Abstract
Background
The serotonin 2A (5-HT2A) receptor has been implicated in neural-processing of emotionally salient information. To elucidate its role in processing of fear and anger, healthy individuals were studied with functional MRI (fMRI) after 5-HT2A receptor blockade, while judging the gender of neutral, fearful and angry faces.
Methods
5-HT2A receptors were blocked with ketanserin to a variable degree across subjects by adjusting the time between ketanserin-infusion and onset of the fMRI protocol. Neocortical 5-HT2A receptor binding in terms of the binding potential (BPp) was assessed prior to fMRI with 18F-altanserin positron emission tomography (PET) and subsequently integrated in the fMRI data analysis. Also functional connectivity analysis was employed to evaluate the effect of ketanserin blocking on connectivity.
Results
Compared to a control session, 5-HT2A receptor blockade reduced the neural response to fearful faces in medial orbitofrontal cortex (OFC), independently of 5-HT2A receptor occupancy or neocortical 5-HT2A receptor BPp. The medial OFC also showed increased functional coupling with left amygdala during processing of fearful faces depending on the amount of blocked 5-HT2A receptors.
Conclusions
5-HT2A receptor mediated signaling increases the sensitivity of OFC to fearful facial expressions and regulates the strength of a negative feedback signal from OFC to amygdala during processing of fearful faces.
Keywords: fMRI, PET, emotion, fearful faces, serotonin, 5-HT2A receptors, ketanserin
Introduction
Facial expressions such as happiness, fear, sadness, anger, disgust, and surprise represent basic human feelings that are readily decoded by members of all human cultures (Ekmann, 1999). The ability to appropriately interpret emotional facial expressions is important for our social interactions and impaired emotion-related processing is associated with an increased risk for affective psychiatric illnesses (Mayberg, 2003; Phillips et al., 2003). Neuroimaging studies in healthy volunteers have identified the amygdala and prefrontal cortex as core-regions of a functional network processing facial emotions (Adolphs, 2002). Evidence indicates that the amygdala receives visual information about facial emotions via cortical projections from the ventral stream of object processing, and from a fast subcortical pathway. The latter includes the superior colliculus and pulvinar as the only relays and is critical for automatic processing of facial emotions (de Gelder et al., 2005). The medial prefrontal cortex (PFC) and orbitofrontal cortex (OFC) are involved in evaluating cognitive aspects such as integrating information about the emotional state of others, derived from face emotion (Bechara et al., 2000; Salzman and Fusi, 2010). OFC and amygdala are strongly interconnected, with OFC exerting inhibitory control over amygdala during processing of emotional faces (Stein et al., 2007). Therefore an effective integration of neuronal activity among these core-regions is likely to be critical for efficient processing of emotions (Fairhall and Ishai, 2007; Liang et al., 2009).
Serotonin (5-HT) signaling plays an important role in the processing and regulation of emotions (Cools et al., 2007). For example, regulation of 5-HT release in the mPFC in response to aversive stimuli has been identified as a crucial mechanism in rats to deal effectively with stressors and to terminate fear-related behavior (Forster et al., 2006). Previous studies have also shown that serotonergic drugs modulate the neural processing of emotional faces in healthy individuals: For instance, Harmer et al. (2003) found that acute tryptophan depletion decreases recognition of fearful facial expressions in healthy women, while Passamonti et al. (2012) found that acute tryptophan depletion modulated the interactions between PFC and amygdala while viewing emotionally salient faces. Further, a single dose of the selective serotonin reuptake inhibitor (SSRI) citalopram, a widely used antidepressant, increased the neural response of amygdala to happy but not to fearful faces in healthy individuals (Norbury et al., 2009).
Several lines of evidence indicate that the serotonin 2 (5-HT2) receptor-family (5-HT2A, 5-HT2B and 5-HT2C) is involved in generation and expression of anxiety. Global disruption of 5-HT2A receptor signaling reduces inhibition in conflict anxiety paradigms in mice (Weisstaub et al., 2006). In humans, there is accumulating evidence that processing of emotionally salient information is modulated by 5-HT2A receptors and that regional expression of the 5-HT2A receptor in the brain, constitutes a trait related to anxiety (Frokjaer et al., 2008). Recently, Fisher et al. (2009) showed that inter-individual variations in 5-HT2A receptor density in mPFC correlated inversely with the activation of right amygdala by angry or fearful faces in a face-matching task compared to a control task. They also reported a positive correlation between amygdala-prefrontal coupling and prefrontal 5-HT2A receptor binding. These neuroimaging data suggest a regulation of amygdala reactivity via feedback inhibition from PFC, which is more pronounced in individuals with greater neocortical 5-HT2A receptor density.
Motivated by these reports, we adopted a multimodal neuroimaging strategy to explore the relation between 5-HT2A receptor signaling and emotional face processing in amygdala and OFC. Our experimental approach integrated pharmacological functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) of 5-HT2A receptor binding. We performed blood oxygen level dependent (BOLD) fMRI while healthy participants made gender-judgments on photographs of male or female faces with fearful, angry, or neutral expressions. 5-HT2A receptors were acutely blocked with intravenous ketanserin infusion. By varying the relative timing between drug intake and the onset of fMRI, we adjusted the relative magnitude of acute 5-HT2A receptor blockage across subjects. In addition, 5-HT2A receptor binding as measured with PET, was used as trait marker of neocortical 5-HT2A receptor dependent neurotransmission. This novel study design enabled us for the first time to study the impact of a gradually increasing 5-HT2A receptor blockage on emotional face processing and to investigate its relation to 5-HT2A receptor binding and occupancy.
We hypothesized that the individuals’ cerebral 5-HT2A receptor binding would have differential effects on neural processing of negative face emotions and that pharmacological blocking of 5-HT2A receptors would suppress neural response in OFC while enhancing neural response in amygdala. We further predicted that the pharmacologically induced activity changes during emotional face processing would depend on the 5-HT2A receptor occupancy level.
Methods
Participants
Twenty-three right-handed adults (9 females), mean age 31.8 ± 6.5, were recruited from a larger cohort of healthy volunteers who have previously undergone 18F-altanserin PET (Erritzoe et al., 2009). All subjects were re-interviewed prior to inclusion of the fMRI study. None of the participants reported a history of stimulant abuse or other psychiatric or neurological disorders. All participants were naïve to antipsychotics and antidepressants. They had a normal neurological examination, heart rate and electrocardiogram. Participants completed a modified Danish version of the Profile of Mood States (POMS) questionnaire (McNair et al., 1971) to assess current mood. On each fMRI session, participants completed the mood questionnaire twice, prior to the start of the fMRI scan (and drug infusion) and immediately after the fMRI scan. Written informed consent was obtained prior to both MRI and PET scanning according to the declaration of Helsinki II. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg, Denmark (KF 01-2006-20).
Behavioral task
During fMRI, participants performed a gender-judgment task on face stimuli taken from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998). Unmasked colored photographs of a male or female face were presented in the middle of the screen for 1800ms, with an inter-trial-interval (ITI) of 200ms. Faces were shown from a frontal perspective and had a neutral, fearful or angry expression. Subjects responded by pressing as quickly as possible one of two buttons with their right index or middle finger.
We employed a mixed fMRI design with alternating blocks showing neutral or aversive male and female faces in equal proportion (NEUTRAL-ANGRY-NEUTRAL-FEARFUL-NEUTRAL…). Each block comprised six events; three to five face stimuli (average of four), and one to three (average of two) null events (fixation cross), which were pseudo-randomly intermixed. In total, 32 blocks of neutral, 16 blocks of fear and 16 blocks of angry faces were presented over two fMRI runs, separated by a short break. All neutral faces were presented twice in total, whereas aversive faces were only presented once. Stimulus presentation and response recordings were performed using E-prime (Psychological Software Tools, Pittsburgh, PA, USA).
Acute blockage of 5-HT2A receptors
Subjects took part in four fMRI sessions. These sessions included ketanserin as below, a control condition with no pharmacological intervention (referred to as the control session throughout), and two other pharmacological interventions; intravenous treatment with the selective serotonin reuptake inhibitor (SSRI) citalopram as well as acute tryptophan depletion (ATD). The order of the drugs were fully counter-balanced across subjects. Apart from the IV line and drug infusion administered while in the scanner, the scanning protocol was identical for the control and ketanserin sessions. To test for drug-related changes in neural response that depended on the receptor occupancy, we systematically varied the interval between the onset of ketanserin administration and the onset of fMRI measurements across subjects. The time interval ranged from 5 to 75 min, leading to a blockade of 5-HT2A receptors of variable degree across subjects. (Fig. 1). Ketanserin was administered intravenously as a 10 mg bolus (time 0) followed by 6 mg/h for the duration of the fMRI scan. This infusion schedule results in a gradual increase in 5-HT2A receptor occupancy (OKET) reaching ~100% occupancy within an hour (Pinborg et al., 2003). OKET is defined as the fraction (%) of a receptor population that is occupied during treatment with an unlabelled drug. The time-dependent estimation of OKET was based on data from our previous PET study with acute ketanserin infusion (Pinborg et al., 2003). First, ketanserin enters the brain from the blood stream and diffuses to the receptor to which the drug then binds, thereby liberating radioligand, which then diffuses back into the bloodstream. To describe this process, we generated a model with two exponentials; one representing the ketanserin binding and liberation of the radioligand from the receptor with a half time of Tk½, the other representing the diffusion of free radioligand out of the brain tissue into the blood with a half time of Tr½. By applying this model to experimental data of time dependent 5-HT2A receptor occupancy following ketanserin injection (Pinborg et al. 2003), an excellent fit was obtained when the following two conditions were met: Tk½ and Tr½ values both were in the range of 5-10 min and the sum of Tk½ and Tr½ amounted to roughly 15 min. This enabled us to estimate the minimum and maximum Tk½ values corresponding to two OKET outcomes termed OKET5 and OKET10. In the absence of actual single subject occupancy measurements we tested the robustness of any observed relation between occupancy and fMRI data using both the estimated maximum and minimum values, OKET5 and OKET10.
Figure 1.
Estimated levels of 5-HT2A receptor blocking over time for each subject shown for both Oket5 (triangles) and Oket10 (squares).
The study reported here was designed to investigate the effect of 5-HT2A receptor blockade on emotion processing, and not to investigate the effects of increasing or decreasing overall serotonin levels in the brain. Results from the ATD and SSRI sessions that address global serotonin changes have been reported elsewhere (Grady et al. 2012). The fMRI sessions were performed on four different scanning days at least one week apart to ensure a proper wash-out period, with session order counterbalanced across subjects. Apart from the pharmacological manipulation, the experimental procedure was the same for all sessions. The study design with four different serotonergic challenges did not make full placebo controlling of the control session practical. We therefore controlled for nonspecific pharmacological effects of ketanserin administration (e.g. IV line present during scan) and indirect effects of drug (e.g. via induced side effects) by directly contrasting the ketanserin behavior and functional data with the behavior and functional data acquired during the SSRI session, which had a similar administration protocol with intravenous administration during the entire MRI session (at a rate of 8 mg/h). The expected neurophysiological effect of citalopram is increased general serotonergic transmission compared with ketanserin that specifically reduces 5-HT2A receptor transmission. Further, the subjects were unaware of the expected effects of the 5-HT manipulations, the differences in probabilities of side effects between the different drug interventions and the degree of 5-HT2A blockade during ketanserin administration.
Measurements of cerebral 5-HT2A receptor binding
18F-altanserin PET was undertaken as described by Pinborg et al. (2003). In short, 18F-altanserin was administered as a combination of a bolus injection followed by continuous infusion to obtain steady state of the tracer in blood and tissue resulting in a maximum dose of 3.7 MBq/kg bodyweight. PET studies were conducted between noon and 6 pm. Individual 18F-altanserin PET had been acquired on average 3.2±1.7 years before the fMRI experiment. Test-retest studies have shown that in healthy individuals, cerebral 5-HT2A receptor binding remains relatively stable over 2 years (Marner et al., 2009), showing that 18F-altanserin PET can be considered a stable trait marker for neocortical 5-HT2A receptor binding.
PET data were acquired in 3D using an eighteen-ring GE-Advance scanner (GE, Milwaukee, WI, USA). 18F-altanserin PET images and the structural T1-weighted MR images were co-registered and the PET images were then normalized to the same anatomical template as that used for MR images (Pinborg et al., 2003). Volumes of Interest (VOI’s) were automatically delineated on each individual transaxial MRI slices in a strictly user-independent fashion (Svarer et al., 2005). Given the extensive co-variation of neocortical 5-HT2A receptor binding across neocortical areas a global neocortical region was defined for each participant as described in Erritzoe et al. (2010). PET images were partial volume corrected using the segmented MRI. A two-tissue model based on gray matter, white matter, and cerebrospinal fluid was used (Muller-Gartner et al., 1992, Quarantelli et al., 2004). The binding potential of specific binding relative to plasma was calculated as:
| (1) |
CT and CND being the radioactive concentration in each region of interest and in the reference region, VT and VND being the distribution volumes in each regions of interest and in the reference region, and CP being the metabolite corrected plasma [18F]altanserin. Cerebellum was used as reference region, as it represents non-displaceable uptake only (Pinborg et al. 2003).
Magnetic resonance imaging
As for the PET scannings, all MRI measurements were carried out between noon and 6 pm. Images were acquired on a 3T Trio scanner with an eight-channel head array coil (Siemens, Erlangen, Germany). Blood oxygen level dependent (BOLD) fMRI uses a T2*-weighted gradient echo spiral echo-planar (EPI) sequence with a repetition time of 2.5s, echo time of 26ms, flip angle of 90°, and 41 slices with a slice thickness of 3 mm and 25% gap between slices.
The EPI sequence was optimized for signal recovery in orbitofrontal cortex by tilting slice orientation from a transverse toward a coronal orientation by about 30° and the use of a preparation gradient pulse (Deichmann et al., 2003). A total of 128 whole-brain volumes were acquired in each of the two sessions (total 12.8 min). We additionally acquired a high-resolution 3D structural brain scan using a T1-weighted spin echo sequence (TI/TE/TR =800/3.93/1540 ms, flip angle 9°, 1 × 1 ×1 mm isotropic resolution).
Analysis of the fMRI data
Data were preprocessed and analyzed using SPM5 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Images were realigned and normalized to MNI (Montreal Neurological Institute) stereotactic space using transformation parameters derived from segmentation of the structural MRI. The normalized images were smoothed using a symmetric 8-mm Gaussian kernel. None of the subjects had at any time head motions that exceeded 3mm (voxel size) in any direction. We tested for differences in head movement between the drug sessions by calculating the root mean square of the movement parameters in x, y and z direction and included the individual values in a repeated measures ANOVA with drug session and movement direction as within-subject factors.
The paradigm was analyzed in an event-related fashion with three event types defined at subject level, corresponding to presentation of neutral, angry, or fearful faces. Each event was modeled as a delta function with onset coinciding with the appearance of the cue. Covariates were then convolved with a canonical hemodynamic response function. A two-stage random effects model was created for each subject, modeling the fMRI runs of the ketanserin and control sessions. Each fMRI run was modeled with the three covariates described above together with a mean (constant) term over scans for each run in order to model the main effects of runs. The within-subject model also included 40 nuisance regressors to account for variance caused by physiological noise, including heart beat (10 regressors), respiration (6 regressors), and head movements (24 regressors) (Glover et al., 2000; Lund et al., 2006).
Parameter estimates for each covariate were calculated and statistical parametric maps of the t-statistic (SPM{t}) resulting from linear contrasts of covariates were generated for each subject. Thus, we generated contrast images for the relative increase in BOLD signal induced by the emotional faces relative to neutral faces in both the control and ketanserin sessions.
At the group level, individual contrast images were entered into separate paired t-test models testing the difference in BOLD response of emotional faces relative to neutral faces in the ketanserin relative to the control session. Additional analog group level models were set up by substituting the control contrasts with the equivalent contrasts of the SSRI session.
We also computed one-sample t-tests for the emotion contrast images from the ketanserin session only, including average neocortical BPP, time-dependent OKET (OKET5 and OKET10), and in order to look at the linear relationship between the two covariates we calculated the product (i.e., BPP times OKET). BPP values were time corrected to comply with the delay between PET and fMRI scannings according to Erritzoe et al. (2009) This model enabled us to identify brain regions where ketanserin-induced changes in emotional face processing depending on the neocortical BPp, OKET, or the product of the two, and was performed for both OKET covariates. In order to test for correlations with mood state, a separate analysis was performed using POMS factor scores (anger/hostility, vigor/activity, and fatigue/inertia) as covariates.
We used the psychophysiological interaction (PPI) method described by Friston et al. (1997) to identify ketanserin-induced changes in OFC connectivity during the processing of fearful faces that can be explained by OKET , BPp or an interaction between the two factors. In the first stage, we defined a spherical region of interest (ROI), 8mm in diameter and centered in the peak OFC region (MNI x,y,z = 4,38,−24) showing an attenuation of the BOLD response in the ketanserin session vs. control during perception of fearful faces and we extracted time-course of the BOLD response from this region. A PPI term was calculated by multiplying the estimated deconvolved time-course from the OFC ROI with the fear vs. neutral contrast. We then computed new subject-specific SPMs where the calculated PPI term and the time-course of the seed region were added as regressors to the initial first level subject model which included the three task regressors (neutral, angry and fearful faces). Individual contrasts based on the PPI term were entered in a second level multiple regression model with BPp, OKET (OKET5 and OKET10), and the product of the two (i.e., BPp times OKET) as covariates. Linear contrasts were specified and SPMs based on one-tailed t-statistics were generated.
As general significance level, we used a threshold of p<0.01 on a voxel-wise level and considered clusters significant at p<0.05 after Family-Wise Error (FWE) correction for multiple non-independent comparisons. All imaging results are reported by the Z score and stereotactic MNI coordinates of the regional maxima. We expected amygdala’s neuronal activity to change directly in response to aversive faces. To define amygdala, we delineated spherical regions of interest (ROI) with a radius of 8 mm (the size of the smoothing kernel) centered in the maximum activation likelihood estimations from the Fusar-poli et al. (2009) meta-analysis for fearful faces. We first converted the estimated voxels for fear vs. neutral contrasts from Talairach to MNI space according to (Lancaster et al., 2007) and then used the resulting coordinates ([−23 −4 −15] and [22, −4, −20]) to perform FWE correction for the voxels using small volume correction (SVC) for fear contrasts as well as angry and aversive.
Analysis of task performance
Behavioral data were analyzed using SPSS (version 18, Chicago, Illinois, USA). Individual scores on mood questionnaires were analyzed using a three-way repeated measures ANOVA with the within-subject factors session (ketanserin versus control), mood factors of the POMS (6 levels), and time of assessment relative to fMRI (before versus after). Reaction time differences were assessed using a two-way repeated measures ANOVA with within-subject factors session (ketanserin versus control, or ketanserin versus SSRI) and emotion of the face stimuli (neutral, anger, fear). The Greenhouse-Geisser method was used to correct for non-sphericity if appropriate. Conditional on significant F-values in the ANOVA, post-hoc paired t-tests were performed. Error rates were analyzed using nonparametric Wilcoxon signed-rank tests, comparing each facial expression from the control session with the same facial expression from the ketanserin session. Behavioral data are given as mean ± standard deviation.
Results
Mood assessment
The effect of ketanserin on mood was evaluated by comparing POMS scores collected before ketanserin was given as well as right after completion of the fMRI session. Compared to the control session, acute ketanserin challenge had a specific effect (F(1.7; 30.9)=44.1; p<0.001). In the ketanserin session, participants reported significant decreases in vigor/activity (1.79±0.62 versus 1.36±0.80, t(21)=4.6; p<0.001), and increases in fatigue/inertia (0.5±0.51 versus 0.91±0.61, t(21)=−3.7; p=0.001) compared to the responses prior to ketanserin infusion. Conversely, in the control sessions the scores for anger/hostility were significantly lower at the end of the session relative to scores at the beginning of the session (0.26±0.23 versus 0.20±0.13), t(18)=2.6; p=0.02). None of these mood changes correlated significantly with the fMRI activation patterns.
Task performance
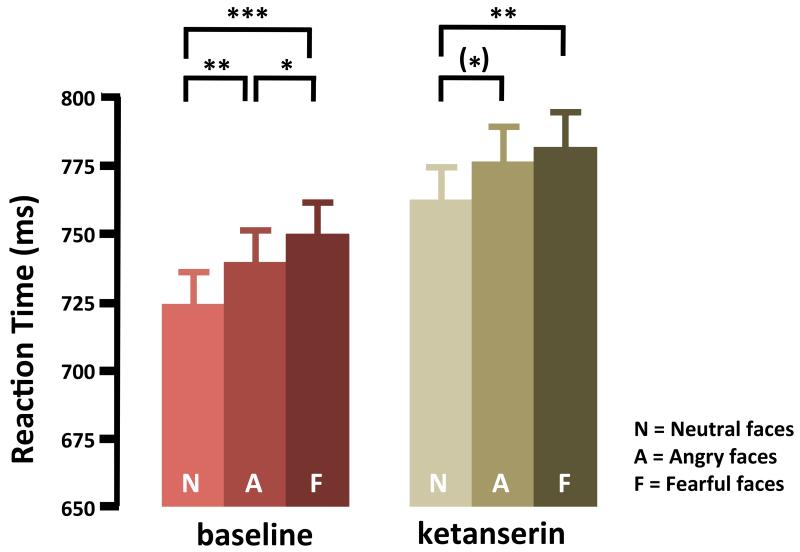
Mean RT was longer when subjects judged the gender of a fearful or angry face relative to a neutral face, showing that gender-judgment was delayed when faces showed an aversive emotion (F(1.8; 38.7) = 16.53; p<0.001). The delay in RT induced by an aversive facial emotion was comparable in size with or without ketanserin treatment (Figure 1). Mean RT was longer for fearful than for neutral faces in both the ketanserin session (t(23)=2.80; p=0.011) and control session (t(23)=6.35; p<0.001). The same was true for RT’s when comparing trials with angry faces versus neutral faces for both ketanserin session (t(23)=2.47; p=0.022) and control session (t(23)=3.,53; p=0.006). In the control session, mean RT’s were also longer in trials with fearful compared to angry faces (t(23)=−2.77; p=0.011).
Ketanserin treatment prolonged mean RT, with an overall increase of approximately 5% compared to the control session (ketanserin session: 774ms±118.6, control session: 741ms±105.5, F(1;23)=18.10; p=0.001). Post-hoc paired t-tests showed this increase was consistent across all facial expressions (p<0.001). Ketanserin had the same effect on RT responses to neutral and aversive faces (Fig. 2) also the ANOVA revealed no interaction between facial emotion and intervention. The relative increase in RT in the ketanserin session correlated with the ketanserin-induced decrease in self-report on vigor (Pearson’s r=0.387, p=0.037), whereas no correlation was found with the reported increase in fatigue (Pearson’s r=0.05, p=0.411).
Figure 2.
Mean reaction time recorded during the gender-judgment task based on facial expressions in the control and ketanserin fMRI sessions. Control session; neutral faces: 724 ±23.55, angry faces: 739±22.23, fearful faces: 750±23.20. Ketanserin session; neutral faces: 762±23.99, angry faces: 776±23.99, fearful faces: 781±25.70, data are presented as mean ± SEM. (*)=p<0.1, *=p<0.05, **=p<0.01, ***=p<0.001. Faces with an aversive emotion delayed the gender-judgment in both sessions relative to neutral faces. Compared to the control session without medication, ketanserin treatment was associated with longer RT.
Error rates did not differ between control and ketanserin session for any of the three facial expressions (Neutral faces: p=0.186. Angry faces: p=0.903. Fearful faces: p=0.613). This shows that the overall slowing of RT found during the ketanserin session was not paralleled by a change in accuracy.
fMRI results
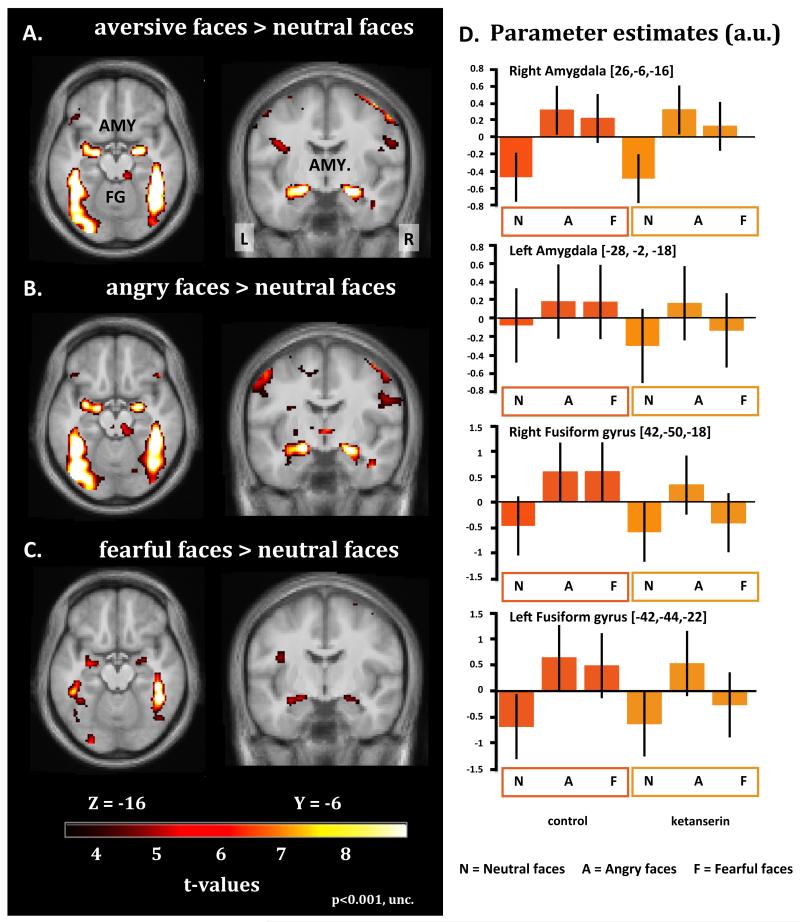
Judging the gender of angry or fearful faces, relative to neutral faces, consistently activated the expected set of brain regions involved in face and emotional processing (Fig. 3, Table 1). Amygdala showed a bilateral increase in neural activity when responses to angry and fearful faces were pooled together (Fig. 3A) or considered separately (Fig. 3B and 3C). Additional bilateral clusters in the fusiform gyrus and visual cortex displayed increases in activity when angry or fearful faces were presented relative to neutral faces (Fig. 3A-C).
Figure 3.
Statistical parametric maps (SPMs) showing brain areas, which are activated by aversive facial expressions relative to neutral faces, as reflected by an increase in BOLD signal. The SPMs are color coded in yellow and red indicating increases in activity, and are thresholded at p<0.001 (uncorrected). The upper left panel gives the activation maps for the contrast aversive faces > neutral faces (A). The middle and lower panel on the left display the activation maps for the two facial emotions separately. Angry faces > neutral faces (B); fearful faces > neutral faces (C). The bar graphs presented in the right panel give statistical estimates (arbitrary units) of face related activity levels in the amygdala and fusiform gyrus for the control session (left column) and ketanserin session (right column) (D). The parameter estimates are taken from the regional maxima showing the strongest increase in regional activity for aversive faces relative to neutral faces. The error bars represent the 90% confidence intervals of the mean.
Table 1.
Coordinates and Z-scores of the voxel showing a peak increase in BOLD signal when viewing aversive (angry/fearful) faces relative to neutral faces.
| Aversive > Neutral | Fear > Neutral | Angry > Neutral | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Area | [x,y,z] | Z-score | Area | [x,y,z] | Z-score | Area | [x,y,z] | Z-score | |||||
| A | Main effect of task | ||||||||||||
| Amygdala | R | 24, −8, −16 | 6.52 | Amygdala | R | 30, −4, −20 | 4.48 | Amygdala | R | 24, −6, −16 | 6.29 | ||
| L | −20, −10, −14 | 6.78 | L | −24, −10, −14 | 4.65 | L | −18, −10, −12 | 6.37 | |||||
| Fusiform Gyrus | R | 40, −52, −14 | inf. | Fusiform Gyrus | R | 44, −44, −16 | 6.80 | Fusiform Gyrus | R | 44, −38, −20 | 7.60 | ||
| L | −40, −66, −12 | 6.80 | L | −32, −56, −12 | 4.22 | L | −42, −54, −14 | inf. | |||||
| Caudate Nucleus | R | 12, 4, 16 | 5.52 | Caudate Nucleus | R | 8, 8, 10 | 4.91 | Thalamus | L | −18, 28, 6 | 4.35 | ||
| L | −12, 0, 22 | 4.31 | L | −6, 4, 10 | 5.78 | ||||||||
|
| |||||||||||||
| B | Main effect of drug | ||||||||||||
| OFC | R | 4, 40, −18 | 4.16 | OFC | R | 4, 38, −24 | 3.71 | ||||||
| Inferior Frontal Gyrus | L | −50, 34, 22 | 3.38 | Inferior Frontal Gyrus | L | −48, 40, 16 | 3.11 | ||||||
|
|
|||||||||||||
| C | BPp*KEToc | ||||||||||||
| Amygdala | R | 20, −8, −14 | 2.78 | Amygdala | R | 26, 4, −26 | 2.51 | ||||||
| L | −24, −6, −12 | 2.51 | L | −20, −6, −18 | 3.08 | ||||||||
| OFC | R | 10, 50, −18 | 3.35 | ||||||||||
Effect of ketanserin on face processing
Orbitofrontal cortex
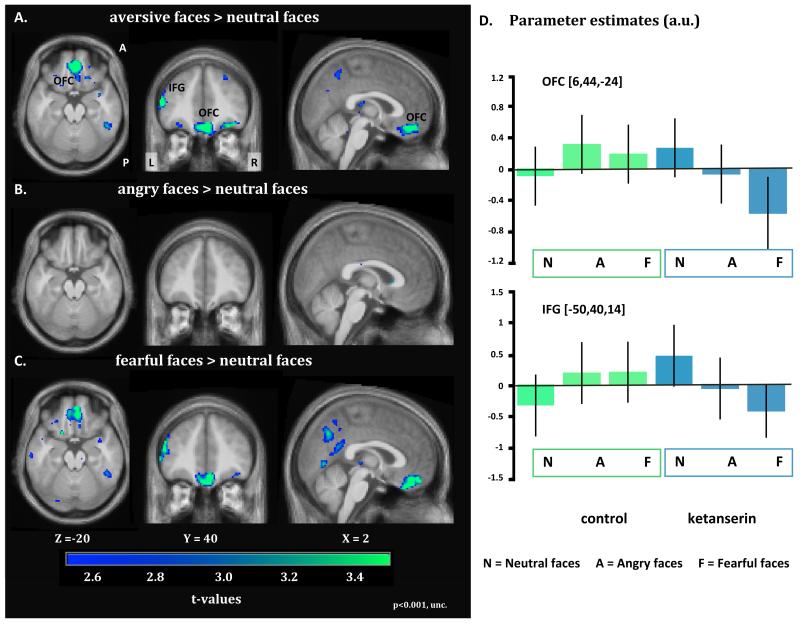
Ketanserin attenuated the regional neuronal response to aversive facial emotions relative to the control session in medial OFC (Fig. 4; peak reduction at x,y,z=4,40,−18, Z=4.12, pFWE=0.005). This attenuation was mainly driven by a reduced responsiveness of the OFC to fearful faces (peak reduction at x,y,z=4,38,−24; Z=4.03, pFWE<0.001). Inspection of the regional response profile in the OFC revealed an interaction with ketanserin having an opposite effect on OFC activity depending on the emotional content. Ketanserin attenuated the OFC response to aversive faces, especially fearful faces (Fig.4D). The ketanserin-related effects on OFC activity were not correlated with inter-individual variations in neocortical BPP. The region in OFC where ketanserin reduced the response to fearful faces also had a stronger influence on the coupling with left amygdala, when the interaction of the two covariates BPP and OKET (i.e. BPP times OKET) was considered. The strength of OFC-to-amygdala connectivity correlated positively with BPP times OKET, meaning that the higher number of 5-HT2A receptors blocked by ketanserin, the stronger the coupling between OFC and left amygdala for both OKET5 and OKET10 (peaked at MNI coordinates; OKET5: x,y,z=−22,4,−18, Z=3.92, pFWE=0.007. OKET10: x,y,z=−20,4,−18, Z=4.38, pFWE=0.002,, corrected within the amygdala ROI, Fig. 5A and B). This also held true when excluding the two extreme values from the analysis. Fig 5B shows the correlation analysis between the BOLD response in left amygdala and the BPp times OKET10 interaction. There was no significant difference in the magnitude of head movements during the fMRI acquisitions between the control and ketanserin sessions (F(1;22)=0.388;p=0.540).
Figure 4.
Statistical parametric maps (SPMs) showing brain regions, which show a decrease in activation for aversive face expressions relative to neutral faces in the ketanserin session as opposed to baseline (control session). The SPMs are color coded in green and blue indicating decreases in BOLD signal and are thresholded at p<0.001 (uncorrected). The upper left panel depicts decreases in regional responsiveness to aversive (angry, fearful) faces under ketanserin treatment (A). The middle and lower panel on the left present the corresponding SPMs for angry (B) and fearful (C) faces. The bar graphs plot the statistical estimates (arbitrary units) of face related activity levels in the amygdala and fusiform gyrus for the control session (baseline) and ketanserin session (D). The parameter estimates are taken from the regional maxima showing the strongest decrease in the regional response to aversive faces relative to neutral faces. The error bars equal the 90% confidence intervals of the mean.
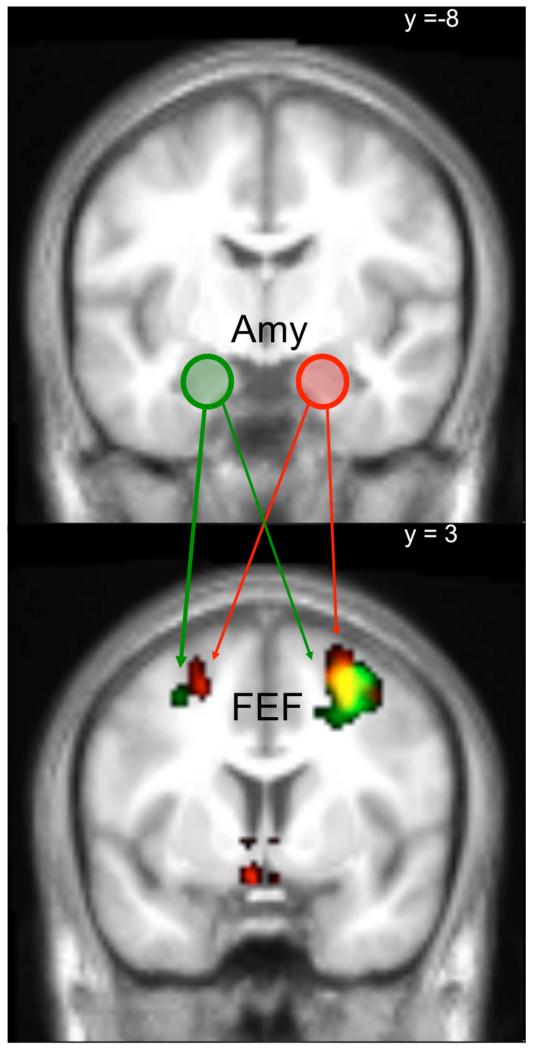
Figure 5.
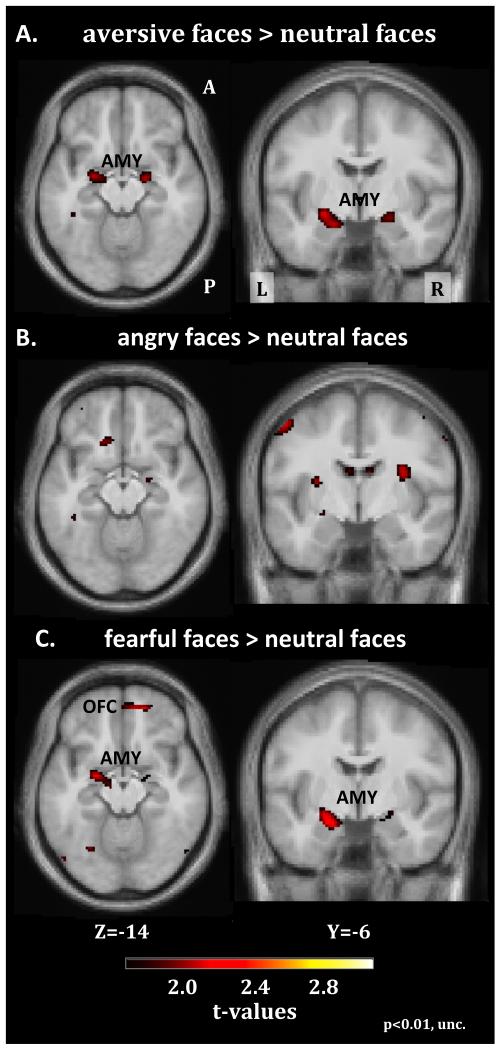
The figure summarizes the results of the PPI connectivity analysis exploring ketanserin related changes in OFC-amygdala connectivity during fear events (for details see methods section). (A) Maps showing the changes in coupling between the OFC seed region and the amygdala following acute 5-HT2A blockade. (B) Positive correlation between the OFC-amygdala connectivity and the interaction between neocortical 5-HT2A receptor binding (BPp) and ketanserin-induced 5-HT2A receptor occupancy (OKET10). The higher the OKET and the higher the neocortical BPP, the stronger was the individual increase in connectivity between the OFC and left amygdala. Values are mean normalized. The extent threshold of the SPMs is set at p<0.01 (uncorrected).
Amygdala
Ketanserin did not change the overall amygdala response to aversive faces, and this was also the case when considering inter-individual variations in either neocortical BPP or any of the OKET covariates (OKET5 and OKET10). When looking at the linear relationship between the two covariates BPP and OKET (i.e. BPP times OKET) we saw a trend towards an increase in activation in left amygdala when processing fearful or aversive faces when using the OKET5 covariates (peak modulation for fearful faces at −24,−6,−8, Z=2.76, pFWE = 0.06, for aversive faces at −26,−6,−10, Z=2.71, p=0.06).
Comparison between the ketanserin and SSRI sessions
In order to control for non-specific effects that could have been induced by administering ketanserin we did a post hoc validation of the observed changes by contrasting the ketanserin session with the SSRI session acquired in the same subjects following a similar IV administration protocol.
Mean RTs from the SSRI session did not differ significantly to the control session (Control session: 741ms±105.46, SSRI session: 745±110.9, F(1.0; 21)=16.622; p=0.760) thus the longer mean RTs found in the ketanserin session compared to control session were also found in the SSRI session (Ketanserin session: 774ms±118.6, SSRI session: 745±110.9, F(1.0; 21)=4.078; p=0.056).
Compared to the ketanserin session, SSRI data confirmed our initial results showing decreased BOLD response in OFC during aversive (peak reduction at x,y,z=2,34,−26; Z=4.30, pFWE<0.001) and fearful face presentation (peak reduction at x,y,z=2,34,−26; Z=3.70, pFWE<0.001).
Discussion
Acute 5-HT2A receptor blockade with ketanserin modulated emotional face processing in the medial orbitofrontal cortex and in amygdala, leading to two main findings. First, ketanserin suppressed the neural response to fearful faces in medial OFC. Second, the more 5-HT2A receptors that were blocked, the stronger the functional coupling between medial OFC and left amygdala in response to fearful faces.
Effect of 5-HT2A blockade on face processing in orbitofrontal cortex
Acute pharmacological 5-HT2A receptor blocking reduced the regional response of the OFC to fearful and to a lesser degree angry faces (Fig. 4) supporting that 5-HT2A receptor signaling is involved in cortical processing of fearful facial expressions. This result corroborates the notion that OFC provides an interface between cognitive and emotional functions (Paulmann et al., 2010). The ability to change behavior based on facial expressions relies partly on OFC (Kringelbach and Rolls, 2003). Patients with uni- or bilateral OFC lesions show an inability to respond appropriately to other people’s emotions and an impaired recognition of emotional features in face and voice (Hornak et al., 1996, 2003). This is probably not related to a failure to recognize facial expressions per se but is rather caused by a deficit in using this social information to guide appropriate actions or decisions (Willis et al., 2010).
The OFC did not express an emotion-specific response pattern in the control session, when participants judged the gender of neutral, angry or fearful faces. The lack of a specific response to aversive as opposed to neutral faces suggests that OFC automatically processes a wealth of face features relevant to social interaction including face identity, gender, and emotional state. Blocking the 5-HT2A receptors attenuated the OFC response to fearful faces and enhanced the response to neutral faces (Fig. 4). This differential effect of 5-HT2A receptor blockade indicates a shift in preferential processing towards non-threat related face features in OFC.
The distribution of the 5-HT2A receptors in the cerebral cortex would allow for such a shift in the relative weight of complementary processing routes within OFC: Immunocytochemical studies in the cortex of macaques have shown that excitatory 5-HT2A receptors are not only expressed in the apical dendritic field proximal to the pyramidal cell soma, but also in GABAergic interneurons known to specialize in the perisomatic inhibition of pyramidal cells (Jakab and Goldman-Rakic, 1998, 2000). A possible scenario is that acute 5-HT2A blockade reduced excitatory signaling in OFC circuits which compute fear related information while increasing neural processing in circuits processing other social stimuli features.
The individual change in emotion-related activity in OFC during acute 5-HT2A receptor blocking was not correlated with individual BPp, OKET or the product of the two. These findings suggest a non-linear relationship between 5-HT2A receptor related signaling and the neural responsiveness of OFC to fearful facial expressions, with a rapid attenuation of the response to threatening facial features as a result of even a relatively small reduction of 5-HT2A receptor signaling.
Effect of 5-HT2A blockade on OFC-amygdala connectivity
The same OFC region in which ketanserin modified facial expression-related neuronal activity also showed a stronger correlation with neural activity in left amygdala during the processing of fearful faces. The ketanserin-induced increase in functional connectivity between medial OFC and left amygdala was related to how many 5-HT2A receptors had been blocked. The more 5-HT2A receptors were blocked, the stronger was the increase in functional connectivity between OFC and left amygdala. Since Stein et al (2007) showed that OFC exerts inhibitory control over amygdala, we now propose that the OFC-to-amygdala projections are under control of orbitofrontal 5-HT2A related neurotransmission and blocking of 5-HT2A receptors enhances the impact of OFC on amygdala responsiveness to fearful faces. Experimental evidence from animal and human studies supports our hypothesis. Forster et al. (2006) showed that in rats, fear correlated negatively with 5-HT levels in OFC. Moreover, Fisher et al (2009) showed an inverse relationship between a greater level of 5-HT2A receptors in OFC, and reduced amygdala activity, as well as a functional coupling between the habituation of amygdala responses with prefrontal regulatory regions, supported by Passamonti et al. (2012) who found an altered connectivity between amygdala and PFC during acute tryptophan depletion. We infer that the efficiency of 5-HT2A receptor blocking (as indexed by the proportion of blocked receptors) had the strongest impact in individuals with a high density of neocortical binding sites as the ketanserin-induced effect on OFC-to-amygdala coupling only became evident when the product between the magnitude of receptors (BPP), and the relative proportion of 5-HT2A receptors blocked by ketanserin (OKET) was considered. These results suggest an important general implication showing that individual variations in regional receptor binding might determine individual susceptibility to drug-induced manipulation of receptor function. If this observation can be replicated in future studies, it will have a large impact on the current view of assessment of receptor drug occupancy as the single most important measure for prediction of drug efficacy.
Methodological considerations
Ketanserin caused a general slowing in RT in the gender-judgment task. This effect on RT accords with the known effects of ketanserin. When given orally, 20 mg of ketanserin may reduce sustained attention (Wingen et al., 2007) or alertness (Koudas et al., 2009), although the clinical effect of ketanserin on arousal is not profound (Herrmann and Baumgartner, 1986). Further, at this dose, ketanserin does not significantly effect cerebral blood flow (Olsen et al., 1992). Participants reported a decrease in vigor and increased fatigue, confirming the known effects of the drug. Importantly, the relative RT cost associated with the gender-judgment of angry or fearful faces relative to neutral faces was not altered by ketanserin. Moreover, individual changes in RT or mood state did not correlate with drug-induced changes in task-related activation or connectivity as revealed by fMRI. Therefore, we argue that effects of ketanserin on task performance and mood state did not account for the observed changes in activation patterns.
Receptor occupancy was not continuously monitored in each individual. Rather, the receptor occupancy was estimated on the basis of time elapsed from the beginning of ketanserin infusion. However, even if the absolute occupancy levels were not precise, the occupancy term nonetheless approximates the normal distribution required for statistical parametric mapping. Further, the robustness of the estimates was supported by the use of two different estimates for the time-dependent occupancy. Another potential limitation of the study was that the pharmacological challenge was not double-blinded. A placebo control would be advantageous in several respects, and prevent the need to consider placebo effects or effects of IV versus no manipulations. However, when the study was designed, and approved by the ethics committee, it was felt that a full placebo control of the oral ATD solution and the IV infusion for ketanserin and SSRI sessions, would be too excessive for a within-subject design. Given the heterogeneity of 5-HT2A, and other genetic or personality factors relevant to inhibition, a between subjects design might have been compromised differently, by uncertainty over the cause of differences between groups and imperfect matching. The no-drug condition without blinded placebo IV/oral solutions was seen as an acceptable choice. Although subjects were made aware of potential side effects within the study, they were not made aware of the specific differences between the interventions. However, the drug effects on neural activity were specific to fearful relative to neutral faces and the changes in amygdala activity depended on the magnitude of 5-HT2A receptor blockade and the individual 5-HT2A receptor density. Given that the volunteers had no prior information about expected effects of the drug given, nor the degree of their individual blocking, these specific effects cannot be accounted for by a simple placebo effect or a lack of blinding. Furthermore, we confirmed the effects of ketanserin by contrasting against both the control session and the SSRI session, the latter sharing the intravenous infusion.
One must also consider potential effects of ketanserin arising from receptors other than 5-HT2A. Ketanserin has some affinity for the 5-HT2C, α1 adrenergic and histamine receptors (Korstanje et al., 1986). However, the affinity of ketanserin is approximately 14-fold higher for the 5-HT2A relative to the 5-HT2C receptor (Glennon et al., 2002). Our hypothesis was entirely based on ketanserin’s modulation of the serotonergic system by blocking the 5-HT2A receptors. We therefore specifically studied the interaction between the estimated 5-HT2A receptor blockade and 5-HT2A receptor density as measured by PET. We consider therefore unlikely that α1 adrenergic, histamine, or 5-HT2C receptor pharmacological effects could have a significant impact on our observations.
Acknowledgements
The authors wish to thank Jon S. Wegener for his valued help with setting up and performing part of the scannings, Lars H. Pinborg for his advise with setting up the pharmacological challenge, Susana Aznar, Patrick Fisher, and Susanne Henningsson for their valuable comments regarding the interpretation of the data, and Sussi Larsen for her help with the drug infusions and Gorm Jensen for going through all electrocardigrams. William Barre and Arnold Skimminge are thanked for their valuable methodological input regarding the analysis of the fMRI data.
Support
The study was funded by a centre grant of the Lundbeck Foundation to Cimbi. The John and Birthe Meyer Foundation donated funding for PET-scanner and cyclotron. The Copenhagen University Hospitals Rigshospitalet and Hvidovre also supported the study. The Spies foundation donated funding to the 3T Trio MRI scanner. James Rowe was supported by the Wellcome Trust (grant 088324). Hartwig R. Siebner was supported by a grant of excellence by the Lundbeck Foundation on the Control of Action (ContAct, Grant no. R59 A5399). None of the authors has any biomedical financial interests or potential conflicts of interest in relation to the study.
References
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2007;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Morris JS, Dolan RJ. Unconscious fear influences emotional awareness of faces and voices. PNAS. 2005;102:18682–18687. doi: 10.1073/pnas.0509179102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, et al. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Ekmann P. Basic Emotions. In: Dalgleish T, Power M, editors. handbook of Cognition and Emotion. John Wiley and Sons, Ltd.; Sussex, U.K.: 1999. [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, et al. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Holst K, Frokjaer VG, et al. A nonlinear relationship between cerebral serotonin transporter and 5-HT(2A) receptor binding: an in vivo molecular imaging study in humans. J Neurosci. 2010;30:3391–3397. doi: 10.1523/JNEUROSCI.2852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, et al. Medial Prefrontal Cortex 5-HT2A Density Is Correlated with Amygdala Reactivity, Response Habituation, and Functional Coupling. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Metwally K, Dukat M, et al. Ketanserin and spiperone as templates for novel serotonin 5-HT(2A) antagonists. Curr Top Med Chem. 2002;2:539–558. doi: 10.2174/1568026023393787. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Grady CL, Siebner HR, Hornbøll B, Macoveanu J, Paulson OB, Knudsen GM. Acute pharmacologically induced shifts in serotonin availability abolish emotion-selective responses to negative face emotions in distinct brain networks. European Neuropsychopharmacology. 2012:1–11. doi: 10.1016/j.euroneuro.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Rogers RD, Tunbridge E, et al. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- Herrmann WM, Baumgartner P. Combined pharmaco-EEG and pharmacopsychological study to estimate CNS effects of ketanserin in hypertensive patients. Neuropsychobiology. 1986;16:47–56. doi: 10.1159/000118296. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Korstanje C, Sprenkels R, Doods HN, et al. Characterization of flufylline, fluprofylline, ritanserin, butanserin and R 56413 with respect to in-vivo alpha 1-,alpha 2-and 5-HT2-receptor antagonism and in-vitro affinity for alpha 1-,alpha 2- and 5-HT2-receptors: comparison with ketanserin. JPharm Pharmacol. 1986;38:374–379. doi: 10.1111/j.2042-7158.1986.tb04590.x. [DOI] [PubMed] [Google Scholar]
- Koudas V, Nikolaou A, Hourdaki E, et al. Comparison of ketanserin, buspirone and propranolol on arousal, pupil size and autonomic function in healthy volunteers. Psychopharmacology (Berl) 2009;205:1–9. doi: 10.1007/s00213-009-1508-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, Aharon I. Effective connectivity between amygdala and orbitofrontal cortex differentiates the perception of facial expressions. Soc Neurosci. 2009;4:185–196. doi: 10.1080/17470910802453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TE, Madsen KH, Sidaros K, et al. Non-white noise in fMRI: does modelling have an impact? Neuroimage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Psychology section KI. 1998. The Karolinska Directed Emotional Faces - KDEF. CD ROM from Department of Clinical Neuroscience. ISBN 91-630-7164-9. [Google Scholar]
- Marner L, Knudsen GM, Haugbol S, et al. Longitudinal assessment of cerebral 5-HT2A receptors in healthy elderly volunteers: an [18F]-altanserin PET study. Eur J Nucl Med Mol Imaging. 2009;36:287–293. doi: 10.1007/s00259-008-0945-4. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Services; San Diego, CA: 1971. [Google Scholar]
- Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- Norbury R, Taylor MJ, Selvaraj S, et al. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology (Berl) 2009;206:197–204. doi: 10.1007/s00213-009-1597-1. [DOI] [PubMed] [Google Scholar]
- Olsen KS, Videbaek C, Schmidt JF, et al. The effect of ketanserin on cerebral blood flow and cerebrovascular CO2 reactivity in healthy volunteers. Acta Neurochir (Wien) 1992;119:7–11. doi: 10.1007/BF01541774. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Crockett MJ, Apergis-Schoute AM, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry. 2012;71:36–43. doi: 10.1016/j.biopsych.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann S, Seifert S, Kotz SA. Orbito-frontal lesions cause impairment during late but not early emotional prosodic processing. Soc Neurosci. 2010;5:59–75. doi: 10.1080/17470910903135668. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Adams KH, Svarer C, et al. Quantification of 5-HT2A receptors in the human brain using [18F]altanserin-PET and the bolus/infusion approach. J Cereb Blood Flow Metab. 2003;23:985–996. doi: 10.1097/01.WCB.0000074092.59115.23. [DOI] [PubMed] [Google Scholar]
- Quarantelli M, Berkouk K, Prinster A, et al. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med. 2004;45:192–201. [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, Cognition, and Mental State Representation in Amygdala and Prefrontal Cortex. Annu Rev Neurosci. 2010 doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Willis ML, Palermo R, Burke D, et al. Orbitofrontal cortex lesions result in abnormal social judgements to emotional faces. Neuropsychologia. 2010;48:2182–2187. doi: 10.1016/j.neuropsychologia.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Wingen M, Kuypers KP, Ramaekers JG. The role of 5-HT1a and 5-HT2a receptors in attention and motor control: a mechanistic study in healthy volunteers. Psychopharmacology (Berl) 2007;190:391–400. doi: 10.1007/s00213-006-0614-x. [DOI] [PubMed] [Google Scholar]