Abstract
Background: Osteoarthritis is a disabling musculoskeletal disease with no definite treatment. This study compared the effect of Platelet-rich plasma (PRP) and Transcutaneous Electrical Nerve Stimulation (TENS) plus exercise in the treatment of patients with knee joint osteoarthritis.
Methods: 54 eligible patients with knee osteoarthritis were randomly allocated into two groups. (IRCT2012110611382N) Group A (27 patients) received 2 injections of PRP (4 weeks apart) and group B (27 patients) received 10 sessions of TENS as well as exercise during the study period. Clinical outcome was evaluated using the Knee injury and Osteoarthritis Outcome Scores (KOOS) questionnaire before the treatment, 4 weeks, and 8 weeks after that the treatment. Pain was also assessed using a visual analog scale (VAS). Time to an intolerable knee pain during treadmill workout was also evaluated using an objective test.
Results: In the PRP group, the mean KOOS symptom score improved significantly from baseline to the end of study, while the change was not significant over this period for the group B. In both groups, significant reductions were observed in VAS scores from baseline till the end of study. The mean time to feel intolerable knee pain during treadmill work out of PRP group increased significantly from baseline to week 4, but no significant changes were found in this parameter over the time of study in the group B.
Conclusion: Intraarticular injection of PRP is an effective, safe method for short-term treatment of patients with knee joint osteoarthritis.
Keywords: Platelet - rich Plasma, PRP, Knee Osteoarthritis, Exercise
Introduction
Osteoarthritis (OA) of knee is one of the most common debilitating conditions associated with pain and limitation in daily living activities which negatively affect quality-of-life. Although different kinds of non-surgical options including analgesics, steroid and nonsteroid anti-inflammatory drugs, glucosamine, chondroitin sulphate and hyaluronic acid are currently available for the treatment of knee joint osteoarthritis, they are mainly aimed at the symptomatic relief, but not joint cartilage regeneration (1). Therefore, there is an urgent need for new procedures that are not only cost effective, but also targeting the biochemical process of OA as well as joint cartilage regeneration. Due to the ability of matrix synthesis enhancement, the use of growth factors has been broadly considered for cartilage repair (2). Autologous Platelet-Rich Plasma (PRP) is the volume of plasma with a high platelet concentration above normal baseline values (3). Platelets are sources of high concentrations of cytokines and a group of growth factors which regulate healing processes as well as tissue regeneration (4). PRP therapy provides a highly concentrated mixture of growth factors to contribute with healing process. Some growth factors in PRP such as transforming growth factor –β seem to be correlated with chondrogenesis in cartilage repair (5, 6). PRP also contains plasmatic proteins, which is believed to boost or stimulate healing process that is very similar to the process which normally occurs in the human body following injury (5, 7). However, most studies on PRP therapy are underpowered, nonrandomized, or engage insufficient sample sizes which make them unreliable (5, 6). Moreover, while there are relatively good evidences (5, 8) regarding the beneficial effects of PRP on chronic, nonhealing tendon injuries including lateral epicondylitis and plantar fasciitis, very few powered studies have pointed toward the safety and efficacy of an intraarticular injection of PRP for the treatment of knee OA. On the other hand the majority of studies (9, 10) used expensive and time-consuming techniques to provide PRP which make it hard to apply these techniques in ordinary clinical settings. Therefore, this clinical trial was conducted to introduce a simple, low-cost protocol for PRP preparation as well as to determine its efficacy and safety for short-term treatment of knee OA.
Methods
Research Design
This study was a randomized clinical trial (IRCT2012110611382N) with 2 groups receiving different kinds of treatment. Prior to study all subjects were asked to sign an informed consent which was approved by the Ethics Committee of the Iran University of Medical Sciences. Besides this clinical trial has the code number (IRCT 2012110611382N) in IRCT. Subjects were randomly allocated to receive a standardized PRP injection protocol or a conventional treatment approach including TENS and exercise therapy. All participants were assessed on a number of subjective and objective variables (Knee injury and Osteoarthritis Outcome Scores (KOOS), visual analog scale (VAS) for pain, time to feel intolerable knee pain during treadmill workout, and adverse effects) before the treatment and at 4 weeks and 8weeks after that.
Sampling
The present study was conducted on patients presenting the sports and exercise medicine outpatient clinic of the Hazrat Rasool-e-Akram hospital. Patients with knee OA as diagnosed by American College of Rheumatology criteria (11) and graded as per Kellgren and Lawrence radiographic scoring system (12) were voluntarily enrolled in this study. 54 patients who had OA of the knee according to the criteria were selected on the basis of predefined inclusion criteria, that were, grade 1, 2 and 3 knee osteoarthritis based on Kellgren and Lawrence radiographic scoring system, no history of corticosteroid injection or consumption within past 6 months, no history of peripheral vascular disease, spinal stenosis, severe disabilities, inflammatory and metabolic diseases and lack of history of anticoagulative drugs consumption or coagulopathies. Exclusion criteria were consumption or intra-articular injection of corticosteroids during the study, anticoagulative drugs consumption during the study, and patient request for leaving the study.
The sample size was calculated based on an assumed study power of 80% (β=0.2), a false-positive rate of 5% (α=0.05), and a predicted difference of 2 points on our VAS (standard deviation, ±2). Using these parameters, and adjusting α for multiple comparisons, as well as including the possible loss of 25% of the samples during the study, we required 27 patients for each group.
The samples were randomly divided into two groups using a computer derived random chart: 27 participants in group A (PRP group) were given two intra-knee injections of PRP 4 weeks apart, 27 participants in group B (TENS group) received 10 sections of TENS, twice a week with a frequency of 100 hertz for 30 minutes in each section. Moreover all patients in group B received a specialized exercise program on a compact disk (CD) as well as in a guided booklet. These subjects were asked to do their daily knee resistance and flexibility exercises in 3 sets of 10 repetitions and 1 set of 5 repetitions, respectively. Among 27 patients in PRP group, 1 patient was excluded as she had an anterior cruciate ligament (ACL) injury due to a trauma. Of the 27 patients in group B, 3 patients refused the treatment, and 24 patients remained in the study (Fig. 1).
Fig. 1 .

Flow chart of recruiting patients in this study
The baseline characteristics of the two groups were similar with respect to age, sex, weight, and height and body mass index (Table 1).
Table 1 . Baseline characteristics of the participants .
| Characteristics |
TENS group (n=27) |
PRP group (n=27) |
p |
| Gender, No. (%) | 0.42 | ||
| Female | 25 (92.6%) | 22 (81.5%) | |
| Male | 2 (7.4%) | 5 (18.5%) | |
| Age (year) | 61.59±8.07(range 44-80) | 62.15±12.14 (range 43-80) | 0.84 |
| Height (cm) | 160.04±6.20 | 160.41±8.21 | 0.85 |
| Weight (kg) | 74.96±10.97 | 73.92±14.66 | 0.77 |
| BMI (kg/m2) | 29.21±3.22 | 28.52±3.83 | 0.48 |
Note: TENS group, exercise therapy with TENS modality group; PRP group, exercise therapy plus PRP group; BMI, Body Mass Index, Quantitative data are presented as Mean ± Standard deviation
PRP Preparation
The PRP required for injection was prepared by the Sports Medicine clinic of the Hazrat Rasool-e-Akram hospital in Tehran. About 22.5 mL of venous blood was drawn under sterile protection from the upper extremities veins meticulouslyin order to avoid any unwanted trauma to the platelets. The blood was collected in a syringe with 2.5 mL ACD (Anticoagulant Citrate Dextrose) as an anticoagulant. The whole blood was slowly transferred using an 18 gauge needle from the syringe into a 25-mL sterile Tubex tube (a PRP kit which is made in South Korea and approved by Iran Health Ministry). The tubes were then centrifuged for 6 minutes at 1600 rpm for women and 1800 rpm for men on the Celex centrifuge, and the blood was separated into PRP and residual red blood cells with the buffy coat. In this step the upper part of tube (plasma) was separated from the lower part (red blood cells) via locking the middle part of tube and then the tubes were centrifuged again for 5 minutes at 2000 rpm. Of 10 to 12 mL remained liquid in the upper part of tube, about 6 mL of plasma in the upper part was extracted via a 14 gauge angio-catheter and the remained liquid was shivered slowly till a homogenous solution was obtained. Finally this platelet enrich homogenous solution was extracted via a 10 mL syringe. Moreover, 0.5 mL of the patient’s peripheral blood as well as the final PRP was sent to the laboratory in order to measure the total platelet count. Platelet count was increased between 3 to 7 times than that of baseline in all samples; therefore, PRP with a platelet count of at least 3 times comparing to the baseline was injected in all.
Intervention
The patient was placed in a supine position with the knee in 45 degree flexion. Under sterile conditions, 5 mL of PRP (which was activated by 0.5 mL calcium gluconate immediately before injection) was slowly injected inside the knee through the infero-medial or infero-lateral approach with a 21- gauge needle without local anesthetic. The patients were discharged after 30 minutes of observation while using a wheelchair in order to leave the clinic. None of the patients reported complications like dizziness, nausea, headache and sweating after injection. During the follow-up period, nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet drugs and ice were not allowed, and paracetamol (500 mg orally three times a day during the first 72 hours after injection) was prescribed in case of discomfort. Also all patients were asked to avoid green tea and cranberry use as well as discontinue NSAIDs 72 hours and antiplatelet drugs such as aspirin 10 days before injection. Patients were strongly recommended to contact with us in case of occurring any adverse effect such as, fever, intolerable pain, redness and swelling.
Outcome Measures
The primary efficacy criteria were change from baseline in terms of pain, other symptoms, function in sport and recreation and knee related quality of life, measured using the KOOS subscales. The KOOS parameters were measured before the first and the second injection, and also at 4 weeks after the second injection. Patients were also assessed for pain using VAS and time to feel intolerable knee pain during treadmill workout (walking on treadmill with a speed of 3 km/h and grade 0 till to fell intolerable knee pain) before the first and the second injection, and also at 4 weeks after the second injection. Moreover all subjects were asked to wear a sports shoe for the treadmill test and avoid vigorous physical activity within 24 hours prior to the test. Adverse effects related to treatment were also recorded in every visit.
Statistical Analysis
To compare baseline demographics of the two groups, independent sample t-test for continuous variables and Pearson chi-square test for nominal variables was applied. The normality assumption of the continuous variables was checked using the Shapiro-Wilk test.
Generalized Estimating Equation (GEE) models were fitted to examine the associations between type of therapy and change in the Knee injury and osteoarthritis outcome scores (KOOS), VAS pain scores and time to feel knee pain over time. GEE models included two main effects (type of therapy and time) and the interaction of these effects. Time points in the analyses included baseline and visits of weeks 4 and 8. Using GEE, the correlation of multiple measurements within one patient is taken into account.
All tests applied were two-sided and a significance level of 0.05 was considered significant. All statistical analysis were performed with statistical software SPSS 16.0.0. (SPSS Inc. Chicago, IL, USA).
Results
Fifty-four eligible patients with knee osteoarthritis were included into the study who were randomly allocated into two groups. The baseline characteristics of the participants are summarized in table 1. Twenty-four patients in TENS group and 26 patients in PRP group attended at least one of the pre-determined visits (visits of weeks 4 and 8) after the baseline visit. The process of enrollment and accomplishment of study is illustrated in Fig.1.
Knee injury and osteoarthritis outcome scores (KOOS)
In the PRP group, the mean KOOS symptom score improved significantly from baseline to week 4, while the change was not significant over this period for the TENS group. In addition, no significant changes were observed in this subscale from week 4 to week 8 in both groups (Table 2). Compared to the TENS group, the PRP group had a greater improvement in the mean KOOS symptom score from baseline to week 4 (mean difference 10.7 points, p=0.010, table 3). However, the pattern of change of this parameter was similar between the two groups from week 4 to week 8 (p=0.060). The patterns of change of the other KOOS subscales’ scores did not differ significantly over time between the two groups (no group×time interactions, Table 2). After 4 weeks of interventions, significant improvements were observed in the KOOS pain scores of both groups. However, the mean pain scores of both groups did not change significantly from week 4 to week 8 (Table 2). Patients in the PRP group experienced a significant improvement in the mean KOOS ADL score from baseline to week 4 and this improvement was maintained up to week 8. In contrast, no significant change was found in the mean scores of this item over time in the TENS group (Table 2). Both interventions had no effects on KOOS Sport/Rec and QOL subscales (Tables 2 and 3).
Table 2 . Knee injury and osteoarthritis outcome scores (KOOS) over time in the two study groups .
| Variables | Time of study | p * | ||
| Baseline | Week 4 | Week 8 | ||
| Pain | 0.59 | |||
| TENS group | 41.3 (3.43) | 46.7 (3.14) £ | 44.2 (3.88) | |
| PRP group | 44.9 (3.56) | 54.4 (4.15) £ | 50.7 (3.24) | |
| Symptoms | 0.047 | |||
| TENS group | 50.3 (3.87) | 51.7 (3.56) | 52.0 (3.96) | |
| PRP group | 51.5 (4.47) | 63.6 (4.23) £ | 61.5 (3.86)* | |
| ADL | 0.44 | |||
| TENS group | 42.4 (4.09) | 46.9 (3.68) | 44.2 (4.36) | |
| PRP group | 48.3 (3.81) | 58.7 (4.08) £ | 54.4 (3.35) | |
| Sport/Rec | 0.99 | |||
| TENS group | 28.4 (6.16) | 27.6 (6.11) | 25.4 (5.31) | |
| PRP group | 23.8 (4.87) | 22.9 (4.68) | 21.3 (4.33) | |
| QOL | 0.12 | |||
| TENS group | 20.6 (3.65) | 18.4 (2.68) | 17.6 (2.58) | |
| PRP group | 17.1 (2.62) | 23.0 (3.14) | 22.6 (2.49) | |
* The P-value for Group×Time interaction (Based on the results of GEE analysis)
The values are expressed as mean (SD).
Note: TENS group: exercise therapy with TENS modality group. KOOS consists of five subscales; pain, other symptoms, function in sport and recreation (Sport/Rec) and knee related quality of life (QOL). A normalized score (range 0 to 100 with 0 indicating extreme problems and 100 indicating no problem) is calculated for each subscale.
£ p<0.05 for statistical difference from baseline to week 4 within the group.
Table 3 . GEE results of the effects of PRP in comparison with the exercise therapy with TENS modality on knee injury and osteoarthritis outcome scores (KOOS) .
| Variables | Group | Week | Estimate (SE) | 95% CI | p | |
| Lower bound | Upper | |||||
| Pain | ||||||
| Group× Week* | PRP group | 4 | 4.1 (4.03) | -3.77 | 12.02 | 0.31 |
| Group× Week Symptoms | PRP group | 8 | 2.9 (5.41) | -7.70 | 13.50 | 0.59 |
| Group× Week | PRP group | 4 | 10.7 (4.35) | 2.17 | 19.22 | 0.01 |
| Group× Week ADL | PRP group | 8 | 8.3 (4.46) | -0.42 | 17.09 | 0.06 |
| Group× Week | PRP group | 4 | 5.9 (4.65) | -3.19 | 15.04 | 0.20 |
| Group× Week Sport/Rec | PRP group | 8 | 4.3 (5.71) | -6.91 | 15.48 | 0.45 |
| Group× Week | PRP group | 4 | -.,2 (8,37) | -16.58 | 16.24 | 0.98 |
| Group× Week QOL | PRP group | 8 | 0.5 (6.74) | -12.73 | 13.68 | 0.94 |
| Group× Week | PRP group | 4 | 8.0 (4.30) | -0.37 | 16.48 | 0.06 |
| Group× Week | PRP group | 8 | 8.5 (4.57) | -0.49 | 17.42 | 0.06 |
* For ease of interpretation, the baseline (week 0) and the TENS group are considered as the reference levels for time and intervention group, respectively.
Note: TENS group, exercise therapy with TENS modality group. KOOS consists of five subscles; pain, other symptoms, function in sport and recreation (Sport/Rec) and knee related quality of life (QOL). A normalized score (range 0 to 100 with 0 indicating extreme problems and 100 indicating no problem) is calculated for each subscale.
Changes of KOOS from baseline to the end of study
In the PRP group, the mean KOOS symptom score improved significantly from baseline to the end of study, while the change was not significant over this period for the TENS group (p=0.010 and p=0.450, respectively). In both groups, no significant change was observed in other KOOS subscales from baseline till the end of study (Table 2).
VAS pain score
The pattern of change of VAS pain scores was not significantly different over the course of study between the two groups (no group×time interaction; p=0.900). Pain relief (according to VAS) was observed at the first month of study in both groups (both p<0.0001). However, no significant improvement was found in VAS scores of the two groups from week 4 till the end of the study (Fig. 2). In both groups, significant reductions were observed in VAS scores from baseline till the end of study (Fig. 2).
Fig. 2 .
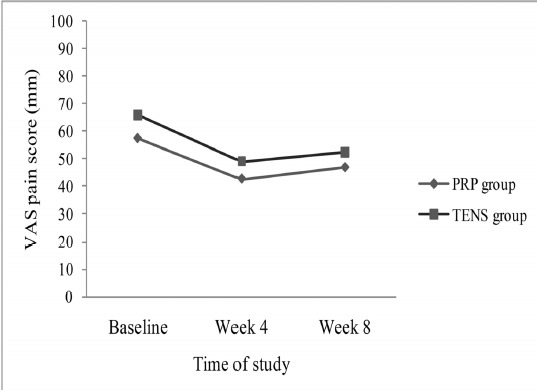
VAS pain score, comparison between PRP and TENS group
Time to feel intolerable pain during treadmill workout
The mean time to feel knee intolerable pain during treadmill workout in the PRP group increased significantly from baseline to week 4 (p<0.001) and remained unchanged till week 8 (mean (SD): 10.07 (1.52) at baseline; 15.86 (1.41) at week 4 and 17.06 (1.51) minutes at week 8). However, no significant change was found in this parameter over the time of study in the TENS group (mean (SD): 13.09 (2.52) at baseline; 13.05 (1.84) at week 4 and 12.72 (1.74) minutes at week 8). The PRP group in comparison with the TENS group had an additional 5.8 minutes increase in the mean time to feel pain from baseline to week 4 (group×time interaction, p=0.04).
Mild complications such as little swelling and pain, were observed in 3 patients (11%) in PRP group and 1 patient (4%) in TENS plus exercise group. No severe complication was observed in both groups.
Discussion
There are limited surveys suggesting clinical effectiveness of PRP for short-term symptomatic relief in patients with knee osteoarthritis. Moreover, these studies are mainly of poor quality and at high risk for bias. The majority of studies on this topic are non- randomized or without a controlled group; those studies with a controlled group, used intra-knee injection of hyaluronic acid or normal saline as the controlled method (13, 14). To our knowledge this is the first study which compared the effect of PRP with TENS and exercise in short-term treatment of patients with knee joint osteoarthritis. The combination of physical therapy modalities such as, Transcutaneous Electrical Nerve Stimulation (TENS) and exercise is a common well known method of treatment in patients with knee osteoarthritis. Pietrosimone et al showed that the combination of TENS and exercise is more effective in short-term treatment of knee osteoarthritis compared with exercise alone (15, 16). Regarding the treatment of knee osteoarthritis, Chen et al reported that TENS plus exercise is more effective than intra-knee injection of hyaluronic acid (17). It seems that there is enough evidence avilable (15-20) in supporting the effectiveness of TENS and exercise in symptomatic relief of patients with knee joint osteoarthritis. The present study showed that PRP is even more effective in short term treatment of knee joint osteoarthritis in comparison with combination of TENS and exercise. Nearly all of previous studies showed that compared with hyaluronic acid or placebo injection, intra-articular PRP injections have more beneficial effects in the short-term treatment of patients with knee OA (10, 13, 21, 22); this can be rational to observe such a result in the present study. Moreover in order to evaluate the effectiveness of PRP objectively, we assessed the time to feel intolerable pain during treadmill workout in addition to the ordinary subjective measured outcomes such as KOOS and VAS. Based on our result, in comparison with TENS and exercise, PRP injections significantly increased the mean time to feel pain during treadmill workout. This finding showed that the patients who received PRP injections for the treatment of KO, not only feel better, but also actually achieve a better performance. To our Knowledge, none of the previous studies used the objective method like this to assess PRP effectiveness in the treatment of knee osteoarthritis. Interestingly we found that, although the mean KOOS symptom score improved significantly from the baseline to week 4 merely in the PRP group, this improvement stopped from week 4 to week 8 even in the PRP group. According to the study of Sundman, et al., (23), this significant short term symptomatic relief in the PRP group seems to be due to suppression of inflammatory mediators; however this phenomenon may decrease after several weeks.
The most important limiting factors in this study were short-term follow-up as well as lack of blindness. However in the present study, we compared the short term effect of PRP with a very common therapeutic approach (TENS plus exercise) for KO in our country, which makes this survey unique.
Conclusion
Intraarticular injection of PRP is an effective, safe method for the short-term treatment of patients with knee joint osteoarthritis. More double blinded randomized clinical trials are needed before widespread use of PRP for the treatment of knee joint osteoarthritis.
Acknowledgements
This research was funded by Iran University of Medical Sciences. The authors highly appreciate the cooperation of all sports medicine assistants who helped us for this project.
Conflict of interests
There are no conflicts of interest declared.
Cite this article as: Angoorani H, Mazaherinezhad A, Marjomaki O, Younespour Sh. Treatment of knee osteoarthritis with platelet-rich plasma in comparison with transcutaneous electrical nerve stimulation plus exercise: a randomized clinical trial. Med J Islam Repub Iran 2015 (27 June). Vol. 29:223.
References
- 1.Handl M, Amler E, Bräun K, Holzheu J, Trc T, Imhoff A B. et al. Positive effect of oral supplementation with glycosaminoglycans and antioxidants on the regeneration of osteochondral defects in the knee joint. Physiol Res. 2007;56:243Y9. doi: 10.33549/physiolres.930917. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O'Keefe RJ. Articular cartilage biology. J Am Acd Orthop Surg. 2003;11:421Y30. doi: 10.5435/00124635-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Pietrzak WS, Eppley BL. Platelet rich plasma: Biology and new technology. J Craniofac Surg. 2005;16:1043–54. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 4.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 5.Sampson S, Gerhardt M, Mandelaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr Rev Musculoskelet Med. 2008;1:165–74. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunziker EB, Driesang IM, Morris EA. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res. 2001;391(suppl):S171–81. doi: 10.1097/00003086-200110001-00017. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E, Sánchez M, Nurden AT, Zalduendo MM, de la Fuente M, Azofra J. et al. Platelet released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology. 2007;46:1769–72. doi: 10.1093/rheumatology/kem234. [DOI] [PubMed] [Google Scholar]
- 8.Barrett S, Erredge S. Growth factors for chronic plantar fasciitis. Podiatry Today. 2004;17:37–42. [Google Scholar]
- 9.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A. et al. Platelet-Rich Plasma Intra-Articular Injection Versus Hyaluronic Acid Viscosupplementation as Treatments for Cartilage Pathology: From Early Degeneration to Osteoarthritis. J Arthro & Relat Surg. 2012;11:1585. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with Platelet-Rich Plasma is more effective than placebo for Knee Osteoarthritis. Am J Sports Med. 2013;41:356–64. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K. et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 12.Kellgren J, Lawrence J. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilvie-Harris D. et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013 Dec;29(12):2037–48. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Dold AP, Zywiel MG, Taylor DW, Dwyer T, Theodoropoulos J. Platelet-Rich Plasma in the Management of Articular Cartilage Pathology: A Systematic Review. Clin J Sport Med. 2014;24:31–43. doi: 10.1097/01.jsm.0000432855.85143.e5. [DOI] [PubMed] [Google Scholar]
- 15.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J Orthop Sports Phys Ther. 2011;41(1):4–12. doi: 10.2519/jospt.2011.3447. [DOI] [PubMed] [Google Scholar]
- 16.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of disinhibitory transcutaneous electrical nerve stimulation and therapeutic exercise on sagittal plane peak knee kinematics and kinetics in people with knee osteoarthritis during gait: a randomized controlled trial. Clinical rehabilitation. 2010;24(12):1091–1101. doi: 10.1177/0269215510375903. [DOI] [PubMed] [Google Scholar]
- 17.Chen WL, Hsu WC, Lin YJ, Hsieh LF. Comparison of intra-articular hyaluronic acid injections with transcutaneous electric nerve stimulation for the management of knee osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(8):1482–9. doi: 10.1016/j.apmr.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R, Wall PD. Pain mechanisms: a new theory. Survey of Anesthesiology. 1967;11(2):89–90. [Google Scholar]
- 19.Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM. et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013 Sep;20:347. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014 Mar;66(3):622–36. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 21.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014 Jul;5(3):351–61. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Merchan EC. Intra-articular Injections of Hyaluronic Acid and Other Drugs in the Knee Joint. HSS J. 2013 Jul;9(2):180–2. doi: 10.1007/s11420-012-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO. et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014 Jan;42(1):35–41. doi: 10.1177/0363546513507766. [DOI] [PubMed] [Google Scholar]


