Abstract
Alzheimer’s disease (AD) is a slowly progressing non-linear dynamic brain disease in which pathophysiological abnormalities, detectable in vivo by biological markers, precede overt clinical symptoms by many years to decades. Use of these biomarkers for the detection of early and preclinical AD has become of central importance following publication of two international expert working group’s revised criteria for the diagnosis of AD dementia, mild cognitive impairment (MCI) due to AD, prodromal AD and preclinical AD. As a consequence of matured research evidence six AD biomarkers are sufficiently validated and partly qualified to be incorporated into operationalized clinical diagnostic criteria and use in primary and secondary prevention trials. These biomarkers fall into two molecular categories: biomarkers of amyloid-beta (Aβ) deposition and plaque formation as well as of tau-protein related hyperphosphorylation and neurodegeneration. Three of the six gold-standard (“core feasible) biomarkers are neuroimaging measures and three are cerebrospinal fluid (CSF) analytes. CSF Aβ1-42 (Aβ1-42), also expressed as Aβ1-42 : Aβ1-40 ratio, T-tau, and P-tau Thr181 & Thr231 proteins have proven diagnostic accuracy and risk enhancement in prodromal MCI and AD dementia. Conversely, having all three biomarkers in the normal range rules out AD. Intermediate conditions require further patient follow-up. Magnetic resonance imaging (MRI) at increasing field strength and resolution allows detecting the evolution of distinct types of structural and functional abnormality pattern throughout early to late AD stages. Anatomical or volumetric MRI is the most widely used technique and provides local and global measures of atrophy. The revised diagnostic criteria for “prodromal AD” and “mild cognitive impairment due to AD” include hippocampal atrophy (as the fourth validated biomarker), which is considered an indicator of regional neuronal injury. Advanced image analysis techniques generate automatic and reproducible measures both in regions of interest, such as the hippocampus and in an exploratory fashion, observer and hypothesis-indedendent, throughout the entire brain. Evolving modalities such as diffusion-tensor imaging (DTI) and advanced tractography as well as resting-state functional MRI provide useful additionally useful measures indicating the degree of fiber tract and neural network disintegration (structural, effective and functional connectivity) that may substantially contribute to early detection and the mapping of progression. These modalities require further standardization and validation. The use of molecular in vivo amyloid imaging agents (the fifth validated biomarker), such as the Pittsburgh Compound-B and markers of neurodegeneration, such as fluoro-2-deoxy-D-glucose (FDG) (as the sixth validated biomarker) support the detection of early AD pathological processes and associated neurodegeneration. How to use, interpret, and disclose biomarker results drives the need for optimized standardization. Multimodal AD biomarkers do not evolve in an identical manner but rather in a sequential but temporally overlapping fashion. Models of the temporal evolution of AD biomarkers can take the form of plots of biomarker severity (degree of abnormality) versus time. AD biomarkers can be combined to increase accuracy or risk. A list of genetic risk factors is increasingly included in secondary prevention trials to stratify and select individuals at genetic risk of AD. Although most of these biomarker candidates are not yet qualified and approved by regulatory authorities for their intended use in drug trials, they are nonetheless applied in ongoing clinical studies for the following functions: (i) inclusion/exclusion criteria, (ii) patient stratification, (iii) evaluation of treatment effect, (iv) drug target engagement, and (v) safety. Moreover, novel promising hypothesis-driven, as well as exploratory biochemical, genetic, electrophysiological, and neuroimaging markers for use in clinical trials are being developed. The current state-of-the-art and future perspectives on both biological and neuroimaging derived biomarker discovery and development as well as the intended application in prevention trials is outlined in the present publication.
Keywords: Alzheimer’s disease, prevention trials, biomarkers, molecular imaging, neuroimaging
Introduction
A first wave of disease-modifying candidate treatments for Alzheimer disease (AD) has so far failed to demonstrate efficacy in systematic clinical trials and therefore have not gained regulatory approval. Part of the reason is considered to be due to an intervention in a too late stage of AD when pathophysiological mechanisms and irreversible neuropathological lesions of AD have largely spread through the brain (1). Therefore, prevention at earlier preclinical stages seems a promising way to decrease the incidence of this age-associated neurodegenerative disease, and its associated burden for society (2). Further roadblocks to successful development are due to shortcomings and challenges in appropriate trial design (3–5).
A biomarker (biological marker) is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (6). Biological and neuroimaging markers of AD are assumed to present central tools for prevention trials and most of them are applied in prevention trials for AD (for an overview, see Table 1). They can be divided into: (i) diagnostic markers, used to enrich, select, and stratify individuals at risk of AD; (ii) endpoint biomarkers, used as outcome measures to monitor the rate of disease progression and detect treatment effects (7), and finally (iii) markers of target engagement, used to target directly the pathophysiology of AD during the preclinical stages (8, 9). Owing to the advances in discovery, development, and validation of AD related neuroimaging and biological markers, it has now become possible to significantly improve the detection and diagnosis of AD by using a combined “multimodal” approach (10, 11). In particular, biomarkers derived from structural/functional/metabolic/molecular neuroimaging and/or neurophysiology (12, 13), and/or neurobiochemistry of cerebrospinal fluid (CSF) (14–16), blood (plasma/serum) and/or (17–19) neurogenetic markers (18, 20, 21) have been introduced. Moreover, the combination of different source biomarkers (22) is believed to make the selection of asymptomatic individuals at risk of AD possible who are a particularly attractive target population for prevention trials. The development of this scenario requires the involvement of regulatory bodies and industry stakeholders providing critical guidance in the area of AD biomarker discovery and application in prevention trials (18, 23).
Table 1.
Biological and imaging markers currently used in prevention trials
| Primary Prevention | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study (clinical Trials.gov Identifier) |
Status | Sample | Intervention | Markers of Incl/Excl criteria |
Stratification | Biomarkers as Secondary Outcome |
Indication | Ref |
| MAPT NCT00672685 | (recruitment completed) | Frail elderly | Omega-3 Fatty Acids and/or Multi-domain Intervention | MRI atrophy FDG-PET | Disease modification | (227) | ||
| SimBio NCT01142336 | (recruiting) | Asymptomatic | Simvastatin | CSF Aβ42, t-tau, p-tau181 and BDNF | Disease modification | |||
| Secondary Prevention | ||||||||
| Study (clinical Trials.gov Identifier) | Status | Sample | Intervention | Markers of Incl/Excl criteria, | Stratification | Biomarkers of Outcome | Use | Ref |
| API NCT01998841 | Phase II (recruiting) | Asymptomatic | Crenezumab | PSEN1 E280A | PSEN1 E280A | MRI atrophy rate FDG-PET amyloid PET | Disease modification | (33, 35) |
| DIAN-TTU NCT01760005 | Phase II/III Trial (ongoing recruiting) | Asymptomatic | Gantenerumab,Solane zumab, LY2886721 | PS1 PS2 carriers | PS1 PS2 carriers | MRI (cortical thickness) FDG-PET amyloid PET | Disease modification | (28) |
| PREVENT-Alzheimer | To be approved | Asymptomatic family history of AD | Naproxen, Nasal Insulin | MRI rate of atrophy | Disease modification | |||
| TOMMORROW NCT01931566 | Phase III (recruiting) | Asymptomatic | Pioglitazone | TOMM40-523 and ApoE ε4 | ||||
| AD-A4 Trial NCT02008357 | Phase III (recruiting) | Asymptomatic | Solanezumab | Positive amyloid PET | volumetric MRI SUVR CSF Abeta42 and Tau levels | Disease modification | (29, 30) | |
| NCT00597376 | Exploratory (completed) | Memory Complaints | Cerefolin NAC | Plasma Aβ42/40 ratio | Memory complaints | |||
| Tertiary Prevention | ||||||||
| Study (clinical Trials.gov Identifier) | Status | Sample | Intervention | Markers of Incl/Excl criteria, | Stratification | Biomarkers of Outcome | Use | Ref |
| NCT01055392 | Phase II (completed) | aMCI | Lithium | CSF Aβ42, total Tau, p-Tau | Target Engagement | (228) | ||
| NCT00438568 | Phase II (completed) | aMCI | Intranasal Insulin | CSF Aβ42, total Tau, p-Tau, 18-FDG-PET “Plasma biological markers” | Disease modification | (229) | ||
| NCT01595646 | Phase II (active, not enrolling) | aMCI | Intranasal Insulin | Plasma Biomarkers of AD (Aβ39, Aβ40, Aβ42), Tau (total and phosphorylated) | Disease modification | |||
| NCT01072812 | Phase 1 (Unknown) | aMCI | Posiphen tartrate | Plasma and CSF APP, A β40, A β42, AChE, BChE | ||||
| NCT01811381 | Phase II (recruiting) | aMCI | Curcumin and Yoga | Clusterin, CRP, NT-proBNP, ApoE, Aβ, VCAM1, BDNF, IL6, IL1b, IL1ra, TNFα, osteopontin. | ||||
| InDDEx study NCT00000174 | Phase IIIb Trial (completed) | MCI | Rivastigmine | Hippocampal and Whole brain atrophy rate, ventricular dilatation rate | Disease modification | (230) | ||
| NCT00620191 | Phase II Completed | MCI | Metformin | 18-FDG-PET Plasma Aβ42 | Disease modification | |||
| NCT00236574 | Phase III Trial (completed) | MCI | Galantamine | Brain and Hippocampal rate of atrophy | Disease modification | |||
| NCT00000173 | Phase III Trial (completed) | MCI | Donepezil and Vitamine E | Brain, Hippocampal, ERC rates of atrophy, ventricular dilatation rate | Disease modification | (67) | ||
| NCT00267163 | Phase IV Trial (completed) | age-associated memory impairment | Donepezil | Change in brain hypometabolism and MRI | Disease modification | |||
| Hippocampus Study | completed | Prodromal AD | Donepezil | Hippocampal rate of atrophy | Disease modification | (231) | ||
| NCT01600859 | Phase I Trial (completed September 2013) | MCI due to AD | E2609 | Positive biomarker for amyloid β | CSF Aβ42 | Target Engagement | ||
| NCT01561430 | Phase II Trial (completed August 2013) | MCI due to AD | LY2886721 | Positive amyloid PET | CSF Aβ40, Aβ42, total Tau, p-Tau and plasma Aβ40, Aβ42 | Target Engagement | ||
| NCT00890890 | Phase II Trial (completed July 2013) | Prodromal AD | BMS-708163 (Avagacestat) | CSF Aβ42 or Total Tau/aβ42 ratio | Aβ40, Aβ42, total Tau, p-Tau MRI rate of atrophy | Disease modification | ||
| NCT01953601 | Phase III Trial (ongoing recruiting) | Prodromal AD | MK-8931 | Positive [18F]flutametamol PET | Hippocampal rate of atrophy, Aβ42, total Tau, p-Tau Positive [18F]flutametamol PET | Disease modification Target Engagement | ||
| NCT01224106 | Phase III Trial (ongoing not recruiting) | Prodromal AD | Gantenerumab (RO4909832) | amyloid PET | Disease modification | |||
| NCT01767311 | Phase II Trial (recruiting) | MCI due to AD | BAN2401 | Positive amyloid PET | Hippocampal rate of atrophy, amyloid PET | Disease modification | ||
| NCT01677572 | Phase I Trial (recruiting) | Prodromal | BIIB037 | Positive at 18F-AV-45 PET scan | MRI, Change in 18F-AV-45 PET scan | Safety Disease modification | ||
Phase I trials on healthy volunteers and study with a sample size <30 were excluded. Abbreviations: MCI, Mild Cogntive Impairment; aMCI, amnestic MCI; AD, Alzheimer’s Disease; A, amyloid-; P-tau, phosphorylated tau; T-tau, total tau; MRI, Magnetic Resonance Imging; 18-FDG-PET, 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography
Here, we review the current and future role of multimodal gold-standard (“core, feasible”) biomarkers –derived from structural, functional, metabolic and molecular neuroimaging, from neurochemistry and genetics – in AD prevention trials, adding some perspectives on biomarker discovery, development, and application in the future prevention trials. In addition, regulatory issues and perspectives related to biomarkers applications in clinical trials will be discussed.
The meaning of prevention in the context of Alzheimer clinical trials
From a public health perspective, treatments as well as clinical trials of therapeutics are classified in terms of primary, secondary, and tertiary prevention interventions (24). Primary prevention aims at reducing the incidence of illness across the broad population by treating the subjects before disease onset, thus promoting the maintenance of good health or eliminating potential causes of disease. Two paradigms of primary prevention approaches are reducing population risk of illness (1) by altering environmental and cardiovascular risk factors, and (2) by using disease-specific mechanistic approaches such as polio vaccination (Figure 1). Secondary prevention aims at preventing disease at preclinical phases of illness, from progressing to clearly diagnosed disease, while tertiary prevention is focused on treating the disease when it has been clinically diagnosed and its consequences.
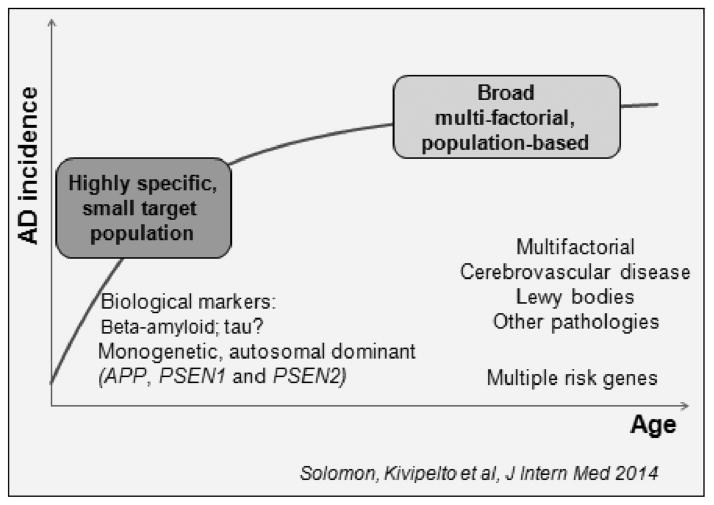
Figure 1.
Prevention approaches. The range of prevention approaches include one targeting highly specific populations (biomarker evidence for AD pathology) with specific targeted interventions (e.g. anti-amyloid). Another approach is broad, multi-factorial, population-based, and non-specific. Both approaches are needed and we should probably work more in the ‘area between’ these approaches, combining potential treatments and interventions and to various at-risk populations. (With permission, Solomon et al 2014) (24)
The above definitions are conceptually direct but they do not practically work well with the developing concepts of AD therapeutics. The traditional diagnosis of AD refers to “Alzheimer disease dementia”, that is when the illness is at the late dementia stage (25). Under these considerations, primary and secondary prevention involve delaying or impeding the onset of dementia, while tertiary prevention involves subjects already diagnosed and treated by cognitive enhancers, psychotherapeutic drugs, as well as psychosocial and environmental approaches.
In this perspective, the difference between primary and secondary prevention is whether individuals to be treated have or not signs of cognitive impairment. The recent use of biomarkers or bioscales to establish population risk or to enrich a treatment sample for those more likely than others to develop AD, together with the related evolution of clinical diagnostic constructs of ‘prodromal Alzheimer disease’ or ‘MCI due to AD (26, 27) has created a milieu in which the meaning of ‘prevention of AD’ becomes more nuanced and complex. Indeed, there is a shared clinical presentation and underlying pathobiology with both prodromal AD and AD (dementia) such that ‘prevention’ might be better considered as delaying the onset of prodromal AD or AD (27).
Secondary prevention may then focus on people who may be at particular, specific risk, have early signs of the illness, or evidence of AD neuropathology that, if further expressed, would lead to the illness. Here, the illness would be represented by the earliest stage of AD that can be accurately diagnosed, and which, currently, is represented by ‘prodromal AD’ or ‘MCI due to AD’ (any attempt to diagnose illness earlier, e.g., ‘pre-clinical’ AD would be far less certain and must rely mainly on the presence of biomarkers of AD neuropathology).
An illustrative exception is the example of the recent Dominantly Inherited Alzheimer Network Trial (DIAN-TU), involving dominantly-inherited AD neuropathology and disease caused by single gene mutations that have nearly 100% penetrance such that it appears that all people with the mutation will sooner or later develop a dementia syndrome (28). In this scenario, the consideration with respect to describing a primary or secondary prevention effort is whether or not the mutation itself without clinical signs can be considered the disease and therefore ‘preclinical AD’.
The concept of ‘primary prevention’ can be taken further by including in clinical trials subjects who are considered to have no evidence of AD pathology based on the absence of clinical signs and negative amyloid biomarker status, assuming that these individuals have a lower risk for AD than the overall population. The complementary approach, however, is selecting a sample with no clinical evidence of AD pathology but that is biomarker positive. This latter sample would have a somewhat higher actuarial risk for illness; and here treatment could be considered either primary or secondary prevention depending on whether the biomarker itself is considered as defining the pathology of AD and diagnosis of the illness (Figure 2) (24). For instance, the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial (http://a4study.org) (29, 30) selects participants with or without a memory complaint and who are PET amyloid positive for randomized treatment with an antibody targeting A β or with placebo. This study may be considered either as primary or secondary prevention trial depending on one’s interpretation of the sample selected for treatment (30–32).
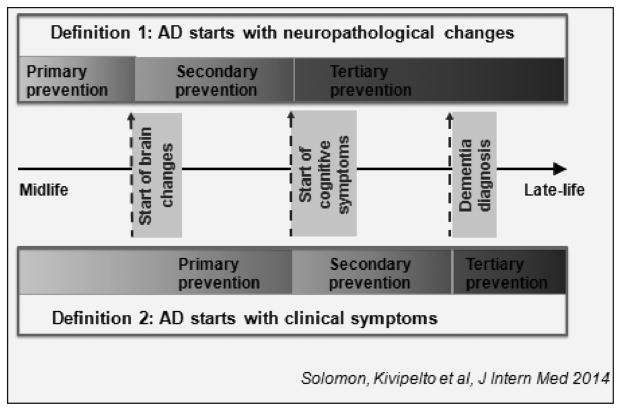
Figure 2.
How disease definition affects prevention. The figure illustrates how two alternative definitions of AD (i.e., definition 1, disease defined as starting with neuropathological changes, and, definition 2, disease starting with clinical symptoms) lead to different definitions of primary, secondary and tertiary prevention. The differences between the definitions may blur distinctions between prevention and treatment strategies. For example, if Abeta-PET positivity is considered and accepted as diagnostic for AD (i.e., pre-clinical AD) then treating such a sample would be an example of secondary prevention rather than primary (237). Alternatively, if Abeta-PET positivity is considered a risk for the future development of cognitive impairment and Alzheimer pathology then treatment would be considered as primary prevention (238, 239). These frameworks show that it is difficult to define pure primary vs secondary prevention. (With permission, Solomon et al 2014) (24)
Several current prevention trials focus on individuals who are cognitively within the normal range but are at increased risk for AD due to a mutation (28, 33), amyloid deposition in the brain (A4 trial) (30), an apolipoprotein E and TOMM40 (ApoE/TOMM40) genotype combination (TOMMORROW trial) (34), or ApoEε4 homozygous status (Alzheimer Prevention Initiative (API), Phoenix) (35). These studies have been developed to prevent the progression from normal or slightly impaired cognition to clear cognitive impairment or, in the TOMMORROW trial, to ‘MCI due to AD’ or AD. Other trials begin with patients in prodromal AD or MCI due to AD and aim at delaying the progression to AD dementia. The majority of these studies are include neuroimaging and biological markers to select target population or as secondary outcome measures. Although biomarkers are potentially useful to select clinical trials sample likely to develop AD, they are not validated as primary surrogate outcomes yet. Thus, clinical outcomes should continue to remain the primary outcomes used in preventive trials.
Finally, preventive interventions should be targeted for those most at risk by determining each individual’s or group’s risk for cognitive impairment and dementia. It may be possible to identify individuals who are relatively more likely than others to benefit from intensive lifestyle or risk-reduction changes and/or pharmacological interventions. Given the heterogeneous and multifactorial etiology of AD, preventive strategies targeting several risk factors simultaneously may be needed for an optimal preventive effect. Many modifiable risk factors (e.g. high blood pressure, obesity, physical inactivity, cigarette-smoking, and unhealthy diet) are shared among dementia/AD and other late-life chronic conditions (36). Thus, prevention agendas linking dementia and other non-communicable diseases should be developed. Because AD develops over decades, an overall life-course approach to prevention is needed. Different preventive interventions may be needed at different ages and in different contexts (37).
Structural, functional and diffusion Magnetic Resonance Imaging (MRI) markers: current applications ad future methods
Structural MRI markers
Magnetic resonance imaging (MRI) is highly versatile and, thus, multi-modality information can be acquired in a single patient examination, including those discussed in the present section. The most widely studied MRI modality is structural MRI (sMRI). In AD, cerebral atrophy – detected by sMRI – occurs in a characteristic topographic distribution (38, 39) which mirrors the Braak (40) and Delacourte (41) neurofibrillary tangles (NFT) staging. Here, atrophy begins in the medial temporal lobe and spreads to the temporal pole, basal and lateral temporal areas, and medial and lateral parietal areas (42). The primary proteinopathies associated with atrophy in AD are tau and TDP43 (43–45). Atrophy, however, does not follow the topography of Aβ nor is atrophy particularly well correlated with plaque counts Aβ or immunostaining in imaging-autopsy correlations (46, 47). Thus, sMRI is correctly viewed as a direct measure of neurodegeneration.
The location and severity of atrophy can be extracted from grey scale images by qualitative visual grading (48), by quantification of the volume of specific structures, or by measuring volume/thickness from multiple regions of interest to form AD-signature composite measures (49, 50). The most common sMRI measure employed in AD is the atrophy of the hippocampus, recently recommended by the revised criteria for AD as one of AD core biomarkers (25–27, 32, 51, 52). For this reason, international efforts to harmonize the definition of the hippocampus were carried out (53–55). Fully automated MR-based hippocampal volumetry seems to fulfill the requirements for a relevant core feasible biomarker for detection of AD associated neurodegeneration in everyday patient care, such as in a secondary care memory clinic for outpatients. Software used is partly freely available, e.g. as an SPM8 toolbox. These methods seem robust and fast and may be easily integrated into routine workflow (56).
In clinical trials, sMRI is or can be used in a variety of capacities. T2-weighted and FLAIR scans can be used to exclude patients with extensive white matter changes, where cognitive impairment might be significantly contributed by or solely due to microvascular disease (57, 58). Hippocampal atrophy has been approved by the European Medicine Agency (EMA) as a means of enriching trials in prodromal AD populations based on the observation in natural history studies that greater hippocampal atrophy predicts more rapid cognitive decline (59–64). Measures of the rate of brain atrophy have been used as endpoints based on the observation in natural history studies that atrophy rates correlate highly with the rate of concurrent clinical decline (65, 66). Of all known outcome measures (including clinical, psychometric, neuroimaging, and biofluid biomarkers), sMRI seems to have the highest measurement precision and thus has been viewed as an attractive outcome measure for clinical trials (67). However, unexpected or counter intuitive results (i.e. more rapid rates of brain shrinkage in treated subjects) in several disease modifying trials (68) have dampened the enthusiasm of some in the pharmaceutical industry for sMRI as an outcome measure. The most rational explanation for such findings, however, is that there may be first wave of short term volume losses associated with amyloid removal perhaps due to a reduction in activated microglia that were associated with plaques. If and when interventions effective on neurodegeneration will be available, sMRI may be able to map a second wave of volume loss sparing that will map onto AD-specific regions of neurodegeneration. Moreover, if/when interventions that target other aspects of the AD pathophysiological pathway (e.g. tau stabilization, or neuroprotection) will be entered into clinical trials, interest in sMRI as an outcome measure might experience a rapid resurgence. In light of this, we believe that sMRI will continue to have a role in AD clinical trials as an outcome measure.
In addition to its role as a measure of AD-related neurodegeneration, sMRI is also an important safety monitor in clinical trials. Both micro bleeds and transient cerebral edema (known as ARIAH and ARIAE respectively) have been reported in some subjects treated with active Aβ immunization and administration of anti Aβ monoclonal antibodies (68–70). ARIAH is best captured by T2* imaging and ARIAE by FLAIR imaging.
Functional MRI markers
The blood oxygenation level dependent (BOLD) signal measured with Functional Magnetic resonance imaging (fMRI) reflects primarily the local vascular response to regional neuronal activation and intracortical processing (71). At the moment the main use for the BOLD signal would be in secondary prevention trials where the signal would be used to predict conversion of MCI subjects to AD dementia. One approach is to use a cognitive paradigm that “stresses” the brain or structure that is known to be affected in the preclinical stages of the disease. For example a learning paradigm will activate the hippocampus and it has been shown to vary linearly from high to low from HC to MCI to AD dementia patient groups, respectively (72, 73). Another learning paradigm (encode face & name pairing) leads to a nonlinear response in hippocampus, with higher activation in MCI subjects compared to HC and AD dementia patients (74–77). Not only memory but also attention-related paradigms may be used as a secondary prevention biomarker such as working memory (78–80) and perceptual tasks (81–83).
Another strategy for BOLD-based biomarkers that could be used for secondary prevention trials are the intrinsic coherent networks (ICN) (84, 85). The biomarkers would be based on measures of neural network integrity, which have been shown to differentiate among HC, MCI subjects and AD dementia groups (86, 87) and also between HC groups with different amyloid loads (88, 89). Functional MRI based biomarkers could provide an approach to select patients for secondary prevention trials and to track progression from preclinical to clinical stages of the disease but also further work needs to be done to better understand the relationship between the BOLD signal and clinical changes.
As a primary prevention biomarker it still needs considerable research and development work, one of the primary issues is the potential confound between normal aging and development of AD-related pathology. Normal aging alters the potential fMRI biomarker (a recent review (90)) and alterations that are seen in MCI group (74–77) are similar due to middle aged HC with different ApoE status (91). The fMRI signal is shown to be dynamic and further investigation is required before the normal aging related changes can be separated from those due to pathology.
Based on these preliminary results, fMRI represents a promising approach for the selection and the stratification of individuals at risk of AD in clinical prevention trials.
Diffusion weighted imaging
Magnetic resonance diffusion weighted imaging quantifies the diffusion characteristics of water molecules in any tissue (92). White matter microstructure integrity can be estimated applying the tensor model to diffusion weighted images. In so doing, monocentric studies report an accuracy between 77% and 98% for diffusion tensor imaging (DTI) metrics of limbic white matter and of whole-brain voxel-based pattern classifiers (such as mean diffusivity and fractional anisotropy) in studies aimed to discriminate MCI individuals who progress and convert to AD dementia and those who remain stable over a follow-up of 1 to 3 years (93–96). DTI measures, however, are more prone to multicenter variability than classical volumetric MRI sequences (97). Despite higher multicenter variability, DTI detected predementia stages of AD with a moderately higher accuracy than volumetric MRI in a multicenter setting using machine learning algorithms (98).
Longitudinal DTI studies are still rare, indeed, individuals with MCI and AD dementia showed declining integrity of intracortically projecting fiber tracts (99–101). One study has reported a moderate effect of treatment with a cholinesterase inhibitor on fiber tract integrity in AD dementia patients (102).
According to the currently available scientific evidence, DTI will be mainly used in secondary prevention trials to predict AD dementia in individuals with MCI. Currently, evidence demonstrating the potential use of DTI to predict cognitive decline and dementia in cognitively healthy elderly individuals is not sufficient for primary prevention trials. On theoretical grounds, based on the early involvement of axonal and dendritic integrity in AD pathology, such a use seems possible but requires multicenter DTI studies to be conducted in preclinical AD. The use of DTI metrics as a surrogate of fiber tract integrity for clinical trials seems questionable to date given the high vulnerability of DTI measures to scanner drift effects over time compared to classical volumetric MRI data. Future studies are needed to further clarify this issue.
In addition to DTI metrics, tractography of diffusion-weighted imaging (DWI) represents a challenging method to study white matter organization in AD prevention trials population.
Given the dense axonal organization of white matter tissues, water molecules will be more likely to diffuse along rather than across them. Hence, by sequentially piecing together discrete estimates of the brain’s water diffusion, one might reconstruct continuous trajectory that follows the subjacent axonal organization. Using this approach, recent tractography studies identified an extended Papez circuit interconnecting essential areas dedicated to memory, emotion, and behavior (103). Indeed, axonal damage is associated with pathological behavioral manifestation (104, 105) and lead to drastic changes in the water diffusion properties that will affect the tractography reconstructions (106). Preliminary evidences have already associated discrete damage to these connections with early behavioral markers in AD (107, 108) and other dementia disorders (109). However, whether some of these anatomical changes occurred before the appearance of any behavioral signs is still unknown. It still needs to be shown if diffusion imaging tractography applied to pre-symptomatic populations may reveal exciting new footprints, which have the potential to model and predict the conversion from cognitive normality to the prodromal symptomatic stages of AD.
Utility of imaging platforms for AD prevention trials
Harmonization of image acquisition and analysis protocols is mandatory for increased statistical power and smaller sample sizes in AD prevention trials. Hence, following the seminal ADNI initiative (http://adni.loni.usc.edu), multiple regional imaging platforms have been set up (110, 111) either in the context of specific multicenter studies or as a service to any study such as the CATI multicenter neuroimaging platform (http://cati-neuroimaging.com), the neuGRID4you (https://neugrid4you.eu), the CBRAIN (http://mcin-cnim.ca/neuroimagingtechnologies/cbrain/), the LONI (https://ida.loni.usc.edu/login.jsp). The service model aims at lowering the cost of imaging technology (http://www.eurobioimaging.eu/). The first objective of these platforms is the harmonization of a network of imaging facilities, data collection, rigorous quality control and standard analysis procedures. ADNI protocols are largely embedded in this kind of activity since they have become a standard (112). The second objective is the emergence of a broader spectrum of potential biomarkers, which can stem from new imaging modalities or from “head-to-head” evaluations of new analytic methods. Finally, these platforms generate normative values for determining trial sample size and for the future clinical use of biomarkers. With regard to the challenges ahead, it is eagerly required to create a superarching organization in charge of globally synchronizing this network of platforms to proceed further with the advent of standard protocols and data sharing. It is all the more crucial that a big data perspective is probably mandatory to generate the ultimate models required for the acceptance of imaging biomarkers as surrogate endpoints.
Molecular Imaging Markers: PET FDG, Amyloid, Tau, Neuroinflammation
Positron emission tomography (PET) provides specific imaging biomarkers for early detection and diagnosis and longitudinal assessment of molecular and functional changes associated with disease progression and therapeutic interventions. An increasing number of 18F-labeled tracers are now available for use at clinical sites, not requiring an on-site cyclotron and thus turning brain PET scans into a widely applicable routine tool in dementia research. This will provide detailed insight into human pathophysiology and the effects of early interventions that until recently could only be studied in experimental animals. In this section we will address current use of molecular markers for amyloid and tau, provide an update on FDG as a functional marker, and provide an outlook on new markers for neuroinflammation and transmitters.
Amyloid-PET imaging
Several tracers with similar properties (113), including 18F-florbetapir, 18F-florbetaben, and 18F-flutemetamol, are now being included into observational studies and intervention trials. Their visual analysis in a binary fashion as amyloid positive or negative has been thoroughly validated by post-mortem pathological assessment in AD (Figure 3 shows an example of PET amyloid uptake in controls and AD) (114). Although results are promising, methods for quantitative analysis have not yet reached the same degree of standardization, and more research is needed to understand inter-individual and longitudinal changes.
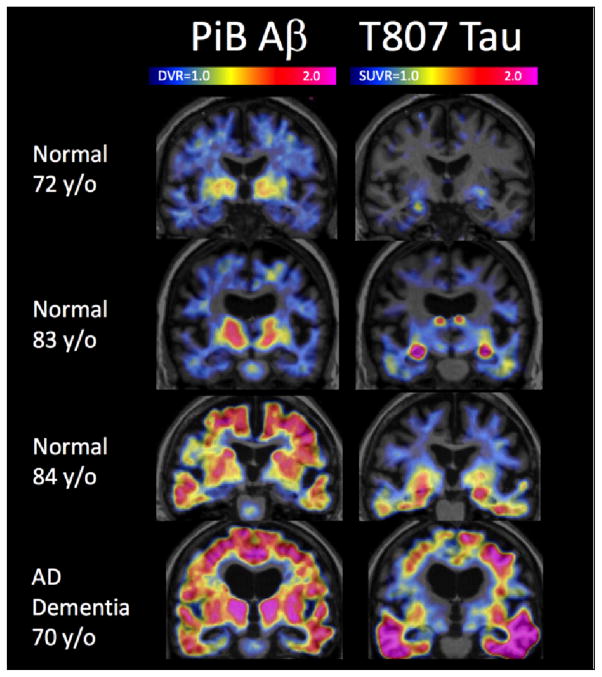
Figure 3.
Positron Emission Tomography Staging of AD pathology. Coronal Positron Emission Tomography images (overlaid with structural Magnetic Resonance) of PiB Aβ (left column) and T807 Tau (right column) acquired from 3 normal individuals (top 3 rows) and a patient with AD dementia (bottom row). Low levels of amyloid are seen in the top 2 cases and high levels in the bottom 2. T807 binding is particularly striking in medial temporal lobe in the middle 2 normal cases, possibly corresponding to Braak Stage III/IV, but is more intense and widespread in the AD dementia case, which is consistent with Braak V/VI
Several important prevention trials on autosomal-dominant AD (ADAD) and late-onset AD (LOAD) incorporating PET amyloid are currently on going (Table 1). The role of PET amyloid in the studies investigating the effect of monoclonal anti-amyloid antibodies varies from that of a primary outcome measure (one arm of DIAN-TU), to secondary outcome measure (API), to screening tool necessary to meet inclusion criteria (A4). In the A4 study, eligible participants must show evidence of elevated amyloid on both a semi-quantitative SUVr measurement and a qualitative binary visual read of a florbetapir PET scan. Amyloid PET is also being utilized as an exploratory outcome measure in A4, along with Tau PET (T807) in a subset of participants in the A4 study. A4 will also include an observational cohort with a group of participants who fell just below the threshold for amyloid eligibility for A4 to determine the factors that predict rapid amyloid accumulation, as these individuals may be ideal candidates for future secondary prevention trials aimed at slowing the production of amyloid-beta.
Tau-PET imaging
In addition to amyloid-beta, deposits of hyperphosphorylated tau are the other main defining neuropathologic feature of AD. Until recently measurement of brain tau deposition has not been possible during life. Several PET ligands highly selective for tau deposits have now been applied to imaging of individuals along the AD spectrum, from cognitively normal to AD dementia. Initial experience with these ligands at a small number of centers (115, 116) indicates that binding is detected in the anatomic areas expected from AD pathology according to the ordinal Braak staging scheme (Figure 3). Thus, binding is observed in medial temporal areas in most cognitively normal older individuals, in additional limbic and neocortical regions among individuals with established AD-like cognitive impairment, and in more widespread neocortical regions among those with AD dementia. While within-subject longitudinal change in tau ligand binding has not yet been reported, the initial experience at the Massachusetts General Hospital in over 200 subjects using 18F-T807 PET suggests that the characteristics of this PET measure are potentially well suited for use in AD prevention trials. This new technology could potentially be used in clinical trials both to stage AD pathology and as a therapeutic endpoint.
FDG-PET imaging
While tracers for amyloid-beta and tau provide images of key pathological protein deposits, 18F-2-fluoro-2-deoxy-D-glucose (FDG) has already been used over many years as a functional marker of cortical synaptic dysfunction for diagnosis (117) and in clinical trials (118). Considerable progress has been made in recent years to derive quantitative biomarkers from FDG scans (119), while further standardization of analysis methods and longitudinal characterization of reference samples is still ongoing.
When applied to Mild Cognitive Impairment (MCI), FDG PET provides a good predictor of progression within the next 2 years (120), while markers of amyloid-beta and tau tend to become positive up to 20 years before actual onset of dementia. Recent studies comparing FDG and amyloid PET have revealed a substantial proportion of patients with amnestic MCI who have impaired FDG uptake while amyloid scans are negative (121). Contrary to the uniform sequential model of disease progression they show a relatively high rate of progression to dementia, and further research is required to clarify which type of dementia they actually suffer from. Considerable heterogeneity of AD subtypes and progression rates is well known from retrospective pathological studies (122), and longitudinal multimodal imaging studies including FDG are expected to provide better predictors and thus improve the efficacy of early intervention studies.
Inflammation- and receptor-PET imaging
Neurodegenerative diseases, including AD, are associated with activation of microglia. This leads to increased mitochondrial expression of the 18-kDa translocator protein (TSPO), which can be imaged using (R)-[11C]PK11195. Recent studies (123, 124) have partially confirmed earlier findings of increased cortical binding potential in AD, but this increase could not be detected in individual patients and was much weaker than the signal on amyloid PET (125). In addition, (R)-[11C]PK11195 was not able to separate clinically stable prodromal AD patients from those who progressed to dementia, and there was no correlation with cognitive function.
More recently, many new TSPO ligands have been developed (126), and TSPO has also been identified as a potential drug target (127). In particular, studies using [11C]PBR28 have shown a signal that correlates with cognitive performance (128), providing a means for detecting changes early in the disease process. However, a major disadvantage of many new TSPO ligands is that, due to genetic polymorphism (129), a subpopulation of subjects will not show binding. There is a need for TSPO ligands that provide high signal, but are insensitive to this polymorphism. In addition, PET ligands for other molecular targets related to neuroinflammation, e.g. monoamine oxidase B located in astrocytes (130), are being investigated. AD is associated with failure of cholinergic neurotransmission, but its relation to clinical symptoms and disease progression is still poorly understood. Thus, ongoing research into development of suitable PET tracers (131) may allow future studies on the relation between pathological protein deposition and their functional interactions and consequences.
Value of multimodal imaging in prevention trials
With regard to preventive strategies of AD, in vivo multi-modal neuroimaging biomarkers may play an important role with regard to early and reliable detection of subjects at risk and to allow measuring of success/improve understanding of failure of therapeutic concepts. In this context, multimodal neuroimaging approaches are expected to be advocated on the basis of several important facts: (i) neurodegeneration in AD cannot be reduced to a singular pathological process in the brain. A number of different neuropathologies are known to be crucially involved in the development of this disorder and the causal interaction between these pathologies is not yet fully understood; (ii) it is well accepted that the onset of development/appearance of the mentioned pathologies in the brain may occur subsequently not simultaneously. Consequently, the presence/detectability of these pathologies depends on the stage of disease; (iii) it has been demonstrated that the temporal development of these different pathologies over time is neither linear, nor parallel to each other (132–134).
These facts explain the potential of multimodal imaging approaches. Several of the characteristic forms of neuropathology known to be involved in AD such as protein aggregation (Aβ and tau), synaptic dysfunction, inflammation and neuronal loss/brain atrophy can be captured using in vivo imaging procedures. However, not a single one out of these pathologies is fully specific for AD (i.e. they can be found in other forms of neurodegeneration as well). Thus, in recent guidelines on the diagnosis of AD, improved diagnostic certainty or increased risk for underlying AD has been proposed for a combination of different disease biomarkers (32). These guidelines divide between markers of Aβ peptides aggregation pathology (including amyloid PET imaging) and markers of neuronal injury (including structural/volumetric MRI and FDG-PET imaging). The authors suggest that cumulative evidence obtained by biomarkers out of these two categories increases the probability for ongoing AD even in preclinical stages. This directly applies to the detection of subjects at risk for AD, e.g. in prevention trials.
It is well accepted that amyloid-pathology may be detectable in the brain of subjects suffering from AD long before clinical symptoms occur and, possibly, also ahead of detectable neuronal injury. However, little is known so far about the time to symptomatic onset in amyloid-positive subjects without cognitive deficits. Furthermore, it has been demonstrated that amyloid-deposition seems to reach a plateau in later stages of AD, whereas markers of neuronal injury seem to better mirror the continued progression of cognitive decline. Consequently, only a multimodal combination of information on amyloid-pathology and neuronal injury may allow a reliable in vivo disease staging, particularly ahead of clinical disease onset.
Generally, the classification of disease biomarkers into only 2 categories may represent an oversimplification (135). Depending on the type of prevention approach, higher resolutions of disease stages may be possible and the spectrum may be completed with other available imaging biomarkers, e.g. of tau-aggregation, inflammation, connectivity or receptor status (136–139).
With regard to therapy monitoring or measuring success of any prevention methods, any one-dimensional biomarker assessment may fall short. With regard to the dynamic non-linear and non-parallel natural courses of the different neurodegenerative pathologies over time, relevant changes may be overlooked and inter-patient differences may be interpreted incorrectly. Furthermore, interventions may influence single parameters without effect on other pathologies, e.g. inhibit amyloid-aggregation pathology without slowing down the ongoing cascade of neuronal injury.
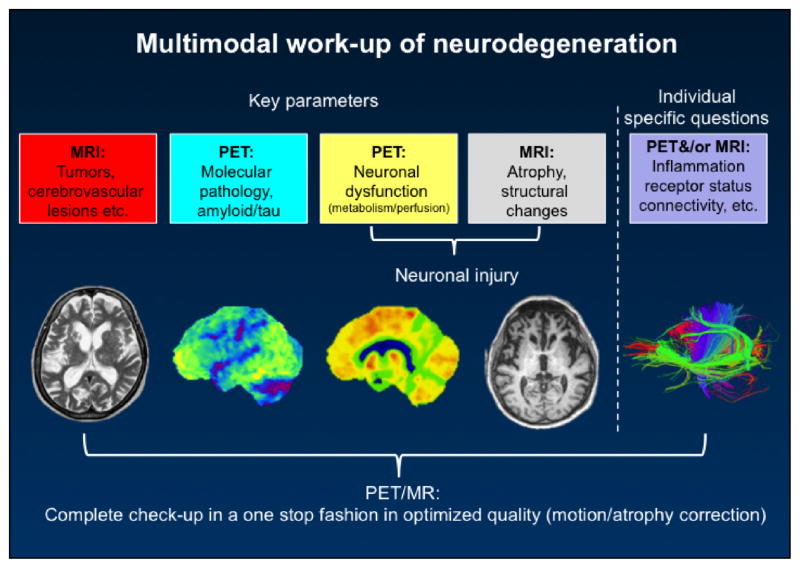
The recent introduction of PET/MR technology may represent the ideal tool for multimodal imaging approaches, particularly in longitudinal prevention trials. The systematic combination of complementary MRI and PET-methods may offer a number of advantages leading to the optimal diagnostic assessment and disease quantification with the least possible burden for the patient (Figure 4). Suitable PET/MR examination work-flow protocols have already been published for the assessment of neurodegenerative disorders (140). In short, such protocols may allow for acquisition of data in high quality (motion and partial volume corrected), providing information on neuronal dysfunction, protein aggregation pathology and atrophy and at the same time exclude non-neurodegenerative diseases in a single patient visit.
Figure 4.
Multimodal work-up of neurodegeneration, opportunities for combined Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI)
In summary, multimodal imaging assessment of different types of neuropathology might be designated as the method of choice for a reliable and specific detection and quantification of AD in vivo, and, thus, represent the approach of choice for prevention strategies.
Established and potential CSF biomarkers
At present, there are three gold standard (“core feasible”) CSF biomarkers for AD molecular pathology: total tau protein (T-tau) that reflects the intensity of neuronal/axonal degeneration, hyperphosphorylated tau protein (P-tau) that probably reflects neurofibrillary tangle pathology and the 42 amino-acid-long form of amyloid β (Aβ1-42) that is inversely correlated with Aβ pathology in the brain (low lumbar CSF levels reflect sequestration of the peptide in the brain parenchyma) (141). The biomarkers detect AD with an overall accuracy of 85–95% in both dementia and MCI stages of AD and appear to switch to pathological levels 10–20 years before the first symptoms become recognizable (142). Recently revised diagnostic criteria for AD suggest that biomarkers for both tau and Aβ pathology should be positive if an AD diagnosis is to be made (27). Here, CSF provides a biomarker source covering both these aspects and the assays for T-tau, P-tau and Aβ1-42 are currently undergoing standardization for such use; the most important international standardization efforts being the Alzheimer’s Association Quality Control program for CSF biomarkers (143, 144), the Alzheimer’s Association Global Biomarkers Standardization Consortium (GBSC) (145) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group for CSF Proteins (WG-CSF) (145). Standard operating procedures (SOPs) for CSF sampling and storage have been published (141). As an outcome from the IFCC WG-CSF and the GBSC, the Single-Reaction Monitoring (SRM) mass spectrometry candidate Reference Measurement Procedures (RMP) for Aβ1-42 has been published (146), and certified reference material is being developed. These will be used to harmonize measurements between assay formats and to assure longitudinal stability and minimize batch-to-batch variations, and thereby serve as the basis for the introduction of uniforms cut-off values and a more general use of CSF biomarkers in clinical routine and trials. Updates on the work within the GBSC are available at: http://www.alz.org/research/funding/global_biomarker_consortium.asp.
Recent data show that it is possible to identify longitudinal changes in CSF A β1-42, T-tau and P-tau in cognitively healthy controls followed with multiple lumbar punctures over several years (147–149), but most studies (with exceptions (147)) show that CSF AD biomarkers are essentially stable in symptomatic AD (150–152). This biomarker stability may be useful in clinical trials to help identify effects of interventions, both on the intended biological target, such as altered Aβ metabolism in response to an anti-Aβ treatment (18). One of the truly longitudinal studies of cognitively normal individuals with repeated CSF samples suggests that Aβ1–42 and T-tau changes occur in parallel and predict upcoming cognitive symptoms better than absolute baseline levels (149). CSF measurements may track trajectories of specific Aβ and APP metabolites (153–156), and down-stream effects on secondary phenomena, such as reduced axonal degeneration in response to a disease-modifying drug as measured by CSF tau levels (157, 158). So far, unfortunately, these changes have not predicted clinical benefit of any anti-AD drug (159).
In addition to T-tau, some CSF biomarkers reflecting neuronal and axonal damage, including visinin-like protein 1 (160) and heart-type fatty acid-binding protein (H-FABP) (161) show a clear increase in AD and correlates with CSF t-tau. Further, a number of novel biomarkers that should be relevant to the disease process in AD are under development. These include markers of synaptic degeneration (e.g. the dendritic protein neurogranin (162)), microglial activation (e.g. chitinase-3-like protein 1, CHI3L1, also called YKL-40 (163)) and protein homeostasis/lysosomal dysfunction (e.g. lysosomal-associated membrane proteins 1 and 2, LAMP-1 and LAMP-2 (164)). An overview of CSF biomarkers and their interpretation in the scenario of AD prevention trials is reported in Table 2.
Table 2.
Cerebrospinal fluid biomarkers in prevention trials
| Application | Method | Biomarkers | Interpretation |
|---|---|---|---|
| Identification of individuals with AD pathology | CSF samples from individuals analyzed during the screening period for enrolment into a clinical trial | Aβ42, T-tau, P-tau | Low Aβ42 is indicative of cortical AD Aβ deposition, and is likely the first CSF biomarker that become positive. The combination of low CSF Aβ42 together with high T-tau and P-tau is indicative of AD, and may thus be used for enrichment of early AD in the trial. |
| Theragnostics | CSF samples taken before study initiation and at time-points during the trial including end-of-study | Aβ42, Aβ40, sAPPβ | The amyloid biomarkers may provide evidence of target engagement of an anti-Ab drug candidate, e.g. a BACE1 inhibitor. |
| P-tau | A change towards normalization in CSF P-tau may provide evidence of an effect of a drug candidate on tau phosphorylation state or tangle formation. | ||
| T-tau, H-FABP, VLP-1 | Downstream biomarkers, e.g. T-tau, may provide evidence of an effect of a drug candidate on the on the intensity of neuronal degeneration. Biomarkers not directly involved in AD pathogenesis, e.g. H-FABP and VLP-1, may give complementary information to T-tau. | ||
| Synaptic proteins | A change in synaptic biomarkers, e.g. neurogranin, may provide evidence of an effect of a drug candidate on synaptic function and degeneration. | ||
| Inflammation and microglial activity | A change in CSF biomarkers reflecting microglial activity, e.g. YKL-40, may additional evidence of downstream drug effects. |
Abbreviations: Ab, amyloid-b AD, Alzheimer disease BACE1, b-site APP cleaving enzyme 1 CSF, cerebrospinal fluid H-FABP, Heart fatty acid-binding protein MRI, magnetic resonance imaging PET, positron emission tomography P-tau, phosphorylated tau sAPP, soluble amyloid precursor protein extracellular domain T-tau, total tau VLP-1, visinin-like protein-1
There is also a critical need for biomarkers to identify co-morbidities, including blood-brain barrier dysfunction, cerebrovascular disease, and Lewy body and TDP-43 pathologies, that could resemble or aggravate AD.
Evolving blood biomarkers
The identification of blood-based biomarkers that have utility in clinical trials for AD is of great importance (165), as they have been recently included as secondary outcome measures in many ongoing trials (Table 1). Blood-based biomarkers and biomarker profiles have been shown to be highly accurate in detecting and discriminating amongst neurodegenerative diseases (19, 166–169) and may serve as a cost-effective first step in a multi-stage screening process for clinical trials (17). As an example, Kiddle (166) and colleagues recently cross-validated the link between 9 markers from previously published studies and AD-related phenotypes across independent cohorts using an independent assay platform (SOMAscan proteomic technology). Recently, O’Bryant and colleagues (168) also cross-validated a serum-based biomarker profile using an independent assay platform (Meso Scale Discovery; 21-protein profile AUC=0.96; 8-protein profile AUC=0.95), across species (mice and humans) and tissues (serum and brain tissue). The proteomic profile approach was also able to extend further and accurately discriminate AD from Parkinson’s disease (168). If demonstrated effective in primary care settings, these blood-based profiles for detection of AD could provide access to clinical trials far beyond what is currently available through specialty clinic settings (168). Additionally, blood-based approaches have been shown capable of detecting Aβ burden (170, 171). Using data from the Australian Imaging, Biomarkers and Lifestyle (AIBL) cohort, a plasma proteomic signature consisting of chemokine 13, IgM-1, PPY, VCAM-1, IL-17, Aβ42, age, ApoE genotype and CDR sum of boxes yielded an AUC=0.88 in AIBL and an AUC=0.85 when applied to the ADNI cohort. The existence of a blood-based screener for Aβ positivity would provide a cost-effective means of screening patients into trials requiring Aβ positivity on PET scans (17, 170).
Preliminary work also suggests that blood-based profiles can identify patients at risk for progression from MCI to AD (172,173) as well as from cognitively normal towards some level of cognitive impairment (174, 175). Along these lines, recent work identified a 10-protein (plasma) algorithm (TTR, clusterin, cystatinC, A1AcidG, ICAM1, CC4, pigment epithelium-derived factor, A1At, RANTES, ApoC3) that when combined with ApoE genotype predicted progression from MCI to AD with an optimal accuracy of 87% (sensitivity = 0.85, specificity = 0.88) (172). Mapstone and colleagues (174) also provided preliminary data suggesting that a set of 10 lipids can predict progression from control to MCI/AD over a 2–3 year period. Kivipelto and colleagues (37) generated a risk score from the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study consisting of ApoE genotype, total cholesterol, systolic and diastolic blood pressure, demographics (age, education, gender), and lifestyle (smoking status, Body Mass Index [BMI], physical inactivity) factors that predicted increased risk for dementia over a 20-year period. Each of these methods has potential use in the identification and selection of patients into novel preventative and therapeutic clinical trials. Blood-based biomarkers can be also employed for patient stratification in trials. For example ApoE ε4 which is the strongest risk factor for AD and correlates well with CSF Aβ1–42 levels and increased amyloid burden and has been used for patient stratification into clinical trials (e.g. ClinicalTrials.gov; identifiers: NCT00574132 and NCT00575055). Recent data also suggests serum/plasma ApoE protein levels are lower among ApoE carriers (169) and that plasma ApoE levels correlate with amyloid PET (176). Therefore, serum/plasma ApoE protein and ApoE genotype may be useful in patient stratification for trials (165). Crenshaw and colleagues (177) generated a patient stratification algorithm based on ApoE ε4 genotype and the TOMM40 gene. Risk stratification per this algorithm assigns all ApoE ε2/ε2 and ε2/ε3 carriers to the low risk group with all ApoE ε4 carriers then assigned to the high risk group. Next, for all non-ApoE ε2 carriers, risk stratification varied by TOMM40 genotype and age. This risk stratification scheme was designed for a preventative trial targeting Pioglitazone for the prevention of cognitive loss (177). Moreover, prior work has suggested that blood-based biomarkers can be utilized for the identification of AD-based endophenotypes (17, 167, 178) with additional work needed to determine if these endophenotypes can predict which groups of patients are more likely to respond to specific interventions (165). Recent findings presented at the Alzheimer’s Association International Conference (AAIC) suggest this is a promising line of investigation. As has been pointed out previously, additional work is needed regarding harmonization of methods for this work to progress (17, 179) with the first guidelines for pre-analytical methods now available (180).
Genetic tests and risk factors for Alzheimer’s disease
AD occurrence and evolution, as for most complex chronic diseases, result from the interactions between environmental factors and an individual susceptibility. The very first genetic determinants have been described for rare hereditary early onset clinical forms almost 25 years ago: the Aβ precursor protein gene (APP), the presenilin 1 (PSEN1) and the presenilin 2 (PSEN2). These three loci were rapidly followed by the discovery of strong and consistent associations of the apolipoprotein E (ApoE) isoforms with late-onset AD. Then, it is only during the last five years, and thanks to large-scale international collaborations such as the AlzGene database (http://www.alzgene.org) (181) and high throughput genotyping progresses, that the deciphering of the genetic susceptibility to sporadic AD has rapidly progressed, leading to the identification of 20 confirmed loci, and of 16 putative others (182). The population attributable risk/preventive fractions of each of these loci vary from 27.1% for the ApoE ε4 allele to less than 2% (Table 3). This allows for the establishment of a more precise picture of the genetic susceptibility background associated with the occurrence of late-onset AD, adding to the list of biomarkers a new tool, useful for AD diagnosis and prognosis.
Table 3.
Population attributable/preventive fractions of AD loci (182)
| SNP | Gene | MAF | PAF(%) | Effect type |
|---|---|---|---|---|
| ε4 allele | ApoE | 0.123 | 27.3 | risk |
| rs6733839 | BIN1 | 0.366 | 8.1 | risk |
| rs10792832 | PICALM | 0.365 | 5.3 | preventive |
| rs9331896 | CLU | 0.398 | 5.3 | preventive |
| rs35349669 | INPP5D | 0.462 | 4.6 | risk |
| rs983392 | MS4A6A | 0.406 | 4.2 | preventive |
| rs6656401 | CR1 | 0.191 | 3.7 | risk |
| rs1476679 | ZCWPW1 | 0.293 | 3.2 | preventive |
| rs9271192 | HLA | 0.277 | 3.2 | risk |
| rs11771145 | EPHA1 | 0.350 | 3.1 | preventive |
| rs28834970 | PTK2B | 0.358 | 3.1 | risk |
| rs2718058 | NME8 | 0.368 | 2.9 | preventive |
| rs4147929 | ABCA7 | 0.162 | 2.8 | risk |
| rs190982 | MEF2C | 0.389 | 2.7 | preventive |
| rs10838725 | CELF1 | 0.312 | 2.4 | risk |
| rs10948363 | CD2AP | 0.255 | 2.3 | risk |
| rs10498633 | SLC24A4/RIN3 | 0.212 | 1.5 | preventive |
| rs17125944 | FERMT2 | 0.079 | 1.5 | risk |
| rs11218343 | SORL1 | 0.044 | 1.1 | preventive |
| rs7274581 | CASS4 | 0.088 | 1.1 | preventive |
However, the use of this information in current clinical practice still remains limited. In the dominant early onset hereditary forms, when a causal mutation can be identified (in half of these early onset forms), presymptomatic genetic testing could be performed following the protocols issued from the Huntington disease experience by the World Federation of Neurology (183). In late-onset AD, despite a high attributable fraction, the ApoE ε4 allele is not recommended for diagnosis because of its low sensitivity and specificity. Conversely, in clinical and translational studies, genomic biomarkers are of the utmost interest. For instance, when studying AD cases, ApoE ε4 allele is now a common risk factor to systematically register, adjust and stratify on, as age, gender and educational level. Today, it is a major requirement to collect DNA in any clinical study or drug trial and the decreased costs of sequencing offer a unique opportunity to access the genetic susceptibility information of each enrolled individual.
The characterization of the 40 known susceptibility locus genotypes constitutes a major biomarker that can be usefully added to CSF biological measurements and PET imaging. This information helps to stratify the heterogeneity of AD clinical forms and identify specific subgroups with different disease evolution and therapeutical answers. This pharmacogenomics stratification based on the potential biological pathways underpinned by the specific genetic background of each patient, helps to better understand the possible mechanism of action of drugs. In primary and secondary AD prevention trials including asymptomatic patients, the identification of this genetic susceptibility allows to select individuals with the highest risk and the very best chances to benefit from these preventive approaches, improving the statistical power of such studies.
The access to genomics information plays also a major role in the discussions about the efficiency of active and passive anti-Aβ immunotherapies in AD treatment (184). Genomics offer the best opportunity to identify presymptomatic individuals with AD causal mutations or at very high risk of developing AD to better appreciate the potential curative interest of these drugs at a stage where the resilience of cognitive functions is still possible. Thus, the DIAN-TU consortium has initiated a phase II/III randomized, double-blind, placebo-controlled multi-center study of two potential disease modifying therapies in presymptomatic mutation carriers and their non-carrier siblings; a prevention trial is also conducted in 300 symptom-free individuals 30 years of age and older from a large Colombian family with a mutant gene (PSEN1 E280A) and another one in volunteers aged 60 to 75, homozygous for the ApoE ε4, without cognitive impairment is in preparation (35). Considering the increasing knowledge and dissemination of these biomarkers based on genetic information, ethical concerns must be carefully taken into account, especially as direct-to-consumer tests develop for diseases as AD where no therapeutic solution is available yet.
Novel Advances and Research Frontiers : High-field MRI, and neurophysiological EEG-MEG markers
High-field of MRI such as (3T and higher) and ultra-high fields (7T and higher) as well as EEG-MEG techniques push further the possibilities of developing new biomarkers able to select and to monitor the disease in primary prevention trials.
High-fields of MRI: 3T MRI is widely available for clinical trials and the number of ultra-highfield 7T scanners is increasing rapidly as well, with about 40 7T scanners for humans currently installed worldwide (185).
An important contribution of high-field MRI to AD biomarkers is the possibility to measure hippocampal subregions. Indeed, hippocampal subparts show distinct vulnerability to the AD pathological process, as demonstrated by neuropathological studies (186). Such measurements are usually based on T2-, T2*- or proton-density-weighted sequences with high in-plane resolution (about 200μm–500μm). At 3T/4T, it is possible to detect atrophy in different hippocampal subfields, such as CA1 and the subiculum (187, 188). 7T MRI provides higher contrasts, increased signal-to-noise ratio and higher spatial resolution, which dramatically improve the visualization of hippocampal subregions. This makes it possible to quantify the atrophy of distinct hippocampal layers associated with AD, such as the stratum pyramidale and the strata radiatum, lacunosum and moleculare (SRLM), and not only subfields (189–191). These measures have the potential to provide more sensitive and specific biomarkers than global hippocampal volumetry but require further validation in larger samples.
Another important area of research is the detection of amyloid plaques using high-fields MRI. Such detection has been demonstrated in transgenic mouse models of AD (192, 193), as well as in non-transgenic mouse lemur primates in which plaques are more similar to those formed in humans (194). In vivo detection in humans of amyloid plaques by high-fields MRI is an important challenge for the upcoming years and might open promising scenario in prevention AD trials.
Ultra-high-field MRI also improves the assessment of vascular burden associated with AD. Cerebral microbleeds are often found in patients with AD and are likely to be due to frequent association between AD and cerebral amyloid angiopathy. 7T MRI, using T2*-weighted sequences or susceptibility weighted imaging (SWI), provides increased sensitivity to detect cerebral microbleeds (195, 196). 7T can also improve in vivo detection of microinfarcts. A recent 7T study reported an increased number of microinfarcts in AD patients compared to controls 197 while another study reported no difference (198).
Electroencephalography (EEG) and magnetoen cephalography (MEG) modalities (199, 200) are complementary techniques to high-field MRI due to their ability to detect the dynamic behavior of neuronal assembly circuits in the brain and to provide non-invasive time-dependent capabilities with sub-millisecond precision, especially in regard to cortical structures. Two main EEG/MEG biomarker approaches have emerged in using these techniques in AD research–evaluation of localized measures and inter-area connectivity indices (201). Localized neurodynamics biomarkers, such as band power or signal strength/phase, can characterize the change of the dynamic state of a brain area either through spontaneous brain oscillations or event-related activity (202). Evidence points to abnormal slowing of faster alpha and beta cortical rhythms especially in posterior regions and increase of slower delta- and theta-band activity in AD (203). Short- and long-range connectivity estimates, on the other hand, offer high sensitivity to evaluate the integrity of brain pathways or reduction of central cholinergic inputs, if employed properly (204). EEG/MEG connectivity biomarkers have revealed the existence of an entire new class of approaches able to manifest, for example, impaired functional synchrony in the upper alpha and beta bands in AD (205), and declining global synchronization in all frequency bands (206). While the full potential of EEG (207) and MEG (208) biomarkers to characterize degenerative brain changes for primary AD prevention has yet to be realized, a substantial number of studies have demonstrated results compatible with secondary prevention trial strategy. Although numerous studies have investigated the feasibility of EEG/MEG biomarkers in varying degrees, they still could be considered an emerging approach in AD trials, and especially in prevention trials, due to the complexity and multidimensionality of the observed dynamic signals, as well as the need to achieve a converging consensus among studies for better understanding of the disease pathology and its time-dependent aspects.
Regulatory Requirements and evolving challenges
As there is now consensus that effective therapies for AD have to start very early in the disease process after the many failures of development programs, European Medicines Agency (EMA) and food and drug administration (FDA) are reacting to these changes. FDA and EMA suggest potential approaches to clinical trial design and execution that allow for regulatory flexibility and innovation (209, 210). It is outlined that clinical diagnosis of early cognitive impairment might be coupled with specific appropriate biomarkers reflecting in vivo evidence of AD pathology. New diagnostic criteria addressing these issues have been established and are under validation by various working groups (18, 26, 27, 211, 212). Most biomarkers include brain Aβ and Tau load, as measured by PET and CSF levels of Aβ and tau proteins (22, 213), however, there is a clear move to update the amyloid hypothesis and to look for new biomarkers for the different disease stages (214, 215).
However, adequate standardization and validation of these biomarkers for regulatory purposes is still lacking as described by Noel-Storr and colleagues (2013) (216). As far as the CSF biomarkers are concerned, it was recently reported that the overall variability of data coming from a total of 84 laboratories remains too high to allow the validation of universal biomarker cut-off values for its intended use (217), which underpins the urgent need for better harmonization and standardization of these methods.
The use of biomarkers as endpoints in earlier stages of drug development is well established for regulators, and there are examples to approve medicinal products on the basis of their effects on validated surrogate markers, eg, anti-hypertensives, or cholesterol-lowering products. However, these examples have been considered as validated surrogate markers as they allow substitution for a clinically relevant endpoint. In their validation a link between a treatment-induced change in the biomarker and long-term outcome of the relevant clinical measure was undoubtedly established. Therefore the regulatory requirements on biomarkers used as endpoints in clinical trials are high as outlined earlier (210). In consequence EU regulators help applicants in their research and development by issuing opinions on the acceptability of using such biomarkers or a distinct methodology in clinical trials. Since 2011, EMA’s Committee for Medicinal Products for Human Use (CHMP) has adopted and published several qualification opinions for use in the development of medicines for AD. In these qualification opinions biomarkers are accepted for identification and selection of patients at the pre-dementia stage of the disease as well as for selection of patients for clinical trials in mild and moderate AD. In September 2013, a qualification opinion for a novel model of disease progression and trial evaluation in mild and moderate AD was adopted by CHMP. The simulation tool is intended to provide a quantitative rationale for the selection of study design and inclusion criteria for the recruitment of patients.
The EMA guideline on the clinical investigation of medicines for the treatment of AD will be updated on the basis of new knowledge obtained from the validation of the new diagnostic criteria, the use of biomarkers in clinical evaluation and other recent trends in research and development. A first draft will be available soon, in a 2-day workshop later this year the draft will be presented and discussed with the involved stakeholders. The final guidance should help regulators and industry to decide on the most appropriate study design for the distinct stages of AD, particularly in its early preclinical/prodromal stage.
Conclusions & perspective on a decade-long initiative on prevention
The discovery-validation of a broad spectrum of interventions, including pharmacologic, behavioral and life-style treatments, remains a crucial global public policy objective (218–222). Although a series of clinical trials for treating AD dementia have failed during the last two decades, these setbacks have not deterred the confidence of investigators in pursuing the strategic goal of acquiring disease-modifying treatments, which would ameliorate the progression of neurodegeneration with the eventual aim of preventing the onset of symptoms. The optimism of the scientific community, regarding the technical feasibility of discovering strategies to slow or halt neurodegenerative process is conditional, predicated by the availability of adequate resources and our capabilities to surmount the major barriers that are hindering progress of research on prevention. In this scenario, as emerged from the current review, the role of neuroimaging and biological markers is crucial. In particular, they are involved in the future development of technologies algorithms identifying the better combination able to detect accurately the early stages of disease or the prognosis in asymptomatic people at elevated risk. Moreover, they could be essential to select sample of prevention trials and, ultimately, they might be employed as surrogate measure to assess drugs treatment efficacy.
Some of the critical challenges need to be addressed in order to accelerate the pace of Research and Development (R&D) of interventions for prevention.
The first challenge refers to the development of new paradigms and conceptual models for R&D on therapies. The sequential failure of clinical trials based on prevailing theories on dementia along with emerging new knowledge about the complexity of the biology underlying the disease has created the need to re-assess our assumptions about its etiology and the adoption of new paradigms for therapy development. At the present, there is growing consensus that AD is a heterogeneous disorder, a syndrome rather than a disease, with polygenic origins where multiple putative risk factors influence the prolonged progression of neurodegenerative processes. These biological features will require radically different thinking and new approaches to therapy development. In particular, the adoption of concepts from ‘systems theory’ might be well suited for guiding the formulation of new conceptual models for teasing out the complexities of this disease.
The second challenge addresses the issue of developing technologies to accurately detect individuals at elevated risk – among asymptomatic populations. Indeed emerging knowledge showed that the cellular and molecular mechanisms leading to neurodegeneration start decades prior the onset of clinical symptoms of AD. For this reason, prospective prevention trials in the future will require the employment of treatments in the earlier asymptomatic or prodromal phases of neurodegeneration. Presently, crucial rate-limiting factors, which hinder the launch of true prevention trials are: (i) the lack of well-validated technologies for identification of asymptomatic people at elevated risk for the disease; (ii) the need for a reliable measure of disease progression – i.e. a surrogate marker allowing for precise tracking of one or more biological indices of the neurodegenerative process.
The third critical challenge to consider is the need for novel original therapeutic targets, new molecules and paradigms for efficacy validation. In this context, the strategic goal is to enrich the drug discovery pipeline by investigating a wide array of options for therapy development. Notably, this issue may have been exasperated by the limitations of current theories, conceptual models, or even ideas about the pathogenesis of AD and dementia disorders, which have provided a dominant framework and paradigm for drug discovery-development efforts thus far.
Finally, taking into account all the above issues, novel/different regulatory requirements for demonstrating efficacy based on revised guidelines or definitions of outcomes measurements are also required.
In conclusion, the major challenge to contend with will be the development of R&D resources for a multinational prevention initiative. The convergence of several unique features of AD (e.g. heterogeneity, complex polygenic etiology, and prolonged asymptomatic pre-clinical phase of neurodegeneration) highlights the need for very large cohorts of well-characterized cohorts from various genetic/cultural backgrounds as potential volunteers for both: a) longitudinal epidemiological studies to discover and/or validate putative risk factors and b) clinical studies for prospective validation of potential preventive interventions. A massive international longitudinal database on health aging and pre-dementia or at risk populations, as a shared R&D resource, is an essential infrastructure to address the future needs of a major prevention initiative. Along with a ‘Big-Data’, the field of therapy development will require novel computational capabilities to not only sort out the complex interactions among multiple etiologic factors but also to discover and validate technologies for the early and accurate detection of the disease (220–222).
In spite of many great strides in understanding AD, the lack of effective interventions for chronic brain disorders along with the rapid expansion of the aging population at risk for dementia pose an ever-increasing threat to the solvency of healthcare systems worldwide. The scope and magnitude of this global health-economic crisis demands a commensurate response; fortunately, many countries have begun to develop national plans to address the scientific, social, economic, and political challenges posed by dementia. There are several parallel efforts that reflect the global concerns and international efforts to formulate strategies for overcoming these challenges - e.g. the Organization for Economic Co-operation and Development (OECD) Expert Conferences/G-8 Dementia Summit/Post G-8 Legacy Meeting (218–221, 223). However, the open question remains whether these prospective plans for action will convince policy-makers worldwide to make the necessary financial commitments to significantly increase R&D resources for prevention.
The first ‘call to arms’ for a global mobilization of all necessary resources to address the looming crisis due to the exponential increases in the prevalence of dementia was made in a 1992 editorial (224). In 1997, nearly two decades ago, in a Congressional Testimony on the ‘Prospects of Prevention’, the Alzheimer’s Association (available at http://www.alz.org/) made the case for a radical shift in therapy development towards a strategy of ‘Prevention’ (225). In 2009, once again, there was a call to launch a major international initiative called The Campaign to Prevent Alzheimer’s Disease by 2020 (PAD2020) (available at http://www.pad2020.org/) (226). Nearly a quarter of a century after the first plea for action, the worldwide scientific community is well poised to make a quantum advance towards the strategic objectives of preventing dementia. The earlier calls for adoption of alternative paradigms to focus for therapies towards prevention were considered untenable goals. To date, however, there is an overwhelming optimism in the field with respects to the prospects of developing disease modifying intervention to delay the onset of disabling symptoms; and eventually to prevent (218, 226). The prevailing consensus is that current symptomatic treatments are woefully inadequate, indicating an urgent need to re-focusing R&D paradigms towards disease-modifying interventions.
Acknowledgments
The work of EC, OC, JFM is supported by CATI project ((cati-neuroimaging.com), Fondation Plan Alzheimer). The work of SL and HH is supported by the program “Investissements d’avenir” (grant number ANR-10-IAIHU-06), by the AXA Research Fund, the Fondation Université Pierre et Marie Curie and the Fondation pour la Recherche sur Alzheimer, Paris, France. The work of PA is supported by the the LABEX (laboratory of excellence program investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2 and the Lille University Hospital ; the International Genomics of Alzheimer’s Project (IGAP) is supported by the French National Foundation on Alzheimer’s disease and related disorders and the Alzheimer’s Association. The work of CRJ is supported by research funding from the National Institutes of Health ((R01-AG011378, U01-HL096917, U01-AG024904, RO1 AG041851, R01 AG37551, R01AG043392, U01-AG06786)), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. The work of KB is supported by the Swedish Research Council. The work of SEO is supported by National Institutes of Health, National Institutes on Aging (AG039389, AG12300). The work of MK is supported by Alzheimer Association, Alzheimer’s Research Prevention Foundation and Sheikha Salama Bint Hamdan Al Nahyan Foundation. The work of WK is supported by The National Institutes of Health (P50 AG005133, R37 AG025516, P01 AG025204). Support to MTS comes from the ‘Agence Nationale de la Recherche’ [grants number ANR-13-JSV4-0001-01]. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. The work of AD is supported by the German Research Foundation (DFG). The work of ALWB is supported by Science Foundation Ireland (grant 11/RFP.1/NES/3194). The work of OC is supported by ANR (project HM-TC, grant number ANR-09-EMER-006) and by France Alzheimer Association (project IRMA7). The work of HZ is supported by Swedish Research Council and the Knut and Alice Wallenberg Foundation.
References
- 1.Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khachaturian ZS, Petersen RC, Gauthier S, et al. A roadmap for the prevention of dementia: the inaugural Leon Thal Symposium. Alzheimers Dement. 2008;4:156–163. doi: 10.1016/j.jalz.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampel H, Lista S. Use of biomarkers and imaging to assess pathophysiology, mechanisms of action and target engagement. J Nutr Health Aging. 2013;17:54–63. doi: 10.1007/s12603-013-0003-1. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H, Broich K. Enrichment of MCI and early Alzheimer’s disease treatment trials using neurochemical and imaging candidate biomarkers. J Nutr Health Aging. 2009;13:373–375. doi: 10.1007/s12603-009-0048-3. [DOI] [PubMed] [Google Scholar]
- 5.Vellas B, Hampel H, Rougé-Bugat ME, et al. Alzheimer’s disease therapeutic trials: EU/US Task Force report on recruitment, retention, and methodology. J Nutr Health Aging. 2012;16:339–345. doi: 10.1007/s12603-012-0044-x. [DOI] [PubMed] [Google Scholar]
- 6.Group. BDW. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Rosa-Neto P, Gauthier S. Use of biomarkers in clinical trials of Alzheimer disease: from concept to application. Mol Diagn Ther. 2011;15:313–325. doi: 10.1007/BF03256467. [DOI] [PubMed] [Google Scholar]
- 8.Vellas B, Aisen PS, Sampaio C, et al. Prevention trials in Alzheimer’s disease: an EU-US task force report. Prog Neurobiol. 2011;95:594–600. doi: 10.1016/j.pneurobio.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Vellas B, Pahor M, Manini T, et al. Designing pharmaceutical trials for sarcopenia in frail older adults: EU/US Task Force recommendations. J Nutr Health Aging. 2013;17:612–618. doi: 10.1007/s12603-013-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 11.Hampel H, Lista S, Teipel SJ, et al. Perspective on future role of biological markers in clinical therapy trials of Alzheimer’s disease: a long-range point of view beyond 2020. Biochem Pharmacol. 2014;88:426–449. doi: 10.1016/j.bcp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Teipel SJ, Sabri O, Grothe M, et al. Perspectives for multimodal neurochemical and imaging biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S329–347. doi: 10.3233/JAD-2012-129030. [DOI] [PubMed] [Google Scholar]
- 13.Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature reviews Neurology. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Shen Y, Walsh DM, et al. Biological markers of amyloid beta-related mechanisms in Alzheimer’s disease. Exp Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Experimental gerontology. 2010;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksen K, O’Bryant SE, Hampel H, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimer’s and Dementia. 2014;10:115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampel H, Frank R, Broich K, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 19.Lista S, Faltraco F, Prvulovic D, Hampel H. Blood and plasma-based proteomic biomarker research in Alzheimer’s disease. Prog Neurobiol. 2013;101–102:1–17. doi: 10.1016/j.pneurobio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Bertram L, Hampel H. The role of genetics for biomarker development in neurodegeneration. Prog Neurobiol. 2011;95:501–504. doi: 10.1016/j.pneurobio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Zetzsche T, Rujescu D, Hardy J, Hampel H. Advances and perspectives from genetic research: development of biological markers in Alzheimer’s disease. Expert Rev Mol Diagn. 2010;10:667–690. doi: 10.1586/erm.10.48. [DOI] [PubMed] [Google Scholar]
- 22.Lista S, Garaci FG, Ewers M, et al. CSF Aβ1–42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers Dement. 2014;10:381–392. doi: 10.1016/j.jalz.2013.04.506. [DOI] [PubMed] [Google Scholar]
- 23.Broich K, Weiergräber M, Hampel H. Biomarkers in clinical trials for neurodegenerative diseases: regulatory perspectives and requirements. Prog Neurobiol. 2011;95:498–500. doi: 10.1016/j.pneurobio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Solomon A, Mangialasche F, Richard E, et al. Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med. 2014;275:229–250. doi: 10.1111/joim.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 28.Mills SM, Mallmann J, Santacruz AM, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–743. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperling R, Donohue M, Aisen P. The A4 Trial: Anti-Amyloid Treatment of Asymptomatic Alzheimer’s Disease. Alzheimer’s and Dementia. 2012;8(4 Supplement):P425–P426. [Google Scholar]
- 30.Sperling RA, Rentz DM, Johnson KA, et al. The A4 Study: Stopping AD Before Symptoms Begin? Science Translational Medicine. 2014;6:228fs213. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling RA, Jack CR, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Assocation workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiman EM, Langbaum JBS, Tariot PN. Alzheimer’s Prevention Initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomarkers in Medicine. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roses AD, Welsh-Bohmer KA, Burns DK, et al. A pharmacogenetics-supported clinical trial to delay onset of mild cognitive impairment due to Alzheimer’s disease. The Journal of Nutrition, Health & Aging. 2012;841 [Google Scholar]
- 35.Reiman EM, Langbaum JB, Fleisher AS, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. The Lancet Neurology. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 37.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 38.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron JC, Chetelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 40.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 41.Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 42.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39:1186–1197. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 52.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frisoni GB, Jack CR. Harmonization of magnetic resonance-based manual hippocampal segmentation: A mandatory step for wide clinical use. Alzheimers Dement. 2011;7:171–174. doi: 10.1016/j.jalz.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Jack CR, Jr, Barkhof F, Bernstein MA, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement. 2011;7:474–485. e474. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frisoni GBJC, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, Preboske G, Swihart T, Blair M, Cavedo E, Grothe MJ, Lanfredi M, Martinez O, Nishikawa M, Portegies M, Stoub T, Ward C, Apostolova LG, Ganzola R, Wolf D, Barkhof F, Bartzokis G, DeCarli C, Csernansky JG, deToledo-Morrell L, Geerlings MI, Kaye J, Killiany RJ, Lehéricy S, Matsuda H, O’Brien J, Silbert LC, Scheltens P, Soininen H, Teipel S, Waldemar G, Fellgiebel A, Barnes J, Firbank M, Gerritsen L, Henneman W, Malykhin N, Pruessner JC, Wang L, Watson C, Wolf H, deLeon M, Pantel J, Ferrari C, Bosco P, Pasqualetti P, Duchesne S, Duvernoy H, Boccardi M for the EADC - European Alzheimer’s Disease Consortium and the ADNI - Alzheimer’s Disease Neuroimaging Initiative. The EADC-ADNI Harmonized Protocol for Hippocampal Segmentation on Magnetic Resonance: Evidence of Validity. Accepted for publication in Alzheimer’s & Dementia 2014. [Google Scholar]
- 56.Suppa P, Anker U, Spies L, et al. Fully Automated Atlas-Based Hippocampal Volumetry for Detection of Alzheimer’s Disease in a Memory Clinic Setting. J Alzheimers Dis. 2014 doi: 10.3233/JAD-141446. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Kim SH, Kim GH, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77:18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- 58.Frisoni GB, Jagust WJ. Subcortical vascular dementia exists! Neurology. 2011;77:12–13. doi: 10.1212/WNL.0b013e318221ad59. [DOI] [PubMed] [Google Scholar]
- 59.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desikan RS, Cabral HJ, Settecase F, et al. Automated MRI measures predict progression to Alzheimer’s disease. Neurobiol Aging. 2010;31:1364–1374. doi: 10.1016/j.neurobiolaging.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 65.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 66.Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000;57:339–344. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- 67.Jack CR, Jr, Petersen RC, Grundman M, et al. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging. 2008;29:1285–1295. doi: 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 69.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sperling RA, Jack CR, Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 72.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in Memory Networks in Mild Cognitive Impairment and Alzheimer’s Disease: An Independent Component Analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamalainen A, Pihlajamaki M, Tanila H, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim HK, Juh R, Pae CU, et al. Altered verbal working memory process in patients with Alzheimer’s disease: an fMRI investigation. Neuropsychobiology. 2008;57:181–187. doi: 10.1159/000147471. [DOI] [PubMed] [Google Scholar]
- 79.Bokde AL, Karmann M, Born C, et al. Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J Alzheimers Dis. 2010;21:103–118. doi: 10.3233/JAD-2010-091054. [DOI] [PubMed] [Google Scholar]
- 80.Olichney JM, Taylor JR, Chan S, et al. fMRI responses to words repeated in a congruous semantic context are abnormal in mild Alzheimer’s disease. Neuropsychologia. 2010;48:2476–2487. doi: 10.1016/j.neuropsychologia.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bokde ALW, Lopez-Bayo P, Born C, et al. Functional Abnormalities of the Visual Processing System in Subjects with Mild Cognitive Impairment: an fMRI study. Psychiatr Res: Neuroimaging. 2008;163:248–259. doi: 10.1016/j.pscychresns.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Bokde AL, Lopez-Bayo P, Meindl T, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- 83.Forster S, Vaitl A, Teipel SJ, et al. Functional representation of olfactory impairment in early Alzheimer’s disease. J Alzheimers Dis. 2010;22:581–591. doi: 10.3233/JAD-2010-091549. [DOI] [PubMed] [Google Scholar]
- 84.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sorg C, Riedl V, Muhlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gili T, Cercignani M, Serra L, et al. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J Neurol Neurosurg Psychiatry. 2011;82:58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- 88.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drzezga A, Becker JA, Van Dijk KR, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Bihan DaEB. Imagerie de diffusion in-vivo par résonance magnétique nucléaire. Comptes rendus de l’Académie des sciences. 1985;301:1109–1112. [Google Scholar]
- 93.Mielke MM, Okonkwo OC, Oishi K, et al. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimers Dement. 2012;8:105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Douaud G, Menke RA, Gass A, et al. Brain Microstructure Reveals Early Abnormalities more than Two Years prior to Clinical Progression from Mild Cognitive Impairment to Alzheimer’s Disease. J Neurosci. 2013;33:2147–2155. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selnes P, Aarsland D, Bjornerud A, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis. 2013;33:723–736. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- 96.Haller S, Nguyen D, Rodriguez C, et al. Individual prediction of cognitive decline in mild cognitive impairment using support vector machine-based analysis of diffusion tensor imaging data. J Alzheimers Dis. 2010;22:315–327. doi: 10.3233/JAD-2010-100840. [DOI] [PubMed] [Google Scholar]
- 97.Teipel SJ, Reuter S, Stieltjes B, et al. Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry research. 2011;194:363–371. doi: 10.1016/j.pscychresns.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Dyrba M, Ewers M, Plant C, Barkhof F, Fellgiebel A, Hausner L, Filippi M, Kirste T, Teipel SJ. Prediction of prodromal AD in MCI subjects using multicenter DTI and MRI data and multiple kernels SVM: an EDSD study. Alzheimer’s & Dementia. 2014;10:P40. [Google Scholar]
- 99.Nowrangi MA, Lyketsos CG, Leoutsakos JM, et al. Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2013;9:519–528. doi: 10.1016/j.jalz.2012.05.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kitamura S, Kiuchi K, Taoka T, et al. Longitudinal white matter changes in Alzheimer’s disease: a tractography-based analysis study. Brain research. 2013;1515:12–18. doi: 10.1016/j.brainres.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 101.Teipel SJ, Meindl T, Wagner M, et al. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- 102.Likitjaroen Y, Meindl T, Friese U, et al. Longitudinal changes of fractional anisotropy in Alzheimer’s disease patients treated with galantamine: a 12-month randomized, placebo-controlled, double-blinded study. Eur Arch Psychiatry Clin Neurosci. 2012;262:341–350. doi: 10.1007/s00406-011-0234-2. [DOI] [PubMed] [Google Scholar]
- 103.Catani M, Acqua FD, de Schotten MT. A revised limbic system model for memory, emotion and behaviour. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Geschwind N. Disconnexion syndromes in animals and man. I Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 105.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 106.Catani M, Thiebaut de Schotten M. Atlas of Human Brain Connections. 2012 [Google Scholar]
- 107.Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O’Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci. 2011;31:13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Metzler-Baddeley C, Hunt S, Jones DK, Leemans A, Aggleton JP, O’Sullivan MJ. Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment. Neurology. 2012;79:2233–2240. doi: 10.1212/WNL.0b013e31827689e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Catani M, Mesulam MM, Jakobsen E, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frisoni GB. Alzheimer’s disease neuroimaging initiative in Europe. Alzheimers Dement. 2010;6:280–285. doi: 10.1016/j.jalz.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Cavedo E, Redolfi A, Angeloni F, et al. The Italian Alzheimer’s Disease Neuroimaging Initiative (I-ADNI): Validation of Structural MR Imaging. J Alzheimers Dis. 2014 doi: 10.3233/JAD-132666. [DOI] [PubMed] [Google Scholar]
- 112.Carrillo MC, Bain LJ, Frisoni GB, Weiner MW. Worldwide Alzheimer’s disease neuroimaging initiative. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012;8:337–342. doi: 10.1016/j.jalz.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 113.Landau SM, Breault C, Joshi AD, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54:70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-? plaques: a prospective cohort study. The Lancet Neurology. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 115.Chien DT, Szardenings AK, Bahri S, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014;38:171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- 116.Villemagne VL, Furumoto S, Fodero-Tavoletti MT, et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41:816–826. doi: 10.1007/s00259-013-2681-7. [DOI] [PubMed] [Google Scholar]
- 117.Bohnen NI, Djang DS, Herholz K, Anzai Y, Minoshima S. Effectiveness and Safety of 18F-FDG PET in the Evaluation of Dementia: A Review of the Recent Literature. J Nucl Med. 2012;53:59–71. doi: 10.2967/jnumed.111.096578. [DOI] [PubMed] [Google Scholar]
- 118.Herholz K, Boecker H, Nemeth I, Dunn G. FDG PET in dementia multicenter studies and clinical trials. Clin Transl Imaging. 2013;1:261–270. [Google Scholar]
- 119.Caroli A, Prestia A, Chen K, et al. Summary Metrics to Assess Alzheimer Disease-Related Hypometabolic Pattern with 18F-FDG PET: Head-to-Head Comparison. J Nucl Med. 2012;53:592–600. doi: 10.2967/jnumed.111.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaffer JL, Petrella JR, Sheldon FC, et al. Predicting Cognitive Decline in Subjects at Risk for Alzheimer Disease by Using Combined Cerebrospinal Fluid, MR Imaging, and PET Biomarkers. Radiology. 2012 doi: 10.1148/radiol.12120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Annals of Neurology. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yokokura M, Mori N, Yagi S, et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2011;38:343–351. doi: 10.1007/s00259-010-1612-0. [DOI] [PubMed] [Google Scholar]
- 124.Schuitemaker A, Kropholler MA, Boellaard R, et al. Microglial activation in Alzheimer’s disease: an (R)-[(1)(1)C]PK11195 positron emission tomography study. Neurobiol Aging. 2013;34:128–136. doi: 10.1016/j.neurobiolaging.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 125.Wiley CA, Lopresti BJ, Venneti S, et al. Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol. 2009;66:60–67. doi: 10.1001/archneurol.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61:10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chua SW, Kassiou M, Ittner LM. The translocator protein as a drug target in Alzheimer’s disease. Expert Rev Neurother. 2014;14:439–448. doi: 10.1586/14737175.2014.896201. [DOI] [PubMed] [Google Scholar]
- 128.Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;17:17. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa Translocator Protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carter SF, Scholl M, Almkvist O, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53:37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- 131.Horti AG, Kuwabara H, Holt DP, Dannals RF, Wong DF. Recent PET radioligands with optimal brain kinetics for imaging nicotinic acetylcholine receptors. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56:159–166. doi: 10.1002/jlcr.3020. [DOI] [PubMed] [Google Scholar]
- 132.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England journal of medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Shapes of the Trajectories of 5 Major Biomarkers of Alzheimer Disease. Archives of neurology. 2012 doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alexopoulos P, Kriett L, Haller B, et al. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014 doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 136.Kendziorra K, Wolf H, Meyer PM, et al. Decreased cerebral alpha4beta2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer’s disease assessed with positron emission tomography. European journal of nuclear medicine and molecular imaging. 2011;38:515–525. doi: 10.1007/s00259-010-1644-5. [DOI] [PubMed] [Google Scholar]
- 137.Villemagne VL, Okamura N. In vivo tau imaging: obstacles and progress. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:S254–264. doi: 10.1016/j.jalz.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 138.Okamura N, Furumoto S, Harada R, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1420–1427. doi: 10.2967/jnumed.112.117341. [DOI] [PubMed] [Google Scholar]
- 139.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. Journal of Alzheimer’s disease : JAD. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 140.Drzezga A, Barthel H, Minoshima S, Sabri O. Potential Clinical Applications of PET/MR Imaging in Neurodegenerative Diseases. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:47S–55S. doi: 10.2967/jnumed.113.129254. [DOI] [PubMed] [Google Scholar]
- 141.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 142.Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6:226ra230. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mattsson N, Andreasson U, Persson S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395. e386. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattsson N, Andreasson U, Persson S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carrillo MC, Blennow K, Soares H, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: An update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement. 2013;9:137–140. doi: 10.1016/j.jalz.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 146.Leinenbach A, Pannee J, Dulffer T, et al. Mass Spectrometry-Based Candidate Reference Measurement Procedure for Quantification of Amyloid-beta in Cerebrospinal Fluid. Clin Chem. 2014 doi: 10.1373/clinchem.2013.220392. [DOI] [PubMed] [Google Scholar]
- 147.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013;126:659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mattsson N, Insel P, Nosheny R, et al. CSF protein biomarkers predicting longitudinal reduction of CSF beta-amyloid42 in cognitively healthy elders. Transl Psychiatry. 2013;3:e293. doi: 10.1038/tp.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moghekar A, Li S, Lu Y, et al. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013 doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zetterberg H, Pedersen M, Lind K, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–260. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- 151.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 152.Mattsson N, Portelius E, Rolstad S, et al. Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J Alzheimers Dis. 2012;30:767–778. doi: 10.3233/JAD-2012-120019. [DOI] [PubMed] [Google Scholar]
- 153.Mattsson N, Rajendran L, Zetterberg H, et al. BACE1 inhibition induces a specific cerebrospinal fluid beta-amyloid pattern that identifies drug effects in the central nervous system. PLoS ONE. 2012;7:e31084. doi: 10.1371/journal.pone.0031084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lannfelt L, Blennow K, Zetterberg H, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 155.May PC, Dean RA, Lowe SL, et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Portelius E, Dean RA, Gustavsson MK, et al. A novel Abeta isoform pattern in CSF reflects gamma-secretase inhibition in Alzheimer disease. Alzheimers Res Ther. 2010;2:7. doi: 10.1186/alzrt30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 158.Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1002–1010. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 159.Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014 doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee JM, Blennow K, Andreasen N, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–1623. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rosen C, Mattsson N, Johansson PM, et al. Discriminatory Analysis of Biochip-Derived Protein Patterns in CSF and Plasma in Neurodegenerative Diseases. Front Aging Neurosci. 2011;3:1. doi: 10.3389/fnagi.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thorsell A, Bjerke M, Gobom J, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 163.Perrin RJ, Craig-Schapiro R, Malone JP, et al. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer’s disease. PLoS ONE. 2011;6:e16032. doi: 10.1371/journal.pone.0016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Armstrong A, Mattsson N, Appelqvist H, et al. Lysosomal Network Proteins as Potential Novel CSF Biomarkers for Alzheimer’s Disease. Neuromolecular Med. 2013 doi: 10.1007/s12017-013-8269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soares HD, Lovestone S. Biomarker utility in Alzheimer’s disease clinical trials. Drug Discovery Today: Therapeutic Strategies. 2014 [Google Scholar]
- 166.Kiddle SJ, Sattlecker M, Proitsi P, et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J Alzheimers Dis. 2014;38:515–531. doi: 10.3233/JAD-130380. [DOI] [PubMed] [Google Scholar]
- 167.O’Bryant SE, Xiao G, Barber R, et al. A serum protein-based algorithm for the detection of Alzheimer disease. Archives of Neurology. 2010;67:1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Bryant SEGX, Zhang F, Edwards M, German D, Yin X, Como T, Reisch J, Huebinger HM, Graff-Radford N, Dickson D, Barber RC, Hall J, O’Suilleabhain P, Grammas P. Validation of a serum screen for Alzheimer’s disease across assay platforms, species and tissues. Journal of Alzheimer’s disease : JAD. 2014 doi: 10.3233/JAD-141041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Doecke J, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Busy AI, Brown B, De Ruyck K, Ellis KA, Fowler C, Gupta VB, Head R, Macaulay L, Pertile K, Rowe CC, Rembach A, Rodrigues M, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Aimes D, Masters CL, Martins RN. Blood-based protein biomarkers for the diagnosis of Alzheimer’s disease. Arch Neurol. 2012 doi: 10.1001/archneurol.2012.1282. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burnham SC, Faux NG, Wilson W, et al. A blood-based predictor for neocortical Aβ burden in Alzheimer’s disease: Results from the AIBL study. Molecular Psychiatry. 2014;19:519–526. doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 171.Thambisetty M, Tripaldi R, Riddoch-Contreras J, et al. Proteome-based plasma markers of brain amyloid-β deposition in non-demented older individuals. Journal of Alzheimer’s Disease. 2010;22:1099–1109. doi: 10.3233/JAD-2010-101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hye A, Riddoch-Contreras J, Baird AL, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimer’s and Dementia. 2014 doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Llano DA, Devanarayan V, Simon AJ. Evaluation of plasma proteomic data for alzheimer disease state classification and for the prediction of progression from mild cognitive impairment to alzheimer disease. Alzheimer Disease and Associated Disorders. 2013;27:233–243. doi: 10.1097/WAD.0b013e31826d597a. [DOI] [PubMed] [Google Scholar]
- 174.Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song F, Poljak A, Crawford J, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Swaminathan S, Risacher SL, Yoder KK, et al. Association of plasma and cortical amyloid beta is modulated by APOE ε4 status. Alzheimer’s and Dementia. 2014;10:e9–e18. doi: 10.1016/j.jalz.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crenshaw DG, Gottschalk WK, Lutz MW, et al. Using genetics to enable studies on the prevention of alzheimer’s disease. Clinical Pharmacology and Therapeutics. 2013;93:177–185. doi: 10.1038/clpt.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thambisetty M, An Y, Kinsey A, et al. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. NeuroImage. 2012;59:212–217. doi: 10.1016/j.neuroimage.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Snyder HM, Carrillo MC, Grodstein F, et al. Developing novel blood-based biomarkers for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Bryant SEGV, Henriksen K, Edwards M, Jeromin A, Lista S, Bazenet C, Soares H, Lovestone S, Hampel H, Montine T, Blennow K, Foroud T, Carrillo M, Graff-Radford N, Laske C, Breteler M, Shaw L, Trojanowski JQ, Schupf N, Rissman R, Fagan AM, Oberoi P, Umek R, Weiner MW, Grammas P, Posner H, Martins R. Guidelines for the standardization fo preanalytic variables for blood-based biomarker studies in Alzheimer’s disease. Alzheimer’s & Dementia. 2014 doi: 10.1016/j.jalz.2014.08.099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 182.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goldman JS, Hahn SE, Catania JW, et al. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13:597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karran E, Hardy J. Antiamyloid therapy for Alzheimer’s disease--are we on the right road? N Engl J Med. 2014;370:377–378. doi: 10.1056/NEJMe1313943. [DOI] [PubMed] [Google Scholar]
- 185.Kraff O, Fischer A, Nagel AM, Mönninghoff C, Ladd ME. MRI at 7 tesla and above: Demonstrated and potential capabilities. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24573. [DOI] [PubMed] [Google Scholar]
- 186.Rössler M, Zarski R, Bohl J, Ohm TG. Stage-dependent and sector-specific neuronal loss in hippocampus during Alzheimer’s disease. Acta Neuropathol. 2002;103:363–369. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- 187.La Joie R, Perrotin A, de La Sayette V, et al. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin. 2013;3:155–162. doi: 10.1016/j.nicl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boutet C, Chupin M, Lehéricy S, Marrakchi-Kacem L, Epelbaum S, Poupon C, Wiggins C, Vignaud A, Hasboun D, Defontaines B, Hanon O, Dubois B, Sarazin M, Hertz-Pannier L, Colliot O. Detection of volume loss in hippocampal layers in Alzheimer’s disease using 7 T MRI: A feasibility study. NeuroImage Clin. 2014:341–348. doi: 10.1016/j.nicl.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kerchner GA, Berdnik D, Shen JC, et al. APOE ε4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology. 2014;82:691–697. doi: 10.1212/WNL.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kerchner GA, Hess CP, Hammond-Rosenbluth KE, et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology. 2010;75:1381–1387. doi: 10.1212/WNL.0b013e3181f736a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee SP, Falangola MF, Nixon RA, Duff K, Helpern JA. Visualization of beta-amyloid plaques in a transgenic mouse model of Alzheimer’s disease using MR microscopy without contrast reagents. Magn Reson Med. 2004;52:538–544. doi: 10.1002/mrm.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Teipel SJ, Kaza E, Hadlich S, et al. Automated detection of amyloid-β-related cortical and subcortical signal changes in a transgenic model of Alzheimer’s disease using high-field MRI. J Alzheimers Dis. 2011;23:221–237. doi: 10.3233/JAD-2010-101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bertrand A, Pasquier A, Petiet A, et al. Micro-MRI study of cerebral aging: ex vivo detection of hippocampal subfield reorganization, microhemorrhages and amyloid plaques in mouse lemur primates. PLoS One. 2013;8:e56593. doi: 10.1371/journal.pone.0056593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Conijn MM, Geerlings MI, Biessels GJ, et al. Cerebral microbleeds on MR imaging: comparison between 1.5 and 7T. AJNR Am J Neuroradiol. 2011;32:1043–1049. doi: 10.3174/ajnr.A2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Theysohn JM, Kraff O, Maderwald S, et al. 7 tesla MRI of microbleeds and white matter lesions as seen in vascular dementia. J Magn Reson Imaging. 2011;33:782–791. doi: 10.1002/jmri.22513. [DOI] [PubMed] [Google Scholar]
- 197.van Rooden S, Goos JD, van Opstal AM, et al. Increased number of microinfarcts in Alzheimer disease at 7-T MR imaging. Radiology. 2014;270:205–211. doi: 10.1148/radiol.13130743. [DOI] [PubMed] [Google Scholar]
- 198.van Veluw SJ, Heringa SM, Kuijf HJ, et al. Cerebral cortical microinfarcts at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis. 2014;39:163–167. doi: 10.3233/JAD-131040. [DOI] [PubMed] [Google Scholar]
- 199.Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 200.Keil A, Debener S, Gratton G, et al. Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51:1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- 201.Hogan MJ, Swanwick GR, Kaiser J, Rowan M, Lawlor B. Memory-related EEG power and coherence reductions in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49:147–163. doi: 10.1016/s0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 202.Drago V, Babiloni C, Bartrés-Faz D, et al. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26(Suppl 3):159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- 203.Babiloni C, Vecchio F, Lizio R, et al. Resting state cortical rhythms in mild cognitive impairment and Alzheimer’s disease: electroencephalographic evidence. J Alzheimers Dis. 2011;26(Suppl 3):201–214. doi: 10.3233/JAD-2011-0051. [DOI] [PubMed] [Google Scholar]
- 204.Stam CJ, Jones BF, Manshanden I, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage. 2006;32:1335–1344. doi: 10.1016/j.neuroimage.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 205.Stam CJ, Montez T, Jones BF, et al. Disturbed fluctuations of resting state EEG synchronization in Alzheimer’s disease. Clin Neurophysiol. 2005;116:708–715. doi: 10.1016/j.clinph.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 206.Lee SH, Park YM, Kim DW, Im CH. Global synchronization index as a biological correlate of cognitive decline in Alzheimer’s disease. Neurosci Res. 2010;66:333–339. doi: 10.1016/j.neures.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 207.Poil SS, de Haan W, van der Flier WM, Mansvelder HD, Scheltens P, Linkenkaer-Hansen K. Integrative EEG biomarkers predict progression to Alzheimer’s disease at the MCI stage. Front Aging Neurosci. 2013;5:58. doi: 10.3389/fnagi.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zamrini E, Maestu F, Pekkonen E, et al. Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:280289. doi: 10.4061/2011/280289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 210.Broich KS-WG, Weiergräber M, Hampel H. Regulatory requirements on clinical trials in Alzheimer’s Disease. In: Basel ABP, editor. Alzheimer’ Disease–Modernizing Concept. Biological Diagnosis and Therapy; Karger: 2012. pp. 168–178. [Google Scholar]
- 211.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet neurology. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 212.Morris JC, Blennow K, Froelich L, et al. Harmonized diagnostic criteria for Alzheimer’s disease: recommendations. J Intern Med. 2014;275:204–213. doi: 10.1111/joim.12199. [DOI] [PubMed] [Google Scholar]
- 213.Hampel HCM. Alzheimer’s Disease – Modernizing Concept, Biological Diagnosis and Therapy. Adv Biol Psychiatry Basel, Karger. 2012:28. [Google Scholar]
- 214.Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-β to tau. Nat Rev Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 215.Feldman HH, Haas M, Gandy S, et al. Alzheimer’s disease research and development: a call for a new research roadmap. Ann N Y Acad Sci. 2014;1313:1–16. doi: 10.1111/nyas.12424. [DOI] [PubMed] [Google Scholar]
- 216.Noel-Storr AH, Flicker L, Ritchie CW, et al. Systematic review of the body of evidence for the use of biomarkers in the diagnosis of dementia. Alzheimers Dement. 2013;9:e96–e105. doi: 10.1016/j.jalz.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 217.Mattsson N, Andreasson U, Persson S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.NAPA AsAEAWo. Workgroup on NAPA’s scientific agenda for a national initiative on Alzheimer’s disease. Alzheimers Dement. 2012;8:357–371. doi: 10.1016/j.jalz.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 219.Milestones AsAWIo. Report on National Alzheimer’s Plan Milestones - FY’ 2014. Alzheimers Dementia. 2014 in press. [Google Scholar]
- 220.OECD. Emerging Trends in Biomedicine and Health Technology Innovation: Addressing the Global Challenge of Alzheimer’s. 2013 [Google Scholar]
- 221.OECD. Unlocking Global Collaboration to Accelerate Innovation for Alzheimer’s Disease and Dementia. Oxford University; 2013. [Google Scholar]
- 222.G8 Health Ministers. G8 Dementia Summit Declaration. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/265869/2901668_G8_DementiaSummitDeclaration_acc.pdf2013 [online]
- 223.Fowler P, Whitwell J, Jeffrey L, Young J, Smith K, Kirkland D. Cadmium chloride, benzo[a]pyrene and cyclophosphamide tested in the in vitro mammalian cell micronucleus test (MNvit) in the human lymphoblastoid cell line TK6 at Covance laboratories, Harrogate UK in support of OECD draft Test Guideline 487. Mutat Res. 2010;702:171–174. doi: 10.1016/j.mrgentox.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 224.ZK The five-five, ten-ten plan for Alzheimer’s disease. Neurobiol Aging. 1992;13:197–198. doi: 10.1016/0197-4580(92)90030-2. [DOI] [PubMed] [Google Scholar]
- 225.Khachaturian ZS. Prospects For Preventing Alzheimer’s Disease. Testimony for a Hearing on Alzheimer’s Disease Research Senate Committee on Labor & Human Resources; Washington, DC. June 5,1997. [Google Scholar]
- 226.Khachaturian ZS, Camí J, Andrieu S, et al. Creating a transatlantic research enterprise for preventing Alzheimer’s disease. Alzheimers Dement. 2009;5:361–366. doi: 10.1016/j.jalz.2009.05.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Carrié I, van Kan GA, Gillette-Guyonnet S, et al. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial) J Nutr Health Aging. 2012;16:355–359. doi: 10.1007/s12603-012-0046-8. [DOI] [PubMed] [Google Scholar]
- 228.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. The British journal of psychiatry : the journal of mental science. 2011;198:351–356. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 229.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 231.Dubois B, Chupin M, Hampel H, Lista S, Cavedo E, Croisile B, Louis Tisserand G, Touchon J, Bonafe A, Ousset PJ, Ameur AA, Rouaud O, Ricolfi F, Vighetto A, Pasquier P, Delmaire C, Ceccaldi M, Girard N, Dufouil C, Lehericy S, Tonelli I, Duveau D, Colliot O, Garnero L, Sarazin M, Dormont D And The “Hippocampus Study Group. Donepezil Decreases Annual Rate Of Hippocampal Atrophy In Suspected Prodromal Ad. 2014 doi: 10.1016/j.jalz.2014.10.003. Submitted. [DOI] [PubMed] [Google Scholar]