Abstract
Risk avoidance is an important determinant of human behavior. The neurotransmitter serotonin has long been implicated in processing aversive outcomes caused by risky decisions. However, it is unclear whether serotonin provides a neurobiological link between making a risk aversive decision and the response to an aversive outcome. Using pharmacological fMRI, we manipulated the availability of serotonin in healthy volunteers while performing a gambling task. The same group of participants was studied in three fMRI sessions: (i) during intravenous administration of the SSRI citalopram to increase the serotonergic tone, (ii) after acute tryptophan depletion (ATD) to reduce central serotonin levels, or (iii) without interventions. ATD and citalopran had opposite effects on outcome related activity in dorsomedial prefrontal cortex (dmPFC) and amygdala. Relative to the control condition, ATD increased and citalopram decreased the neural response to aversive outcomes in dmPFC. Conversely, ATD decreased and citalopram increased the neural response to aversive outcomes in left amygdala. Critically, these pharmacological effects were restricted to aversive outcomes that were caused by low-risk decision and led to a high missed reward. ATD and citalopram did not alter the neural response to positive outcomes in dmPFC, but relative to ATD, citalopram produced a bilateral increase in the amygdala response to large wins caused by high-risk choices. The results show a selective involvement of the serotonergic system in neocortical processing of aversive outcomes resulting from risk-averse decisions, thereby linking risk aversion and processing of aversive outcomes in goal-directed behaviors.
Keywords: pharmacological fMRI, reward, serotonin, SSRI, tryptophan depletion
Introduction
When confronted with choices involving a potential for undesirable outcomes, people show a strong tendency towards risk avoidance even when the utility of these choices is less favorable than the utility of more risky alternatives. For instance, people reject 50/50 offers unless the potential gain is twice in size as the potential loss (Tom et al., 2007). On the other hand, when making risky decisions, people generally overweight unlikely events and underweight likely events (Hsu et al., 2009). These deviations from simple utility estimates have been described in behavioral economics by e.g. prospect theory (Kahneman and Tversky, 1979; Tversky and Kahneman, 1992; Glimcher et al., 2008). The proposed models introduce the idea of non-linear “decision weights” which bias objective probabilities. Recent studies have started to unveil neural systems that code the utility of “unchosen” options, i.e. alternative choices that were not selected (Boorman et al., 2009, 2011). These findings highlight an additional component in risky decision making which contributes to the “decision weights”.
The neural substrate of risk aversion has recently started to be explored (Huettel et al., 2006; Tobler et al., 2007; Christopoulos et al., 2009). Several lines of research have implicated the monoamine neurotransmitter serotonin (5-HT) in processing and avoiding aversive outcomes (Rogers et al., 2003; Tanaka et al., 2007), predicting future punishment (Daw et al., 2002; Cools et al., 2008b), loss aversion (Deakin, 1983; Deakin and Graeff, 1991; Cools et al., 2008a), and forming retrospective associations between punishments and past actions (Tanaka et al., 2007). Animal studies have shown that 5-HT neurons in the raphe nucleus are activated (Grahn et al., 1999; Takase et al., 2004, 2005) and 5-HT is released (Bland et al., 2003) in response to aversive events. The role of 5-HT in processing and avoiding aversive outcomes is clinically important because excessive aversive processing has been identified as a key feature of mood and anxiety disorders which are associated with dysfunctional 5-HT signaling (Elliott et al., 1997; Mathews and Mackintosh, 2000; Murphy et al., 2003).
Manipulations of 5-HT in humans have provided somewhat inconsistent results. Rogers et al. (1999) showed that acute tryptophan depletion (ATD) in healthy volunteers significantly reduced the propensity to choose the more likely outcome. The group observed the same pattern in patients with focal lesions of the ventromedial and orbital regions of prefrontal cortex. On the other hand, using the same paradigm Talbot et al. (2006) found the reverse effect with increased choice of the more likely outcome. In monkeys, ATD reduced the preference for safe options and also reduce the premium required to make the monkey switch from choosing the safe towards the risky option (Long et al., 2009). 5-HT manipulations also modulated the sensitivity to negative feedback in a probabilistic reversal learning task performed by rats (Bari et al., 2010), with acute 5-HT depletion increasing the sensitivity to negative feedback, whereas increasing 5-HT levels with citalopram had the opposite effect.
Despite the clear evidence for a significant contribution of 5-HT in risk aversion, it remains unclear whether and how 5-HT provides a neurobiological link between making a risk aversive decision and the resulting aversive outcome. In this study, we assessed changes in regional brain activity with blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) to elucidate the role of 5-HT in processing aversive outcomes. During fMRI, participants performed a newly designed gambling task in which wining probabilities and associated outcomes were parametrically modulated from trial to trial while matching the expected values and loss magnitudes of the different risk options. The same group of subjects performed four fMRI sessions in a counter-balanced fashion that differed only in 5-HT manipulation: (a) increased 5-HT neurotransmission by intravenous administration of the selective serotonin reuptake inhibitor (SSRI) citalopram; (b) reduced brain 5-HT synthesis via acute dietary depletion of the 5-HT precursor tryptophan (acute tryptophan depletion, ATD) (Williams et al., 1999); (c) selective blockade of 5-HT2A receptors with ketanserin or (d) without any intervention, to act as control condition. Compared with the effects of ATD and citalopram, ketanserin has a receptor-specific effect and these results will be reported separately. The control condition did not include a placebo drink or intravenous placebo infusion and was primarily used to estimate normal brain function in the absence of any manipulation. The main focus here was to contrast increased 5-HT transmission following citalopram with decreased 5-HT transmission following ATD.
Given the role of 5-HT in processing aversive outcomes we hypothesized that acute manipulations of 5-HT levels would preferentially modulate the neural response to aversive events when the subject failed to receive the reward and lost the bet. We further predicted that the 5-HT manipulations would have the highest impact on aversive outcomes that resulted from low-risk choices because the value of the “missed reward” (the reward of the alternative unselected option) would be highest (Boorman et al., 2009, 2011). We expected that the 5-HT manipulations to affect the BOLD signal in frontopolar and dorsomedial frontal cortex (dmPFC) (Boorman et al., 2009, 2011) and amygdala which has previously been found sensitive to serotonergic manipulations (McKie et al., 2005; Bigos et al., 2008)
Experimental procedures
Participants
24 right-handed healthy adults (8 females) were recruited for the fMRI study. None reported a history of stimulant abuse, neurological or psychiatric disorders. All subjects were naïve for antipsychotics and antidepressants according to self-report. Written informed consent was obtained prior to the MRI scanning sessions. The study was approved by the Copenhagen Ethics Committee (KF 01-2006-20). One subject was excluded because he showed extreme risk avoiding behavior while performing the gambling task in the MR scanner. A second participant was excluded because he did not complete all three sessions of the study. 22 participants (8 females) with a mean age ± SD of 31.5 ± 6.2 years were included in the final analysis.
The gambling task
During fMRI participants performed a novel gambling task (Figure 1) that required subjects to make a choice between two sets of playing cards displayed face down. One of the sets included the “ace of hearts” and subjects were required to choose in which set it was hidden. If the subject chose correctly they won the associated reward. If not, they lost the bet. The objective was to maximize the profit, which was subsequently paid to them in Danish Kroner (DKK). Each gamble had a stable trial structure consisting of an information, decision and outcome phase (Figure 1 A). In the information phase, participants saw the accumulated sum, starting with 50 DKK (Approx. 10 USD) and a variable bet (scaled to the accumulated sum: 3-5 DKK). In the decision phase, two sets of cards were presented facedown together with the associated reward and subjects made their choice. The outcome phase revealed the “ace of hearts”, giving the subjects feedback about whether they had won or lost.
Figure 1. Gambling task.
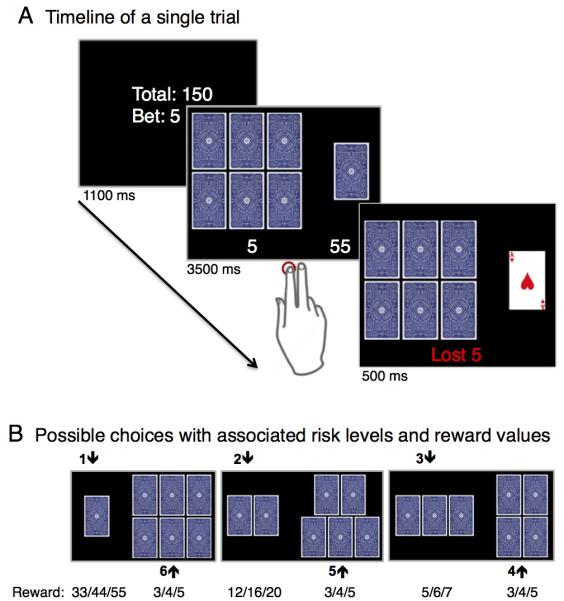
(A) Temporal structure of a single gambling trial. Each trial was divided into 3 phases: an INFORMATION, DECISION and OUTCOME phase. Subjects first received INFORMATION about the sum of money they had accumulated and the bet size, which could be lost. In the DECISION phase, two sets of cards facedown were presented together with the associated monetary reward. Participants chose the set of cards where they believed the “ace of hearts” would be hidden. In the OUTCOME phase, the “ace of hearts” was revealed, providing the subjects a feedback whether they had chosen the right set and won the associated reward or lost the bet. (B) Possible choices with associated risk levels.
In each gambling trial, seven cards were divided in two sets (Figure 1 B), resulting in six possible risk scenarios with a parametric variation of the odds, ranging from 1/7 (low probability to win) to 6/7 (high probability to win). Choosing the set with the lower number of cards was associated with a higher risk but also with a correspondingly higher reward when the subjects had chosen correctly. For choices with winning probability of more than 50% (i.e., odds of 6/7, 5/7 or 4/7), the reward was matched to the amount of the bet. For choices with a winning probability of less than 50%, the possible reward exceeded the bet by the factor 11 for a winning probability of 1/7, 4 for bets with a winning probability of 2/7, or 1.66 for a winning probability of 3/7, respectively. The magnitude of losses was matched to the bet independent of the chosen risk.
The task was tuned to stimulate an even distribution of choices across all risk levels by varying the reward value with the size of the assumed risk so that the expected value (i.e., the sum of probabilistically weighted wins and losses) would match across all possible choices. The experimental design enabled us to associate neural activity related to aversive outcomes to the riskiness of choice behavior. In particular, we were able to assess differential responses to aversive outcomes depending on whether the decision preceding it was risk-averse (i.e., playing it safe but being punished for it) or risk-taking (i.e., taking a risk and being punished for it).
Serotonergic challenges
Subjects took part in multiple fMRI sessions that only differed in terms of pharmacological manipulation: a “no drug” condition, referred to as control, ATD, acute administration of citalopram and acute blockage of 5-HT2A receptors with ketanserin Because the protocol for drug administration differed substantially between ATD, citalopram,. and ketanserin treatment, we decided against giving placebo during the control session. fMRI sessions were performed at least one week apart in a fully counterbalanced order with subjects being assigned a specific 5-HT challenge order e.g. control, ATD, citalopram, ketanserin. As there were 16 possible combinations and 24 subjects, eight of the sequences were used twice. This design allowed us to control for task and scan repetition effects. In contrast to the global effects on the serotonergic tone induced by ATD and SSRI, ketanserin has a receptor-specific effect. These results will be reported in a separate paper. For the citalopram session, citalopram was administrated intravenously at a rate of 20 mg/h starting two hours before scanning, with maintenance dose during the fMRI session at 8 mg/h (~50 mg in total). The ATD session was performed based on the description of Young et al. (1985). The day before scanning participants followed a low protein diet. Upon arrival on the scanning day, participants ingested 75 g tryptophan-free powdered mixture of essential and non-essential amino acids dissolved in water (XLYS, TRY Glutaridon, SHS International Ltd.) and performed the fMRI session five hours later.
In the citalopram session, blood samples were taken before citalopram was administrated, right before the start of the scanning session, and after the scanning session to assess serum prolactin levels as a proxy for cerebral 5-HT level changes. In the ATD session, blood samples were taken before the ingestion of the amino acid drink, before and after the scanning session. A second blood sample was taken at the same time points to measure changes in plasma levels of tryptophan and the other large neutral amino acids (Williams et al., 1999).
Mood assessment
Participants completed a modified Danish version of the Profile of Mood States (POMS) questionnaire (McNair PM, Lorr M, 1971) to assess current mood based in six domains: tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia and confusion/bewilderment. For the control session, participants completed the mood questionnaire twice, prior to the start of the fMRI scan and immediately after the fMRI scan. For the ATD and SSRI sessions the participants were requested to report mood on three occasions, upon arrival, right before and right after the fMRI scan.
Behavioral data analysis
The frequency of risk choices and reaction times were entered into repeated measures analyses of variance (ANOVA) models (PASW-SPSS17 statistics software, Chicago) with the factors of type of intervention (3 levels, ATD, citalopram and control) and “risk level” (3 levels, odds of 4/7, 5/7 and 6/7) as within subject factors. We examine the frequencies of low-risk choices because each high-risk option was paired to a corresponding low-risk option in a forced-choice design (Figure 1 B). Significance threshold was set at p<0.05 using the Greenhouse-Geisser correction for non-sphericity when appropriate. Conditional on significant F-values, pair-wise post-hoc t-tests were performed to further explore significant main effects and interactions.
Magnetic resonance imaging
All MRI measurements were performed on a 3 Tesla MR scanner (Siemens Trio, Erlangen, Germany) using an eight-channel head array coil. The same MRI protocol was performed during the control, ATD and citalopram sessions. BOLD-sensitive fMRI used a T2*-weighted gradient echo spiral echo-planar imaging (EPI) sequence with a repetition time (TR) of 2.5 s, echo time (TE) of 26 ms, and flip angle of 90 degrees. The fMRI measurements were obtained in two fMRI runs, each run lasting 11 minutes. A total of 128 brain volumes were acquired in a single fMRI session. Each brain volume consisted of 41 slices with a slice thickness of 3 mm, between-slice gap of 25%, and a field of view (FOV) of 256 × 256 mm using a 64 × 64 grid. The EPI sequence was optimized for signal recovery of frontal cortex close to the base of the skull by tilting slice orientation from a transverse toward a coronal orientation by about 30° and the use of a preparation gradient pulse (Deichmann et al., 2003). In addition, high-resolution 3D structural T1-weighted spin echo images were obtained after the first session of BOLD fMRI (TI = 800, TE= 3.93, TR = 1540 ms, flip angle 9°; 256 × 256 FOV; 192 slices). After the BOLD fMRI measurements, we assessed regional blood perfusion at rest using arterial spin labeling (ASL). The ASL measurements were performed to test whether any differences in the regional BOLD signal between ATD, SSRI and control sessions resulted from a real difference in regional neural activity induced by 5-HT challenge rather than a mere difference in baseline blood perfusion levels. ASL-based perfusion measurements used FAIR Q2TIPS (Luh et al., 1999) sequences with 3D GRASE (Günther et al., 2005) single-shot readout with background suppression (TR = 3.4s, TE = 19.3 ms, TI = 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800, 2000, 2200, 2400, 2600, 2800, 3000 ms, 2 averages per TI, Q2TIPS saturation duration = 150 ms, 26 slices, voxel size = 5.0 × 5.0 × 4.0 mm, FOV = 320 × 160 × 104 mm, vessel suppression with bipolar gradients, b = 6 s/mm2). The duration of the ASL measurements was 4 minutes and the sequence was run right after the fMRI session.
Functional MRI data analysis
The preprocessing and statistical analysis of the acquired images used SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5). The structural images were segmented and the resulting parameters were used during the normalization of the functional images. The functional images were realigned to the first image, normalized and smoothed using a symmetric 8-mm Gaussian kernel.
The statistical analyses focused on the outcome phase of the trials. For the first level subject models we implemented an event related design with six different regressors for aversive outcomes separating different type of events by the size of the risk the subject took (odds) during the decision making phase (from the lowest odds 1/7, to the highest odds 6/7). The different choices are illustrated in Figure 1 B. The model also included six regressors for the positive outcomes according to the risk level, one regressor for the decision making, one for the information phase, and 40 additional nuisance regressors to correct for physiological noise related to pulse (10) respiration (6) and movement (24) (Glover et al., 2000; Lund et al., 2006).
The impact of risk level and challenge on neural activity related to aversive outcomes was modeled at the group level (second level) using a flexible-factorial design with the factors “subject” (22 levels), “type of pharmacological challenge” (3 levels; ATD, SSRI, and control) and “risk level”. Two complementary models were specified which differed in the number of risk levels. In a first model, the parametric change in odds was modeled separately, resulting in six risk levels (odds of 1/7, 2/7, 3/7, 4/7, 5/7 and 6/7). This model was used to identify brain regions showing a linear increase or decrease in aversive outcome related activity depending on the riskiness of the choice that caused the aversive outcome, independent of the pharmacological challenge. We performed a one-sample t-test to assess the main effect of aversive outcomes by collapsing the estimates across pharmacological challenges and risk levels. In the second model, the aversive events were grouped: choices with odds of 1/7 and 2/7 were modeled together as high-risk choices, choices with odds of 3/7 and 4/7 as medium-risk choices and choices with odds of 5/7 and 6/7 as low-risk choices. This model was used to test for an interaction between the riskiness of choice behavior and pharmacological challenge to identify brain regions where ATD and SSRI had opposite effects on aversive outcome related activity depending on the risk level of the decision causing the aversive outcome. We also constructed an analogous model to assess increases in BOLD signal in response to rewarding outcomes. The model consisted of separate regressors modeling the BOLD response to positive outcomes following low-risk, medium-risk and high-risk decisions. This enabled us to identify brain regions where the 5-HT challenges modified the functional response to both, aversive and rewarding outcomes.
Using a voxel-wise extent threshold of p<0.01, all clusters were considered to be significant at p<0.05 after Family-Wise Error (FWE) correction for multiple non-independent comparisons. Voxels showing a change in BOLD response at an uncorrected p<0.001 but without surviving FWE correction are reported as trend changes.
We expected that 5-HT would influence the regional neural response to aversive outcomes in the amygdala and dmPFC for two reasons. First, the amygdala (Kahn et al., 2002; Yacubian et al., 2006) and dmPFC (Taylor et al., 2006; Liu et al., 2007) have been shown to be involved in processing aversive outcomes. Secondly, pharmacological manipulations of 5-HT levels during fMRI have been successfully used to modulate the processing of aversive stimuli in both amygdala and dmPFC. In healthy volunteers, the amygdala showed an increased BOLD response to aversive emotional stimuli after acute administration of the SSRI citalopram (McKie et al., 2005; Bigos et al., 2008). An acute decrease in brain 5-HT synthesis with ATD increased the neural response to negative outcomes during a probabilistic reversal learning task in dmPFC (Evers et al., 2005) and to fearful faces in the amygdala (Cools et al., 2005; van der Veen et al., 2007). We therefore defined spherical regions of interest (ROI) for left and right amygdala with a radius of 8 mm (the smoothing kernel size) centered in [−27, −3, −18] and [30, −3, −12] the peak activity voxels in amygdala found by Yacubian et al. (2006) for loss related expected value. For the dmPFC region, we choose an ROI with a radius of 8 mm centered in the peak dmPFC voxel from the main effect of aversive outcomes contrast [14, 54, 36]. We performed FWE correction for the voxels within the spherical ROIs using small volume correction. All imaging results are reported by the Z-score and stereotactic MNI coordinates of the regional maxima.
Blood perfusion analysis
The ASL-based brain perfusion measurements were analyzed using FABBER with spatial priors (www.fmrib.ox.ac.uk/fsl/fabber). Perfusion differences between control, ATD and citalopram sessions were evaluated using permutation-based statistics. We performed a small volume correction of the perfusion contrasts using regions of interest calculated from the fMRI contrasts that showed significant differences between the 5-HT challenges.
Results
Choice behavior
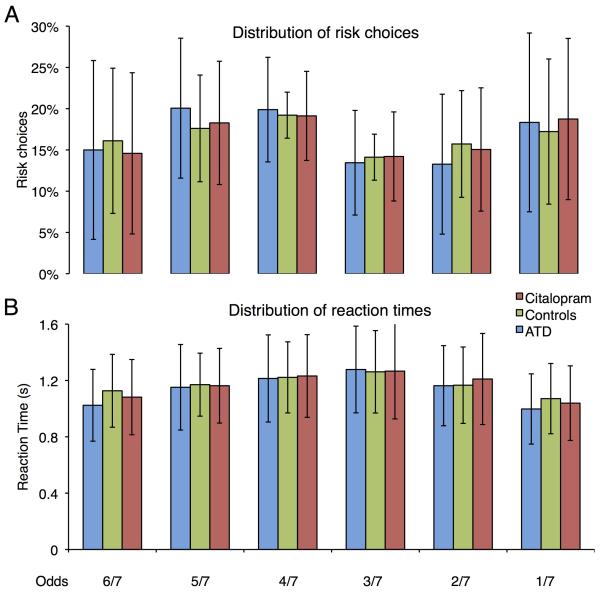
Subjects distributed their choices evenly across the six different risk levels and none of the serotonergic challenges influenced the distribution of choices (Figure 2 A). ANOVA showed no significant main effects or interactions between the factors challenge and risk level (p=0.6). There were no differences in reaction times between the risk choices, and the reaction times were not influenced by the serotonergic challenges (Figure 2 B). Using reaction time as the dependent variable, the ANOVA yielded neither significant main effects nor interactions between the factors challenge and risk level (p=0.8).
Figure 2. Choice behavior.
(A) Distribution of choices across the six risk levels (odds). We found no significant change in risk distribution due to pharmacological challenges. (B) Distribution of reaction times across the six risk levels. There were no significant differences in reaction times between the risk choices, or the serotonergic challenges. The columns represent the group mean and error bars give the standard deviation from the mean (n = 22).
Mood assessment
We analyzed POMS scores collected before and after the pharmacological interventions during all fMRI scan days. The POMS scores allowed us to identify mood changes caused by the scanning session by itself as well as changes potentially induced by the ATD and citalopram challenges. An ANOVA analyses with two factors, 5-HT intervention (ATD, citalopram and control) and time (before and after fMRI session) yielded an effect of time for anger/hostility with lower scores at the end of the scanning session as compared to pre-scanning baseline (F1,11=5.98, p=0.033). Importantly, there was no significant effect of the intervention × time interaction in any of the reported mood states. Because the fMRI analyses focused on the differential effects of ATD and citalopram, we also set up a second ANOVA model with two factors, 5-HT intervention (ATD and citalopram) and time (arrival time, before and after the fMRI session). Again, we found a main effect of time, with a decrease in vigor/activity scores after both pharmacological interventions (F2,19=6.61, p=0.009), but neither a main effect of the type of intervention nor an intervention × time interaction for any of the mood states.
Biochemical results
Interindividual baseline prolactin levels across sessions were highly correlated (r=0.80, n=19, p<0.001). ANOVA including the factor challenge (citalopram vs. ATD) and time (baseline vs. pre-scanning) yielded no main effect of drug (F<1) or time (F1,17=2.86, ns), but a significant interaction (F1,17=3.0, p<0.1) with increased prolactin levels after intravenous application of citalopram. The ATD protocol reduced the plasma ratio of tryptophan to the sum of competing large neutral amino acids (found to determine the uptake of tryptophan in the brain) by 90% (paired t-test, t21=11.2, p<0.001).
Neuroimaging results
Regional brain activity related to aversive outcomes
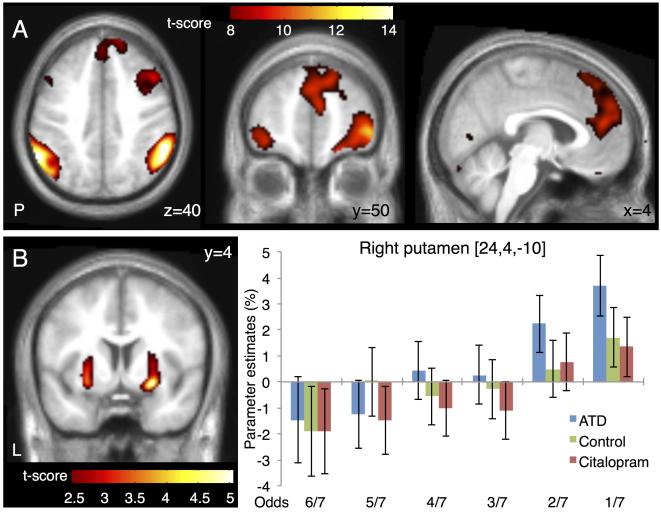
Independent of serotonergic challenge or riskiness of choice (main effect of aversive outcomes), an extensive bilateral fronto-parietal network showed an increased BOLD response for aversive outcomes (Figure 3 A). This network included inferior frontal gyrus, (regional peak in right inferior frontal gyrus at MNI coordinates x, y, z =46, 36, −10; Z>7.8; pFWE<0.001 and left inferior frontal gyrus x, y, z =−42, 36, −10; Z>7.8; pFWE<0.001), lateral frontopolar cortex (regional peak in right frontopolar cortex at MNI coordinates x, y, z =34, 56, −4; Z>7.8; pFWE<0.001 and left frontopolar cortex at MNI coordinates x, y, z =−42, 44, −10; Z>7.8; pFWE<0.001), anterior cingulate cortex, (regional peak in anterior cingulate cortex at MNI coordinates x, y, z =6, 46, 16; Z>7.8; pFWE<0.001), dmPFC (regional peak in dmPFC at MNI coordinates x, y, z =14, 54, 36; Z>7.8; pFWE<0.001), inferior parietal cortex (regional peak in right inferior parietal cortex at MNI coordinates x, y, z =58, −44, 40; Z>7.8; pFWE<0.001 and left inferior parietal cortex x, y, z =−60, 52, 36; Z>7.8; pFWE<0.001) and caudate (regional peak in right caudate at MNI coordinates x, y, z =8, 0, 6; Z>7.8; pFWE<0.001)
Figure 3. Changes in regional brain activity related to aversive outcomes.
(A) Main effect of aversive outcomes. The panels show representative slices of the statistical parametric map thresholded at p<0.01 FWE corrected. (B) Putamen showed a linear increase in aversive outcome activity with the risk choice (odds). The left panel show map thresholded at p<0.01 uncorrected. Note that only the cluster in the right putamen was significant after correction for multiple comparisons. The right panel gives the parameter estimates of the regional peak in right putamen for the six risk choices (odds) and the three fMRI sessions. Error bars represent the 90% confidence intervals of the mean.
Since we parametrically manipulated the risk level from trial to trial, we were able to test for brain regions where aversive outcome related activity showed a linear relationship with the riskiness of the choice made during the decision phase. The putamen showed a linear increase in aversive outcome related activity with the risk choices (Figure 3 B). The strongest increase in correlation was found in the ventral portion of the anterior putamen. This linear increase was expressed bilaterally in the putamen, but only reached significance on the right side (regional peak in right putamen at MNI coordinates x, y, z =24, 4, −10; Z=4.66; pFWE=0.048), whereas the positive relationship between aversive outcome related activity and the riskiness of choice only reached trend significance in left putamen (regional peak in left putamen at MNI coordinates x, y, z = −26, 0, −10; Z=4.13; pFWE = 0.323). When testing for a linear decrease in loss related activity with the riskiness of choice, the left anterior insula showed a trend correlation (regional peak in left insula at MNI coordinates x, y, z = −30, 22 −2; Z=3.10). Of note, the relationship between activity related to aversive outcomes in putamen and insula and the riskiness of the choices was not modulated by citalopram or ATD (Figure 3 B).
Impact of changes in 5-HT transmission on regional brain activity related to aversive outcomes
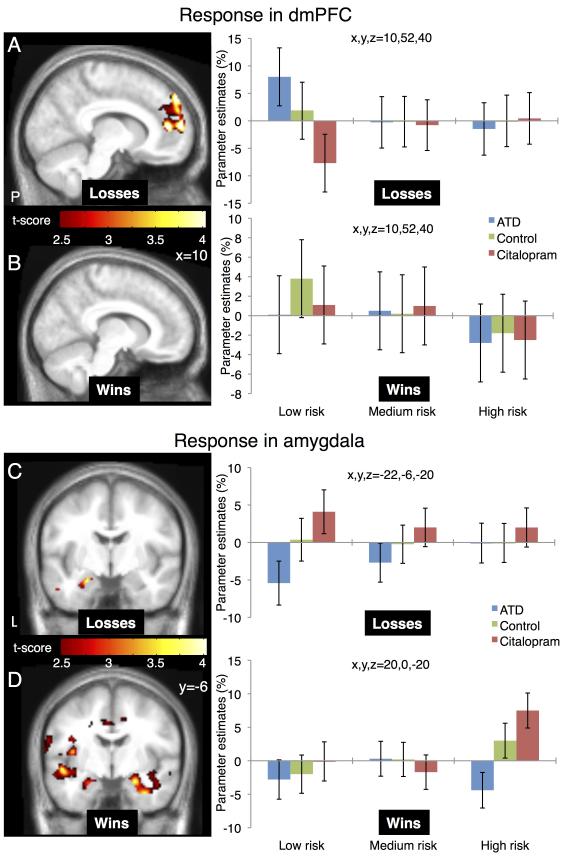
The two pharmacological interventions had opposite effects on aversive outcome related activity in the dmPFC and amygdala (Figure 4). In the dmPFC, ATD increased and citalopram decreased aversive outcome related activity relative to the baseline condition (peak difference at MNI coordinate x, y, z = 10,52,40; Z=3.18; pFWE = 0.025 corrected within the dmPFC ROI). This region was part of a larger medial PFC cluster that showed increased activity with aversive outcomes. The opposite pattern emerged in left amygdala. Here citalopram increased and ATD decreased the BOLD response to aversive outcomes (peak difference at MNI coordinate x, y, z = −22,−6,−20; Z = 3.80; pFWE = 0.004 corrected within the amygdala ROI). We also observed trend differences in right amygdala but below the significance threshold. Critically, the changes in aversive outcome related activity in both regions were significant only when the aversive event was caused by a low-risk but not by a medium or high-risk choice (Figure 4 A and C).
Figure 4. 5-HT induced changes in outcome related activity.
The figure illustrates changes in regional BOLD response to aversive and positive outcomes depending on the risk choice and the type of serotonergic challenge. Choices with odds of 1/7 and 2/7 were pooled together as high-risk, choices with odds of 3/7 and 4/7 as medium-risk and choices with odds of 5/7 and 6/7 as low-risk choices. (A) For unsuccessful low-risk choices, ATD increased and citalopram decreased dmPFC activity compared to the control condition. (B) Serotonergic challenges have no significant influence on dmPFC during positive outcomes. (C) For unsuccessful low-risk choices amygdala’s response decreased with ATD and increased with citalopram. (D) For high-risk positive outcomes amygdala’s response showed a bilateral decrease with ATD and increase with citalopram. The maps are thresholded at p<0.01 (uncorrected) for illustrative purposes. The right panels give the parameter estimates of the regional peaks for the low, medium and high-risk choices and the three pharmacological challenges. Error bars represent 90% confidence interval of the mean.
Regional brain activity related to positive outcomes
We did not find any effects of the serotonergic challenges in dmPFC during positive outcomes (Figure 4 B). However, the amygdala showed a bilateral increase in activity in the citalopram session compared to the ATD session when subjects made large wins following high-risk choices (peak difference at MNI coordinate x, y, z = −28,2,−14; Z = 3.49; pFWE = 0.011 and 26,2,−16; Z=3.40; pFWE = 0.014 corrected within the amygdala ROI, for left and right amygdala respectively - Figure 4 D). These amygdala regions overlapped with regions showing a general increase in activity for positive outcomes.
Regional brain perfusion at rest
Whole-brain analysis of the ASL data revealed no significant differences in regional cerebral perfusion when perfusion levels during the citalopram or ATD sessions were contrasted with brain perfusion measured in the control session. Additionally, there was no difference in regional brain perfusion when contrasting the ASL data of the citalopram and ATD sessions. Critically, we found no pharmacologically induced changes in regional brain perfusion in the amygdala and dmPFC after correcting for multiple comparisons within respective predefined ROIs.
Discussion
The present study discloses two main findings: Analysis of BOLD signal changes revealed that the two pharmacological manipulations had opposite effects on activity related to aversive outcomes in two brain regions. In the dmPFC, ATD increased and citalopram decreased activity to the control condition. The inverse activation pattern was found in left amygdala with citalopram increasing and ATD decreasing the BOLD response to aversive outcomes. This differential effect of the pharmacological challenges was only observed when the aversive outcome was caused by a low-risk decision.
Our new gambling task parametrically varied winning probabilities and associated outcomes and matched expected values and loss magnitudes. This has two implications. First, the selective 5-HT effect on processing aversive outcomes in dmPFC and amygdala following low-risk choices was not caused by differences in the magnitude of losses, because the loss values for all risk choices were matched. Second, matching the expected values prompted participants to distribute their choices in a balanced manner across all different levels of risk. We argue that the observed changes in BOLD response to low-risk aversive outcomes cannot be explained by a 5-HT mediated change in behavioral inhibition, as subjects always had to make a choice and neither of the pharmacological interventions resulted in a shift in choice behavior.
A cluster in the dmPFC showed an increase in neural response to aversive outcomes following unsuccessful low-risk choices for ATD, while citalopram had the opposite effect. This cluster formed a subset of the larger medial PFC region that was activated during aversive outcomes. This finding is in line with previous studies that have implicated the dmPFC in processing monetary losses (Taylor et al., 2006; Kim et al., 2006; Liu et al., 2007). Of particular interest, ATD has been shown to increase the neural response in dmPFC to negative feedback during probabilistic reversal learning, irrespective of whether the errors were followed by behavioral reversal (Evers et al., 2005). The present study confirms a modulatory role of 5-HT on performance monitoring in dmPFC by suppressing the neuronal response to aversive outcomes. Extending previous work, we show that the suppressive effect of 5-HT is also present in a gambling task with stable reward-punishment rules and without any need to learn or adjust reward or punishment contingencies. The critical new finding is that the suppressive effect of 5-HT on the processing of aversive outcomes in dmPFC is restricted to events caused by risk-averse decisions.
Our design did not allow to directly test whether the sensitivity to 5-HT transmission in dmPFC and amygdala relates to the magnitude of missed reward (i.e. the reward of the alternative unselected choice) or to the aversiveness of unsuccessful low-risk choices. However, previous neuroimaging studies have found that activity of dmPFC and frontopolar cortex codes for the missed rewards of the best unselected choice (Boorman et al., 2009, 2011). Another possibility is that the 5-HT challenges exerted a general modulatory effect on the processing of surprising events. However, a general surprise effect is unlikely because the acute manipulations of cerebral 5-HT levels did not modulate the neural response of dmPFC to equally surprising high positive outcomes following high-risk choices. Instead, we propose that in the present gambling task, the dmPFC processed the magnitude of the missed reward of unselected choices, when subjects “played it safe” but still lost and that a higher availability of 5-HT “dampens” the sensitivity of dmPFC to missed reward.
The lateral frontopolar cortex responded during both aversive and positive outcomes. This suggests that the lateral frontopolar cortex processed both the outcomes of the selected option and the alternative unselected option. However, contrary to our hypothesis, the frontopolar cortex was not sensitive to the global changes in serotonergic transmission. This negative finding does not imply that the processing of low risk aversive outcomes in the frontopolar cortex is not influenced by 5-HT. It is worth to keep in mind that ATD and citalopram alter 5-HT transmission through many types of receptors which can have competing actions in specific cortical regions and thus may not result in a change in the overall neural response as captured by the BOLD signal.
The left amygdala also showed a selective change in BOLD response to aversive outcomes following low-risk decisions: reduction in cerebral 5-HT with ATD decreased the BOLD response to aversive outcomes in the left amygdala, whereas increasing 5-HT levels with citalopram had the opposite effect. ATD and citalopram also had a selective effect on amygdala’s response to positive outcomes. ATD bilaterally decreased, but citalopram increased the regional response of the amygdala to positive outcomes caused by high-risk decisions. Of note, amygdala displayed a general response to positive, but not negative outcomes. These findings shed new light on the neuromodulatory effects of 5-HT on reward processing in amygdala (Yacubian et al., 2006). The observed pharmacological effects in the amygdala are compatible with the hypothesis that in the context of aversive outcomes, amygdala labels these events as unfair when a low-risk choice is punished (Crockett et al., 2008, 2010). Conversely, in the context of positive outcomes, amygdala enhances a positive surprise effect. In this regard, our data point to a possible functional lateralization with the left amygdala showing stronger 5-HT modulation during aversive outcomes and the right amygdala during positive outcomes. An alternative explanation might be that 5-HT related processes in amygdala involve categorizing the salience of the events regardless of whether outcomes are positive or aversive.
The effects of ATD and citalopram on the neural response to low-risk aversive outcomes in dmPFC and amygdala were opposite in sign. During aversive outcomes, higher 5-HT levels increased the amygdala neural response while reducing the response in dmPFC. This finding points to complementary or even antagonistic roles of dmPFC and amygdala in processing aversive outcomes. In accordance with our findings, an inverse response pattern of medial prefrontal cortex and amygdala has been repeatedly demonstrated with invasive methods in animals (Grace and Rosenkranz, 2002; Quirk et al., 2003) and functional neuroimaging in humans (Ochsner et al., 2002; Kim et al., 2003; Urry et al., 2006; Passamonti et al., 2008). For instance, Ochsner et al. (2002) found increased activation of lateral and medial frontal regions paralleled by decreased orbitofrontal and amygdala activations during cognitive control of negatively valenced stimuli.
The ventral putamen showed a positive correlation between neural activity related to aversive outcomes and risk taken during the decision phase with highest activity for the most risky choice. This linear relationship between response to aversive outcomes and riskiness of the gamble was expressed bilaterally in the putamen, but only reached significance in the right putamen. An opposite trend was found in the left anterior insula where aversive outcome related activity decreased linearly with increased riskiness of the choice. These findings extend previous work on the processing of positive and aversive outcome information (Liu et al., 2007), pointing to a differential role of putamen and insula in the evaluation of unsuccessful choices. The ventral putamen seems to process primarily aversive outcomes caused by highly risky choices motivated by a potentially high reward, while the anterior insula seems to preferentially process aversive outcomes caused by highly risk-avoiding choices resulting in a high missed reward. Interestingly, the activation profile of aversive outcomes in ventral putamen and anterior insula were not modulated by the serotonergic challenges in the present study. Our results show that aversive outcome activity in ventral putamen and anterior insula do not rely on serotonergic signaling. Rather, our findings are compatible with the notion that 5-HT seem to be more involved in emotional aspects of loss processing (Bechara et al., 1999) by modulating structures such as amygdala and dmPFC.
A potential limitation of the study design was that the pharmacological challenges were not blinded. The main goal of the study was to compare two serotonergic challenges with opposite effects and to use the session without pharmacological challenges as reference. All volunteers handled waiting times, scanning and drug infusions without any complaint or difficulty. In particular, no subject reported any discomfort due to the infusion line. They were not informed about any expected effects of 5-HT manipulation and we could not find any measurable effects of the 5-HT challenges on reaction times, choice behavior or self reported mood states. The only exception was the scores related to vigor/activity that showed a similar decrease in both the citalopram and ATD sessions. Given these factors, the regionally specific drug-by-task interactions are unlikely to be due to simple placebo effects or a lack of blinding. The results support our predictions of context-specificity (affecting only aversive outcomes after low-risk choices, or positive outcome after high-risk choices) and region-specificity (amygdala, dmPFC). We also verified using a subsequent ASL analysis that there were no challenge-induced changes in cerebral perfusion in these regions, suggesting that in dmPFC and amygdala the observed modulation of BOLD response is most likely to reflect altered serotonergic neurotransmission induced by the challenges, in the specific context of the gambling task.
Conclusions
We show that 5-HT transmission preferentially modulates the neural response in dmPFC and amygdala to the specific events when people play it safe but are punished anyway. The effect of 5-HT level on the regional response to aversive outcomes following low-risk choices was opposite in sign for dmPFC and amygdala, pointing to a reciprocal inhibitory interaction between the two regions. Further, only in the dmPFC, the 5-HT induced change in response to low-risk losses was not paralleled by a change in responsiveness to positive outcomes, suggesting a unique role of the dmPFC in the selective processing of aversive outcomes in the context of risk avoidance. Together, these findings have important implications for understanding the role of 5-HT in mood and anxiety disorders. For instance, dysfunctional 5-HT signaling may bias cognitive and emotional processes towards risk-averse decisions, result in excessive behavioral inhibition or render patients more susceptible to uncontrollable stress.
REFERENCES
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–5. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003;49:206–8. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Rushworth MF. Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLoS Biol. 2011;9:e1001093. doi: 10.1371/journal.pbio.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–43. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574–83. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology. 2005;180:670–9. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008(a);12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cools R, Robinson OJ, Sahakian B. Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology. 2008(b);33:2291–9. doi: 10.1038/sj.npp.1301598. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci U S A. 2010;107:17433–8. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–16. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Deakin J. Roles of serotonergic systems in escape, avoidance and other behaviours. In: Cooper S, editor. Theory in psychopharmacology. Vol. 2. Academic Press; London: 1983. pp. 149–193. [Google Scholar]
- Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. J Psychopharm. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EAT, Cools R, Clark L, Der Veen FM van, Jolles J, Sahakian BJ, Robbins TW. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–47. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Camerer C, Poldrack R. Neuroeconomics: Decision Making and the Brain. Academic Press; London: 2008. [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–93. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Günther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54:491–8. doi: 10.1002/mrm.20580. [DOI] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer C. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci. 2009;29:2231–7. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–75. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Kahn I, Yeshurun Y, Rotshtein P, Fried I, Ben-Bashat D, Hendler T. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33:983–994. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometria. 1979;47:263–291. [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS biology. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–97. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AB, Kuhn CM, Platt ML. Serotonin shapes risky decision making in monkeys. Soc Cogn Affect Neurosci. 2009;4:346–56. doi: 10.1093/scan/nsp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–54. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lund TE, Madsen KH, Sidaros K, Luo W-L, Nichols TE. Non-white noise in fMRI: does modelling have an impact? NeuroImage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. Induced emotional interpretation bias and anxiety. J Abnorm Psychol. 2000;109:602–15. [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, Vai N del, Anderson I, Deakin JFW. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology. 2005;180:680–6. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- McNair PM, Lorr MDL. Profile of mood states manual. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–67. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cog Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Ewbank M, Hampshire A, Keane J, Calder AJ. Connectivity from the ventral anterior cingulate to the amygdala is modulated by appetitive motivation in response to facial signals of aggression. NeuroImage. 2008;43:562–70. doi: 10.1016/j.neuroimage.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD. The Roles of Dopamine and Serotonin in Decision Making: Evidence from Pharmacological Experiments in Humans. Neuropsychopharmacology. 2010;36:114–32. doi: 10.1038/npp.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, BJ E, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable Deficits in the Decision-Making Cognition of Chronic Amphetamine Abusers, Opiate Abusers, Patients with Focal Damage to Prefrontal Cortex, and Tryptophan-Depleted Normal Volunteers Evidence for Monoaminergic Mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–62. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Sharpe WF. Capital Asset Prices: A Theory of Market Equilibrium under Conditions of Risk. J Finance. 1964;19:425–442. [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav Brain Res. 2004;153:233–9. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Watson DR, Barrett SL, Cooper SJ. Rapid tryptophan depletion improves decision-making cognition in healthy humans without affecting reversal learning or set shifting. Neuropsychopharmacology. 2006;31:1519–1525. doi: 10.1038/sj.npp.1300980. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Schweighofer N, Asahi S, Shishida K, Okamoto Y, Yamawaki S, Doya K. Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PloS one. 2007;2:e1333. doi: 10.1371/journal.pone.0001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–70. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–32. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack R a. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect theory: Cumulative representation of uncertainty. J Risk Uncertainty. 1992;5:297–323. [Google Scholar]
- Urry HL, Reekum CM van, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen FM van der, Evers E a T, Deutz NEP, Schmitt J a J. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology. 2007;32:216–24. doi: 10.1038/sj.npp.1301212. [DOI] [PubMed] [Google Scholar]
- Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila M. Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem. 1999;72:1641–7. doi: 10.1046/j.1471-4159.1999.721641.x. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87:173–7. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]