Abstract
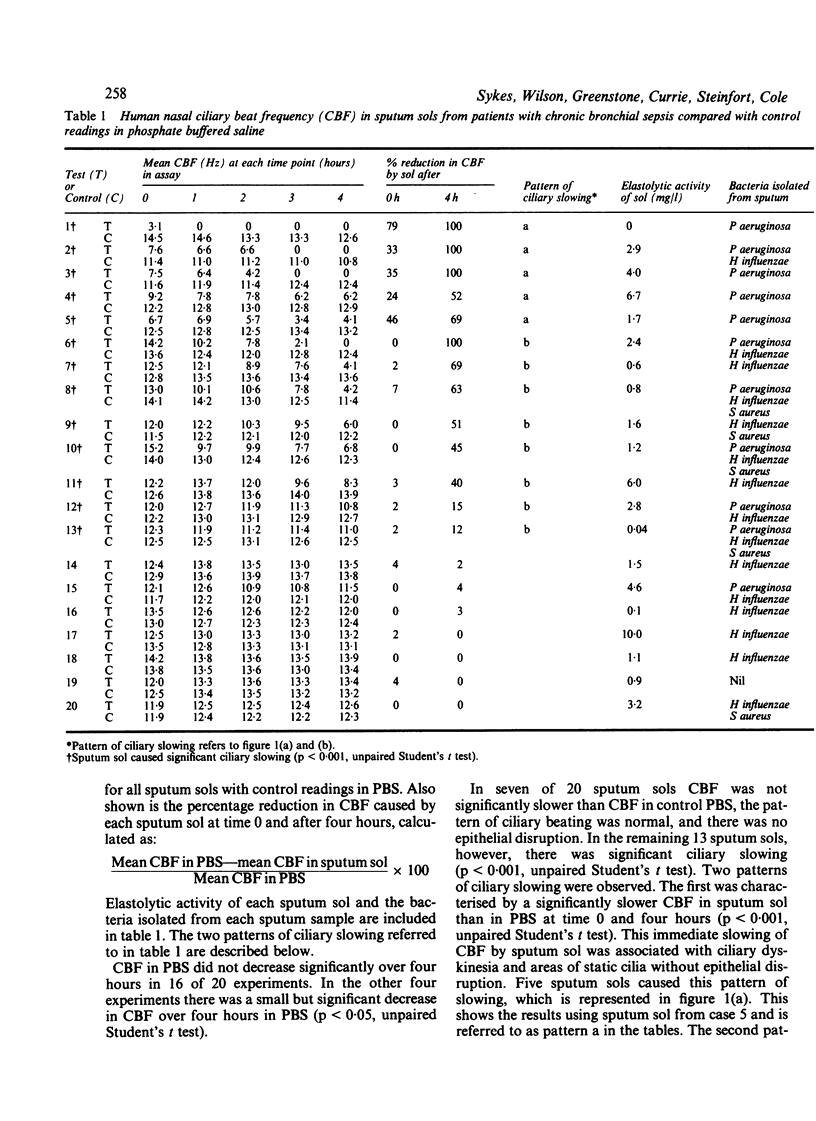
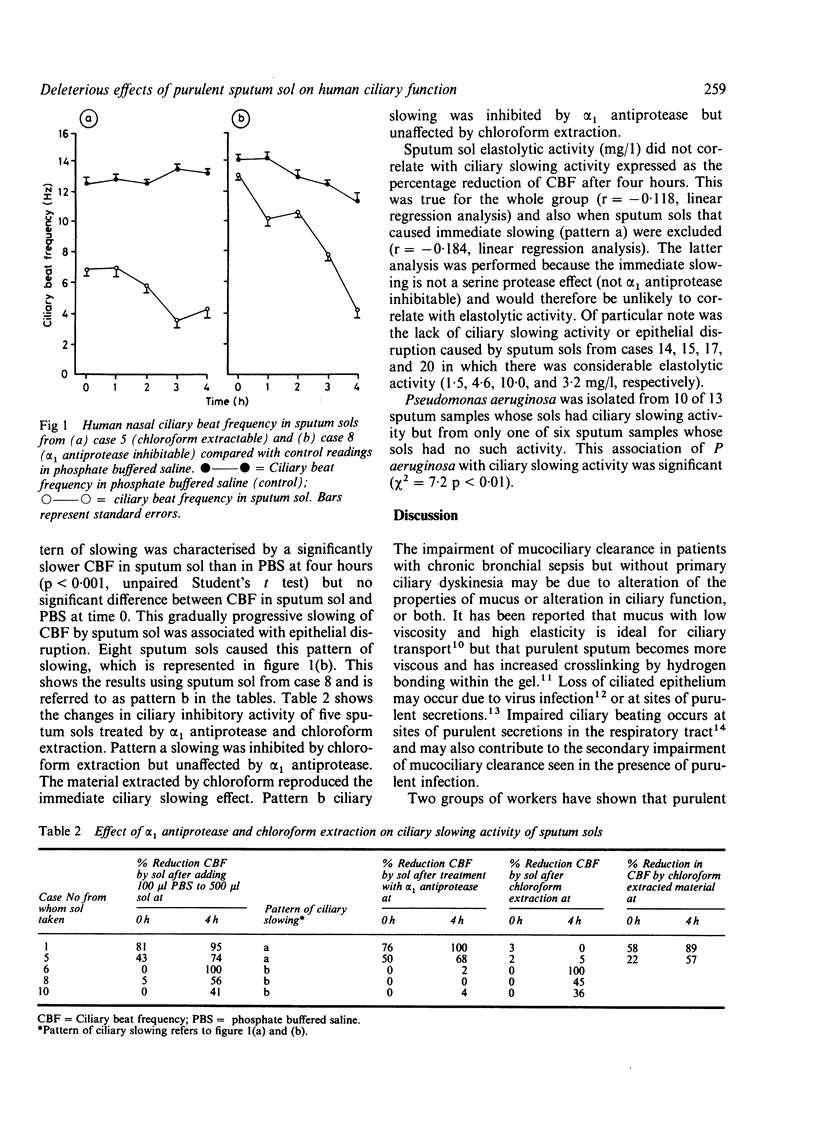
Patients with chronic bronchial sepsis have impaired mucociliary clearance. A study was carried out on the effect of sputum sol (obtained by rapid centrifugation of purulent sputum) from 20 patients with chronic bronchial sepsis on the beating of human nasal cilia in vitro by a photometric technique. Thirteen sols caused significant (p less than 0.001) ciliary slowing. Two patterns of slowing were observed: firstly, a gradual onset associated with epithelial disruption (inhibited by alpha 1 antiprotease) and, secondly, an immediate onset associated with ciliary dyskinesia and ciliostasis (inhibited by chloroform extraction). The ciliary slowing activity of sputum sols was associated with the isolation of Pseudomonas aeruginosa (p less than 0.01). It is concluded that purulent sputum contains at least two factors that impair ciliary beating--one a serine protease, which is probably a product released by the host's phagocytic defences, and the other, which is chloroform extractable and probably a bacterial product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denny F. W. Effect of a toxin produced by Haemophilus influenzae on ciliated respiratory epithelium. J Infect Dis. 1974 Feb;129(2):93–100. doi: 10.1093/infdis/129.2.93. [DOI] [PubMed] [Google Scholar]
- Dulfano M. J., Adler K. B. Physical properties of sputum. VII. Rheologic properties and mucociliary transport. Am Rev Respir Dis. 1975 Sep;112(3):341–347. doi: 10.1164/arrd.1975.112.3.341. [DOI] [PubMed] [Google Scholar]
- Eliasson R., Mossberg B., Camner P., Afzelius B. A. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med. 1977 Jul 7;297(1):1–6. doi: 10.1056/NEJM197707072970101. [DOI] [PubMed] [Google Scholar]
- GODBER G. E. Health services, past, present and future. Lancet. 1958 Jul 5;2(7036):1–6. doi: 10.1016/s0140-6736(58)90001-1. [DOI] [PubMed] [Google Scholar]
- Kollberg H., Mossberg B., Afzelius B. A., Philipson K., Camner P. Cystic fibrosis compared with the immotile-cilia syndrome. A study of mucociliary clearance, ciliary ultrastructure, clinical picture and ventilatory function. Scand J Respir Dis. 1978;59(6):297–306. [PubMed] [Google Scholar]
- Lourenço R. V., Loddenkemper R., Carton R. W. Patterns of distribution and clearance of aerosols in patients with bronchiectasis. Am Rev Respir Dis. 1972 Dec;106(6):857–866. doi: 10.1164/arrd.1972.106.6.857. [DOI] [PubMed] [Google Scholar]
- McGillivray D. H., Burnett D., Afford S. C., Stockley R. A. An evaluation of four methods for the measurement of elastase activity. Clin Chim Acta. 1981 Apr 9;111(2-3):289–294. doi: 10.1016/0009-8981(81)90200-x. [DOI] [PubMed] [Google Scholar]
- Puchelle E., Tournier J. M., Petit A., Zahm J. M., Lauque D., Vidailhet M., Sadoul P. The frog palate for studying mucus transport velocity and mucociliary frequency. Eur J Respir Dis Suppl. 1983;128(Pt 1):293–303. [PubMed] [Google Scholar]
- Reimer A., Klementsson K., Ursing J., Wretlind B. The mucociliary activity of the respiratory tract. I. Inhibitory effects of products of Pseudomonas aeruginosa on rabbit trachea in vitro. Acta Otolaryngol. 1980 Nov-Dec;90(5-6):462–469. doi: 10.3109/00016488009131749. [DOI] [PubMed] [Google Scholar]
- Reimer A., von Mecklenburg C., Toremalm N. G. The mucociliary activity of the upper respiratory tract. III. A functional and morphological study on human and animal material with special reference to maxillary sinus diseases. Acta Otolaryngol Suppl. 1978;356:1–20. [PubMed] [Google Scholar]
- Roberts D. E., Cole P. Use of selective media in bacteriological investigation of patients with chronic suppurative respiratory infection. Lancet. 1980 Apr 12;1(8172):796–797. doi: 10.1016/s0140-6736(80)91295-7. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordelli D. O., Cerquetti M. C., Morris Hooke A., Bellanti J. A. Effect of chemotactins released by Staphylococcus aureus and Pseudomonas aeruginosa on the murine respiratory tract. Infect Immun. 1985 Aug;49(2):265–269. doi: 10.1128/iai.49.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. J., Wilson R., Greenstone M. A., Mackay I. S., Cole P. J. Abnormal nasal mucociliary clearance in patients with rhinitis and its relationship to concomitant chest disease. Br J Dis Chest. 1985 Jan;79(1):77–82. doi: 10.1016/0007-0971(85)90010-5. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M., Starkie C. M. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984 Jun;39(6):408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegner H., Ohlsson K., Toremalm N. G., von Mecklenburg C. Effect of human leukocyte enzymes on tracheal mucosa and its mucociliary activity. Rhinology. 1979 Sep;17(3):199–206. [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Currie D., Cole P. J. Beat frequency of cilia from sites of purulent infection. Thorax. 1986 Jun;41(6):453–458. doi: 10.1136/thx.41.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Wanner A., Hirsch J., Farrell P. M. Tracheal mucociliary transport in patients with cystic fibrosis and its stimulation by terbutaline. Am Rev Respir Dis. 1975 Jun;111(6):733–738. doi: 10.1164/arrd.1975.111.6.733. [DOI] [PubMed] [Google Scholar]



