Abstract
Background: Oral lichen planus (OLP) has been classified as a pre-malignant condition. Matrix metalloproteinase-13 (MMP-13) or collagenase-3 may play a key role in cancer development. The aim of this study was to compare serum and saliva MMP-13 between patients with oral lichen planus (OLP) and oral squamous cell carcinoma (OSCC).
Methods: This cross sectional study was performed on 30 patients with OLP (8 reticular and 22 erosive forms) and 20 patients with OSCC (6 in low stage and 14 in advanced stage) who were selected randomly. The study was conducted at the Cancer Department, Clinic of Oral Medicine, Tehran University of Medical Sciences. The serum and saliva MMP-13 were assayed by ELISA method. Statistical analysis of the Student’s t-test, ANOVA and Pearson correlation coefficient was performed.
Results: There were no significant differences in mean saliva and serum levels of MMP-13 between patients with OSCC and OLP and their subgroups. Serum MMP-13 correlated significantly with unstimulated (r = 0.307, p= 0.048), but not with stimulated, saliva MMP-13.
Conclusion: Serum and saliva MMP-13 levels appear to be statistically similar in OLP and OSCC.
Keywords: MMP-13, Oral lichen planus, Squamous cell carcinoma, Saliva
Introduction
Lichen planus is a chronic, recurrent inflammatory disease which may affect both skin and mucosa. The most involved mucosa is the oral and/or genital (1). The pathogenesis of Oral Lichen Planus (OLP) is not clear, and both antigen specific and non-specific mechanisms maybe involved (2,3). OLP is a common disease with a prevalence of 1-2% in the general adult population (2,4); it is also the most common non-infectious oral mucosal disease, affects women more than men, and occurs predominantly in adulthood (4). Breaks, branches, and duplications are common epithelial basement membrane changes in OLP and, basement membrane disruption may trigger keratinocyte apoptosis. Since the apoptotic keratinocytes may not be able to repair the disruption of basement membrane, such a cyclic mechanism may cause the chronicity of the disease (5). Studies have estimated the malignant potential of OLP in the range of 0.4 to 6.5%, and WHO has classified OLP as a pre-malignant condition. Underlying molecular mechanisms that initiate the development of oral cancer in OLP patients have not been clearly identified yet (6-8). Oral squamous cell carcinoma (OSCC) is one of the most common cancers in the world. At the present time survival rates are extremely poor, although there are advances in surgery, radiation and chemotherapy (9). The epidemiologists of cancer believe that smoking, daily diet and inflammation/infection are the three major factors for causing cancers. OSCC can develop from apparently normal epithelium or pre-cancerous lesions. Prognosis of OSCC is not good. After curative treatment, about 50% of patients suffer recurrence, 80% within 2 years and 20% within four years (10).
It has been accepted that host-related factors may have a key role in a fundamental understanding of the diseases processes. One of these host factors is the matrix methaloproteinases (MMPs) (11). MMPs are often up-regulated in groups forming activation cascades both in inflammatory and malignant diseases (11). MMPs are a large family of zinc-dependent endopeptidases which are capable of degrading basement membrane components and extracellular matrix (12). The importance of MMPs is that, as the MMPs increase, the tumor cell can invade the surrounding tissue, following which many MMPs are able to play vital roles in growth, cell migration and angiogenesis. Matrix metalloproteinase-13 (MMP-13) or collagenase-3 was originally cloned from a breast tumor and cleaves preferentially type II collagen. It is expressed during pathological situations, such as in squamous cell carcinomas of different organs. Some authors have demonstrated elevation of MMP-13 in head and neck SCC, and have suggested that it may be a prognostic biomarker in cancer (13). In the oral cavity, potentially abnormal markers/cells are sloughed off directly or indirectly into saliva; therefore its use as a diagnostic fluid could have diagnostic and prognostic significance. Saliva is an ideal diagnostic tool for biomarker assessment, due to several advantages – it is a cost-effective means to monitor; its collection is safe, simple and non- invasive; and it may be collected repeatedly without any discomfort to the patient (9). The purpose of the current study was to examine the relationship between serum and saliva level of MMP-13, and to compare saliva and serum MMP-13 among patients with OLP and OSCC.
Methods
Patient selection
Twenty patients with SCC (13 male, 7 female, aged 28–77), proven by biopsy and pathological examination, referred to the Cancer Department of Emam Khomeini Hospital of Tehran University of Medical Sciences (TUMS), were selected according to inclusion and exclusion criteria of the study. The most frequent complications in patients with OSCC were exophytic mass in mouth (buccal, marginal of tongue, floor of the mouth) interfering with eating, moderate or severe pain, and ill-fitting dentures. Mean time of presence of the complication was about six months.
Based on WHO modified lichen planus diagnostic criteria, 20 patients with OLD (6 men, 24 women; aged 32–77 years) were selected. Patietns were referred to the Department of Oral Medicine, Faculty of Dentistry, TUMS. Inclusion criteria consisted of being clinically diagnosed with lichen planus (presence of bilateral lesions, and presence of reticular lesions elsewhere in the oral); and the presence of well-defined band-like zones of inflammatory infiltration confined to the superficial part of the connective tissue, consisting mainly of mature lymphocytes – vacuolar alteration of the basal layer of the epithelium, identified through histopathological examination. Exclusion criteria were histological sign of dysplasia, lichenoid drug reactions, drug consumption in the past month, and any kind of localized or systemic disease. The inclusion and exclusion criteria in this study were the same as previous studies (10).
The protocol was approved by the Review Board of TUMS, and written informed consent was obtained from all of patients.
Saliva collection
Stimulated and unstimulated whole saliva were collected under resting conditions in a quiet room between 10.00 AM and 12.00 PM, and at least 120 minutes after the last intake of food, drink or smoking. Five milliliterof unstimulated whole salivary samples were obtained by expectoration in the absence of chewing movements. One of the best methods to collect whole saliva is spitting method, in which the subject expectorates saliva into a test tube.
Pre-stimulation was accomplished by asking patients to chew a piece of standard-size paraffin for 60 seconds before swallowing the saliva present in the mouth. Thereafter, whole stimulated saliva was collected for about 5 minutes in a dry, de-ionized and sterilized plastic tube. Blood specimens were obtained by venipuncture, collected in 10 ml glass vacuum tubes without additive, and allowed to clot. The blood and saliva were sampled after the diagnosis had been established, and before any therapy undertaken. The blood and saliva were then centrifuged (2500 g, 10 min), and the serum and supernatants of saliva were separated and immediately stored at -70°C for later assessment.
Laboratory measurements
Serum and saliva concentrations of MMP-13 were determined by Enzyme-linked immunosorbent assay using ELISA kits (Boster biological technology, USA). Determination of MMP-13 levels was carried out according to the manufacturer's instruction.
Statistical analysis
Data are presented as a mean±SEM. Comparison of means among groups were carried out with unpaired two-tailed Student’s t-test or ANOVA followed by the Student-Newman-Keuls as post-hoc test. The Pearson correlation test was applied to determine association between serum and salivary concentration of MMP-3. Results were considered statistically significant if p< 0.05. Analyses were performed using SPSS 16.
Results
The study involved two groups. Group I consisted of 30 patients suffering from OLP (6 males, 24 females), with a mean ±SD age of 51.4+11.60 years. A sub-group of 8 had the reticular form and a sub-group of 22 the erosive form. The buccal mucosa was the most common site for OLP (50% (n=15), followed by the gingiva (30% (n=9)) and tongue (20% (n=6)). Group II consisted of 20 patients suffering from OSCC (13 males, 7 females) with a mean ±SD age of 50±12.9 years. A sub-group of 6 were in the low stages (1 and 2), and a sub-group of 14 patients in the advanced stage (3 and 4). The tongue was the most common site (45% (n=9)), followed by the lip (30% (n=6)), buccal (15% (n=3)), the floor of the mouth (5% (n=1)) and the palate (5% (n=1)).
Student’s t-test showed that there were no significant differences in the serum, unstimulated and stimulated saliva MMP-13 levels between OLP and OSCC groups (p>0.05; Fig. 1).
Fig. 1 .
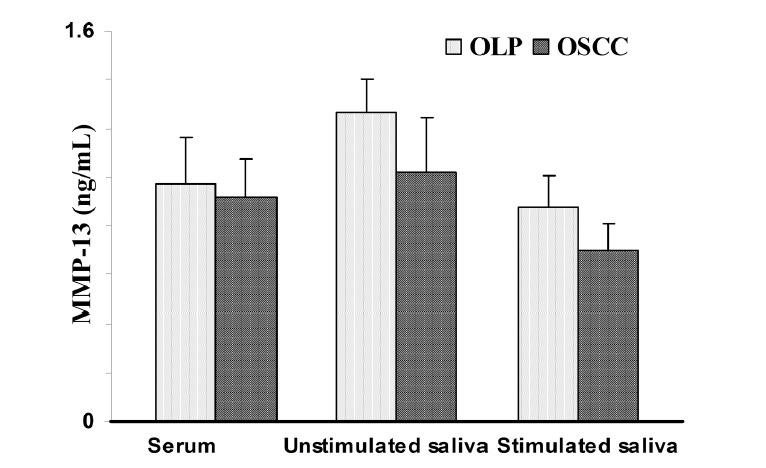
Matrix metalloproteinase-13 (MMP-13) concentration in patients with oral lichen planus (OLP) and oral squamous cell carcinoma (OSCC). Data are expressed as mean ± SEM.
One-way ANOVA indicated that there were no significant differences in serum and saliva MMP-13 concentrations among erosive and reticular forms of OLP, stages 1,2 and stages 3,4 of OSCC groups (p>0.05; Fig. 2).
Fig 2 .
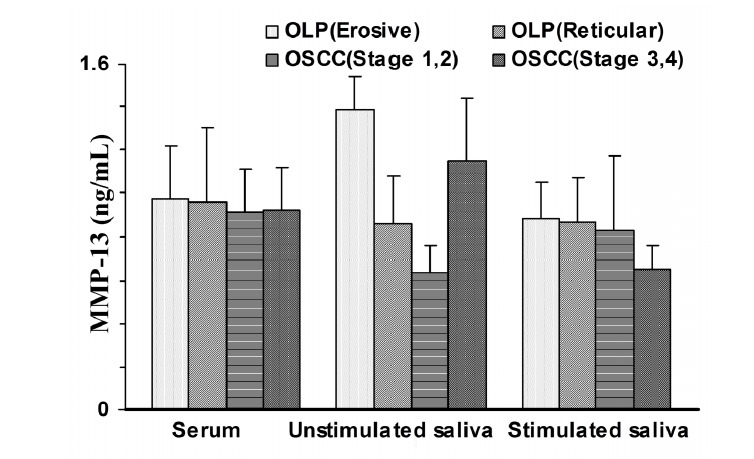
Matrix metalloproteinase-13 (MMP-13) concentration in patients with reticular and erosive form of oral lichen planus (OLP) and oral squamous cell carcinoma (OSCC) at stages 1,2 and stages 3,4. Data are expressed as mean ± SEM.
Statistical evaluation of data using Pearson analysis indicated that serum MMP-13 weakly correlated with unstimulated salivary MMP-13 (r= 0.307, p= 0.048), but not with stimulated salivary MMP-13.
There were no significant differences in serum and saliva MMP-13 between OLP and OSCC patients in each gender; and also between male and female (Table 1).
Table 1 . Matrix metalloproteinase-13 (MMP-13) concentration in male and female patients with oral lichen planus (OLP) and oral squamous cell carcinoma (OSCC) .
| Parameter | Male | Female | ||
| OLP* | OSCC | OLP | OSCC | |
| Serum (ng/mL) | 1.08 ± 0.24 | 0.64 ± 0.07 | 0.57 ± 0.03 | 1.10 ± 0.25 |
| Unstimulated saliva (ng/mL) | 1.29 ± 0.16 | 0.97 ± 0.43 | 1.17 ± 0.33 | 1.05 ± 0.27 |
| Stimulated saliva (ng/mL) | 0.94 ± 0.16 | 0.54 ± 0.06 | 0.64 ± 0.03 | 0.62 ± 0.17 |
*Data are expressed as Mean±SEM.
Discussion
The key finding of this study is that serum MMP-13 concentrations did not differ between patients with OLP and OSCC. Moreover, saliva levels of MMP-13 at resting, and stimulated saliva flow rates did not show any difference between the two groups and between male and female. To the best of our knowledge, this is the first study to assess MMP-13 in stimulated and unstimulated saliva in OSCC and OLP.
MMP-13, a catalytically very competent protease, is expressed by malignantly transformed epidermal keratinocytes - for example, squamous carcinoma cells in culture and in vivo - but it is not expressed by normal epidermal keratinocytes in culture or in vivo (14). This indicates that MMP-13 expression serves as a marker for malignant transformation of squamous epithelial cells, and suggests an important role for MMP-13 in the invasion of SCC cells. MMP-13 has also an essential role in other invasive malignancies.WHO has described OLP as a pre-malignant condition, with a possible progression to oral squamous cell carcinoma (13,15-18). Our results show that there is no significant difference between OLP and OSCC regarding MMP-13 levels, which is consistent with the WHO description.
It is clear that MMPs are often up-regulated in groups forming activation cascades both in inflammatory and malignant diseases (11). OLP is a chronic and inflammatory disease with the potential ability to undergo malignant transformation. In addition, chronic infection and tissue inflammation are now undoubtedly recognized as risk factors in various types of human cancers, as more evidence of the close link between inflammation and cancer accumulates. Therefore, the MMPs might play multiple roles in pathogenesis of OLP and its cancerization (12). However, the process from OLP to OSCC is complicated and regulated by many factors (12).
We found merely a poor correlation between serum concentration of MMP-13 and resting saliva of this biochemical. Additionally, serum MMP-13 was not associated with stimulated salivary parameters. Furthermore, saliva levels of MMP-13 were similar to its serum concentrations. This might be due to the ubiquitous expression of MMP-13. If serum MMP-13 was the only source of salivary MMP-13, we would expect to find a close connection between the serum and saliva values of this peptide. It is possible that MMP-13 is produced by the salivary gland and released into oral fluids. Owing to its peptide structure, MMP-13 seems unlikely to be actively transported from plasma and accumulated into salivary secretions. Our observation, therefore, is in favor of salivary production and secretion of MMP-13.
The rationale of our study for measuring MMP-13 in salivary secretions is that saliva is being considered as a diagnostic fluid of the future. Saliva is believed to be a mirror of the body and may be acknowledged as a promising medium for monitoring the health and disease states of an individual in healthcare programs. Several lines of evidence have consistently validated and proposed using salivary assays for diagnosing, monitoring or predicting prognosis of diseases. In this regard, it has been shown that several biochemical molecules can be measured in oral fluids of diseased patients; for example, steroid hormones, such as cortisol (19), progesterone (20) and 17β-estradiol (21); and protein/polypeptide hormones, such as CA15-3 (22), CA125 (23), creatine kinase MB (24), creatine phosphokinase (25) and parathyroid hormone (26). Much of the attention saliva receives as a biological specimen is due to the quick, uncomplicated and non-invasive nature of sample collection. Furthermore, oral fluid sampling is safe for both the operator and the patient and has easy and low-cost storage. To establish saliva as an alternative medium to plasma for various biological assays, there must be a high correlation between plasma and saliva levels of measured parameters (24). The lack of such a close association between serum and saliva measures of MMP-13 implies that salivary MMP-13 may not be a good alternative for serum MMP-13 in diagnostic assays, screening programs or epidemiological studies.
Conclusion
Serum and saliva MMP-13 levels appear to be statistically similar in OLP and OSCC.
Cite this article as: Agha-Hosseini F, Mirzaii-Dizgah I. Serum and saliva collagenase-3 (MMP-13) in patients with oral lichen planus and oral squamous cell carcinoma. Med J Islam Repub Iran 2015 (7 June). Vol. 29:218
References
- 1.Nylander E, Ebrahimi M, Wahlin YB, Boldrup L, Nylander K. Changes in miRNA expression in sera and correlation to duration of disease in patients with multifocal mucosal lichen planus. J Oral Pathol Med. 2012;41(1):86–9. doi: 10.1111/j.1600-0714.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 2.Agha-Hosseini F, Mirzaii-Dizgah I, Mikaili S, Abdollahi M. Increased salivary lipid peroxidation in human subjects with oral lichen planus. Int J Dent Hyg. 2009;7(4):246–50. doi: 10.1111/j.1601-5037.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 3.Agha-Hosseini F, Mirzaii-Dizgah I, Abdollahi M, Akbari-Gillani N. Efficacy of IMOD in the treatment of oral lichen planus. Open Journal of Stomatology. 2011;1:13–17. [Google Scholar]
- 4.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39(10):729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A. et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13(4):350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 6.Giacomelli L, Oluwadara O, Chiappe G, Barone A, Chiappelli F, Covani U. Relationship between human oral lichen planus and oral squamous cell carcinoma at a genomic level: a datamining study. Bioinformation. 2009;4(6):258–62. doi: 10.6026/97320630004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha-Hosseini F, Khalili M, Rohani B. Immunohistochemistry Analysis of P53 and Ki-67 proteins in oral lichen planus and normal oral mucosa. Iranian J Publ Health. 2009;38(2):37–43. [Google Scholar]
- 8.Agha-Hosseini F, Borhan-Mojabi K, Monsef-Esfahani HR, Mirzaii-Dizgah I, Etemad-Moghadam S, Karagah A. Efficacy of purslane in the treatment of oral lichen planus. Phytother Res. 2010;24(2):240–4. doi: 10.1002/ptr.2919. [DOI] [PubMed] [Google Scholar]
- 9.Stott-Miller M, Houck JR, Lohavanichbutr P. et al. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2628–36. doi: 10.1158/1055-9965.EPI-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agha-Hosseini F, Mirzaii-Dizgah I, Farmanbar N, Abdollahi M. Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med. 2012;41:736–40. doi: 10.1111/j.1600-0714.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 11.Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10(6):311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhang W, Geng N, Tian K, Jack Windsor L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck. 2008;30(9):1237–45. doi: 10.1002/hed.20869. [DOI] [PubMed] [Google Scholar]
- 13.Mäkinen LK, Häyry V, Atula T. et al. Prognostic significance of matrix metalloproteinase-2, -8, -9, and -13 in oral tongue cancer. J Oral Pathol Med. 2012;41(5):394–9. doi: 10.1111/j.1600-0714.2011.01110.x. [DOI] [PubMed] [Google Scholar]
- 14.Ala-aho R, Ahonen M, George SJ. et al. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene. 2004;23(30):5111–23. doi: 10.1038/sj.onc.1207678. [DOI] [PubMed] [Google Scholar]
- 15.Culhaci N, Metin K, Copcu E, Dikicioglu E. Elevated expression of MMP-13 and TIMP-1 in head and neck squamous cell carcinomas may reflect increased tumor invasiveness. BMC Cancer. 2004;4:42. doi: 10.1186/1471-2407-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corte MD, Gonzalez LO, Corte MG. et al. Collagenase-3 (MMP-13) expression in cutaneous malignant melanoma. Int J Biol Markers. 2005;20(4):242–8. doi: 10.1177/172460080502000407. [DOI] [PubMed] [Google Scholar]
- 17.Luukkaa H, Klemi P, Hirsimäki P. et al. Matrix metalloproteinase (MMP)-1, -9 and -13 as prognostic factors in salivary gland cancer. Acta Otolaryngol. 2008;128(4):482–90. doi: 10.1080/00016480801922895. [DOI] [PubMed] [Google Scholar]
- 18.Marcos CA, Martínez DA, de Los Toyos JR. et al. The usefulness of new serum tumor markers in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140(3):375–80. doi: 10.1016/j.otohns.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Agha-Hosseini F, Mirzaii-Dizgah I, Mirjalili N. Relationship of unstimulated saliva cortisol level with severity of oral dryness feeling in menopausal women. Aust Dent J. 2011;56(2):171–4. doi: 10.1111/j.1834-7819.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 20.Mirzaii-Dizgah I, Agha-Hosseini F. Stimulated and unstimulated saliva progesterone in menopausal women with oral dryness feeling. Clin Oral Investig. 2011;15(6):859–62. doi: 10.1007/s00784-010-0449-z. [DOI] [PubMed] [Google Scholar]
- 21.Agha-Hosseini F, Mirzaii-Dizgah I, Mansourian A, Khayamzadeh M. Relationship of stimulated saliva 17 beta-estradiol and oral dryness feeling in menopause. Maturitas. 2009;62(2):197–99. doi: 10.1016/j.maturitas.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A. Correlation of serum and salivary CA15-3 levels in patients with breast cancer. Med Oral Patol Oral Cir Bucal. 2009;4(10):e521–e24. doi: 10.4317/medoral.14.e521. [DOI] [PubMed] [Google Scholar]
- 23.Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A, Seilanian-Toosi M. Correlation of serum and salivary CA125 levels in patients with breast cancer. J Contemp Dent Pract. 2009;10(6):E001–E008. [PubMed] [Google Scholar]
- 24.Mirzaii-Dizgah I, Hejazi SF, Riahi E, Salehi MM. Saliva-based creatine kinase MB measurement as a potential point-of-care testing for detection of myocardial infarction. Clin Oral Investig. 2012;16(3):775–9. doi: 10.1007/s00784-011-0578-z. [DOI] [PubMed] [Google Scholar]
- 25.Mirzaii-Dizgah I, Jafari-Sabet M. Unstimulated whole saliva creatine phosphokinase in acute myocardial infarction. Oral Dis. 2011;17(6):597–600. doi: 10.1111/j.1601-0825.2011.01817.x. [DOI] [PubMed] [Google Scholar]
- 26.Agha-Hosseini F, Mirzaii-Dizgah I, Mansourian A, Zabihi-Akhtechi G. Serum and stimulated whole saliva parathyroid hormone in menopausal women with oral dry feeling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(6):806–10. doi: 10.1016/j.tripleo.2009.01.024. [DOI] [PubMed] [Google Scholar]


