Abstract
The neural crest is a transient structure of vertebrate embryos that initially generates neural crest stem cells (NCSCs) which then migrate throughout the body to produce a diverse array of mature tissue types. Due to the rarity of adult NCSCs as well as ethical and technical issues surrounding isolation of early embryonic tissues, biologic studies of human NCSCs are extremely challenging. Thus, much of what is known about human neural crest development has been inferred from model organisms. In this study, we report that functional NCSCs can be rapidly generated and isolated from in vitro—differentiated human embryonic stem cells (hESCs). Using the stromal-derived inducing activity (SDIA) of PA6 fibroblast co-culture we have induced hESCs to differentiate into neural crest. Within 1 week, migrating cells that express the early neural crest markers p75 and HNK1 as well as numerous other genes associated with neural crest induction such as SNAIL, SLUG, and SOX10 are detectable. Fluorescence-activated cell sorting (FACS)-based isolation of the p75-positive population enriches for cells with genetic, phenotypic, and functional characteristics of NCSCs. These p75-enriched cells readily form neurospheres in suspension culture, self-renew to form secondary spheres, and give rise under differentiation conditions to multiple neural crest lineages including peripheral nerves, glial, and myofibroblastic cells. Importantly, these cells differentiate into neural crest derivatives when transplanted into developing chick embryos in vivo. Thus, this SDIA protocol can be used to successfully and efficiently isolate early human NCSCs from hESCs in vitro. This renewable source of NCSCs provides an invaluable source of cells for studies of both normal and disordered human neural crest development.
Introduction
The neural crest is a transient structure that originates from the dorsal neural tube at the early stages of vertebrate embryonic development. It gives rise to a vast array of histologically and geographically diverse cell types including, among others, skeletal cells, myofibroblasts, melanocytes, and nearly all the cells of the peripheral nervous system including sympathetic and parasympathetic ganglia, the adrenal medulla, autonomic and sensory neurons, and supporting glial cells (i.e., Schwann and satellite) (reviewed in ref. 1). Given the diversity of its progeny, it is not surprising that disruption of the neural crest adversely affects human development and several complex human disorders such as DiGeorge, Waardenburg and Apert syndromes, neurofibromatosis, and Hirschsprung's disease arise because of aberrations in neural crest development [2,3]. In addition, several aggressive malignancies including malignant melanoma, neuroblastoma, and peripheral primitive neuroectodermal tumors—also known as Ewing sarcoma family tumors—have been proposed to arise because of genetic mutations in neural crest–derived cells [2,4–7]. Thus, elucidation of human neural crest development and the mechanisms by which it is disrupted in human disease has the potential to profoundly impact our understanding of disease pathogenesis and, ultimately, afford opportunities for novel therapeutic interventions.
To date, knowledge of human neural crest development has largely been acquired from studies of model organisms that have defined the genetic and phenotypic characteristics of neural crest cells at various developmental stages [8]. Through a complex series of tightly regulated molecular signals, ectodermal cells are first induced to create neural crest stem cells (NCSCs). Multipotent NCSCs then respond to differentiation and migration signals and undergo progressive fate restrictions, giving rise to progenitor cells (NCPCs) with varying degrees of self-renewal and differentiation potential that are determined by both cell autonomous as well as environmental factors (reviewed in ref. [1]).
Induction and differentiation of embryonic neural crest occurs very early, within only a few weeks of fertilization [9,10]. Thus, the feasibility of acquiring primary human neural crest cells from embryonic tissue is severely challenged by both ethical and technical issues—in fact, neural crest induction and migration is underway long before most women even realize that they are pregnant. Although NCSCs have been isolated from some human adult tissues, they are exceedingly rare and do not possess the same self-renewal and pluripotency of early embryonic NCSCs [1,11,12]. Therefore, to fully advance in vitro studies of human neural crest development alternate renewable sources of precursor cells will be required.
In recent years, pluripotent embryonic stem cells have been shown to be an invaluable source of cells for studies of developmental biology and regenerative medicine. Embryonic stem cells can be differentiated into cell types from all three germ layers (endo-, meso-, and ectoderm) and differentiation along specific lineages can be directed by altering growth conditions in defined media [13]. Importantly, culture of murine, primate, and human embryonic stem cells (hESCs) on PA6 murine stromal fibroblasts can be used to selectively induce terminal differentiation of neural crest derivatives as a result of so-called stromal-derived inducing activity (SDIA) of the PA6 feeder layer [14,15]. In this study, we report that SDIA-treated hESC transition through a neural crest precursor stage on the path to terminal differentiation and that isolation of p75-positive cells after 1 week successfully enriches for multipotent cells with genetic, phenotypic, and functional characteristics of NCSCs. This p75-enriched cell population forms neurospheres in suspension culture and gives rise to peripheral nerves, glial and myofibroblastic cells under differentiation conditions. Thus, hESCs represent a renewable source of NCSCs that can be readily and rapidly accessed for studies of both normal and disordered human neural crest development.
Materials and Methods
Cell culture
Undifferentiated hESCs H9 (WA-09) and H1 (WA-01) were cultured as described previously [16] on irradiated mouse embryo fibroblast (MEF) feeder layers in medium consisting of a 1:1 mixture of DMEM and nutrient mixture F-12 supplemented with 20% knockout serum replacement, 1% nonessential amino acids stock, 1 mM l-glutamine, 4 ng/mL basic fibroblast growth factor (bFGF) (all from Invitrogen, Carlsbad, CA), and 0.1 mM -mercaptoethanol (Sigma Chemical Corp., St. Louis, MO). All hESC studies were performed with the assurance of the CHLA Stem Cell Research Oversight Committee. The cells were passaged by semi-weekly splits of 1:3 and replated on fresh irradiated MEF feeder layers to maintain an undifferentiated state. Human ESCs were monitored weekly by fluorescence-activated cell sorting (FACS) analysis and only cultures with >90% SSEA-4+ (R&D Systems Inc., Minneapolis, MN) and <1% SSEA-1+ (R&D, Minneapolis, MN) cells were used. The mouse stromal PA6 cell line (Riken BSI Research, Japan), was cultured on gelatin-coated dishes in 90% α-MEM and 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA).
Neural crest differentiation of hESCs was induced as described [15] with some modification. Briefly, hESC colonies were treated with collagenase (Invitrogen, Carlsbad, CA), mechanically sectioned into clumps and transferred into PA6-coated dishes at densities of up to 1,000 colonies per 3-cm dish. Media was then changed to induction medium containing 90% BHK-21 medium/Glasgow modified Eagle's medium (MEM), 10% knockout serum replacement, 2 mM glutamine, 1 mM pyruvate, 0.1 mM nonessential amino acid solution, and 0.1 mM β-mercaptoethanol. On day 6, the media was changed to induction medium with 1X N2 supplement (Invitrogen) and replaced every 2 days thereafter.
FACS analysis and purification
Human ESC colonies were dissociated by incubating them for 5 min at 37°C in Accumax (Chemicon, Temecula, CA). Cells were gently triturated and filtered through a 30 μm cell strainer to obtain a single-cell suspension. The dissociated hESCs were then blocked with anti-human Fc-receptor (Miltenyi Biotec, Bergisch Gladbach, Germany) in staining medium containing L15 medium with 10 mM HEPES and 1 mg/mL bovine serum albumin (BSA). Following Fc blocking, cells were incubated with the phycoerythrin (PE)-conjugated anti-p75 (Miltenyi Biotec) for 15 min in the dark at 4°C. For HNK-1 and p75 double staining, the cells were stained with HNK-1 (Abcam, Cambridge, MA) first, followed by anti-mouse IgG FITC (Jackson ImmunoResearch Laboratories, West Grove, PA), and then the conjugated antip75-PE. Cells were washed with staining media before analysis using a FACS Calibur (BD Biosciences, San Jose, CA). Cell isolation was conducted on a FACS Vantage or FACS Aria (BD Biosciences). Compensation for FITC and PE was performed using compensation beads (BD Pharmingen, San Jose, CA). Analysis was done using the Flow-Jo program (Tree Star, Ashland, OR). Positive and negative gates were determined using IgG stained and unstained controls.
Limiting dilution analysis
Limiting dilution analysis was performed as described [17]. Cells were plated in either 96-microwell or six-well ultralow attachment plates (Costar, Corning, Lowell, MA) in media consisting of DMEM-F12 (1:1), 7.5% chick embryo extract (US Biological, Swampscott, MA), N2, B27, 20 ng/mL bFGF, 20 ng/mL EGF, 20nM IGF-1, and 0.1 mM β-mercaptoethanol. For microwell plates, cell numbers were adjusted to 5,000 cells/mL from which serial dilutions were prepared. Final cell dilutions ranged from 1,000 cells per well to 1 cell per well in 200 μL aliquots. After 7 days the fraction of wells not containing spheres was determined. The number of cells required to form one sphere, which reflected the proportion of stem cells in the entire population, was then determined from the point at which the line crossed 0.37 level. That is, F0 = e−x, where F0 is the fraction of wells without spheres and x is the mean number of cells per well. Based upon a Poisson distribution of cells, F0 = 0.37 corresponds to the dilution at which there is one stem cell per well.
In other experiments, cells were re-suspended and plated onto six-well ultra-low-attachment plates at serial dilutions (5–100 × 103 cells/well). The number of spheres was counted after 1 week and the data presented are mean ± standard deviations (SD) of three independent experiments.
Subcloning and differentiation assays
For subcloning p75+ and p75− primary spheres were cultured in low attachment plates for 1 week and then single colonies were handpicked and dissociated with Accumax. The dissociated cells were immediately transferred into centrifuge tubes containing L15 medium with 0.2% BSA and 10 mM HEPES, triturated, counted by hemocytometer, and spun down at 1,000 rpm for 4 min at 4°C. The re-suspended single cells were then inoculated in low attachment plates at clonal densities (10–30 cells/cm2). After 7–10 days, each well was scored for the number of secondary spheres.
To assess differentiation potential, single p75+ or p75− spheres were handpicked as above and directly plated on four-well or eight-well chamber slides pre-coated with 10 μg/mL Poly-d-lysine and 150 μg/mL fibronectin. The cells were switched to differentiation medium containing DMEM/F12 (1:1) supplemented with N2, B27, and 5% FBS for another 2 weeks before immunocytochemical characterization of colonies.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from sorted and unsorted cells using the RNeasy kit and DNAse I treatment (Qiagen, Valencia, CA). RNA (500 ng) was reverse transcribed using iScript Reverse Transcriptase kit (Bio-Rad, Hercules, CA) in a 20-μL reaction and 2 μL of reverse-transcription product was amplified by polymerase chain reaction (PCR) using standard protocols. Primer sequences, product length and annealing temperatures are provided (Supplementary Table 1; Supplementary materials are available online at http://www.liebertpub.com/scd). Primers were chosen for human specificity wherever possible and PA6 only and no-RT controls were included in all experiments.
Immunocytochemistry
Expression of NCSC markers, neuronal markers, and neural crest lineage markers was examined by immunocytochemistry. For glial fibrillary acidic protein (GFAP) staining, cultures were fixed with a solution of 95% ethanol and 5% glacial acetic acid at −20°C for 20 min. For other antibodies the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After a rinsing in phosphate-buffered saline (PBS), the slides were incubated for 1 h in blocking solution containing 1% bovine serum albumin and 5% donkey serum in PBS. The slides were then incubated with the primary antibodies for 1 h at 37°C or overnight at 4°C. The following antibodies were used at the indicated dilutions: β-III-tubulin/Tuj1 (1:500; CRP Inc., Berkley, CA), peripherin (1:500; Chemicon International Inc., Billerica, MA), Brn3a (1:100; Chemicon), TH (1:100; Santa Cruz Biotech, Santa Cruz, CA), NCAM (1:200; Chemicon), p75 (1: 200; Upstate, Lake Placid, NY), AP2 (1:100; Santa Cruz Biotech), HNK-1 (1:100; Abcam, Cambridge, MA), SMA (1:400; Sigma, Saint Louis, MO), GFAP (1:100; Chemicon). Triple-labeling experiments were performed by simultaneously incubating samples in appropriate combinations of primary antibodies followed by non-cross-reactive secondary antibodies (Alexa fluorophore-conjugated secondary antibodies, 1:1,000; Molecular Probes- Invitrogen, Eugene, OR) or cy-conjugated secondary antibodies (1:500; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). In some samples, nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). The specificity of the light microscopic immunocytochemical procedures was validated by omitting the primary antibodies or by replacing them with nonimmune serum. Stained slides were viewed with a Leica DM RXA Upright Fluorescence Microscope and photographed using a SKY camera on the system (Applied Spectral Imaging, Inc., Carlsbad, CA) and no features were removed or added digitally. For quantitative analysis, differentiated colonies were counted at different time points as indicated. At least three separate experiments were performed for quantification purposes.
In vivo transplantation
Fertilized chicken eggs were obtained from AA Enterprises (AA Enterprises, Ramona, CA) and incubated on their sides at 38°C in humidified incubators (Lyon Electric Co., Chula Vista, CA). The FACS-isolated p75+ cells were transduced with a green fluorescent protein (GFP) lentiviral vector (pCCL-MND-X-PGK-EGFP) and transduction efficiency was ∼90%. Three to four days after transduction, EGFP-labeled p75+ cells were transplanted into the area adjacent to the midbrain of H&H stage 10–12 chick embryos as described [18]. Eggs were incubated for 3 days after transplantation and the embryos were cryoprotected, embedded in gelatin, and cryosectioned at 10 μm. The cryostat sections of chick embryos were then stained with the primary antibodies at the following concentrations: GFP (Abcam, Cambridge, MA; 1:500); SMA (Sigma, Saint Louis, MO; 1:400); Tuj1 (CRP Inc., Cumberland, VA; 1:500); HuC/HuD (Molecular Probes, Eugene, OR; 1:250).
Statistical analysis
Values are reported as means ± SD if not indicated otherwise. Paired t test was used for comparing the sphere formation, subcloning efficiency and clonogenicity of p75+ and p75− cells.
Results
PA6 fibroblast co-culture induces peripheral neuronal differentiation from hESCs
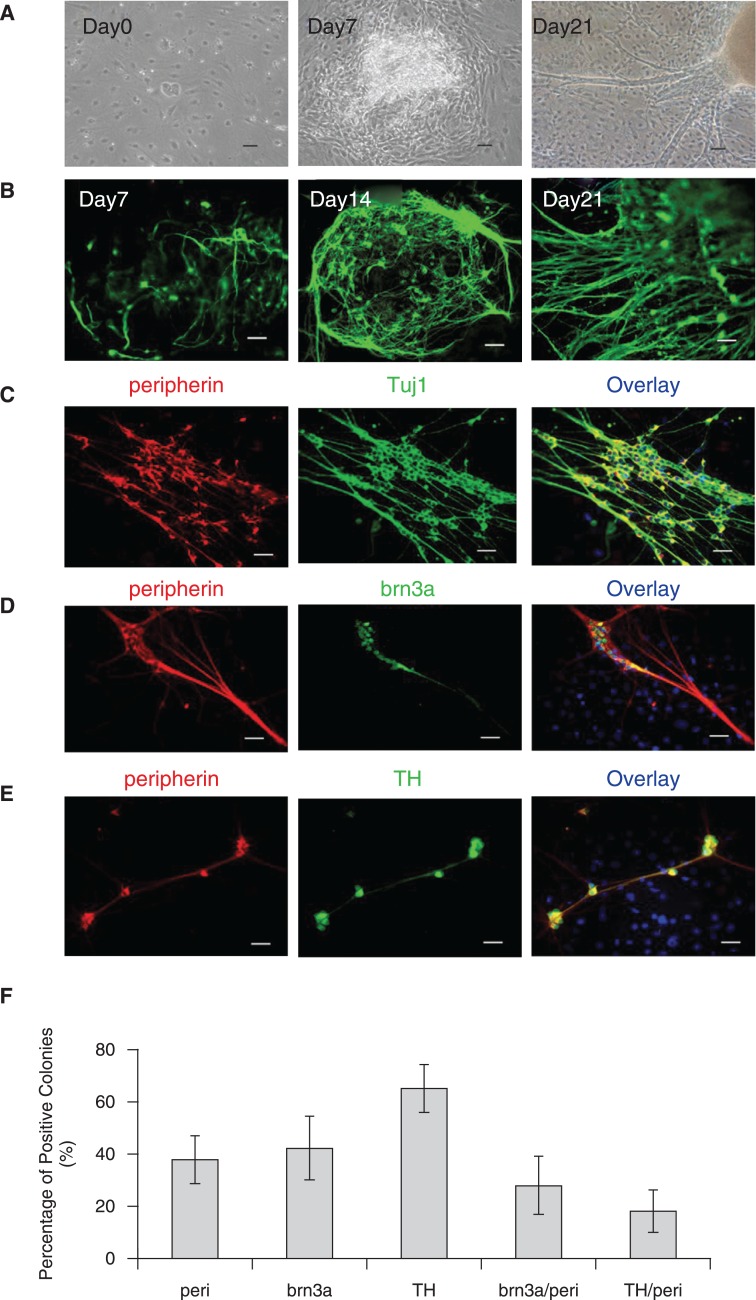
Neural differentiation of undifferentiated SSEA4+/SSEA1− hESCs (H1 and H9 lines; Supplementary Fig. 1) was initiated by culturing on a feeder layer of PA6 stromal cells. By 7 days of culture on PA6 cells, most hESC colonies had generated an outgrowth of elongated cells (Fig. 1A, day 7). After 3 weeks of differentiation, cells that migrated out of the colonies formed a monolayer and displayed morphology characteristic of neurons, with extensive development of fine processes. Prominent fiber bundles formed by processes emanating from the colonies were frequently observed (Fig. 1A, day 21). Colonies positive for the early neural marker TuJ1 first appeared between day 5 and 7 (Fig. 1B, day 7), and the proportion of positive colonies increased during the following weeks of differentiation. Three weeks after seeding hESCs on PA6 cells, massive neuronal differentiation with extensive networks of stained axons was observed using immunocytochemisty (Fig. 1B, day 21). By 4 weeks of differentiation, >90% of the colonies were Tuj1+ (data not shown).
FIG. 1.
Induction of peripheral neurons from human embryonic stem cells (hESCs) by the stromal cell line PA6. For neural induction, H1 or H9 cells were plated at 5–10 × 102/cm2 on a confluent layer of PA6 cells in six-well plates or chamber slides in induction medium as described in Materials and Methods. (A) Phase-contrast photographs of neural differentiation of H9 induced by PA6 over 3 weeks (Scale bar = 200 μm). (B) Expression of the general neuronal marker Tuj1 by H9 colonies on PA6 for 7, 14, or 21 days (Scale bar = 50 μm). (C–E) Immunostaining of H9 colonies after 3 weeks of culture on PA6 (Scale bars = 50 μm). (C) Co-expression of peripherin, an intermediate filament found in peripheral axons (red) and Tuj1 (green). (D) Co-expression of peripherin (red) and the transcription factor Brn3a (green) found primarily in peripheral sensory (PS) neurons. (E) Co-expression of peripherin (red) and tyrosine hydroxylase (TH, green) expressed in putative sympathetic neurons. (F) Quantification of H9 colonies containing Brn3a, TH, and/or peripherin-immunoreactive cells after 4 weeks of culture on PA6. Results are the average of three experiments and error bars = SD.
Next, to ascertain whether peripheral nervous system neurons are present in SDIA-induced cultures, we co-immunostained Tuj1 with peripherin, a class III intermediate neurofilament subunit expressed by peripheral sensory and autonomic neurons [19]. After 4 weeks of SDIA treatment, ∼30% of the colonies contained Tuj1+/peripherin+ peripheral neurons (Fig. 1C). In addition, cells that co-expressed both peripherin and the transcription factor Brn3a, a combination of markers that is characteristic of peripheral sensory (PS) ganglion neurons [20], were apparent in 3-week co-cultures (Fig. 1D). Cells that co-expressed tyrosine hydroxylase (TH) and peripherin, a combination characteristic of sympathetic ganglion (SG) neurons, were also detected (Fig. 1E). Quantitative analysis of the cultures revealed that by 4 weeks of co-culture, approximately one quarter of the colonies contained putative PS neurons while SG neurons were detected in only 18% of colonies (Fig. 1F). However, the number of peripherin+/Brn3a+ cells within each colony was widely variable and very few TH+/peripherin+ double-positive cells were observed in most of the colonies (Fig. 1E). The low yield of SG neurons relative to PS neurons may be due to an increased requirement of bone morphogenic proteins (BMPs) for catecholaminergic compared with differentiation [14]. Further study with addition of BMPs to the induction media will help to resolve if SG differentiation is enhanced under these conditions.
In summary, SDIA co-culture of the hESC cell lines H1 and H9 reliably induces terminal differentiation to peripheral neurons within 3–4 weeks. These data are consistent with earlier studies [15] in which SDIA was used to successfully induce terminal neural crest differentiation from HES-1 and HUES 7 cell lines. Together these studies show that this rapid and straightforward approach to neural crest differentiation is broadly applicable to multiple different hESC cell lines. In our hands, roughly one third of SDIA-differentiated colonies contain cells that are positive for peripherin and 18 and 25% of colonies demonstrate differentiation to SG and PS neurons, respectively—although the number of positive cells within each colony is highly variable. Thus, although feasible, the efficiency and reproducibility of terminal neural crest differentiation under nonenriched SDIA conditions is suboptimal for focused study of human neural crest development.
Generation of putative NCSCs from hESCs
In vivo, all neural crest structures, including peripheral neurons, develop from populations of NCSCs that initiate in the developing neuroepithelium and then migrate throughout the body (reviewed in ref. 1). We therefore reasoned that the terminally differentiated neurons in our hESC cultures must have transitioned through a NCSC phase on their transition to PS and SG nerves. Further, we hypothesized that isolation of these cells from the cultures would enable enrichment and improvement of directed neural crest differentiation of hESCs in vitro. To identify populations of putative NCSCs within our differentiating hESC cultures we first assayed expression of NCSC markers by both immunostaining and reverse transcription (RT)-PCR. Because there is no single gene, or even combination of two genes, whose expression unequivocally marks these important cells, we examined our cultures for the temporal expression of several different molecules used as early neural crest markers in several species.
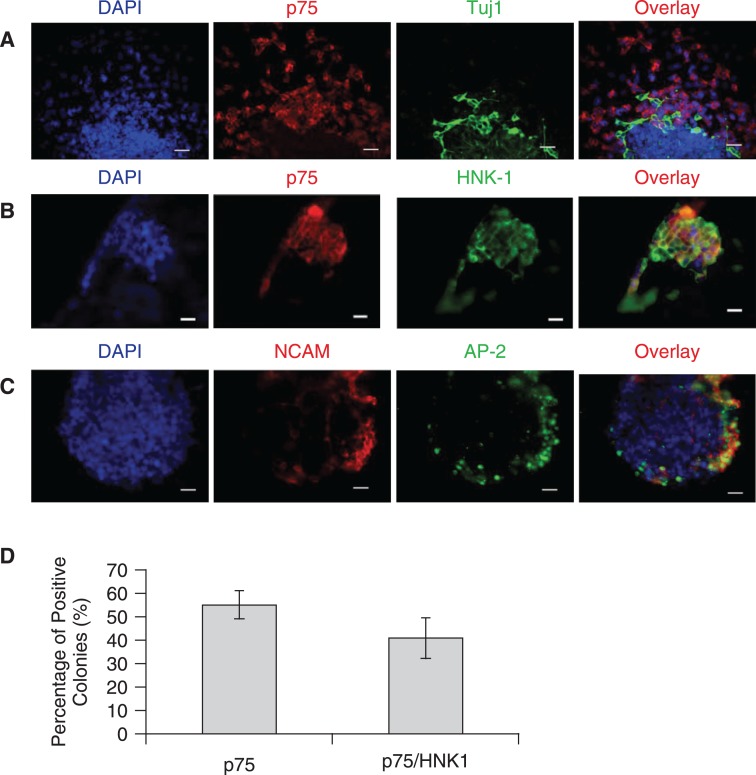
By 1 week of co-culture, expression of low-affinity neurotrophin receptor p75, which is highly expressed in migrating NCSCs [21,22], could be observed in cell clusters and these were predominantly localized at the periphery of hESC colonies (Fig. 2A). In addition, consistent with a migratory phenotype, many of these cells were also identified as individual cells at sites distant from p75+ colonies (Fig. 2A). The HNK-1 carbohydrate epitope is a residue on various glycoproteins and glycolipids, and is recognized by monoclonal antibody HNK-1. The HNK-1 epitope has been used as a general marker for neural crest cells in the avian embryo [23] and in human NCSCs [21]. Therefore, we co-immunostained cells for both HNK1 and p75. As shown in Fig. 2B, of the majority of p75+ cells also expressed HNK1 providing further evidence to support their identity as NCSCs. Quantitative analysis revealed that by 1 week of co-culture, 55.3 ± 10.6% of the hESC colonies contained p75+ cells and the vast majority of these colonies were also positive for HNK1 with virtually complete overlap between HNK1+ and p75+ cells (Fig. 2D). In addition, we used co-expression of the transcription factor AP2α and the cell-surface molecule NCAM as an indicator of neural crest differentiation [14,15]. Consistent with p75/HNK1 staining, after 1 week of co-culture, almost half of the hESC colonies contained AP2+/NCAM+ cells and the majority of these double-positive cells were localized at the edge of the colonies (Fig. 2C).
FIG. 2.
Induction of putative neural crest stem cells (NCSCs) from human embryonic stem cells (hESCs). (A–C) Immunostaining of H9 colonies after 1 week of culture on PA6. (Scale bars = 50 μm). (A) A colony of H9 hESCs double stained for p75 (red) and Tuj1 (green). Note that the expression of p75 is outside or at the edge of the hESC colony (asterisk). (B) A colony of H9 hESCs double stained with p75 (red) and HNK1 (green). (C) A colony of H9 double stained for NCAM (red) and AP-2 (green), indicative of early migrating neural crest cells. (D) Quantification of H9 colonies expressing p75 and /or HNK1 after 1 week of culture on PA6. Results are the average of three experiments and error bars = SD.
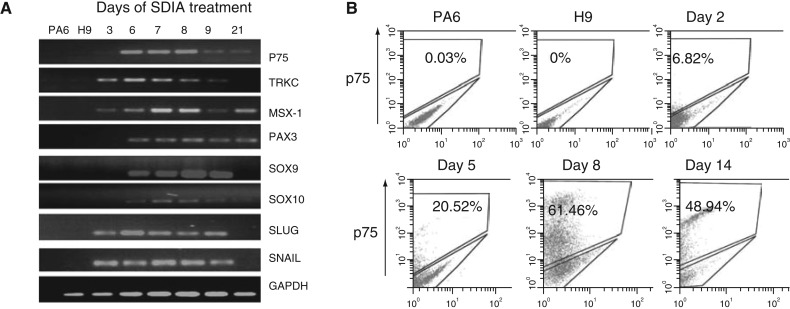
Next, we used RT-PCR to examine the expression of early neural crest markers in PA6 cells, undifferentiated hESCs and hESCs over the course of SDIA exposure (Fig. 3A). Semi-quantitative evaluation of the expression of many of these genes showed a temporal pattern with induction at 1 week followed by downregulation by 3 weeks, consistent with the putative transient appearance of NCSCs in SDIA-differentiating hESCs. For example, expression of the early neural crest cell markers SNAIL and SLUG was first detected at day three, peaked at ∼1 week, and dramatically decreased by 3 weeks. Other neural crest development—associated genes that were also induced by 1 week and diminished by 3 weeks include SOX9, SOX10, and TRKC. However, in keeping with their known expression in other more differentiated cell types, a few genes that are highly but not specifically expressed by NCSCs showed the expected induction at week 1 but little or no subsequent downregulation. The p75 gene, e.g., which is expressed both in migrating neural crest cells and many postmitotic neurons in dorsal root ganglia [24], was expressed by both 1 week and 3 week co-cultures. In addition, the continued expression of MSX1 at 3 weeks may be explained by its expression in nonneural ectoderm and mesoderm derivatives in addition to neural crest cells [25]. Finally, PAX3 is normally expressed in the dorsal neural tube, the region from which neural crest cells emerge [26] but it is also detected in the somites and in mature Schwann cells of murine embryos after E8.5 [27].
FIG. 3.
Expression of neural crest stem cell (NCSC) markers in PA6-induced human embryonic stem cell (hESC) cultures. (A) Reverse transcription-polymerase chain reaction analysis (RT-PCR) of neural crest–associated genes demonstrates temporal induction of these genes over the first 6–7 days. In contrast, PA6 cells and undifferentiated H9 cells do not express these neural crest markers. (B) Analysis of H9-derived p75+ cells by fluorescence-activated cell sorting (FACS) analysis. Cells were incubated with FcR blocking reagent and the phycoerythrin (PE)-conjugated anti-p75 for 10 min in the dark at 4°C. Cells were run through a FACS can flow cytometer with standard equipment. A minimum of 10,000 events was collected and acquired in list mode using the CellQuest software. The dot plots are representative of three independent experiments. P75+ cells appeared as early as Day 2 of the culture period, and the level of p75 increased dramatically from Day 5 to 8.
Therefore, immunostaining and RT-PCR assays support the temporal induction of putative NCSCs/NCPCs from hESCs after 1 week of SDIA treatment. Together, these findings infer that neural crest precursors capable of differentiating into peripheral neurons including PS and SG neurons emerge from SDIA-induced hESCs very rapidly upon PA6 co-culture.
Isolation and molecular characterization of hESC-derived NCSCs
To further characterize the putative NCSCs induced from hESCs, we used FACS to isolate these target cells from the bulk cultures. Among the various cell-surface proteins known to be expressed by the neural crest, p75 has been widely used to characterize and isolate NCSCs [21,22,28]. Thus, we used FACS analysis to evaluate the emergence of p75+ cells in our differentiating hESC cultures. As shown (Fig. 3B), p75+ cells appeared as early as day two of the culture and continued to increase in number until day 8. Together with the aforementioned RT-PCR data (Fig. 3A), these findings suggested to us that the emerging NCSC population peaks at day 7–8 of SDIA induction. In addition, similar temporal analysis of HNK1 expression by FACS showed that most hESC-derived p75+ cells also express HNK1 (data not shown). This is consistent with our immunocytologic findings and supportive of their identification as early NCSCs (Fig. 2).
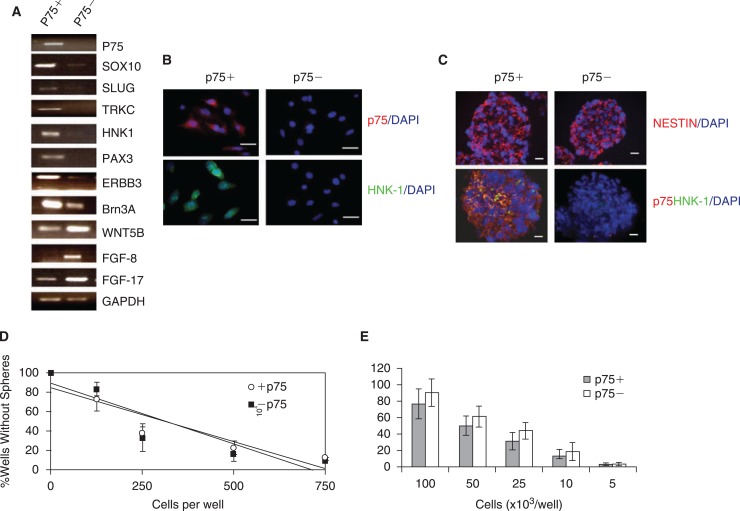
Next, we proceeded to test whether these p75+ cells demonstrate genetic and phenotypic characteristics of human NCSCs. First, we sorted p75+ and p75− cells by FACS and evaluated the expression of neural crest marker genes by RT-PCR. As shown in Fig. 4A, the p75+ fraction transcripts were highly enriched for NCSCs (p75, SOX10, HNK1, and ERBB3) or key neural crest development markers (SLUG, TRKC, PAX3, and POU4F1). In contrast, markers of central nervous system (CNS) precursors including WNT5B, FGF8, and FGF17 [21] were more highly expressed in the p75− population suggesting that FACS sorting of p75+ cells differentially isolates NCSCs from CNS stem cells as well as other p75− cell populations. Gene expression data was confirmed by immunocytochemistry which showed that markers of neural crest identity were limited to p75+ cell fractions (Fig. 4B).
FIG. 4.
Molecular characterization and sphere formation analysis of neural crest stem cells (NCSCs). (A) Different gene expression profiles are displayed by p75+ and p75− populations as assessed by RT-PCR with p75+ cells expressing markers of putative NCSCs and p75− cells expressing higher levels of CNS stem cell genes (see text). (B) Immunocytochemistry analysis of FACS-purified p75+ and p75− cells identifies markers of neural crest identity in only the p75+ fraction (Scale bar = 100 μm). (C) Immunostaining of p75+ and p75− spheres generated after 1 week in neurosphere induction media in anchorage-independent conditions. While both spheres contain cells that express the stem-cell marker nestin, only FACS-purified p75+ spheres are positive for neural crest stem cell markers HNK1 and p75. (D) Limiting dilution analysis of p75+ and p75− cells. Cells were inoculated into microwell plates at concentrations ranging from 1,000 cells/well to 1 cell/well and maintained in vitro for 7 days. The frequency with which at least one cell formed a sphere (37% mark on the y axis) was not significantly different between p75+ and p75− cells. (E) Quantification of sphere formation in FACS-enriched p75+ and p75− populations. Freshly sorted cells were plated onto six-well ultra-low-attachment plates at serial dilutions (5–100 × 103 cells/well) and grown in media as described in Materials and Methods. The number of spheres was counted 1 week later and the data presented are mean ± SD of three independent experiments.
In summary, FACS analysis of differentiating hESCs demonstrates peak emergence of p75+/HNK1+ cells 1 week after SDIA induction. Sorting based on p75 expression enriches for cells that possess both genetic and phenotypic characteristics of NCSCs and separates putative neural crest precursors from CNS precursors.
Characterization and multilineage differentiation of hESC-derived NCSCs
Having established that p75+ cells express genetic and phenotypic features of NCSCs we next sought to evaluate their functional characteristics. Freshly isolated p75+ and p75− cells were cultured on ultra-low attachment plates in N2 medium containing EGF and bFGF. Twenty-four hours after plating, cell clusters were found in both p75+ and p75− cultures. These cells continued to proliferate and formed spheres by 6–7 days. Antibody staining revealed that both p75+ and p75− spheres expressed the neural stem cell marker nestin, however, only spheres derived from p75+ cells were positive for neural crest markers p75, HNK1, and AP2 (Fig. 4C). These data again indicate that FACS sorting enriches for NCSCs in the p75+ cell fractions while CNS stem cells, which also express nestin and possess sphere-forming capability, remain predominantly in the p75− fraction.
To further characterize these putative stem cells, we performed clonal analysis of freshly isolated cells. Notably, neither p75+ nor p75− cells could proliferate at clonal density in vitro immediately after isolation. Because we were not able to generate colonies from freshly isolated single cells, we performed a limiting dilution analysis [17]. In so doing, we determined that the number of cells required to generate at least one sphere/well was 432 cells/well in p75+ cells, and 420 cells/well in p75− cells (Fig. 4D). In addition, we found that after 1 week, p75+ and p75− cells formed spheres at similar efficiencies when cultured in the presence of growth factors on ultra-low attachment plates (Fig. 4E). Finally, mechanical dissociation and passaging of the spheres revealed that both p75+ and p75− cells could be maintained in these in vitro culture conditions for at least three passages with continued expansion (data not shown). Importantly, whereas differentiation of p75+ spheres resulted in bias toward neural crest derivatives including peripherin+ neurons and GFAP+/MBP+ Schwann cells (Fig. 5A and Supplementary Fig. 2), cultures derived from p75− populations were enriched in MAP2+ CNS neurons and GFAP+/MBP- astrocytes (Supplementary Fig. 2).
FIG. 5.
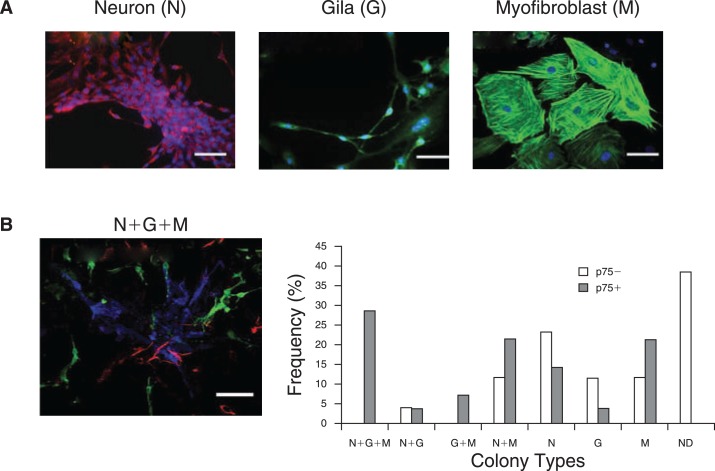
Human ESC–derived NCSCs give rise to multilineage neural crest derivatives. (A) p75+ spheres in bulk culture were plated on poly-d-lysine- and fibronectin-coated coverslips in differentiation media for 2 weeks and then immunostained with individual neural crest lineage markers separately (peripherin as peripheral neuronal marker; GFAP as glial marker; and SMA as myofibroblastic marker). Three major neural crest–derived cell types were detectable after this differentiation period: peripherin+ neurons, GFAP+ glial cells, and SMA+ myofibroblasts. (B) Single clonally derived spheres from FACS-sorted p75+ or p75− cells were handpicked and allowed to differentiate on poly-d-lysine- and fibronectin-coated coverslips in neural crest differentiation media for 2 weeks and then assessed for evidence of tri-lineage differentiation using tri-labeling immunocytochemistry. The left panel shows a representative example of a multipotent colony (3 lineages) generated from an individual sphere. The histogram in the right panel demonstrates the quantification of different types of colonies generated from clonal p75+ and p75− spheres. The frequencies of total colonies in each category are shown. Abbreviations: ESC, embryonic stem cell; NCSC, neural crest stem cell; SMA, smooth muscle α-actin; NGM, multipotent colonies (positive staining for neurons, glia, and myofibroblasts); NM, bipotent colonies with neurons and myofibroblasts; GM, bipotent colonies with glia and myofibroblasts; NG, bipotent colonies with neurons and glia; G, unipotent glial colonies; M, unipotent myofibroblast colonies; N, unipotent neuronal colonies; ND, no terminal neural crest differentiation detected.
To address whether these hESC-derived putative NCSCs could differentiate into multilineage neural crest derivatives in vitro, multiple spheres were plated on poly-d-lysine/fibronectin-coated plates for 2 weeks in the presence of defined medium plus 5% FBS. As illustrated in Fig. 5A, three major neural crest–derived cell types were detectable after this differentiation period: peripherin+ neurons, glial fibrillary acidic protein (GFAP)+ glial cells and smooth muscle actin (SMA)+ myofibroblasts. Clonal analysis of cells derived from individual spheres (Fig. 5B) showed that in colonies derived from p75+ cells, up to 28.6% showed differentiation into cells from all three lineages including neurons, glial cells, and myofibroblasts, suggesting that these colonies were derived from multipotent precursors. In addition, p75+ cells also generated colonies that comprised only two cell types (i.e., 21.4% contained neurons and myofibroblasts, 7.1% contained myofibroblasts and glia, and 3.6% contained neurons and glia) indicating the possibility that these cells had derived from bipotent precursors. Finally, in 39.3% of colonies derived from a single sphere only a single cell type was identified, suggesting derivation from unipotent precursors. In contrast, although p75− cells formed spheres and these spheres could be induced to differentiate, in nearly half of the colonies no evidence of neural crest differentiation was observed (Fig. 5B). While occasional colonies with two cell types could be seen (15.3%), evidence of terminal neural crest differentiation from p75− spheres was primarily limited to cells of a single lineage (Fig. 5B). Thus, FACS-based sorting of p75+ cells from differentiating hESCs after 1 week of SDIA co-culture significantly enriches for putative NCSCs/NCPCs with multilineage differentiation capacity.
Because peripherin is expressed by primary spinal motoneurons as well as peripheral neurons, we examined SDIA-induced cells for expression of the early motoneuron-associated transcription factor HB9. As shown (Supplementary Fig. 3), HB9 was not present in undifferentiated hESCs, but was expressed after 1 week of co-culture. The level of HB9 expression then declined dramatically by 3 weeks of co-culture, suggesting that motoneurons do not persist in large numbers in PA6-differentiated cells. We also examined the expression of a specific marker for cholinergic neurons, ChAT in PA6-H9 co-culture. Again, expression of ChAT was detected at 1 week of co-culture, but was undetected in 3 week cultures. These results suggest that PA6 does not support the survival of spinal motoneurons or other nondopaminergic neurons. In addition, we demonstrated that neither HB9 nor ChAT transcript (Supplementary Fig. 3) nor protein (immunostaining, data not shown) were expressed by differentiated progeny derived from FACS-sorted p75+ cells. Together these data support the conclusion that peripherin staining cells are peripheral neurons and not CNS-derived motoneurons.
To establish whether FACS-isolated hESC-derived, putative NCSCs are capable of self-renewal, we tested whether individual cells derived from the primary spheres could form secondary spheres. Primary spheres were dissociated into single-cell suspensions and reseeded at clonal density in cloning medium. In all cases, clonally derived spheres were visible within 2 weeks. As shown in Table 1, p75+ cells exhibited a higher subcloning efficiency than p75− cells (11.6 ± 3.7% vs. 6.7 ± 3.2%), indicating that although CNS progenitors are likely to exist within the p75− population, the p75+ neural crest fraction is more highly enriched for self-renewing stem cells.
Table 1.
Self-Renewal Potential of Primary Neurospheres Generated From hESC-Derived p75+ and p75− Cells
| p75– | Mean ± SD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary sphere identity | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Cell numbers | 476 | 310 | 250 | 582 | 530 | 380 | 350 | 568 | 360 | 390 | 280 | 168 | 246 | 250 | 420 | 240 | 210 | ||
| Number of secondary spheres | 35 | 30 | 27 | 13 | 25 | 56 | 44 | 37 | 48 | 30 | 32 | 14 | 6 | 6 | 8 | 22 | 4 | 8 | |
| Cloning efficiency (%) | 7.4 | 9.7 | 10.8 | 6.2 | 4.3 | 10.6 | 11.6 | 10.6 | 8.5 | 8.3 | 8.2 | 5.0 | 3.6 | 2.4 | 3.2 | 5.2 | 1.7 | 3.8 | 6.7 ± 3.2 |
| p75+ | Mean ± SD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary sphere identity | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Cell numbers | 560 | 385 | 202 | 310 | 270 | 250 | 108 | 150 | 346 | 258 | 220 | 105 | 120 | 264 | 120 | 246 | 380 | 278 | |
| Number of secondary spheres | 68 | 47 | 17 | 55 | 30 | 32 | 12 | 15 | 44 | 30 | 22 | 18 | 6 | 18 | 24 | 30 | 36 | 24 | |
| Cloning efficiency (%) | 12.1 | 12.2 | 8.4 | 17.7 | 11.1 | 12.8 | 11.1 | 10.0 | 12.7 | 11.6 | 10.0 | 17.1 | 5.0 | 6.8 | 20.0 | 12.2 | 9.5 | 8.6 | 11.6 ± 3.7a |
Single cells dissociated from individual primary spheres inoculated in low attachment plates at clonal densities (10–30 cells/cm2). The number of secondary spheres was counted 7–10 days later and cloning efficiency was calculated (% of total no. of seeded cells). Presented data are compiled from two independent experiments.
Significant difference in secondary sphere formation between p75+ and p75− cells (P < 0.01, t-test).
Finally, to address the issue of lineage specificity in SDIA-induced hESCs we also examined the expression of several mesoderm and endoderm differentiation markers in PA6-hESCs co-cultures and FACS-isolated p75+ and p75− populations. As shown (Supplementary Fig. 3), the mesoderm marker Brachyury was induced early in co-culture but disappeared by 3 weeks while GATA1 and MyoD were never detected in differentiating, SDIA-induced H9 cells. Similarly, the endodermal marker AFP was detected at early timepoints only and PDX-1 was never induced. These results confirm that terminal differentiation of hESCs into mesodermal and endodermal lineages is not supported by PA6 stromal cells without the addition of specific survival factors. In addition, after FACS isolation, neither mesodermal nor endodermal markers were detectable in FACS-sorted cells (Supplementary Fig. 3) further supporting the neuroectodermal lineage specificity of SDIA-induced, FACS-sorted hESCs.
In vivo analysis of hESC-derived NCSCs
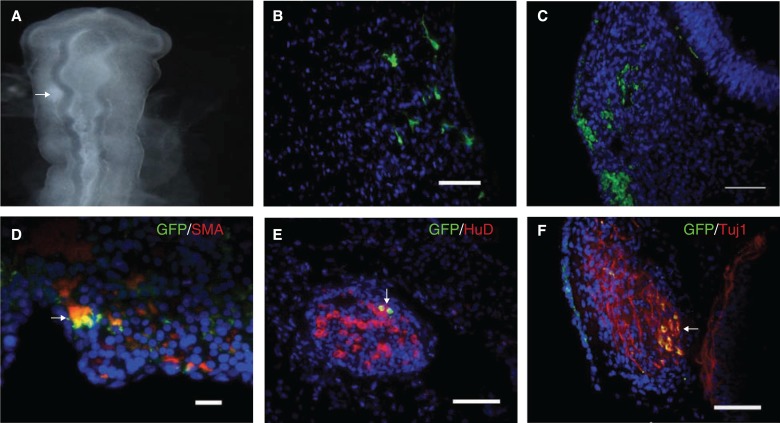
To determine whether hESC-derived NCSCs demonstrate functionality in vivo we evaluated integration and migration of these cells in the developing chick embryo. FACS-purified hESC-derived NCSCs were transduced to express GFP and grafted into the space adjacent to the midbrain of H&H stage 10–12 chick embryos (Fig. 6A). Human NCSCs were identified in embryo sections 3–5 days after cell injection by the anti GFP antibody. Three days after transplantation, we observed massive migration of hESC-derived NCSC progeny to peripheral cranial neural crest target sites. Many transplanted cells had migrated into the cranial mesenchyme (Fig. 6B) and trigeminal ganglion (Fig. 6C). Interestingly, some of the GFP+ cells in the cranial mesenchyme were positive for SMA (Fig. 6C). Moreover, we found that in the host trigeminal ganglion, some human NCSCs had differentiated into HuC/HuD+ (Fig. 6E) and Tuj1+ (Fig. 6F) neurons. Thus, these hESC-derived p75+ human cells demonstrate functional properties of NCSCs in vivo in that they survive, migrate along neural crest migratory pathways, and differentiate into derivatives compatible with neural crest identity.
FIG. 6.
Human embryonic stem cell–derived neural crest stem cells (NCSCs) give rise to NC derivatives in chick embryo. (A) Freshly isolated p75+ cells were transduced with a GFP-lentivirus. Three days after transduction, cells were grafted into the space between midbrain and hindbrain in H&H stage 10–12 chick embryos. White arrow shows location of the injection site. (B and C) Three days after injections embryos were harvested and stained with GFP (Green). GFP+ cells migrated to the cranial mesenchyme (B) and trigeminal ganglion (C). (D–F) Human NCSCs differentiate into neural crest derivatives in chick embryo. (D) GFP+/SMA+ cells are detected in the cranial mesenchyme. (E) Two GFP+/Huc/HuD+ (pan early neuronal marker) cells as indicated by the arrowhead are localized in the trigeminal ganglion. (F) GFP+/Tuj1+ cells in the trigeminal ganglion. Scale bar = 100 μm.
Discussion
The ability to generate terminally differentiated neural crest cells from hESCs using SDIA co-culture has been previously described [15]. However, we have found that the efficiency of this process is inadequate for focused study of neural crest as only 30% of differentiated colonies contain peripherin+ peripheral nerves and many colonies show little to no evidence of neural crest generation after 4 weeks. To improve efficiency it has been recently shown that combining SDIA co-culture with manual isolation of precursor neurosphere colonies after 2 weeks can further increase the yield of neural crest cells from hESCs [29,30]. In addition, FACS-based enrichment of p75+ cells from differentiating hESCs has been shown to be a viable means of NCSC isolation, although this protocol is again both labor- and time-intensive [21]. In this study, we have demonstrated that the efficiency of neural crest differentiation can be dramatically improved by combining SDIA-co-culture with FACS-based enrichment. Moreover, this protocol can be used to successfully and efficiently isolate functional early human NCSCs from hESCs.
Studies in a variety of animal models suggest that the newly formed neural crest is comprised of cells that are largely multipotent and that these cells become progressively restricted with further development, though the presence of some early restricted subpopulations cannot be ruled out. Conversely, adult NCSC populations appear to be retained in neural crest derivatives and other tissues, like the epidermis (reviewed in ref. 1). Although progressively restricted in their differentiation potential, these cells maintain self-renewal potential at least through several generations thereby earning them the stem cell designation [31,32]. Thus, the neural crest represents a somewhat unique structure in which downstream progeny cells maintain the stem cell property of self-renewal while simultaneously undergoing fate restriction (reviewed in ref. 33).
In our studies, we have used FACS to enrich for neural crest cells that express p75. The ability to generate multiple neural crest lineages from nearly 30% of clonally derived p75+—spheres suggests that this FACS-based enrichment successfully isolates early multipotent NCSCs. However, in addition to tri-lineage (neural, glial, myofibroblast) differentiation, dual- and single-lineage colonies were also detected in abundance (32 and 39% of colonies, respectively). Thus, it is likely that sorting on the basis of p75 alone isolates not only multipotent NCSCs but also more restricted bipotent and unipotent NCSCs. In particular, although nearly all p75+ cells also expressed the early migratory marker HNK1, small numbers of p75+/HNK1− cells were present at the time of sorting and these cells may be representative of post-migratory NCSCs. Alternatively, isolated multipotent NCSCs may undergo progressive fate restriction in vitro culture. In an attempt to address whether further enrichment of p75+/HNK1+ cells would result in increased generation of tri-lineage colonies we isolated dual positive cells by FACS at day 6 of differentiation. Unfortunately, the prolonged incubation and sorting times required for dual staining resulted in a high degree of cell death that precluded quantitative analysis of differentiation capacity (data not shown). Optimized studies using multiple markers and lineage tracing are now required to fully address the issue of fate restriction in these SDIA-induced, hESC-derived NCSCs. In the meantime, we propose that this rapid approach using bulk sorting with a single antibody can be reliably and reproducibly used to isolate large numbers of human NCSCs/NCPCs for biologic study.
Importantly, we have confirmed that SDIA-induced, hESC-derived NCSCs not only survive when transplanted into the developing chick embryo, but also differentiate into neural crest progeny including peripheral neurons and smooth muscle in the cranial neural crest target sites. Interestingly, although glial differentiation was observed in our in vitro studies we did not detect any human Schwann cells in developing chick embryos 3 days after NCSC transplantation. This result is in agreement with previous studies which showed that hESC-derived NCSCs did not differentiate into Schwann cells in either developing or adult vertebrate hosts [21]. The lack of differentiation toward glial fates in vivo in both studies may be due to the fact that the generation of glial derivatives during peripheral nervous system development follows a stereotypic sequence, with neurogenesis preceding gliogenesis and the specification toward glial fates is a late event. Therefore, optimized experiments using multiple time points and different transplantation sites will be required to fully assess the in vivo differentiation potential of hESC-derived NCSCs.
Neural crest precursors with differentiation potential toward melanocytes, neurons and GFAP+ cells have been isolated from mouse embryonic stem cells using ST2 stromal cells and cell sorting for c-KIT [34]. In contrast, PA6 co-cultured cells do not give rise to melanocytic derivatives in the absence of cell sorting [14]. c-KIT is a marker previously used in the identification of embryonic stem cell–derived melanocytes [35] and is considered as a marker of committed migratory melanocyte precursors in vivo [36]. To assess c-KIT status in differentiating hESCs, we used RT-PCR to examine the expression of c-KIT in hESCs over the course of SDIA exposure. c-KIT expression appeared as early as day 1 of the culture, peaked at day 3 and remained at high level until 3 weeks (data not shown). Thus, although human NCSCs with melanocytic lineage potential may also express c-KIT it will not be useful as a single marker for NCSC enrichment protocols given its persistent expression in terminally differentiated cultures. Whether combining c-KIT with p75 and/or HNK-1 will permit selective isolation of SDIA-induced human NCSCs that have maintained melanocytic differentiation capacity remains to be determined.
Recently, the feasibility of generating NCSCs from hESCs using an alternate protocol has also been reported [21]. In these studies, NCSCs were successfully isolated from hESC-derived neural rosettes. However, this rosette-intermediate process requires nearly 4 weeks of directed differentiation and is substantially more complex than the simple 1-week protocol we describe herein. Given the disparity in the two protocols, it is conceivable that the two approaches yield cells that correspond to in vivo–derived cells of different developmental stages. In particular, whether or not rosette-derived and SDIA-derived NCSCs possess equivalent differentiation and self-renewal capacities in vitro and in vivo will be of great interest to determine and direct comparison of these different techniques is now warranted. In addition, comparison of rosette- or SDIA-derived cells with adult tissue-derived NCSCs will provide insights into the presumed ontogeny of hESC-derived neural crest cells.
There can be no doubt that knowledge of human neural crest development will be profoundly enhanced by the unique opportunities provided by in vitro–differentiated hESCs. In addition, the ability to rapidly and reproducibly generate large numbers of NCSCs for biologic study will be invaluable for studies of disrupted neural crest development as we endeavor to elucidate the pathogenesis of both benign and malignant human neural crest disorders.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dinithi Senadheera, Xingchao Wang, Lora Barsky, and the Saban Research Institute Stem Cell and FACS Cores at Childrens Hospital Los Angeles for their invaluable assistance. Support for this work was provided by CIRM SEED grant (RS1–00249) and by a Stop Cancer Foundation Career Development Award (E.R.L.). X.J. was supported by a CIRM Post-doctoral Scholarship through Training Grant T2–00005. S.J.M. was supported by an NH&MRC CJ Martin fellowship. Additional financial support from the TJ Martell and My Brother Joey Foundations is also gratefully acknowledged. This work was presented in part at the 6th Annual Meeting of the ISSCR, Philadelphia, PA, June 2008.
References
- 1.Delfino-Machin M, Chipperfield TR, Rodrigues FS. and Kelsh RN. (2007). The proliferating field of neural crest stem cells. Dev Dyn 236:3242–3254 [DOI] [PubMed] [Google Scholar]
- 2.Bolande RP. (1974). The neurocristopathies: a unifying concept of disease arising in neural crest maldevelopment. Hum Pathol 5:409. [DOI] [PubMed] [Google Scholar]
- 3.Taneyhill LA. and Bronner-Fraser M. (2005). Recycling signals in the neural crest. J Biol 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsokos M, Scarpa S, Ross RA. and Triche TJ. (1987). Differentiation of human neuroblastoma recapitulates neural crest development. Study of morphology, neurotransmitter enzymes, and extracellular matrix proteins. Am J Pathol 128:484–496 [PMC free article] [PubMed] [Google Scholar]
- 5.Vance KW. and Goding CR. (2004). The transcription network regulating melanocyte development and melanoma. Pigment Cell Res 17:318–325 [DOI] [PubMed] [Google Scholar]
- 6.Meltzer PS. (2007). Is Ewing's sarcoma a stem cell tumor?. Cell Stem Cell 1:13–15 [DOI] [PubMed] [Google Scholar]
- 7.Coles EG, Lawlor ER. and Bronner-Fraser M. (2008). EWS-FLI1 causes neuroepithelial defects and abrogates emigration of neural crest stem cells. Stem Cells 26:2237–2244 [DOI] [PubMed] [Google Scholar]
- 8.Meulemans D. and Bronner-Fraser M. (2004). Gene-regulatory interactions in neural crest evolution and development. Dev Cell 7:291–299 [DOI] [PubMed] [Google Scholar]
- 9.Basch ML, Bronner-Fraser M. and Garcia-Castro MI. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441:218–222 [DOI] [PubMed] [Google Scholar]
- 10.O'Rahilly R. and Muller F. (1999). Minireview: summary of the initial development of the human nervous system. Teratology 60:39–41 [DOI] [PubMed] [Google Scholar]
- 11.Almond S, Lindley RM, Kenny SE, Connell MG. and Edgar DH. (2007). Characterisation and transplantation of enteric nervous system progenitor cells. Gut 56:489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie IA, Biernaskie J, Toma JG, Midha R. and Miller FD. (2006). Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci 26:6651–6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spagnoli FM. and Hemmati-Brivanlou A. (2006). Guiding embryonic stem cells towards differentiation: lessons from molecular embryology. Curr Opin Genet Dev 16:469–475 [DOI] [PubMed] [Google Scholar]
- 14.Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, Murakami F. and Sasai Y. (2003). Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci USA 100:5828–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pomp O, Brokhman I, Ben-Dor I, Reubinoff B. and Goldstein RS. (2005). Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells 23:923–930 [DOI] [PubMed] [Google Scholar]
- 16.Jang JE, Shaw K, Yu XJ, Petersen D, Pepper K, Lutzko C. and Kohn DB. (2006). Specific and stable gene transfer to human embryonic stem cells using pseudotyped lentiviral vectors. Stem Cells Dev 15:109–117 [DOI] [PubMed] [Google Scholar]
- 17.Bellows CG. and Aubin JE. (1989). Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol 133:8–13 [DOI] [PubMed] [Google Scholar]
- 18.Bronner-Fraser M. and Garcia-Castro M. (2008). Manipulations of neural crest cells or their migratory pathways. Methods Cell Biol 87:75–96 [DOI] [PubMed] [Google Scholar]
- 19.Escurat M, Djabali K, Gumpel M, Gros F. and Portier MM. (1990). Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (NF-L), during the development of the rat. J Neurosci 10:764–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedtsova NG. and Turner EE. (1995). Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev 53:291–304 [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V. and Studer L. (2007). Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 25:1468–1475 [DOI] [PubMed] [Google Scholar]
- 22.Stemple DL. and Anderson DJ. (1992). Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71:973–985 [DOI] [PubMed] [Google Scholar]
- 23.Bronner-Fraser M. (1986). Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol 115:44–55 [DOI] [PubMed] [Google Scholar]
- 24.Rifkin JT, Todd VJ, Anderson LW. and Lefcort F. (2000). Dynamic expression of neurotrophin receptors during sensory neuron genesis and differentiation. Dev Biol 227:465–480 [DOI] [PubMed] [Google Scholar]
- 25.Catron KM, Wang H, Hu G, Shen MM. and Abate-Shen C. (1996). Comparison of MSX-1 and MSX-2 suggests a molecular basis for functional redundancy. Mech Dev 55:185–199 [DOI] [PubMed] [Google Scholar]
- 26.Goulding M, Lumsden A. and Paquette AJ. (1994). Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 120:957–971 [DOI] [PubMed] [Google Scholar]
- 27.Kioussi C, Gross MK. and Gruss P. (1995). Pax3: a paired domain gene as a regulator in PNS myelination. Neuron 15:553–562 [DOI] [PubMed] [Google Scholar]
- 28.Morrison SJ, White PM, Zock C. and Anderson DJ. (1999). Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96:737–749 [DOI] [PubMed] [Google Scholar]
- 29.Brokhman I, Gamarnik-Ziegler L, Pomp O, Aharonowiz M, Reubinoff BE. and Goldstein RS. (2008). Peripheral sensory neurons differentiate from neural precursors derived from human embryonic stem cells. Differentiation 76:145–155 [DOI] [PubMed] [Google Scholar]
- 30.Pomp O, Brokhman I, Ziegler L, Almog M, Korngreen A, Tavian M. and Goldstein RS. (2008). PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res 1230:50–60 [DOI] [PubMed] [Google Scholar]
- 31.Trentin A, Glavieux-Pardanaud C, Le Douarin NM. and Dupin E. (2004). Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA 101:4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T. and Morrison SJ. (2002). Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35:657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane JF. and Trainor PA. (2006). Neural crest stem and progenitor cells. Ann Rev Cell Dev Biol 22:267–286 [DOI] [PubMed] [Google Scholar]
- 34.Motohashi T, Aoki H, Chiba K, Yoshimura N. and Kunisada T. (2007). Multipotent cell fate of neural crest-like cells derived from embryonic stem cells. Stem Cells 25:402–410 [DOI] [PubMed] [Google Scholar]
- 35.Yamane T, Hayashi S, Mizoguchi M, Yamazaki H. and Kunisada T. (1999). Derivation of melanocytes from embryonic stem cells in culture. Dev Dyn 216:450–458 [DOI] [PubMed] [Google Scholar]
- 36.Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ. and Murphy M. (2004). Neural crest cell lineage segregation in the mouse neural tube. Development 131:6153–6162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.