK2 siRNAs, that were characterized by a partial reduction in ROCK1/2 expression as was evidenced by immunohistochemistry. Similarly, in haplo-insufficient ROCK1 mice thrombin-induced ICAM1 expression was completely inhibited [46]. These findings indicate a critical threshold for Rho kinase expression and/or activity below which Rho kinase-dependent responses are severely hampered.
It should be emphasized that ROCK2 was not necessary for the thrombin-induced formation of F-actin stress fibers and force enhancements. Similarly, ROCK2, but not ROCK1 has been shown to be dispensable for stress fiber formation in vascular smooth muscle cells [25]. Stress fibers are considered as contractile F-actin filaments formed upon Rho kinase activation to prolong MLCK-initiated contraction [47]. As stress fibers are a characteristic feature accompanying many in vitro hyperpermeability responses, it is a wide-held assumption that they mediate the cellular contractile response leading to gap formation between ECs [9]. Our finding that a ROCK2 inhibitor reduces thrombin-induced permeability, while leaving the formation of F-actin stress fibers intact, challenges the paradigm that these stress fibers are necessary for prolonged Rho kinase-mediated endothelial permeability. Interestingly, recent data indicate that central F-actin fibers distinguish the endothelial phenotype of adult arteries from veins [48]. Moreover, in intact microvessels in situ thrombin-induced F-actin fibers are smaller in size and in number [30], and in vivo a PAR1-peptide hardly induced the formation of stress fibers, whereas both stimuli resulted in enhanced vascular permeability [30].
In epithelial cells, ROCK2 is complexed to adaptor protein cingulin in junctional complexes where it regulates tight junction formation [49]. It is tempting to speculate that ROCK2 in endothelium also enforces such interactions in the junctions. An attractive scenario for how ROCK2 could establish basal endothelial tension or prestress in the endothelium is recruitment to cortical F-actin through Shroom2 (Shrm2). Recent data indicate that Shrm2 is expressed within the endothelium, is localized to cortical actin and cell–cell adhesions, and contains a conserved Rho kinase binding domain [50].
In conclusion, this is the first report addressing the individual contributions of ROCK1 and ROCK2 to in vivo vascular leak. It points to a major role of ROCK2 in vascular hyperpermeability responses through regulation of baseline tension in the adherens junctions independent from the involvement of F-actin stress fibers and reinforces the notion that Rho kinase is an attractive target in future anti-vascular leak therapy.
4. Materials & methods
Sources of reagents are listed in the expanded Materials and methods section in the online data supplement section.
4.1. Animal studies
All animal studies were approved by the Institutional Animal Care Committee of the University of Illinois.
To deliver siRNA in the mouse lung, cationic liposomes were made using a mixture of dimethyldioctadecyl-ammonium bromide (DDAB) and cholesterol in chloroform, as described previously [51]. Control, ROCK1 or ROCK2 siRNA (75 µg) or ROCK1 + ROCK2 (37.5 µg each siRNA) were mixed with 100 µL of liposomes. The mixture of liposomes and siRNA was injected intravenously (via retro-orbital injection) into C57/Bl6 mice. After 48 h mice were used for determining lung microvascular permeability or were used for immunoblotting and immunohistochemistry analysis.
To assess lung capillary leakage, PAR1 specific peptide (TFRLLN) (1 mg/kg) was injected retroorbitally followed by injection of Evans blue conjugated albumin (EBA) (20 mg/kg) as described [52]. After 30 min, mice were sacrificed and blood was collected from the right ventricle into heparinized syringes. The right lung lobe was homogenized as described [52]. Lung homogenates and plasma were incubated with 2 volumes of formamide (18 h, 60 °C), centrifuged at 5000 ×g for 30 min, and the optical density of the supernatant was determined spectrophotometrically at 620 nm and at 740 nm (to correct for hemoglobin). EBA extravasation was calculated as the ratio of EBA extravasated in the lung versus that in plasma. To determine lung weights, the left lungs from the same mice used for EBA extravasation were excised and completely dried in an oven at 60 °C overnight for calculation of lung wet–dry ratio.
4.2. In vitro studies
Human pulmonary microvascular endothelial cells (HPMVECs) isolated from human lung tissue and human umbilical vein endothelial cells (HUVECs) were isolated, cultured, and characterized as previously described [8],. ECs were transfected with short interfering (si) RNA duplexes using Amaxa technology.
Barrier function was evaluated by the transfer of HRP across EC monolayers grown on polycarbonate filters of the Transwell system [8]. Alternatively, electrical cell impedance sensing (ECIS) was used to measure trans-endothelial electrical resistance (TEER) in confluent monolayers. For 3D-digital fluorescence imaging microscopy, HUVECs were examined with a ZEISS Axiovert 200 Marianas inverted microscope.
Endothelial monolayer contraction was measured by monolayer traction microscopy [31]. Intercellular stresses were mapped by monolayer stress microscopy [34].
4.3. Statistical analysis
All data are reported as mean ± SD. Comparisons between 2 experimental groups were made by Student's t-test and between >2 groups by one way ANOVA with Bonferroni post-hoc test. Differences in mean values were considered significant at p < 0.05.
Supplementary Material
Acknowledgments
Sources of funding
GPvNA was supported by the Netherlands Heart Foundation (NHF) (The Hague, grants 2003T032 and 2011T072), RK in part by the Parker B. Francis Foundation, DM by National Institute of Health (HL71784) and CCH in part by the National Institutes of Health (NIH, 1K25HL111212).
Abbreviations
- ARDS
acute respiratory distress syndrome
- DDAB
dimethyldioctadecyl-ammonium bromide
- EBA
Evans blue conjugated albumin
- EBAE
EBA extravasation
- EC
endothelial cell
- ECIS
electrical cell impedance sensing
- HRP
horseradish peroxidase
- HPMVECs
human pulmonary microvascular endothelial cells
- HSA
human serum albumin
- HUVEC
human umbilical vein endothelial cell
- ICAM
intercellular adhesion molecule
- LPS
endotoxin
- MLC
myosin light chain
- MYPT1
myosin phosphatase target subunit 1
- PAR
protease-activated receptor
- PH domain
Pleckstrin homology domain
- RMS
root mean square
- ROCK
Rho kinase
- Shrm2
Shroom2
- TEER
trans-endothelial electrical resistance
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None.
Appendix A. Supplementary
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.vph.2015.03.017.
References
- 1.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011 Jun 22;3(88) doi: 10.1126/scitranslmed.3002011. 88ps25. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006 Jan;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003 Apr 17;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010 Jul 15;87(2):243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004 Jan;67(1):64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 6.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000 Aug 18;87(4):335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol. 2007 Oct;293(4):C1309–C1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]
- 8.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007 Nov;27(11):2332–2339. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 9.Gudermann T, Steinritz D. STIMulating stress fibers in endothelial cells. Sci Signal. 2013 Mar;6(267):e8. doi: 10.1126/scisignal.2004051. %19. [DOI] [PubMed] [Google Scholar]
- 10.Baumer Y, Burger S, Curry FE, Golenhofen N, Drenckhahn D, Waschke J. Differential role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem Cell Biol. 2008 Feb;129(2):179–191. doi: 10.1007/s00418-007-0358-7. [DOI] [PubMed] [Google Scholar]
- 11.Uhlig S, Yang Y, Waade J, Wittenberg C, Babendreyer A, Kuebler WM. Differential regulation of lung endothelial permeability in vitro and in situ. Cell Physiol Biochem. 2014;34(1):1–19. doi: 10.1159/000362980. [DOI] [PubMed] [Google Scholar]
- 12.Huveneers S, Oldenburg J, Spanjaard E, van der KG, Grigoriev I, Akhmanova A, et al. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012 Mar 5;196(5):641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Hahn CN, Parsons M, Drew J, Vadas MA, Gamble JR. Role of protein kinase Czeta in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood. 2004 Sep 15;104(6):1716–1724. doi: 10.1182/blood-2003-11-3744. [DOI] [PubMed] [Google Scholar]
- 14.van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000 Dec;20(12):E127–E133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- 15.Gorovoy M, Neamu R, Niu J, Vogel S, Predescu D, Miyoshi J, et al. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ Res. 2007 Jul 6;101(1):50–58. doi: 10.1161/CIRCRESAHA.106.145847. [DOI] [PubMed] [Google Scholar]
- 16.Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D, et al. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005 Mar;7(3):251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Ding RY, Zhao DM, Zhang ZD, Guo RX, Ma XC. Pretreatment of Rho kinase inhibitor inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in mice. J Surg Res. 2011 Dec;171(2):e209–e214. doi: 10.1016/j.jss.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.McGown CC, Brown NJ, Hellewell PG, Brookes ZL. ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase. Microvasc Res. 2011 May;81(3):281–288. doi: 10.1016/j.mvr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003 Jun;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 20.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003 Jul;23(14):5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005 Mar 14;168(6):941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006 Feb 17;98(3):322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011 Mar;32(3):167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013 Jan;13(1):205–219. doi: 10.2174/1566524011307010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009 Feb 27;104(4):531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005 Jul 1;280(26):25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 27.Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, et al. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis. 2008 Oct;19(7):709–718. doi: 10.1097/MBC.0b013e32830b2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breyer J, Samarin J, Rehm M, Lautscham L, Fabry B, Goppelt-Struebe M. Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochem Pharmacol. 2012 Mar 1;83(5):616–626. doi: 10.1016/j.bcp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Chuang HH, Yang CH, Tsay YG, Hsu CY, Tseng LM, Chang ZF, et al. ROCKII Ser1366 phosphorylation reflects the activation status. Biochem J. 2012 Apr 1;443(1):145–151. doi: 10.1042/BJ20111839. [DOI] [PubMed] [Google Scholar]
- 30.van Nieuw Amerongen GP, Musters RJ, Eringa EC, Sipkema P, van Hinsbergh VW. Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am J Physiol Cell Physiol. 2008 May;294(5):C1234–C1241. doi: 10.1152/ajpcell.00551.2007. [DOI] [PubMed] [Google Scholar]
- 31.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, et al. Universal physical responses to stretch in the living cell. Nature. 2007 May 31;447(7144):592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van BJ, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011 Jan;300(1):C146–C154. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goeckeler ZM, Bridgman PC, Wysolmerski RB. Nonmuscle myosin II is responsible for maintaining endothelial cell basal tone and stress fiber integrity. Am J Physiol Cell Physiol. 2008 Oct;295(4):C994–C1006. doi: 10.1152/ajpcell.00318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011 Jun;10(6):469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010 Sep;24(9):3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell–ECM traction force modulates endogenous tension at cell–cell contacts. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno V, Gonzalo P, Gomez-Escudero J, Pollan A, cin-Perez R, Breckenridge M, et al. An EMMPRIN-gamma-catenin-Nm23 complex drives ATP production and actomyosin contractility at endothelial junctions. J Cell Sci. 2014 Sep 1;127(Pt 17):3768–3781. doi: 10.1242/jcs.149518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013 Jun 3;23(11):1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, et al. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell–cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huveneers S, de RJ. Mechanosensitive systems at the cadherin–F-actin interface. J Cell Sci. 2013 Jan 15;126(Pt 2):403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009 Feb 15;182(4):2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 43.Bogatcheva NV, Zemskova MA, Poirier C, Mirzapoiazova T, Kolosova I, Bresnick AR, et al. The suppression of myosin light chain (MLC) phosphorylation during the response to lipopolysaccharide (LPS): beneficial or detrimental to endothelial barrier? J Cell Physiol. 2011 Dec;226(12):3132–3146. doi: 10.1002/jcp.22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein AM, Dioum EM, Cobb MH. Exposing contingency plans for kinase networks. Cell. 2010 Dec 10;143(6):867–869. doi: 10.1016/j.cell.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 45.van Wageningen S, Kemmeren P, Lijnzaad P, Margaritis T, Benschop JJ, de C, et al. Functional overlap and regulatory links shape genetic interactions between signaling pathways. Cell. 2010 Dec 10;143(6):991–1004. doi: 10.1016/j.cell.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008 May;118(5):1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. J Cell Biol. 2013 Jan 7;200(1):9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Geemen D, Smeets MW, van Stalborch AM, Woerdeman LA, Daemen MJ, Hordijk PL, et al. F-actin-anchored focal adhesions distinguish endothelial phenotypes of human arteries and veins. Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):2059–2067. doi: 10.1161/ATVBAHA.114.304180. [DOI] [PubMed] [Google Scholar]
- 49.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa CS, IV, Balda MS, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011 Feb;13(2):159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farber MJ, Rizaldy R, Hildebrand JD. Shroom2 regulates contractility to control endothelial morphogenesis. Mol Biol Cell. 2011 Mar;22(6):795–805. doi: 10.1091/mbc.E10-06-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006 Jan 27;281(4):2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 52.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008 Nov 7;103(10):1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daneshjou N, Sieracki N, Schwartz MA, Conway DE, Van Nieuw Amerongen GP, Komarova YA, et al. Rac1 as a reversible tension modulator of adherens junctions in endothelial cells. J Cell Biol. 2015;208(1):23–32. doi: 10.1083/jcb.201409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.