Abstract
We describe the first report of temporally related cases of Bordetella holmesii bacteremia. Demographic and clinical data were collected through chart abstraction and case-patient interviews. Twenty-two cases were identified from 6 states. Symptom onset dates ranged from April 2010 to January 2011. Median age of patients was 17.1 years and 64% had functional or anatomic asplenia. Pulsed-field gel electrophoresis profiles of a sample of isolates were identical. These cases occurred during a peak in pertussis outbreaks with documented cases of B. holmesii/Bordetella pertussis respiratory coinfection; whether there is a link between B. holmesii respiratory and bloodstream infection is unknown.
Keywords: Bordetella holmesii, bacteremia, asplenia, pertussis
Bordetella holmesii is a gram-negative, nonoxidizing, slow-growing organism first identified as a species in 1995 [1]. Relatively little is known about the epidemiology, clinical manifestations, or natural history of B. holmesii. Unlike Bordetella pertussis, B. holmesii has been associated with invasive disease in immunocompromised persons, primarily bacteremia in young adults with sickle-cell anemia [2–6]. Although B. holmesii has not been historically associated with a cough illness, more recently the pathogen has been associated with a pertussis-like respiratory syndrome in healthy individuals [7–9]. Prior knowledge about B. holmesii bacteremia has come from single case reports or small case series [3–6, 10]. The largest published series described all B. holmesii bacteremia cases (n = 30) identified by the Centers for Disease Control and Prevention (CDC) over a 17-year period (1983–2000) [2].
Between 12 June 2010 and 13 August 2010, the New York City Department of Health and Mental Hygiene (NYC DOHMH) Public Health Laboratory received 4 Bordetella holmesii blood isolates submitted for confirmatory testing from 4 New York City hospitals. On 23 August 2010, B. holmesii was isolated from a fifth blood specimen at a New York City hospital laboratory. This number of cases was above baseline for that time period based on historic records.
We describe the first reported investigation of temporally related B. holmesii bacteremia cases.
METHODS
On 7 and 8 September 2010, NYC DOHMH issued laboratory and clinician alerts requesting the report of all cases of B. holmesii isolated from a sterile site or respiratory specimen in the previous 2 years. Additionally, laboratories were asked to report unidentified gram-negative coccobacilli biochemically compatible with B. holmesii from patients with invasive or respiratory disease, and clinicians were asked to conduct prospective surveillance for B. holmesii in febrile immunocompromised patients (particularly asplenic).
Finally, a nationwide notification was issued by the CDC in collaboration with NYC DOHMH on 16 September 2010 to identify additional cases of invasive B. holmesii. The notification was distributed through the EPI-X (CDCs Web-based communications portal for public health professionals) requesting reporting of additional cases of B. holmesii identified since 1 January 2010.
For this investigation, cases were defined as patients with B. holmesii isolated from a normally sterile site (blood; cerebrospinal, pleural, peritoneal, pericardial, or joint fluid; bone; surgical aspirate; or internal body site [eg, lymph node, brain]) between 1 January 2010 and 1 February 2011. Laboratory-confirmed cases detected as a result of the New York City and national EPI-X alerts were reported to CDC or NYC DOHMH. Patients with B. holmesii isolated only from nasopharyngeal specimens were not included in these analyses.
A standardized data collection form was developed and included patient demographic characteristics, clinical signs and symptoms, laboratory results, and diagnosis, treatment, and outcome variables. Data were collected through a combination of chart abstraction and telephone interviews with case patients or case-patient representatives. Data collection forms were distributed to all health departments that reported B. holmesii cases to NYC DOHMH or CDC. Completed de-identified forms were sent to CDC. Data were analyzed using Excel 2010. Human subjects review at CDC determined this study to be public health practice, not research; informed consent was not obtained for this study.
Microbiologic confirmation of B. holmesii isolates was performed by the NYC DOHMH Public Health Laboratory and reporting hospital laboratories. Pulsed-field gel electrophoresis (PFGE) was performed at the NYC DOHMH Public Health Laboratory and CDC on a sample of available case isolates. At CDC, PFGE with XbaI enzyme was performed on 9 isolates using methods previously described [11]. At NYC DOHMH Public Health Laboratory, 8 isolates were characterized by PFGE using XbaI and SpeI as previously described [7]. Three B. holmesii isolates from the NYC DOHMH Public Health Laboratory were sent to CDC for comparison with the 9 isolates, as well as a reference database of 47 B. holmesii strains, using BioNumerics software (version 5.0, Applied Maths, Austin, Texas). Of the 47 reference strains, 13 (28%) were isolated from respiratory specimens, 18 (38%) were from blood specimens, and 16 (34%) were of unknown origin.
RESULTS
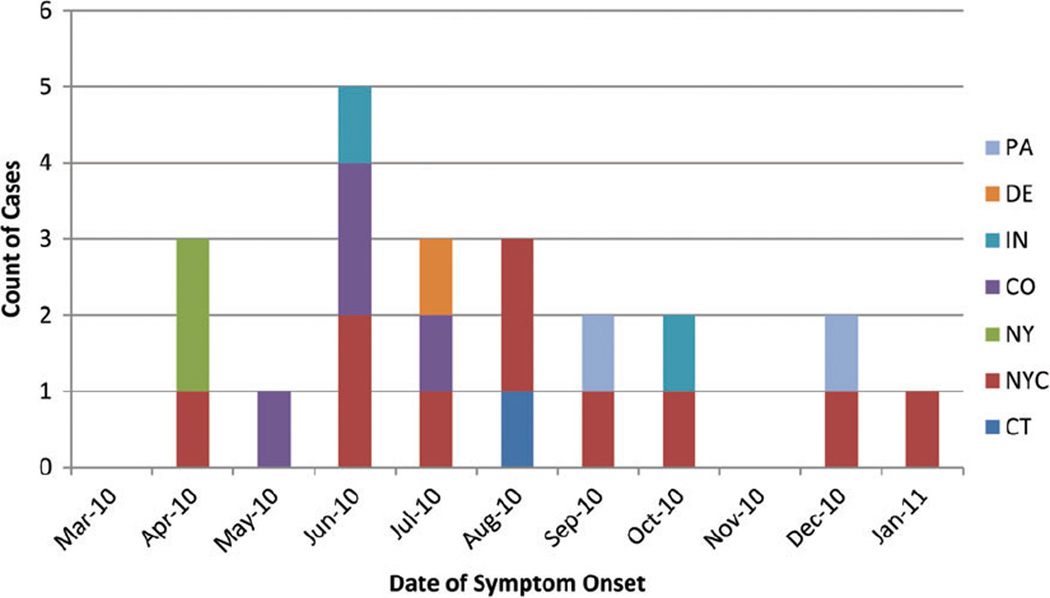
A total of 22 cases of invasive B. holmesii infection were identified from 6 states. Bordetella holmesii was isolated from blood specimens in 21 patients and from both blood and synovial fluid specimens in 1 patient. Symptom onset dates ranged from 1 April 2010 to 19 January 2011 (Figure 1). Eleven (50%) cases were clustered between June and August of 2010. Twelve (55%) patients were from New York and of those, 10 (83%) were from New York City. Colorado reported 4 cases, Indiana and Pennsylvania each reported 2 cases, and Connecticut and Delaware each reported 1 case. In New York City, 2 separate hospitals reported 2 cases each. Two cases were reported from the same hospital in Philadelphia. All other cases were single cases identified in separate hospitals. No epidemiologic links between the patients were identified.
Figure 1.
Bacteremic Bordetella holmesii cases by month of onset and reporting site (N = 22). Abbreviations: CO, Colorado; CT, Connecticut; DE, Delaware; IN, Indiana; NY, New York; NYC, New York City; PA, Pennsylvania.
Data collection forms were completed or partially completed for 18 (82%) of the patients (New York City = 10, New York State = 2, Indiana = 2, Pennsylvania = 2, Connecticut = 1, Delaware = 1). Some clinical or demographic data were obtained for the 4 remaining patients. Twelve (55%) patients were male, and the median patient age at onset was 17.1 years (range, 1.5–77.3 years) (Table 1). Of 18 patients with data on race/ethnicity, 10 (56%) were black, 5 (28%) were white, and 2 (17%) reported “other” race.
Table 1.
Demographic and Clinical Characteristics of Patients With Bordetella holmesii Bacteremia—April 2010 to January 2011
| Patient No |
State or NYC |
Age at Onset, y |
Sex | Race | Functional/Anatomic Asplenia |
Presenting Symptoms |
Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | NYC | 2 | Female | Black | Unknown | Fever, cough | Hemolytic uremic syndrome |
| 2 | NYC | 5 | Male | Black | Sickle cell | Fever, headache, cough, leg/foot pain | Bacteremia |
| 3 | NYC | 7 | Female | Black | No | Chest pain | Bacteremia |
| 4 | NYC | 13 | Female | White | Sickle cell, splenectomy | Fever, bilateral leg pain, throat pain | Vasoocclusive crisis, streptococcal pharyngitis |
| 5 | NYC | 14 | Male | Black | Sickle cell | Fever, knee/back pain | Vasoocclusive crisis |
| 6 | NYC | 14 | Female | Other | No | Fever, unilateral jaw swelling | Infected facial hematolympangioma |
| 7 | NYC | 16 | Female | Black | Sickle cell | Fever, headache | Vasoocclusive crisis, bacteremia |
| 8 | NYC | 35 | Female | Black | Sickle cell | Fever, back/leg pain, cough | Vasoocclusive crisis |
| 9 | NYC | 74 | Female | Other | Sickle cell | Fever, headache | Meningitis, bacteremia |
| 10 | NYC | 77 | Male | White | Splenectomy | Fever | Cellulitis, bacteremia |
| 11 | NY | 25 | Male | Black | Sickle cell | Fever, headache, nausea | Vasoocclusive crisis |
| 12 | NY | 41 | Male | White | No | Fever, unilateral facial numbness | Acute sinusitis |
| 13 | CT | 62 | Male | White | No | Shortness of breath | Pleural effusion |
| 14 | PA | 6 | Female | Black | Sickle cell | Fever, knee pain, dysuria | Bacteremia |
| 15 | PA | 15 | Male | Black | Sickle cell | Fever, headache | Bacteremia |
| 16 | DE | 14 | Male | Black | Sickle cell | Fever, abdominal pain | Bacteremia |
| 17 | IN | 18 | Male | White | Splenectomy | Fever, chest pain | Pneumonia |
| 18 | IN | 19 | Male | Other | Sickle cell, splenectomy | Unknown | Vasoocclusive crisis |
| 19 | CO | 9 | Female | Missing | Unknown | Bump on chest/neck | Bacteremia |
| 20 | CO | 52 | Male | Missing | Splenectomy | Fever, headache, myalgias | Bacteremia |
| 21 | CO | 61 | Female | Missing | Unknown | Weakness, altered mental status | Bacteremia |
| 22 | CO | 63 | Male | Missing | Unknown | Lower extremity pain/ swelling | Cellulitis |
Abbreviations: CO, Colorado; CT, Connecticut; DE, Delaware; IN, Indiana; NY, New York; NYC, New York City; PA, Pennsylvania.
In total, 20 (91%) patients reported at least 1 underlying medical condition. Fourteen (64%) patients had functional or anatomic asplenia, of whom 11 (79%) had sickle cell disease and 3 had splenectomies without reported reasons (Table 1). Reported underlying conditions that could result in immunosuppression included diabetes (14%), history of kidney transplant (9%), cancer (9%), end-stage renal disease (9%), and IgA nephropathy (5%). One patient who did not report an immunocompromising condition had facial hematolymphangioma. One patient reported no underlying conditions.
The majority of patients reported fever (89%); among 10 patients with a highest temperature recorded, the median temperature was 39.4°C (range, 38.1°C–40.2°C). The most commonly reported presenting symptoms included headache (44%), joint pain (28%), cough (28%), and difficulty breathing (22%). Among 5 patients reporting cough, none reported paroxysmal cough, whoop, or posttussive vomiting. Three of 11 (27%) patients with chest radiographic results had abnormal findings (2 with possible consolidation, 1 with pleural effusion); of these, 1 reported cough as a presenting symptom. Among the 11 patients with sickle cell disease, 9 (82%) were diagnosed with vasoocclusive crisis on presentation; the tenth patient reported fever, chills, and abdominal pain, which may also represent vasoocclusive crisis (Table 1). The median white blood cell count (n = 17) on presentation was 13 700 cells/µL (range, 4600–29 600 cells/µL), and the mean peak lymphocyte percentage (n = 13) was 23.3% (range, 7.6%–48.3%).
Of 21 cases with data available, 2 patients had B. holmesii identified in cultures obtained on 2 separate days, with a period of 2 and 6 days between collections, respectively. The median time between symptom onset and date of first specimen collection was 1.5 days (range, 0 to 30 days; n = 16). No patients had blood cultures identifying a pathogen other than B. holmesii. Of 21 patients with data, 18 (86%) were hospitalized for this illness. Data were incomplete for length of stay or antibiotic treatment. There were no reported deaths, and 2 of 10 (20%) patients with data on highest level of care were admitted to the intensive care unit.
Eight isolates analyzed by PFGE at the NYC DOHMH Public Health Laboratory had indistinguishable PFGE profiles. Nine isolates (Colorado [4], Delaware [1], New York State [2], and Pennsylvania [2]) analyzed by the CDC laboratory also showed indistinguishable PFGE profiles. These 9 strains were compared with 3 randomly selected isolates from NYC DOHMH Public Health Laboratory using CDC PFGE running conditions and all presented identical profiles. These 12 strains were also indistinguishable by PFGE typing from 37 of the 47 (79%) reference strains maintained at CDC. The remaining 10 reference strains were categorized into 7 different PFGE types.
DISCUSSION
We describe the first reported investigation of temporally related B. holmesii bacteremia cases. We are unaware of any widespread changes in diagnostic capacity, contamination concerns, heightened laboratory or clinician suspicion or testing prior to the alert, or increases in populations known to be at risk for B. holmesii bacteremia that would have explained the increased number of cases.
The cases we describe occurred in the same time period as the historic peak in US pertussis disease in 2010 [12]. Two pertussis outbreak investigations conducted during the study time period (November 2010–January 2011 in Jefferson County, New York; and May 2010–May 2011 in Franklin County, Ohio) used 4-probe multiplex polymerase chain reaction (PCR) methods to demonstrate that B. holmesii was circulating with B. pertussis in 12%–16% of samples tested (personal communication, Kimberlee A. Musser, New York State Department of Health Wadsworth Center [13]). These are higher proportions than were seen in a study from Massachusetts in 1999, when B. holmesii was isolated from 0.6% of 2508 respiratory specimens [8].
Respiratory infections caused by B. holmesii are clinically indistinguishable from those by B. pertussis [7, 8]. Whether there is a link between B. holmesii respiratory and bloodstream infection is unknown. In our study, respiratory symptoms (cough and difficulty breathing) were reported by one-third and one-fifth of case patients, respectively, but we do not know if these symptoms were caused by B. holmesii, and nasopharyneal specimens were not taken. Given that these cases of B. holmesii bacteremia emerged in the same geographic area as several pertussis outbreaks with documented cases of B. holmesii coinfection, the cases may reflect the exposure of susceptible populations, such as those with asplenia and other immunocompromising conditions, to B. holmesii circulation. However, because there is no routine surveillance for B. holmesii disease, we do not know the true burden of disease in the United States or if these cases represent a true increase in circulation. Although efforts were made to capture all cases during the study period, it is likely that some cases may have been missed. Further work is needed to evaluate the link between B. holmesii respiratory and blood infections and to estimate the burden of B. holmesii disease in the United States and how it relates to circulation of pertussis disease.
Similar to previous reports, the majority of patients in this investigation were functionally or anatomically asplenic [2, 3, 5, 6]. Asplenia is associated with an increased risk of morbidity and mortality from some bacterial infections, most commonly polysaccharide encapsulated bacteria [14]. While the presence of polysaccharide capsule has not yet been definitively demonstrated in B. holmesii, preliminary investigations provide evidence supporting the presence of polysaccharide capsule in other Bordetella species [15, 16]. Further studies are needed to confirm the role of encapsulation in the pathogenesis of B. holmesii among asplenic patients.
Identification of B. holmesii, even in high-risk patients, is difficult. Routine diagnostic tools such as conventional methods using biochemical patterns and automated diagnostic machines for blood specimens are used to identify B. holmesii. However, these tests, particularly automated blood specimen microbial identification systems, are not always capable of distinguishing B. holmesii from other pathogenic species [10]. In fact, of the 8 isolates sent by hospital laboratories to NYC DOHMH Public Health Laboratory for confirmatory testing, 1 was initially suspicious for Francisella tularensis, 1 for Acinetobacter lwoffii, and 1 for Kingella species based on automated diagnostic machine results [17]. Cellular fatty acid analysis (CFA) performed by NYC DOHMH Public Health Laboratory conclusively identified all 8 isolates as B. holmesii [17]. Specialized reference methods such as CFA, 16S ribosomal RNA sequence analysis, multitarget real-time PCR assays, and PFGE are useful for more definitive identification of B. holmesii [2, 10, 17, 18]. While these specialized methods are increasingly available at state health department reference laboratories, these techniques are not widely available in hospital diagnostic laboratories. As such, B. holmesii may be frequently misidentified by routine clinical laboratories.
In conclusion, these cases of B. holmesii bacteremia occurred in the same time period as pertussis outbreaks in which B. holmesii was isolated in >10% of suspected cases. While the transmission route and true disease burden of B. holmesii is not definitively known, there may have been increased circulation when this series of cases appeared. Routine use of diagnostic tests that can differentiate B. pertussis and B. holmesii in nasopharyngeal specimens—such as the multitarget PCR protocol developed by CDC—would allow for a better understanding of B. holmesii transmission and epidemiology [18]. While the CDC PCR protocol is currently in use by some state health departments, it generally is not used by commercial or hospital clinical diagnostic laboratories. Furthermore, improving automated identification algorithms that distinguish B. holmesii from Francisella tularensis, Acinetobacter lwoffii, or Kingella species would limit misclassification. Finally, physicians treating immunocompromised or asplenic patients should be aware of this pathogen and consider it as a possible cause of bacteremia. These diagnostic improvements will have the potential to enhance future studies of B. holmesii epidemiology, including the role of respiratory transmission in invasive disease, risk factors for colonization and disease, and opportunities for prevention.
Acknowledgments
Financial support. This work was supported by internal funding from the CDC.
Bordetella holmesii Working Group
Susan Whittier, Department of Pathology and Cell Biology, New York Presbyterian/Columbia University Medical Center, New York; Glenn Fennelly, Department of Pediatrics, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, New York; Benyang Zhao, Public Health Laboratory, New York City Department of Health and Mental Hygiene, New York; Paula del Rosso and Marie S. Dorsinville, Bureau of Communicable Disease, New York City Department of Health and Mental Hygiene, Long Island City, New York; Maria L. Tondella, Meningitis and Vaccine Preventable Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia; Denise Woods-Stout, retired from Disease Control and Environmental Epidemiology Division, Colorado Department of Public Health and Environment, Denver; Marjorie W. Shannon, Health Information and Science, Delaware Division of Public Health, Dover; Angela Cierzniewski, Epidemiology Resource Center, Indiana State Department of Health, Indianapolis; Joan Baumbach, Bureau of Infectious Disease Epidemiology, New Mexico Department of Health, Santa Fe; Ami S. Patel, Office of Public Health Preparedness and Response, Philadelphia Department of Public Health, Pennsylvania; Kathy Kudish, Immunization Program, Connecticut Department of Public Health, Hartford; and Angela Maxted, Division of Environmental Health, New York State Department of Health, Albany, New York.
Footnotes
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weyant RS, Hollis DG, Weaver RE, et al. Bordetella holmesii sp. nov., a new gram-negative species associated with septicemia. J Clin Microbiol. 1995;33:1–7. doi: 10.1128/jcm.33.1.1-7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard CW, Daneshvar MI, Kaiser RM, et al. Bordetella holmesii bacteremia: a newly recognized clinical entity among asplenic patients. Clin Infect Dis. 2004;38:799–804. doi: 10.1086/381888. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist SW, Weber DJ, Mangum ME, Hollis DG, Jordan J. Bordetella holmesii sepsis in an asplenic adolescent. Pediatr Infect Dis J. 1995;14:813–815. [PubMed] [Google Scholar]
- 4.Greig JR, Gunda SS, Kwan JTC. Bordetella holmesii bacteraemia in an individual on haemodialysis. Scand J Infect Dis. 2001;33:716–717. doi: 10.1080/00365540110026826. [DOI] [PubMed] [Google Scholar]
- 5.Njamkepo E, Delisle F, Hagege I, Gerbaud G, Guiso N. Bordetella holmesii isolated from a patient with sickle cell anemia: analysis and comparison with other Bordetella holmesii isolates. Clin Microbiol Infect. 2000;6:131–136. doi: 10.1046/j.1469-0691.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 6.McCavit TL, Grube S, Revell P, Quinn CT. Bordetella holmesii bacteremia in sickle cell disease. Pediatr Blood Cancer. 2008;51:814–816. doi: 10.1002/pbc.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazengia E, Silva EA, Peppe JA, Timperi R, George H. Recovery of Bordetella holmesii from patients with pertussis-like symptoms: use of pulsed-field gel electrophoresis to characterize circulating strains. J Clin Microbiol. 2000;38:2330–2333. doi: 10.1128/jcm.38.6.2330-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yih WK, Silva EA, Ida J, Harrington N, Lett SM, George H. Bordetella holmesii-like organisms isolated from Massachusetts patients with pertussis- like symptoms. Emerg Infect Dis. 1999;5:441–443. doi: 10.3201/eid0503.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiya H, Otsuka N, Ando Y, et al. Transmission of Bordetella holmesii during pertussis outbreak, Japan. Emerg Infect Dis. 2012;18:1166–1169. doi: 10.3201/eid1807.120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagopoulos MI, Saint Jean M, Brun D, et al. Bordetella holmesii bacteremia in asplenic children: report of four cases initially misidentified as Acinetobacter lwoffii. J Clin Microbiol. 2010;48:3762–3764. doi: 10.1128/JCM.00595-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwick TH, Cassiday P, Weyant RS, Bisgard KM, Sanden GN. Changes in predominance and diversity of genomic subtypes of Bordetella pertussis isolated in the United States, 1935 to 1999. Emerg Infect Dis. 2002;8:44–49. doi: 10.3201/eid0801.010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Summary of notifiable diseases—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 13.Rodgers L, Martin SW, Cohn A, et al. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with co-circulating Bordetella holmesii and Bordetella pertussis—Ohio, 2010–2011. Clin Infect Dis. 2013;56:322–331. doi: 10.1093/cid/cis888. [DOI] [PubMed] [Google Scholar]
- 14.Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician. 2001;63:499–506. 508. [PubMed] [Google Scholar]
- 15.Neo Y, Li R, Howe J, et al. Evidence for an intact polysaccharide capsule in Bordetella pertussis. Microbes Infect. 2010;12:238–245. doi: 10.1016/j.micinf.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Preston A, Parkhill J, Maskell DJ. The bordetellae: lessons from genomics. Nat Rev Microbiol. 2004;2:379–390. doi: 10.1038/nrmicro886. [DOI] [PubMed] [Google Scholar]
- 17.Lee LV, Zhao B, Kornblum J, et al. Bordetella holmesii: an emerging pathogen?. Poster # 122, Section 017: 017, Unusual Organisms and Case Studies (Division C); 111th General Meeting of the American Society for Microbiology; 21–24 May 2011; New Orleans, LA. [Google Scholar]
- 18.Tatti KM, Sparks KN, Boney KO, Tondella ML. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J Clin Microbiol. 2011;49:4059–4066. doi: 10.1128/JCM.00601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]