Abstract
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide and mounting evidence indicates that toxicant exposures can profoundly impact on CVD risk. Epidemiologic studies have suggested that arsenic (As) exposure is positively related to increases in blood pressure (BP), a primary CVD risk factor. However, evidence of whether genetic susceptibility can modify the association between As and BP are lacking. In this study, we used mixed effects models adjusted for potential confounders to examine the interaction between As exposure from well water and potential genetic modifiers on longitudinal change in BP over approximately 7 years of follow-up in 1137 subjects selected from the Health Effects of Arsenic Longitudinal Study (HEALS) cohort in Bangladesh. Genotyping was conducted for 235 SNPs in 18 genes related to As metabolism, oxidative stress and endothelial function. We observed interactions between 44 SNPs with well water As for one or more BP outcome measures (systolic, diastolic, or pulse pressure (PP)) over the course of follow-up. The interaction between CYBA rs3794624 and well water As on annual PP remained statistically significant after correction for multiple comparisons (FDR-adjusted p for interaction = 0.05). Among individuals with the rs3794624 variant genotype, well water As was associated with a 2.23 mmHg (95% CI: 1.14-3.32) greater annual increase in PP, while among those with the wild type, well water As was associated with a 0.13 mmHg (95% CI: 0.02-0.23) greater annual increase in PP. Our results suggest that genetic variability may contribute to As-associated increases in BP over time.
Keywords: Arsenic, HEALS, blood pressure, CYBA, longitudinal, SNPs
Introduction
Arsenic (As) is a widespread, naturally occurring toxicant to which millions are exposed worldwide, primarily via contaminated drinking water sources [1-3]. In Bangladesh alone, an estimated 50 million individuals have been chronically exposed to drinking water containing As levels exceeding the World Health Organization limit of 10μg/L [1-3]. A growing body of evidence, including prospective studies, has linked As exposure to increased cardiovascular disease (CVD) risk, even at a lower levels of exposure (<100 μg/L) [4-6]. High blood pressure (BP) is a major risk factor for coronary heart disease and cerebrovascular disease and according to recent reports from the WHO, is a contributing factor in an estimated 7.5 million deaths annually worldwide [7,8]. When left uncontrolled, hypertension can cause a range of health effects including heart failure, renal impairment, peripheral vascular disease and visual impairment through damage to retinal blood vessels [7,8]. Recent work has supported associations between As exposure and preclinical indicators of CVD, including carotid intima media thickness, hypertension, and plasma markers of oxidative stress and endothelial dysfunction [9-12]. A recent case-control study reported that exposure to moderate levels of water arsenic (>40μg/L) was associated with a 4-fold increased risk of CVD [13]. Evidence from longitudinal analyses and several cross-sectional studies suggested that As exposure at both high and low levels increase risk of high BP [9,14].
However, evidence of whether genetic susceptibility can modify the association between As and BP are lacking. Arsenic likely influences a number of cellular pathways and mechanisms that may lead to increased BP [6,12]. Work from a growing number of both experimental and human studies have indicated that As can induce oxidative stress, which in turn, may alter gene expression, inflammatory responses, and endothelial nitric oxide production [15,16]. Greater As exposure has also been linked to increases in markers of endothelial dysfunction [10,17] indicative of a loss of vasomotor tone in the blood vessels that may increase risks of atherosclerosis, hypertension and CVD. Further, an individual’s ability to efficiently metabolize As is likely key to mitigating As’s potential adverse health effects. Thus, genetic variation in these cardiovascular-related physiological responses or As detoxification mechanisms could influence As-related increases in BP.
We sought to investigate the contribution of environmental As exposure to elevated BP, a primary risk factor for CVD. In 2000, we established the Health Effects of As Longitudinal Study (HEALS), a large prospective study in Araihazar, Bangladesh to assess the long-term health effects of As exposure. In this study, we analyzed BP exposure in relation to repeated BP measurements in a subset of 1137 HEALS participants and used a panel of 235 cardiovascular, oxidative stress and As metabolism SNPs in 18 genes to examine genetic susceptibility loci that may contribute to As-related increases in BP.
Methods
Study population
The parent study, the Health Effects of As Longitudinal Study (HEALS) is an ongoing prospective cohort study in Araihazar, Bangladesh described in detail previously [18]. Between October 2000 and May 2002, 11,746 married men and women ages 18-75 years were recruited into the original cohort. Individuals were required to be a resident of the study area for at least 5 years and drinking water primarily from one of the local tube wells. Baseline interviews were conducted to gather information regarding history of water well use, demographics, and lifestyle characteristics. The cohort has been actively followed approximately every two years since baseline, with the first three follow-up assessments occurring September 2002-May 2004, September 2004-May 2006, and June 2007-March 2009, respectively. Follow-up assessments include a physical examination, collection of a urine sample, and a structured interview, following the same procedures used in the baseline interview. Informed consent was obtained from study participants and all study procedures were approved by the ethics committee of the Bangladesh Medical Research Council and the Institutional Review Boards of Columbia University and the University of Chicago.
At the time of this analysis, participants in this study (n= 11,746) had been followed on average for 6.6 years (range 0.9-8.3 years). Individuals who died before the first follow-up (n=107) were excluded, leaving a total of 11,639 subjects. Among these, we focused only on those on whom we had obtained a blood sample and had genotyped as part of a previous case-cohort study (n=1804) [11,19]. We excluded 553 individuals who did not have a baseline well water As measurement, in addition to 3 individuals who did not have a BP measurement at baseline and 69 without at least one follow-up BP measurements. We also excluded 42 individuals undergoing treatment for hypertension at baseline, for a final study population of 1,137 subjects. Of the 1,137 individuals who did not have CVD at baseline, a total of 311 incident cases of any CVD (including 169 coronary heart disease cases and 128 stroke cases) occurred over the course of follow-up.
Measurements of As exposure
At baseline, water samples from local tube wells were collected in 20mL polyethylene scintillation vials after rinsing several times with groundwater. Samples were acidified to 1% with high purity Optima hydrochloric acid (Fisher Scientific) for at least 48 h before analysis. Total As concentration was analyzed by high-resolution inductively-coupled plasma mass spectrometry (HR ICPMS), with a detection limit of <0.2 μg/L. The long-term reproducibility determined from consistency standards included with each run is relatively stable over time [20-22]. Baseline well water As data were available for 100% of participants. Spot urine samples were collected in 50mL acid washed tubes from 1129 (99.3%) of the participants in this substudy at baseline. Total As concentration was measured by graphite furnace atomic absorption (GFAA), with a detection limit of 2 μg/L, as previously described [23]. Previous work from this study population has shown that fish intake, the primary source of arsenobetaine, is very low. In the HEALS, the correlations of total urinary arsenic concentration with urinary arsenobetaine and arsenocholine concentrations were 0.13 and 0.06, respectively, and the correlation between total urinary arsenic and water arsenic was 0.70. These data suggest that seafood intake contributes very little to total urinary arsenic concentration in this study population and that arsenobetaine concentration is not a major contributor to total arsenic concentrations [24]. Urinary creatinine was analyzed by a colorimetric Sigma Diagnostics Kit to adjust for urinary dilution.
Blood pressure measurements
BP was measured at baseline and at each follow-up by trained clinicians using an automatic sphygmomanometer (HEM 712-C; Omron Healthcare GmbH), which has been validated to have 85 percent of readings falling within 10 mmHg of the mercury standard [25]. Measurements were taken with participants in a seated position after 5 minutes of rest, with the cuff around the upper left arm, in accordance with recommended guidelines. Two consecutive measurements were taken and the average of the two were used in our analyses. The reliability of BP measurements was high, with intraclass correlation coefficients ranging from 0.92 to 0.94 [26].
BP was measured at the first, second and third follow-ups with overall participation rates for the full cohort of 96.9%, 93.6%, and 92.2%. Information on medication use was collected at baseline and during each of the follow-ups. Study participants were asked about all medicines they were taking regularly, and anti-hypertensive medications were extracted and coded by trained interviewers. Measurements from individuals who began anti-hypertensive medication during the follow-up period were treated as missing for the visit when the use of anti-hypertension treatment was reported and for all subsequent follow-up measurements. The prevalence of anti-hypertensive use was relatively low with 7.5% (n=85), 9.2% (n=105) and 13.2% (n=150) reporting anti-hypertensive at the first, second and third follow-ups in the study population, respectively.
Selection of genes and single nucleotide polymorphisms (SNPs)
Candidate genes were selected based on their: 1) involvement in As metabolism, or 2) if they have been shown to modify associations between CVD and As exposure in previous epidemiologic studies, or 3) if As exposure has been associated with gene products (i.e. plasma sICAM-1 and sVCAM-1) identified as CVD predictors or risk factors in epidemiologic studies, as previously described [11,19]. A total of 18 candidate genes were chosen, including As metabolism genes Glutathione S-transferase Mu 1 (GSTM1), glutathione S-transferase theta 1 (GSTT1), glutathione S-transferase omega 1 (GSTO1), glutathione S-transferase pi 1 (GSTP1), methylenetetrahydrofolate reductase (MTHFR), cystathionine beta-synthase (CBS), purine nucleoside phosphorylase (PNP), and arsenite methyltransferase (AS3MT); oxidative stress genes heme oxygenase 1 (HMOX1), nitric oxide synthase 3 (NOS3), superoxide dismutase 2 (SOD2), and cytochrome b-245, alpha polypeptide (CYBA); and inflammation and endothelial dysfunction genes apolipoprotein E (APOE), tumor necrosis factor (TNF), interleukin 6 (IL6), intercellular adhesion molecule 1 (ICAM1), sphingosine-1-phosphate receptor 1 (S1PR1), and vascular cell adhesion molecule 1 (VCAM1). As previously detailed [11,19], we used a comprehensive approach to select SNPs in the candidate genes of interest, including tag SNPs from International Hapmap Project [27] and SeattleSNPs [28], functional SNPs from the F-SNP database [29,30] and SNPs related to CVD risk and/or phenotypic markers of interest in the literature. A total of 384 SNPs were genotyped using an Illumina GoldenGate assay; 27 SNPs were excluded due to assay failure. For quality control, 26 duplicate samples from 6 subjects were randomly distributed in the genotyping plate. Concordance rates for all assays were >99%. Of the 357 SNPs, we removed 122 SNPs with a MAF <0.02 in the study population (under the assumption that only SNPs with an MAF ≥0.02 will maintain stability of effect estimates and statistical validity for interaction tests), leaving 235 SNPs in 18 genes for analysis.
Statistical analysis
We first conducted descriptive analyses to compare the distribution of demographic and lifestyle characteristics, As exposure and BP measurements, using Chi-square tests and analysis of variance (ANOVA) for categorical and continuous variables, respectively. In these descriptive analyses, we categorized participants by baseline SBP (i.e. <120 mmHg, normal or ≥120mmHg, pre-hypertensive to hypertensive).
We used longitudinal mixed effect models with a random slope and intercept for each subject to assess the association between baseline As exposure from well water and annual change in BP over time. The mixed effect model is a flexible modeling method for repeated measures data that accounts for within-subject correlation between baseline and follow-up BPs and that can account for imbalances in the number of follow-up measurements. We modeled BP as a continuous variable for each of the three outcomes; systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse pressure (PP). The latter is the difference between systolic and diastolic measurements and previously has been associated with CVD risk [31].
In order to investigate whether individual’s baseline water As level was associated with differences in longitudinal BP change, the mixed effect model was specified as follows:
| [Model 1] |
where As0j is baseline water As as a continuous variable, with 100 μg/L as the unit of increase for ease of interpretation of the effect estimate. The standard deviation is 112 μg/L for water As. TIME is years since baseline at the time of BP measurement; β2 is the change in mean baseline BP for every unit increase in baseline water As levels; β12 is the difference in annual BP change over time for every unit increase in baseline water As level (i.e. the estimated effect of baseline As water levels on annual BP change; αT is a row vector of regression coefficient estimates for covariates at baseline (T denotes vector transpose); and Z0j is a vector of potential confounders. The terms in the first and second brackets are the fixed and random parts of the model, respectively.
We then investigated whether the effects of As exposure on annual increase in BP differ by individual SNPs, by adding a three-part interaction term to the model, which was specified as follows:
| [Model 2] |
where the term β3 represents the change in mean baseline BP by SNP genotypes, corresponding to additive, dominant, or recessive genetic models, β13 represents the difference in annual BP change over time associated with SNP genotypes, and β123 denotes the extent by which the effect of arsenic exposure on annual BP change differs by genotypes. In the additive genetic model, genotypes of each SNP were coded with values 0, 1, or 2, corresponding to genotypes AA (wild-type homozygote), Aa (heterozygote), and aa (variant homozygote), respectively. In the dominant genetic model, genotypes Aa and aa were combined and compared to genotype AA, while in the recessive model, genotypes AA and Aa were combined and compared to genotype aa. Thus, we estimated the beta coefficients and their 95% confidence intervals (CIs) for the difference in annual BP change over the course of follow-up in relation to 1) a 100 μg/L increase in baseline well-water As, in the absence of a genetic variant (β12), 2) the presence of a genetic variant in the absence of As exposure (β13), and 3) the interaction effect of a 100 μg/L increase in As exposure and a genetic variant (β123). The statistical significance of the interaction for the additive, dominant and recessive models was the p-value for coefficient associated with the cross-product term of time with each SNP and As exposure (β123). When the estimate for a cross-product term for time, SNP and As exposure (β123) was negative, we reversed the coding to give a positive estimate, allowing for easier interpretation of the results. We focused on baseline water arsenic as our primary exposure variable because well water arsenic is the main source of exposure in the population and has been shown to be highly correlated with total urinary arsenic (0.70) in HEALS participants [24]. Further, baseline well water arsenic is a reflection of exposure to the index wells that participants had used for 8 years on average prior to baseline measurements [32]. Most of the participants did not change wells during the follow-up, although exposure was reduced in some highly exposed participants [32,33]. We chose covariates to include in our models based on a priori hypotheses and informed by our previous publications on the main effects of arsenic on longitudinal BP [32]. We adjusted our models for potential confounders including sex, age (years), body mass index (kg/m2) at baseline, educational attainment (years), smoking status (never, former, current), and diabetes status (yes/no) at baseline. We used smoking status at baseline in our models, as prior analyses have observed that smoking behavior does not change much over time in this population. Most (83-88%) of the current smokers at baseline remained as current smokers during follow-up, and current smoking status was not related to arsenic exposure in the overall population and use of ever smoking versus current smoking did not alter estimates of blood pressure change over time [32]. We used the false-discovery rate (FDR) correction [34] to account for multiple comparisons of all SNPs and under all three models (additive, dominant, and recessive), although only results from dominant and recessive models are shown, as the results for the additive model were similar to those observed under the dominant models, but less robust overall. We did not present analyses for which 10 or fewer individuals possessed the variant genotype of interest (i.e. recessive models for some rarer variants). For each of the SNPs with an interaction tested at p < 0.01, we further estimated the annual change in BP associated with a 100μg/L increase in baseline well water As by different SNP genotype strata (Figures 1-3), by graphically representing the estimates for effect of arsenic in those without at-risk genotypes [β12 As0j (TIME)ij] and effect of arsenic in those with at-risk genotypes [β12 As0j (TIME)ij + β123 As0j Gij (TIME)ij] (See Model 2 above) with their corresponding 95% confidence intervals for non-susceptible (SNP=0) versus susceptible (SNP=1) genotypes. All analyses were performed with SAS 9.3 (SAS Institute).
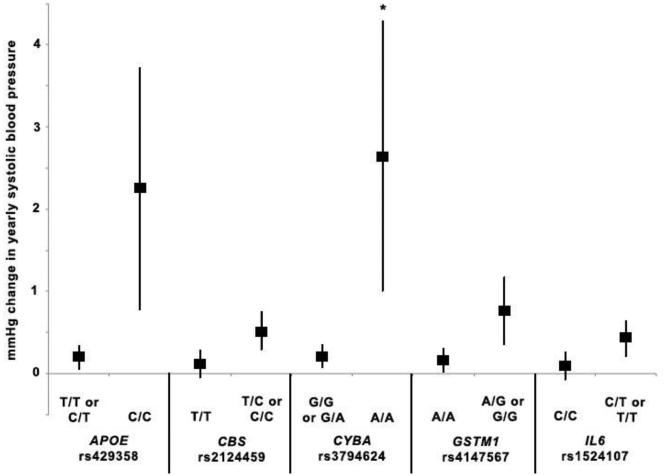
Figure 1.
Yearly change in systolic blood pressure in relation to arsenic exposure and variant SNP genotypes. For each SNP, the symbol to the left represents the estimate of yearly change in systolic blood pressure in relation to arsenic for reference genotype(s) and the symbol on the right represents the estimate of yearly change in systolic blood pressure in relation to arsenic for the variant genotype under either a dominant (CBS rs2124459, GSTM1 rs4147567, IL6 rs 15241107) or recessive model (APOE rs429358, CYBA rs3794624). Vertical lines represent 95% CI for each estimate. We chose SNPs that had a significant interaction with arsenic exposure p≤0.01 in relation to systolic blood pressure over time. SNP-arsenic interactions that were significant at p<0.01 prior to FDR correction are indicated with an asterisk (*).
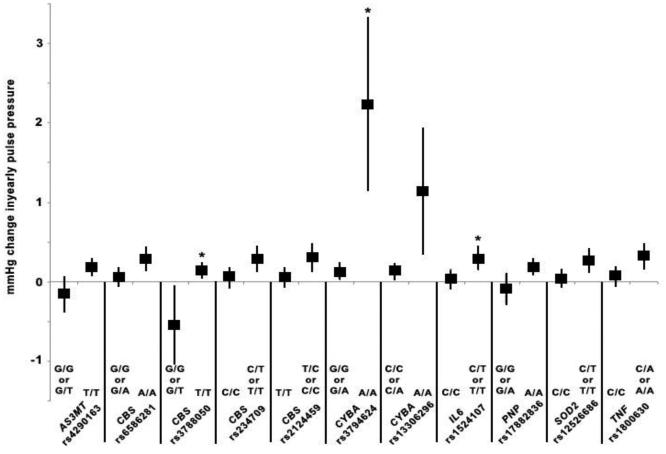
Figure 3.
Yearly change in pulse pressure in relation to arsenic exposure and variant SNP genotypes. For each SNP, the symbol to the left represents the estimate of yearly change in pulse pressure in relation to arsenic for reference genotype(s) and the symbol on the right represents the estimate of yearly change in pulse pressure in relation to arsenic for the variant genotype under either a dominant (CBS rs234709 and rs2124459, IL6 rs1524107, SOD2 rs12526686, TNF rs1800630) or recessive model (AS3MT rs4290163, CBS rs6586281 and rs3788050, CYBA rs3794624 and rs13306296, PNP rs17882836). Vertical lines represent 95% CI for each estimate. We chose SNPs that had a significant interaction with arsenic exposure p≤0.01 in relation to pulse pressure over time. SNP-arsenic interactions that were significant at p<0.01 prior to FDR correction are indicated with an asterisk (*).
As a sensitivity analysis, we excluded individuals with incident CVD during the follow-up period and used the same models as described above for SNPs that were found to have statistically significant results (p-values prior to FDR adjustment) in the overall population. The overall trends and directionality were very similar and many of the p-values for the estimates remained statistically significant (data not shown). We also repeated the analyses using urinary As levels at baseline (in place of baseline water As levels) as our exposure variable and compared the main effect estimates for longitudinal BP change.
Results
The final study population was 1,137 participants, with available water As levels and data on BP at baseline and at least one follow-up visit. The median follow-up time was 6.6 years, ranging from 1.0 to 8.3 years. Of the study population, 1,069 participants had all four BP measurements (baseline and 3 follow-ups), 60 had three measurements (baseline and 2 follow-ups) and 8 individuals had two measurements (baseline and 1 follow-up) in total.
Individuals with baseline SBP equal to or above 120mmHg tended to be older, male, and have slightly more years of education (Table 1). These individuals were also more likely to have been former or current smokers, have a history of diabetes and a slightly higher BMI. No differences were observed between these groups for water As or creatinine-adjusted urinary As at baseline or over the course of follow-up (Table 1).
Table 1.
Baseline and follow-up characteristics of cohort by systolic blood pressure at baseline (n=1137).
| SBP at Baseline |
|||||
|---|---|---|---|---|---|
| Characteristics | Normal (<120 mmHg) (n=705) |
Pre-hypertensive to hypertensive (≥120 mmHg) (n=432) |
|||
|
| |||||
| N | Mean (SD) or % | N | Mean (SD) or % | P-value | |
| Age, years | 705 | 37.4 (10.4) | 432 | 43.1 (10.7) | <0.001 |
| Male, % | 334 | 47.4 | 244 | 56.5 | 0.003 |
| Ever smoker, % | 287 | 40.7 | 208 | 48.2 | 0.02 |
| Diabetes history, % | 15 | 2.1 | 27 | 6.3 | <0.001 |
| Education, years | 705 | 3.3 (3.6) | 432 | 3.9 (4.1) | 0.009 |
| BMI baseline, kg/m2 | 702 | 19.4 (3.2) | 429 | 20.6 (3.6) | <0.001 |
|
| |||||
| Systolic Blood Pressure, mmHg | |||||
| Baseline | 705 | 105.11 (9.5) | 432 | 135.4 (16.3) | <0.001 |
| Follow up 1 | 698 | 110.5 (15.1) | 426 | 130.7 (22.2) | <0.001 |
| Follow up 2 | 665 | 114.8 (13.4) | 353 | 133.5 (20.0) | <0.001 |
| Follow up 3 | 642 | 108.9 (14.0) | 292 | 123.2 (20.3) | 0.56 |
|
| |||||
| Diastolic Blood Pressure, mmHg | |||||
| Baseline | 704 | 69.3 (8.7) | 432 | 84.5 (10.5) | 0.27 |
| Follow up 1 | 698 | 71.1 (9.2) | 426 | 81.2 (12.0) | 0.50 |
| Follow up 2 | 665 | 74.6 (9.0) | 353 | 83.5 (11.1) | 0.54 |
| Follow up 3 | 642 | 71.7 (9.6) | 292 | 79.1 (11.6) | 0.65 |
|
| |||||
| Baseline Urinary Arsenic, μg/g creatinine | 704 | 279.2 (263.6) | 425 | 268.1 (458.3) | 0.61 |
|
| |||||
| Baseline Water Arsenic, μg/L | 705 | 102.0 (115.9) | 432 | 91.9 (104.5) | 0.14 |
We first examined the main effects of water As at baseline on annual BP outcomes over time. Similar to previously reported results [14], we observed that well water As was related to longitudinal increases in each of the three BP outcomes; a 100 μL increase in baseline water As was related to a greater increase in annual SBP (β12: 0.22mmHg, 95% CI: 0.08, 0.36, p=0.002), annual DBP (β12: 0.10mmHg, 95% CI: 0.01, 0.19, p=0.04), and annual PP (β12: 0.13, 95% CI: 0.03, 0.24, p=0.01). We likewise observed a greater increase in annual BP in analyses using total urinary As at baseline as the exposure measure in mixed models adjusted for confounders including urinary creatinine, although the estimate for PP was somewhat attenuated in comparison to estimates from water As. A 100 μL increase in baseline urinary As was related to a greater increase in annual SBP (β12: 0.13mmHg, 95% CI: 0.02, 0.24, p=0.02), annual DBP (β12: 0.12mmHg, 95% CI: 0.05, 0.19, p=0.001) and annual PP (β12: 0.02, 95% CI: −0.06, 0.10, p=0.62).
We found interactions for As and time (β12), each SNP and time (β13) and the interaction effect of As, time and SNP (β123) on SBP (Table 2), DBP (Table 3) and PP (Table 4), respectively. Overall, we observed interactions between 44 SNPs with As under either dominant or recessive models for at least one of the three BP outcomes over the course of follow-up with p-values <0.05 prior to FDR correction (Tables 2, 3, 4).
Table 2.
Interaction between SNPs and well arsenic over the course of follow-up in relation to systolic blood pressure.
| Gene | db SNP ID | MAF (%) |
Genetic modela |
Arsenic*Timeb
(95% CI) |
SNP*Timec
(95% CI) |
Arsenic*SNP*Timed
(95% CI) |
p-valuee | q-valuef |
|---|---|---|---|---|---|---|---|---|
| APOE | rs429358 | 9.6 | Dominant | 0.17 (0.02, 0.32) | −0.35 (−0.90, 0.19) | 0.26 (−0.08, 0.59) | 0.14 | 0.78 |
| Recessive | 0.20 (0.06, 0.34) | −0.91 (−3.23, 1.42) | 2.05 (0.57, 3.54) | 0.01 | 0.34 | |||
|
| ||||||||
| CBS | rs234709 | 17.7 | Dominant | 0.10 (−0.08, 0.27) | −0.52 (−0.98, −0.07) | 0.34 (0.06, 0.62) | 0.02 | 0.54 |
| Recessive | 0.20 (0.05, 0.36) | −0.75 (−2.21, 0.71) | 0.49 (−1.00, 1.97) | 0.52 | 0.94 | |||
| rs2014564 | 47.6 | Dominant | 0.02 (−0.22, 0.27) | −0.21 (−0.69, 0.27) | 0.28 (0.01, 0.56) | 0.04 | 0.67 | |
| Recessive | 0.18 (0.02, 0.34) | −0.06 (−0.58, 0.46) | 0.17 (−0.12, 0.46) | 0.25 | 0.86 | |||
| rs2124459 | 18.3 | Dominant | 0.12 (−0.06, 0.30) | −0.51 (−0.98, −0.04) | 0.40 (0.12, 0.68) | 0.01 | 0.34 | |
| Recessive | 0.24 (0.09, 0.40) | −0.62 (−2.19, 0.95) | 0.41 (−1.12, 1.95) | 0.60 | 0.96 | |||
| rs234701 | 47.1 | Dominant | 0.02 (−0.23, 0.26) | −0.26 (−0.73, 0.21) | 0.29 (0.02, 0.57) | 0.04 | 0.67 | |
| Recessive | 0.20 (0.04, 0.36) | 0.00 (−0.52, 0.52) | 0.12 (−0.18, 0.41) | 0.44 | 0.93 | |||
|
| ||||||||
| CYBA | rs3794624 | 10.9 | Dominant | 0.22 (0.07, 0.38) | −0.10 (−0.61, 0.41) | 0.02 (−0.29, 0.33) | 0.90 | 0.99 |
| Recessive | 0.22 (0.07, 0.36) | −0.67 (−2.41, 1.09) | 2.43 (0.78, 4.08) | 0.004 | 0.34 | |||
|
| ||||||||
| GSTM1 | rs4147567 | 4.0 | Dominant | 0.16 (0.02, 0.32) | −0.69 (−1.50, 0.12) | 0.60 (0.17, 1.03) | 0.01 | 0.34 |
|
| ||||||||
| GSTP1 | rs749174 | 18.0 | Dominant | 0.13 (−0.04, 0.30) | −0.45 (−0.89, 0.00) | 0.29 (0.02, 0.52) | 0.04 | 0.67 |
| Recessive | 0.22 (0.07, 0.37) | −0.70 (−2.19, 0.79) | 0.43 (−1.11, 1.97) | 0.58 | 0.96 | |||
|
| ||||||||
| GSTT1 | rs4630 | 7.1 | Dominant | 0.25 (0.09, 0.41) | −0.34 (−1.14, 0.46) | 0.10 (−0.33, 0.52) | 0.66 | 0.98 |
| Recessive | 0.24 (0.09, 0.39) | −0.91 (−2.19, 0.38) | 1.07 (0.11, 2.03) | 0.03 | 0.67 | |||
|
| ||||||||
| IL6 | rs1524107 | 20.4 | Dominant | 0.09 (−0.08, 0.27) | −0.34 (−0.76, 0.09) | 0.34 (0.08, 0.60) | 0.01 | 0.47 |
| Recessive | 0.22 (0.07, 0.36) | 0.54 (−0.76, 1.83) | 0.10 (−0.81, 1.01) | 0.83 | 0.98 | |||
|
| ||||||||
| NOS3 | rs743506 | 17.3 | Dominant | 0.26 (0.09, 0.42) | 0.11 (−0.33, 0.55) | −0.10 (−0.36, 0.16) | 0.45 | 0.93 |
| Recessive | 0.20 (0.05, 0.34) | −0.97 (−2.04, 0.10) | 0.72 (0.04, 1.40) | 0.04 | 0.67 | |||
|
| ||||||||
| S1PR1 | rs3753194 | 30.6 | Dominant | 0.24 (0.04, 0.45) | 0.07 (−0.35, 0.49) | −0.04 (−0.29, 0.22) | 0.78 | 0.98 |
| Recessive | −0.19 (−0.60, 0.23) | −0.90 (−1.65, −0.15) | 0.45 (0.02, 0.88) | 0.04 | 0.67 | |||
|
| ||||||||
| SOD2 | rs5746123 | 11.8 | Dominant | 0.15 (0.00, 0.31) | −0.02 (−0.52, 0.48) | 0.33 (0.02, 0.64) | 0.04 | 0.67 |
| Recessive | 0.22 (0.07, 0.36) | 0.27 (−1.22, 1.77) | 0.18 (−0.75, 1.12) | 0.70 | 0.98 | |||
Abbreviations: SNPs, single nucleotide polymorphisms; CI, confidence interval; SBP, systolic blood pressure.
Recessive models are not shown for variants where fewer than 10 individuals possessed the homozygous recessive genotype
Coefficient in relation to interaction between well arsenic level and time
Coefficient in relation to interaction between each SNP in the dominant or recessive genetic models and time.
Coefficient in relation to the three-way interaction between each SNP, time and well arsenic in the dominant, or recessive genetic models.
P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, prior to FDR correction for multiple comparisons.
Q values are adjusted P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, after FDR correction for multiple comparisons.
Table 3.
Interaction between SNPs and well arsenic over the course of follow-up in relation to diastolic blood pressure.
| Gene | db SNP ID | MAF (%) |
Genetic modela |
Arsenic*Timeb
(95% CI) |
SNP*Timec
(95% CI) |
Arsenic*SNP*Timed
(95% CI) |
p-valuee | q-valuef |
|---|---|---|---|---|---|---|---|---|
| APOE | rs429358 | 9.6 | Dominant | 0.08 (−0.02, 0.18) | −0.02 (−0.37, 0.33) | 0.06 (−0.15, 0.27) | 0.56 | 0.99 |
| Recessive | 0.08 (−0.01, 0.18) | 0.77 (−0.72, 2.25) | 0.95 (0.03, 1.88) | 0.04 | 0.82 | |||
|
| ||||||||
| AS3MT | rs9527 | 7.1 | Dominant | 0.14 (0.04, 0.24) | 0.13 (−0.26, 0.52) | 0.22 (0.02, 0.42) | 0.03 | 0.74 |
| Recessive | 0.10 (0.01, 0.19) | 1.22 (−0.71, 3.14) | −1.30 (−2.65, 0.04) | 0.06 | 0.88 | |||
| rs4290163 | 8.1 | Dominant | 0.13 (0.03, 0.23) | −0.20 (−0.59, 0.18) | 0.26 (0.03, 0.49) | 0.03 | 0.74 | |
| Recessive | 0.10 (0.01, 0.19) | 1.36 (−0.16, 2.87) | −0.60 (−1.63, 0.42) | 0.25 | 0.88 | |||
|
| ||||||||
| GSTM1 | rs4147567 | 4.0 | Dominant | 0.07 (−0.03, 0.16) | −0.47 (−0.99, 0.05) | 0.36 (0.09, 0.63) | 0.01 | 0.74 |
|
| ||||||||
| GSTP1 | rs6591256 | 32.7 | Dominant | 0.01 (−0.10, 0.12) | −0.16 (−0.43, 0.11) | 0.24 (0.08, 0.40) | 0.003 | 0.70 |
| Recessive | 0.08 (−0.01, 0.18) | −0.13 (−0.58, 0.32) | 0.20 (−0.06, 0.46) | 0.14 | 0.88 | |||
| rs1695 | 44.0 | Dominant | 0.36 (0.10, 0.61) | −0.04 (−0.47, 0.40) | 0.30 (0.04, 0.57) | 0.02 | 0.74 | |
|
| ||||||||
| HMOX1 | rs5755718 | 17.1 | Dominant | 0.03 (−0.08, 0.15) | −0.04 (−0.40, 0.31) | 0.26 (0.04, 0.48) | 0.02 | 0.74 |
| Recessive | 0.07 (−0.05, 0.18) | −0.20 (−0.67, 0.26) | 0.16 (−0.11, 0.43) | 0.24 | 0.88 | |||
| rs8139532 | 8.5 | Dominant | 0.05 (−0.05, 0.15) | −0.13 (−0.49, 0.24) | 0.29 (0.07, 0.52) | 0.01 | 0.74 | |
| rs16995662 | 8.2 | Dominant | 0.06 (−0.04, 0.16) | −0.09 (−0.46, 0.28) | 0.25 (0.03, 0.48) | 0.03 | 0.74 | |
| rs2269534 | 8.1 | Dominant | 0.06 (−0.04, 0.16) | −0.12 (−0.50, 0.25) | 0.25 (0.03, 0.48) | 0.03 | 0.74 | |
|
| ||||||||
| IL6 | rs2069840 | 14.3 | Dominant | 0.12 (0.02, 0.23) | 0.21 (−0.09, 0.51) | −0.11 (−0.29, 0.07) | 0.24 | 0.88 |
| Recessive | −0.48 (−0.99, 0.03) | −1.08 (−2.07, −0.10) | 0.59 (0.07, 1.10) | 0.02 | 0.74 | |||
| rs13306435 | 10.9 | Dominant | 0.16 (0.05, 0.26) | 0.27 (−0.06, 0.60) | 0.19 (0.01, 0.36) | 0.04 | 0.82 | |
| Recessive | 0.12 (0.02, 0.21) | 0.03 (−1.12, 1.18) | −0.37 (−1.12, 0.37) | 0.33 | 0.92 | |||
| rs1554606 | 7.8 | Dominant | 0.13 (0.03, 0.23) | −0.22 (−0.61, 0.17) | 0.28 (0.04, 0.52) | 0.02 | 0.74 | |
| Recessive | 0.10 (0.00, 0.19) | 1.36 (−0.16, 2.87) | −0.60 (−1.63, 0.42) | 0.25 | 0.88 | |||
| rs1474347 | 20.8 | Dominant | 0.20 (0.08, 0.31) | −0.26 (−0.54, 0.01) | 0.19 (0.03, 0.34) | 0.02 | 0.74 | |
| Recessive | 0.12 (0.02, 0.21) | 0.13 (−0.47, 0.74) | 0.02 (−0.46, 0.49) | 0.94 | 0.99 | |||
|
| ||||||||
| NOS3 | rs3918198 | 8.2 | Dominant | 0.06 (−0.04, 0.16) | −0.09 (−0.46, 0.28) | 0.25 (0.02, 0.47) | 0.03 | 0.74 |
| rs1799983 | 8.8 | Dominant | 0.05 (−0.05, 0.15) | −0.12 (−0.48, 0.24) | 0.30 (0.07, 0.52) | 0.01 | 0.74 | |
|
| ||||||||
| PNP | rs17882836 | 49.2 | Dominant | 0.05 (−0.10, 0.21) | −0.14 (−0.45, 0.18) | 0.04 (−0.13, 0.21) | 0.65 | 0.99 |
| Recessive | −0.08 (−0.26, 0.10) | −0.27 (−0.59, 0.05) | 0.21 (0.02, 0.40) | 0.03 | 0.74 | |||
|
| ||||||||
| TNF | rs4248159 | 5.6 | Dominant | 0.14 (0.04, 0.24) | −0.37 (−0.80, 0.05) | 0.21 (0.02, 0.40) | 0.03 | 0.74 |
Abbreviations: SNPs, single nucleotide polymorphisms; CI, confidence interval; DBP, diastolic blood pressure.
Recessive models are not shown for variants where fewer than 10 individuals possessed the homozygous recessive genotype
Coefficient in relation to interaction between well arsenic level and time
Coefficient in relation to interaction between each SNP in the dominant or recessive genetic models and time.
Coefficient in relation to the three-way interaction between each SNP, time and well arsenic in the dominant, or recessive genetic models.
P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, prior to FDR correction for multiple comparisons.
Q values are adjusted P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, after FDR correction for multiple comparisons.
Table 4.
Interaction between SNPs and well arsenic over the course of follow-up in relation to pulse pressure.
| Gene | db SNP ID | MAF (%) |
Genetic modela |
Arsenic*Timeb
(95% CI) |
SNP*Timec
(95% CI) |
Arsenic*SNP*Timed
(95% CI) |
p-valuee | q-valuef |
|---|---|---|---|---|---|---|---|---|
| APOE | rs429358 | 9.6 | Dominant | 0.10 (−0.01, 0.20) | −0.39 (−0.77, −0.02) | 0.22 (−0.01, 0.44) | 0.06 | 0.62 |
| Recessive | 0.12 (0.02, 0.22) | −1.77 (−3.38, −0.16) | 1.08 (0.11, 2.06) | 0.03 | 0.52 | |||
|
| ||||||||
| AS3MT | rs4290163 | 8.1 | Dominant | 0.18 (0.07, 0.28) | −0.14 (−0.56, 0.27) | 0.34 (0.10, 0.58) | 0.01 | 0.45 |
| Recessive | 0.14 (0.03, 0.24) | 0.24 (−1.41, 1.89) | −0.30 (−1.39, 0.78) | 0.58 | 0.90 | |||
| rs10509761 | 28.8 | Dominant | 0.07 (−0.07, 0.21) | −0.01 (−0.30, 0.28) | 0.11 (−0.05, 0.28) | 0.18 | 0.79 | |
| Recessive | 0.10 (−0.01, 0.21) | −0.14 (−0.66, 0.37) | 0.32 (0.06, 0.58) | 0.02 | 0.49 | |||
|
| ||||||||
| CBS | rs6586281 | 45.0 | Dominant | 0.29 (0.14, 0.44) | −0.33 (−0.64, 0.02) | 0.23 (0.05, 0.40) | 0.01 | 0.46 |
| Recessive | 0.15 (0.04, 0.26) | −0.04 (−0.39, 0.31) | −0.10 (−0.31, 0.12) | 0.38 | 0.88 | |||
| rs234701 | 47.1 | Dominant | −0.01 (−0.17, 0.15) | −0.37 (−0.70, −0.04) | 0.21 (0.03, 0.39) | 0.02 | 0.49 | |
| Recessive | 0.13 (0.01, 0.24) | −0.15 (−0.51, 0.22) | 0.06 (−0.13, 0.25) | 0.54 | 0.90 | |||
| rs706208 | 49.3 | Dominant | 0.26 (0.09, 0.44) | −0.25 (−0.59, 0.09) | 0.19 (0.01, 0.38) | 0.04 | 0.57 | |
| Recessive | 0.13 (0.02, 0.25) | 0.14 (−0.22, 0.50) | −0.06 (−0.27, 0.15) | 0.59 | 0.91 | |||
| rs3788050 | 7.3 | Dominant | 0.11 (0.00, 0.22) | 0.04 (−0.39, 0.48) | 0.12 (−0.10, 0.34) | 0.27 | 0.85 | |
| Recessive | −0.54 (−1.03, −0.05) | −1.75 (−2.81, −0.68) | 0.69 (0.19, 1.18) | 0.007 | 0.45 | |||
| rs234709 | 17.7 | Dominant | 0.06 (−0.06, 0.18) | −0.36 (−0.68, −0.04) | 0.23 (0.05, 0.41) | 0.01 | 0.47 | |
| Recessive | 0.13 (0.02, 0.24) | −0.70 (−1.72, 0.33) | 0.32 (−0.69, 1.33) | 0.53 | 0.90 | |||
| rs2124459 | 18.3 | Dominant | 0.06 (−0.07, 0.18) | −0.33 (−0.67, 0.00) | 0.26 (0.07, 0.44) | 0.01 | 0.45 | |
| Recessive | 0.14 (0.03, 0.25) | −0.54 (−1.65, 0.57) | 0.17 (−0.88, 1.21) | 0.76 | 0.95 | |||
| rs2849727 | 17.3 | Dominant | 0.07 (−0.04, 0.19) | −0.27 (−0.58, 0.04) | 0.20 (0.02, 0.37) | 0.03 | 0.53 | |
| Recessive | 0.13 (0.02, 0.23) | −0.90 (−1.91, 0.11) | 0.53 (−0.47, 1.53) | 0.30 | 0.86 | |||
| rs9983620 | 21.4 | Dominant | 0.12 (−0.01, 0.25) | 0.02 (−0.28, 0.32) | 0.01 (−0.16, 0.17) | 0.95 | 0.98 | |
| Recessive | −0.24 (−0.61, 0.13) | −0.49 (−1.24, 0.26) | 0.39 (0.01, 0.76) | 0.04 | 0.57 | |||
|
| ||||||||
| CYBA | rs3794624 | 10.9 | Dominant | 0.13 (0.02, 0.24) | −0.07 (−0.43, 0.28) | 0.01 (−0.19, 0.21) | 0.90 | 0.98 |
| Recessive | 0.13 (0.02, 0.23) | −1.00 (−2.23, 0.23) | 2.10 (1.01, 3.20) | <0.001 | 0.05 | |||
| rs13306296 | 10.1 | Dominant | 0.13 (0.02, 0.24) | −0.12 (−0.49, 0.24) | 0.03 (−0.21, 0.15) | 0.74 | 0.94 | |
| Recessive | 0.13 (0.03, 0.23) | −0.26 (−1.55, 1.03) | 1.02 (0.22, 1.81) | 0.01 | 0.47 | |||
|
| ||||||||
| GSTM1 | rs4147567 | 4.0 | Dominant | 0.10 (0.00, 0.21) | −0.25 (−0.82, 0.32) | 0.30 (0.01, 0.58) | 0.04 | 0.57 |
|
| ||||||||
| GSTP1 | rs749174 | 18.0 | Dominant | 0.07 (−0.04, 0.19) | −0.28 (−0.59, 0.03) | 0.20 (0.02, 0.38) | 0.03 | 0.49 |
| Recessive | 0.14 (0.03, 0.24) | −0.78 (−1.82, 0.25) | 0.49 (−0.54, 1.52) | 0.35 | 0.88 | |||
|
| ||||||||
| IL6 | rs2069845 | 17.6 | Dominant | 0.07 (−0.05, 0.19) | −0.09 (−0.40, 0.21) | 0.18 (0.004, 0.35) | 0.04 | 0.57 |
| Recessive | 0.13 (0.02, 0.23) | 0.63 (−0.54, 1.80) | −0.05 (−0.89, 0.78) | 0.90 | 0.98 | |||
| rs1524107 | 20.4 | Dominant | 0.04 (−0.08, 0.16) | −0.36 (−0.66, −0.06) | 0.26 (0.08, 0.43) | 0.004 | 0.45 | |
| Recessive | 0.13 (0.03, 0.24) | 0.21 (−0.69, 1.11) | −0.02 (−0.63, 0.58) | 0.94 | 0.98 | |||
| rs1554606 | 7.8 | Dominant | 0.17 (0.07, 0.28) | −0.13 (−0.55, 0.29) | 0.29 (0.04, 0.54) | 0.02 | 0.49 | |
| Recessive | 0.14 (0.04, 0.24) | 0.25 (−1.39, 1.90) | −0.32 (−1.40, 0.76) | 0.56 | 0.90 | |||
|
| ||||||||
| MTHFR | rs9651118 | 16.9 | Dominant | 0.14 (0.02, 0.26) | 0.03 (−0.28, 0.34) | −0.04 (−0.21, 0.14) | 0.69 | 0.93 |
| Recessive | 0.11 (0.01, 0.22) | −0.54 (−1.34, 0.25) | 0.48 (0.03, 0.94) | 0.04 | 0.55 | |||
|
| ||||||||
| NOS3 | rs743506 | 17.3 | Dominant | 0.15 (0.03, 0.26) | 0.02 (−0.29, 0.32) | −0.03 (−0.20, 0.14) | 0.72 | 0.94 |
| Recessive | 0.12 (0.01, 0.22) | −0.44 (−1.20, 0.32) | 0.49 (0.04, 0.94) | 0.03 | 0.53 | |||
| rs1808593 | 12.2 | Dominant | 0.08 (−0.04, 0.19) | 0.00 (−0.34, 0.34) | 0.22 (0.03, 0.40) | 0.02 | 0.49 | |
|
| ||||||||
| PNP | rs17882836 | 49.2 | Dominant | 0.06 (−0.11, 0.22) | −0.21 (−0.56, 0.13) | 0.10 (−0.09, 0.29) | 0.30 | 0.86 |
| Recessive | −0.09 (−0.28, 0.10) | −0.35 (−0.70, −0.01) | 0.28 (0.08, 0.48) | 0.01 | 0.45 | |||
| rs1617940 | 17.3 | Dominant | 0.15 (0.03, 0.27) | 0.03 (−0.28, 0.34) | −0.05 (−0.22, 0.12) | 0.58 | 0.90 | |
| Recessive | 0.12 (0.02, 0.22) | −0.45 (−1.23, 0.34) | 0.47 (0.01, 0.92) | 0.04 | 0.57 | |||
|
| ||||||||
| SOD2 | rs10370 | 29.9 | Dominant | 0.11 (−0.03, 0.25) | 0.10 (−0.20, 0.40) | 0.04 (−0.13, 0.22) | 0.61 | 0.91 |
| Recessive | 0.10 (−0.01, 0.21) | −0.32 (−0.83, 0.20) | 0.32 (0.06, 0.58) | 0.02 | 0.47 | |||
| rs12526686 | 43.7 | Dominant | 0.27 (0.11, 0.42) | −0.30 (−0.60, 0.01) | 0.23 (0.05, 0.40) | 0.01 | 0.46 | |
| Recessive | 0.14 (0.03, 0.25) | −0.03 (−0.38, 0.33) | −0.16 (−0.38, 0.07) | 0.17 | 0.78 | |||
|
| ||||||||
| TNF | rs1800630 | 39.6 | Dominant | 0.33 (0.17, 0.49) | −0.42 (−0.10, −0.73) | 0.26 (0.08, 0.43) | 0.01 | 0.45 |
| Recessive | 0.17 (0.06, 0.28) | −0.09 (−0.51, 0.34) | −0.08 (−0.34, 0.17) | 0.52 | 0.90 | |||
| rs3179060 | 12.7 | Dominant | 0.08 (−0.04, 0.19) | 0.01 (−0.32, 0.34) | 0.20 (0.02, 0.38) | 0.03 | 0.53 | |
|
| ||||||||
| VCAM1 | rs3176877 | 45.8 | Dominant | 0.28 (0.12, 0.44) | −0.34 (−0.03, −0.66) | 0.22 (0.04, 0.39) | 0.02 | 0.47 |
| Recessive | 0.15 (0.04, 0.26) | −0.03 (−0.38, 0.32) | −0.13 (−0.34, 0.09) | 0.26 | 0.85 | |||
| rs3783617 | 17.1 | Dominant | 0.14 (0.03, 0.26) | 0.02 (−0.30, 0.33) | −0.02 (−0.20, 0.15) | 0.78 | 0.95 | |
| Recessive | 0.12 (0.02, 0.22) | −0.54 (−1.34, 0.26) | 0.48 (0.03, 0.94) | 0.04 | 0.55 | |||
| rs3917016 | 18.7 | Dominant | 0.20 (0.06, 0.33) | −0.26 (−0.63, 0.10) | 0.24 (0.03, 0.46) | 0.02 | 0.49 | |
| Recessive | 0.12 (0.00, 0.24) | −0.32 (−1.24, 0.59) | −0.20 (−0.95, 0.56) | 0.60 | 0.91 | |||
Abbreviations: SNPs, single nucleotide polymorphisms; CI, confidence interval; PP, pulse pressure.
Recessive models are not shown for variants where fewer than 10 individuals possessed the homozygous recessive genotype
Coefficient in relation to interaction between well arsenic level and time
Coefficient in relation to interaction between each SNP in the dominant or recessive genetic models and time.
Coefficient in relation to the three-way interaction between each SNP, time and well arsenic in the dominant, or recessive genetic models.
P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, prior to FDR correction for multiple comparisons.
Q values are adjusted P values for interaction adjusted for sex, age at enrollment, body mass index, smoking status (never, past, and current), educational attainment and diabetes at baseline, after FDR correction for multiple comparisons.
In particular, we detected several SNPs interactions with As exposure on annual SBP over the course of follow-up (Table 2). Under the dominant model, interactions with a total of 8 SNPs in CBS, GSTM1, GSTP1, IL6, and SOD2 were observed with As exposure and annual increase in SBP. Under the recessive model, an additional 5 SNPs in APOE, CYBA, GSTT1, NOS3, and S1PR1 were found to have interactions with As exposure and annual SBP over time (Table 2). However, none of these associations remained significant after FDR adjustment for multiple comparisons.
Fifteen SNPs in the AS3MT, GSTM1, GSTP1, HMOX1, IL6, NOS3, and TNF genes were found to have interactions with As exposure and annual DBP increase over the course of follow-up in the dominant model (Table 3). We observed interactions for three additional SNPs in APOE, IL6, and PNP with As exposure and annual DBP increase in the recessive model (Table 3). None of these associations remained significant after FDR adjustment for multiple comparisons.
Interactions with 18 different SNPs in 9 genes of interest including AS3MT, CBS, GSTM1, GSTP1, IL6, NOS3, SOD2, TNF, and VCAM1 and water As for annual PP over time were statistically significant prior to FDR correction under the dominant model (Table 4). Additionally, interactions with 12 SNPs in 10 genes and water As under the recessive model were related to annual PP over time, including APOE, AS3MT, CBS, CYBA, MTHFR, NOS3, PNP, SOD2, TNF and VCAM1 (Table 4). These interactions did not remain statistically significant after correction for multiple testing with one exception -- a CYBA gene SNP (rs3794624) (β123: 2.10 mmHg, 95% CI: 1.01, 3.20) in the recessive model (Table 4).
In general, the additive models were less informative than the dominant or recessive models. However, under the additive model, we observed interactions between some SNPs and water As with annual SBP (CBS rs2124459, GSTM1 rs4147567), annual DBP (GSTM1 rs4147567, HMOX1 rs8139532, NOS3 rs1799983) and annual PP (AS3MT rs4290163, CBS rs2124459 and rs234709, IL6 rs1524107, SOD2 rs12526686) over time at the p<0.01 level (data not shown).
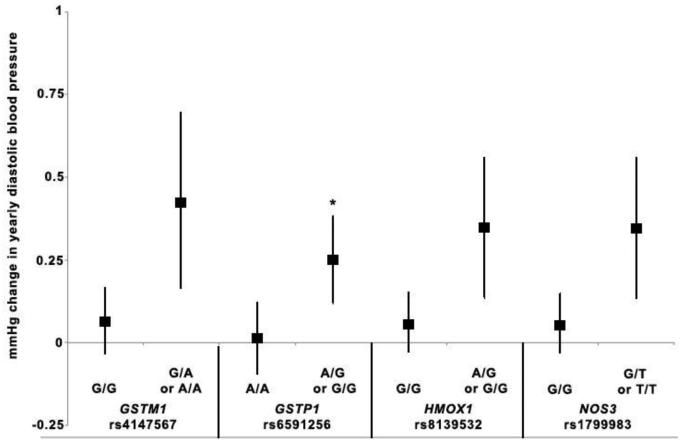
We also graphically represented the association of As with annual increase in BP outcomes by genotype for interactions that were nominally significant at the p=0.01 level. We identified 5 interactions between As and SNPs that met these criteria and were associated with increases in SBP over time that were signficant at the p=0.01 level prior to FDR correction, including APOE rs429358, CBS rs2124459, CYBA rs3794624, GSTM1 rs4147567 and IL6 rs1524107. Four As-SNP interactions significantly increased DBP over time at the p=0.01 level prior to FDR correction including GSTM1 rs4147567, GSTP1 rs6591256, HMOX1 rs8139532 and NOS3 rs1799983. Additionally, we identified 11 As-SNP interactions that significantly increased PP over time at the p=0.01 level prior to FDR correction, including AS3MT rs4290163, CBS rs6586281, rs3788050, rs234709, and rs2124459, CYBA rs3794624 and rs13306296, IL6 rs1524107, PNP rs17882836, SOD2 rs12526686 and TNF rs1800630. The graphs present the As-related change in annual in SBP (Figure 1), DBP (Figure 2) and PP (Figure 3) for individuals with either the wild-type/reference genotype or the variant genotype, for those SNPs identified as significantly interacting with As to increase BP over time. For all SNPs, the annual As-related increase in BP was greater among those with the variant genotype, although the effects were greater, and statistically significant, among individuals with the CYBA rs3794624 variant genotype (Figures 1 and 3). Among individuals with the CYBA rs3794624 variant genotype, well As was associated with a 2.23 mmHg (95% CI: 1.14-3.32) greater annual increase in PP, while among those with the wild type, well As was associated with a 0.13 mmHg (95% CI: 0.02-0.23) greater annual increase in PP (Figure 3).
Figure 2.
Yearly change in diastolic blood pressure in relation to arsenic exposure and variant SNP genotypes. For each SNP, the symbol to the left represents the estimate of yearly change in diastolic blood pressure in relation to arsenic for reference genotype(s) and the symbol on the right represents the estimate of yearly change in diastolic blood pressure in relation to arsenic for the variant genotype under a dominant model (GSTM1 rs4147567, GSTP1 rs6591256, HMOX1 rs8139532, NOS3 rs1799983). Vertical lines represent 95% CI for each estimate. We chose SNPs that had a significant interaction with arsenic exposure p≤0.01 in relation to diastolic blood pressure over time. SNP-arsenic interactions that were significant at p<0.01 prior to FDR correction are indicated with an asterisk (*).
Discussion
To our knowledge, the present study is among the first large epidemiologic studies to examine the relationship between As exposure from drinking water, genetic susceptibility factors and change in BP over time. In this study, we tested whether the association between water As and increases in BP over time differed by 235 SNPs in 18 genes related to As metabolism, oxidative stress, inflammation and endothelial function in a subset of 1,137 HEALS participants. Our findings suggest that the joint effects of As exposure and genetic variability may significantly increase the risk of developing elevated BP over time. A total of 44 SNPs were observed to have nominally significant interactions with well water As in relation to elevations in BP parameters over the course of follow-up. In particular, the interaction between well water As and CYBA (rs3794624) in relation to PP over time remained significant after adjustment for multiple testing.
There is growing evidence that As exposure is associated with increases in BP. Several cross-sectional studies reported associations between As exposure and BP, but until recently, prospective data were lacking. A recent longitudinal study published by HEALS researchers reported that among nearly 11,000 individuals followed over nearly 7 years of follow-up, water and urinary As levels were associated with increases in BP over time [14]. The study reported that each 100 μg/L increase in baseline water As was significantly associated with a greater annual increase of 0.11 (95% CI: 0.05, 0.16) mmHg/year in SBP and 0.05 (95% CI: 0.02, 0.08) mmHg/year in DBP, with similar increases associated with urinary As. We observed similar trends in increasing BP over time in our smaller dataset, although with less statistical precision.
We found a significant interaction between CYBA variant rs3794624 and As exposure that was associated with increases PP over the course of follow-up that was unlikely due to chance as a result of multiple comparisons. Elevated PP is thought to indicate increased vascular stiffness and endothelial dysfunction [35] and is an independent predictor of CVD risk [31,36]. In a previous cross-sectional study, As exposure was positively related to PP [26]. In our data there also was a suggestion of positive interactions between As exposure and CYBA SNPs rs3794624 for SBP and rs13306296 for PP over time. The CYBA gene encodes the key p22(phox) subunit of the NADPH oxidase enzyme that is involved in the generation of reactive oxygen species. The SNP rs3794624 is located in an intron and not yet well characterized. However, this variant allele has been associated with an increased risk of ankle-brachial index, a noninvasive measure of peripheral arterial disease [37]. A number of SNPs have been identified in the CYBA promoter and exonic sequences, some of which can impact gene expression and NADPH activation [38,39] and could potentially increase production of harmful oxidants, increasing oxidative stress and thus impacting endothelial function. Although published reports have been conflicting, some studies have suggested that other SNPs in CYBA are associated with increased risks of atherosclerosis, essential hypertension and increases in carotid intima media thickness [38,40]. Furthermore, in vitro studies have shown that As exposure can stimulate NADPH oxidase activity and alter its subcellular localization and phosphorylation [41-45], increasing vascular smooth muscle DNA damage and cell migration [41] and in turn could affect in endothelial function. Given the literature supporting a role for As in the promotion of oxidative imbalance demonstrated both in vitro and in vivo [6,45], as well as its ability to deregulate NADPH oxidase [45], our observed interaction may represent a biologically relevant mechanism underlying alterations in endothelial function and in turn, long-term changes in BP, but will need to be validated in other studies.
Many of the SNP interactions we observed with As-related BP changes are consistent with findings that As can alter mediators of inflammation [6,12,46]. Multiple SNPs in key inflammatory mediators, including IL6, appeared to modify the association between As exposure and all three BP outcomes. Further, we observed significant positive interactions between water As and genes independently related to CVD risk, including the APOE variant rs429358, and each of the three BP outcomes. The APOE rs429358 variant C-allele has been associated with an increased risk of type 2 diabetes and altered lipid profiles [47-49] and when co-inherited with rs7412, has been associated with an elevated risk of heart disease [50]. We observed potential interactions with four NOS3 variants with As exposure and BP increase over time. NOS3 generates nitric oxide (NO) to promote vascular smooth muscle relaxation and some variants have been associated with increased risk of hypertension and coronary artery disease, with heterogeneity across ethnic groups [51,52]. Thus, the possibility that SNPs that increase inflammation or result in suboptimal vascular function may confer greater susceptibility to As’s adverse cardiovascular effects should be considered further.
Arsenic’s ability to generate reactive oxygen species (ROS) and increase oxidative stress has been experimentally demonstrated both in vitro and in vivo [6,45] and increases in ROS production are thought to aid the progression of CVD and atherosclerosis. SNPs in proteins that help to regulate ROS production may disrupt the balance between oxidizing and reducing species, increasing the likelihood of endothelial injury, inflammation and altered vascular tone [53]. In addition to CYBA rs3794624, we identified gene-As interactions for BP with other oxidative stress related genes. Multiple SNPs in SOD2, an antioxidant defense enzyme that helps clear superoxide, impacted annual SBP and PP over time. Four HMOX1 variants, which encodes the heme oxygenase (HO) protein that influences vascular tone, nitric oxide synthase function, and protects against inflammation and oxidative tissue damage [54], interacted with As exposure to increase annual DBP. Epidemiological studies have reported associations between As exposure and biomarkers of increased oxidative stress [55,56] and one cross-sectional study found greater risk of hypertension among As exposed individuals with polymorphisms in oxidative stress genes manganese superoxide dismutase (MnSOD) and 8-oxoguanine DNA glycosylase (OGG1), which were not among genes studied here [57]. Together, these data suggest that the reported oxidative stress SNPs-As interaction effects on BP are biologically plausible, although the SNPs reported here were only nominally significant and require further investigation.
SNPs in genes that play a role in As metabolism may change an individual’s susceptibility to As’ toxic effects, by altering the rate of methylation and excretion. Three AS3MT variants, which catalyzes the conversion of iAs to methylated metabolites, appeared to modify the association between As and DBP and PP changes over time, but we could not exclude the possibility of chance associations. AS3MT SNPs have previously been associated with decreased gene expression, impaired As metabolism, and cancer risk [58,59]. In a recent GWAS, multiple AS3MT SNPs were associated with impaired metabolism of MMA to DMA and rs9527 was associated with increased risk of skin lesions, a classic sign of As toxicity [60]. For all three measures of BP over the follow-up, we also identified As-SNP interactions for GSTP1 and GSTM1, members of the glutathione-S-transferase family of enzymes that is involved in xenobiotic metabolism and has also been implicated in As metabolism [61]. Several variants in other As metabolism genes PNP, an enzyme that reduces arsenate, and CBS, involved in one-carbon metabolism and As methylation, were associated with As-related BP outcomes. Previous work identified associations between CBS variants and altered As methylation profiles in urine [62]. While it is plausible that variation in As metabolism genes could alter or enhance As’s impact on BP, further replication studies are needed.
Although the absolute difference in the rate of blood pressure change that we report here may be modest, there is evidence that small increases in blood pressure are important [63,64]. Evidence suggests that CVD risk rises continuously as both SBP and DBP increase from 115 mmHg and 75 mmHg, respectively [63]. Even a reduction in usual SBP of 2 mmHg would potentially lower stroke mortality by 10% and lower mortality from IHD or other vascular causes in middle age by nearly 7% [63]. Therefore, although the differences in the rate of BP change associated with arsenic exposure, over time these increases may have a cumulative effect on the risk of clinical events, particularly among genetically susceptible subpopulations. Further, evidence is beginning to suggest that results from Bangladesh may be translated to populations with lower levels of exposure, such as the US. Within the HEALS population, we have reported that the relationships between conventional CVD risk factors and longitudinal change in BP were consistent with those of the literature [32]. Although no longitudinal studies have been done to assess the impact of arsenic on blood pressure change in the US population, there is growing evidence to support a role for arsenic in this context and at lower doses than those found in Bangladesh. A recent cross-sectional analysis of 2009-2012 NHANES data found that arsenic was associated with an increased risk of high blood pressure [65]. Work from the Normative Aging Study found that increases in toenail arsenic were associated with increases in SBP and pulse pressure [66]. Further, a cross-sectional study among 405 villagers exposed to drinking water with low level arsenic (<50 μg/L), found that water arsenic was related to abnormal mean arterial pressure and pulse pressure [67]. Lastly, recent evidence from a prospective pregnancy cohort in New Hampshire reported an association between low-level arsenic exposure and greater pregnancy-related increases in blood pressure over time [68].
Strengths of this study include the relatively long follow-up period of ~7 years on average, over which we have multiple BP measurements and individual environmental exposure assessments for drinking water As at baseline. The prevalence of anti-hypertensive medication use is low in this population, so we were able to investigate BP changes in the relative absence of intervention. Further, we were able to assess a large number of genetic variants, including tagSNPs and functional SNPs that a priori might be expected to modify associations between As and blood pressure. The ethnic homogeneity of the population is an advantage, as it reduces bias from population stratification. On the other hand, our study has limitations. First, we performed many interaction analyses in order to test each SNP under multiple genetic models. Thus, it was necessary to correct for multiple testing to avoid detecting interactions due to chance. Due to the number of tests we performed, the p-values adjusted for multiple comparisons were much higher than the original p-values, thus only one remained significant after FDR correction. Second, it is possible that other genes or SNPs may play a role in BP and that other variants may interact with As exposure. Although BP measurements were obtained in nearly all participants, only a small subset of individuals had genetic information available for this study. However, the subset was randomly selected, and the distributions of demographic, lifestyle, and As exposure variables in our study population and in the overall cohort were very similar (data not shown). Third, it is possible that other risk factors, such as smoking behavior and diet, may have additional impacts on blood pressure over time. To account for these effects, we included smoking status in our model as a possible confounder. We did not include dietary factors such as western diet or sodium intake in this study, as other work from our group has shown that although these factors are related to increases in blood pressure over time, arsenic does not interact with dietary factors to increase longitudinal blood pressure in this population [69]. Fourth, the HEALS population is different from the US population and may have different underlying risk factors that could impact blood pressure changes over time, such as differences in lifestyle, dietary habits and other environmental factors. Despite these differences, a growing number of studies have suggested that arsenic exposure is a contributor to incidence and mortality from CVD [5,70,71], as well as blood pressure changes [65,68,71] in US populations. Further, the majority of HEALS subjects have been exposed to levels of As <100 μg/L, which are relevant to prevalent exposure levels in the US.
Lastly, approximately 75% of the study population was exposed to relatively low to moderate levels of well As (<150μg/L) and it is possible that genetic susceptibility may vary across doses.
Conclusions
In a large prospective study, we found evidence of potential gene-environment interactions between As metabolism, oxidative stress, inflammation and endothelial function gene polymorphisms and As exposure from contaminated well water on longitudinal changes in BP, with potential consequences for cardiovascular health. Rates of CVD are predicted to continue to rise and given the high incidence of CVD particularly in low to middle income countries, the contribution of even a small increased risk associated with environmental exposures in combination with genetic susceptibility factors may translate to a large number of excess cases.
Highlights.
Arsenic (As) exposure has been associated with blood pressure increases over time
Genetic polymorphisms may modify the association between As and blood pressure
An interaction between CYBA rs3794624 and well As increased annual pulse pressure
Genetic variants may contribute to As-related blood pressure increases over time
Acknowledgments
Funding sources: This work is supported by the National Institutes of Health grants: R01ES017541, R01CA107431, P42ES010349, P30ES000260, P30ES009089, R01CA107431, P42ES0073737 and 1K99ES024144. The funding agencies had no role in the study design, data collection, analysis or interpretation of data; or in the writing of the report or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Research Council N . Critical Aspects of EPA's IRIS Assessment of Inorganic Arsenic: Interim Report. National Research Council; Washington, DC: 2014. [Google Scholar]
- 2.WHO WHO Guidelines for drinking-water quality. (fourth) 2011 [Google Scholar]
- 3.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, et al. Association Between Exposure to Low to Moderate Arsenic Levels and Incident Cardiovascular DiseaseA Prospective Cohort Study. Ann Intern Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stea F, Bianchi F, Cori L, Sicari R. Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int. 2014;21:244–251. doi: 10.1007/s11356-013-2113-z. [DOI] [PubMed] [Google Scholar]
- 7.WHO WHO . A global brief on hypertension: Silent killer, global public health crisis. Geneva; Switzerland: 2013. [Google Scholar]
- 8.Heilpern K. Pathophysiology of hypertension. Ann Emerg Med. 2008;51:S5–6. doi: 10.1016/j.annemergmed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental Health Perspectives. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Jasmine F, Kibriya MG, Liu M, Wojcik O, et al. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. Am J Epidemiol. 2012;175:1252–1261. doi: 10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, et al. Interaction between arsenic exposure from drinking water and genetic susceptibility in carotid intima-media thickness in Bangladesh. Toxicol Appl Pharmacol. 2014;276:195–203. doi: 10.1016/j.taap.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F, Molinaro P, Chen Y. Arsenic Exposure and Subclinical Endpoints of Cardiovascular Diseases. Curr Environ Health Rep. 2014;1:148–162. doi: 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade TJ, Xia Y, Mumford J, Wu K, Le XC, et al. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: a case control study. Environ Health. 2015;14:35. doi: 10.1186/s12940-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Liu M, Parvez F, Wang B, Wu F, et al. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among HEALS Cohort Participants. Environmental Health Perspectives. 2015 doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 16.imeonova PP, Luster MI. Arsenic and atherosclerosis. Toxicol Appl Pharmacol. 2004;198:444–449. doi: 10.1016/j.taap.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Santella RM, Kibriya MG, Wang Q, Kappil M, et al. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ Health Perspect. 2007;115:1415–1420. doi: 10.1289/ehp.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, et al. Interaction between Arsenic Exposure from Drinking Water and Genetic Polymorphisms on Cardiovascular Disease in Bangladesh: A Prospective Case-Cohort Study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Z, van Geen A, Seddique AA, Ahmed KM. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environ Sci Technol. 2005;39:4759–4766. doi: 10.1021/es048065f. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 22.Van Geen A, Cheng Z, Seddique AA, Hoque MA, Gelman A, et al. Reliability of a commercial kit to test groundwater for arsenic in Bangladesh. Environ Sci Technol. 2005;39:299–303. [PubMed] [Google Scholar]
- 23.Nixon DE, Mussmann GV, Eckdahl SJ, Moyer TP. Total Arsenic in Urine - Palladium Persulfate Vs Nickel as a Matrix Modifier for Graphite-Furnace Atomic-Absorption Spectrophotometry. Clinical Chemistry. 1991;37:1575–1579. [PubMed] [Google Scholar]
- 24.Chen Y, Ahsan H, Slavkovich V, Peltier GL, Gluskin RT, et al. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect. 2010;118:1299–1305. doi: 10.1289/ehp.0901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165:541–552. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 27. International Hapmap Project.
- 28. SeattleSNPs.
- 29.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. F-SNP database Queen's University, Ontario, Canada.
- 31.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Liu M, Parvez F, Wang B, Wu F, et al. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among HEALS Cohort Participants. Environ Health Perspect. 2015 doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, van Geen A, Graziano JH, Pfaff A, Madajewicz M, et al. Reduction in urinary arsenic levels in response to arsenic mitigation efforts in Araihazar, Bangladesh. Environ Health Perspect. 2007;115:917–923. doi: 10.1289/ehp.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 35.Safar ME, Levy BI, Struijker-Boudier H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 36.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murabito JM, White CC, Kavousi M, Sun YV, Feitosa MF, et al. Association between chromosome 9p21 variants and the ankle-brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ Cardiovasc Genet. 2012;5:100–112. doi: 10.1161/CIRCGENETICS.111.961292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauer H, Shah AM, Laurindo FRM. Studies on cardiovascular disorders. Humana Press; New York: 2010. p. 587. xvii. [Google Scholar]
- 39.San Jose G, Fortuno A, Beloqui O, Diez J, Zalba G. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci (Lond) 2008;114:173–182. doi: 10.1042/CS20070130. [DOI] [PubMed] [Google Scholar]
- 40.Moreno MU, San Jose G, Fortuno A, Beloqui O, Redon J, et al. A novel CYBA variant, the −675A/T polymorphism, is associated with essential hypertension. J Hypertens. 2007;25:1620–1626. doi: 10.1097/HJH.0b013e3281ac211d. [DOI] [PubMed] [Google Scholar]
- 41.Qian Y, Liu KJ, Chen Y, Flynn DC, Castranova V, et al. Cdc42 Regulates Arsenic-induced NADPH Oxidase Activation and Cell Migration through Actin Filament Reorganization. Journal of Biological Chemistry. 2005;280:3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- 42.Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L442–449. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- 43.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, et al. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med. 2009;47:381–388. doi: 10.1016/j.freeradbiomed.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radical Biology and Medicine. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci. 2005;85:541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 47.Alharbi KK, Khan IA, Syed R. Association of apolipoprotein E polymorphism with type 2 diabetes mellitus in a Saudi population. DNA Cell Biol. 2014;33:637–641. doi: 10.1089/dna.2014.2461. [DOI] [PubMed] [Google Scholar]
- 48.Chang MH, Yesupriya A, Ned RM, Mueller PW, Dowling NF. Genetic variants associated with fasting blood lipids in the U.S. population: Third National Health and Nutrition Examination Survey. BMC Med Genet. 2010;11:62. doi: 10.1186/1471-2350-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhary R, Likidlilid A, Peerapatdit T, Tresukosol D, Srisuma S, et al. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovasc Diabetol. 2012;11:36. doi: 10.1186/1475-2840-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H, Li H, Liu J, Zhu D, Wang Z, et al. Meta-analysis of apolipoprotein e gene polymorphism and susceptibility of myocardial infarction. PLoS One. 2014;9:e104608. doi: 10.1371/journal.pone.0104608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rai H, Parveen F, Kumar S, Kapoor A, Sinha N. Association of endothelial nitric oxide synthase gene polymorphisms with coronary artery disease: an updated meta-analysis and systematic review. PLoS One. 2014;9:e113363. doi: 10.1371/journal.pone.0113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugamura K, Keaney JF., Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbagallo I, Galvano F, Frigiola A, Cappello F, Riccioni G, et al. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid Redox Signal. 2013;18:507–521. doi: 10.1089/ars.2011.4360. [DOI] [PubMed] [Google Scholar]
- 55.Pei Q, Ma N, Zhang J, Xu W, Li Y, et al. Oxidative DNA damage of peripheral blood polymorphonuclear leukocytes, selectively induced by chronic arsenic exposure, is associated with extent of arsenic-related skin lesions. Toxicol Appl Pharmacol. 2013;266:143–149. doi: 10.1016/j.taap.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 56.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013;121:1068–1074. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen SC, Chen CC, Kuo CY, Huang CH, Lin CH, et al. Elevated risk of hypertension induced by arsenic exposure in Taiwanese rural residents: possible effects of manganese superoxide dismutase (MnSOD) and 8-oxoguanine DNA glycosylase (OGG1) genes. Arch Toxicol. 2012;86:869–878. doi: 10.1007/s00204-011-0797-8. [DOI] [PubMed] [Google Scholar]
- 58.Engstrom K, Vahter M, Mlakar SJ, Concha G, Nermell B, et al. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119:182–188. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung CJ, Hsueh YM, Bai CH, Huang YK, Huang YL, et al. Polymorphisms in arsenic metabolism genes, urinary arsenic methylation profile and cancer. Cancer Causes Control. 2009;20:1653–1661. doi: 10.1007/s10552-009-9413-0. [DOI] [PubMed] [Google Scholar]
- 60.Pierce BL, Kibriya MG, Tong L, Jasmine F, Argos M, et al. Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS genetics. 2012;8:e1002522. doi: 10.1371/journal.pgen.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agusa T, Iwata H, Fujihara J, Kunito T, Takeshita H, et al. Genetic polymorphisms in glutathione S-transferase (GST) superfamily and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol Appl Pharmacol. 2010;242:352–362. doi: 10.1016/j.taap.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Porter KE, Basu A, Hubbard AE, Bates MN, Kalman D, et al. Association of genetic variation in cystathionine-beta-synthase and arsenic metabolism. Environ Res. 2010;110:580–587. doi: 10.1016/j.envres.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 64.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, et al. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease. New England Journal of Medicine. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 65.Shiue I, Hristova K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3-19% of the population attributable risk for high blood pressure: US NHANES, 2009-2012. Hypertens Res. 2014;37:1075–1081. doi: 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- 66.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. 2012;120:98–104. doi: 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Mao G, He S, Yang Z, Yang W, et al. Relationship between long-term exposure to low-level arsenic in drinking water and the prevalence of abnormal blood pressure. J Hazard Mater. 2013;262:1154–1158. doi: 10.1016/j.jhazmat.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 68.Farzan SF, Chen Y, Wu F, Jiang J, Liu M, et al. Blood Pressure Changes in Relation to Arsenic Exposure in a U.S. Pregnancy Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang J, Liu M, Parvez F, Wang B, Wu F, et al. Association of major dietary patterns and blood pressure longitudinal change in Bangladesh. J Hypertens. 2015;33:1193–1200. doi: 10.1097/HJH.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mordukhovich I, Wright RO, Amarasiriwardena C, Baja E, Baccarelli A, et al. Association between low-level environmental arsenic exposure and QT interval duration in a general population study. Am J Epidemiol. 2009;170:739–746. doi: 10.1093/aje/kwp191. [DOI] [PMC free article] [PubMed] [Google Scholar]