Abstract
Interleukin (IL)-10-producing B cells (B10 cells) have emerged as important regulatory players with immunosuppressive roles. Chronic lymphocytic leukemia (CLL) B cells also secrete IL-10 and share features of B10 cells, suggesting a possible contribution of CLL B cells to immunosuppression in CLL patients. Factors controlling the emergence of B10 cells are not known. B cell-activating factor of the tumour necrosis factor (TNF) family (BAFF) is critical for B cell maturation and survival, and is implicated in the development and progression of CLL. We sought to investigate the role of BAFF in the emergence of IL-10-producing B regulatory cells in healthy donors and CLL patients. Here, we report that BAFF signaling promotes IL-10 production by CLL B cells in a mouse model of CLL and in CLL patients. Moreover, BAFF-mediated IL-10 production by normal and CLL B cells is mediated via its receptor TACI. Our work uncovered a major targetable pathway important for the generation of regulatory B cells that is detrimental to immunity in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia of adults in the developed world (1). It is characterized by the accumulation of monoclonal neoplastic CD5+CD23+CD19+ B cells (CLL B cells) over time, in the peripheral blood and secondary lymphoid organs including the spleen (2). CLL B cells share phenotypic features with several normal B cell subsets including marginal zone (MZ) B cells, B1 B cells (3) and memory B cells (4). Characteristics such as unmutated immunoglobulin (Ig) variable heavy chain (IGVH) genes (5), ZAP70 (6), CD38 (5) are broadly associated with a poor prognosis. As is common in many hematological malignancies, systemic immunosuppression is associated with a more aggressive disease course (7).
Expression of T cell leukemia gene 1 (TCL1) has been described as a molecular marker of aggressive disease and poor outcome in patients with CLL (8). Transgenic (Tg) mice overexpressing TCL1 under the B cell specific μ enhancer (EμTCL1-Tg) develop a disease similar to progressive CLL. EμTCL1-Tg mice display cumulative expansion of circulating CD5+CD19+ B cells beginning at 3-4 months of age with consequent splenomegaly, hepatomegaly and lymphadenopathy, as seen in patients with progressive CLL (9). Additionally, the EμTCL1-Tg mice display T cell dysregulation, resulting in decreased T cell activation, increased regulatory T cell (Treg) numbers and attenuated effector function (10). Increased Treg numbers in EμTCL1-Tg mice (11) and in CLL patients (12, 13) contribute to active immunosuppression, which facilitates disease progression.
Multiple immunosuppressive mechanisms have been described in CLL, including indoleamine 2,3-dioxygenase (IDO) production (12), disruption of effector T cell synapses (14) and evasion of perforin-mediated CD4+ T cell killing by cellular sequestration in stromal niches (15). Incidence of hypogammaglobulinemia increases with advanced disease as the consequence of the extensive breakdown of many immune functions, and has been associated with increased infectious complications (16).
Interleukin (IL)-10 is also a well-known immunosuppressor (reviewed in (17)) and numerous studies have implicated IL-10-secreting B (B10) cells as strong immuno-suppressive drivers facilitating the progression of malignancy (reviewed in (18, 19)). Indeed, the frequency of B10 cells was significantly increased in EμTCL1-Tg mice and correlated with TCL1 expression (20). Moreover, the proportion of B10 cells increased in EμTCL1-Tg mice treated with specific Toll-like receptor (TLR) ligands (20). However, factors facilitating IL-10 production by this subset of B cells remain unknown.
B cell-activating factor of the tumour necrosis factor (TNF) family (BAFF) is an indispensable survival factor necessary for the maturation and maintenance of B2 B cells (reviewed in (21)). BAFF mediates class switching, anti-apoptotic activity and maintenance of long-lived plasma cells residing in the bone marrow, via three cognate receptors, namely transmembrane activator and cyclophilin ligand interactor (TACI), BAFF receptor (BAFF-R) and B cell maturation antigen (BCMA), respectively (reviewed in (22)). A paralogue of BAFF, a proliferation-inducing ligand (APRIL) also mediates survival effects via cognate receptors, TACI and BCMA (22). Both BAFF and APRIL are implicated in the development and maintenance of leukemic B cells, including CLL (reviewed in (21)). Autocrine production of BAFF in CLL patients is a key driver of tumor persistence (23). Early reports suggested a role for BAFF in inducing the emergence of B10 cells in the context of autoimmunity (24). To date, the role of the BAFF system in CLL has primarily been studied in the context of tumor survival, yet a role for this factor in active immunosuppression has not been investigated.
Here, we report that BAFF stimulation enhanced IL-10 production by leukemic B cells in CLL patients and EμTCL1-Tg mice. Furthermore, splenic B cells from TACI-deficient (TACI−/−) mice were unable to secrete IL-10 following TLR stimulation, and TACI−/− mice had undetectable basal serum concentrations of IL-10. These findings indicate that TACI signaling is important for IL-10 production by normal and leukemic B cells. This work expands our understanding of the BAFF system and highlights the dual functions of BAFF in supporting CLL B cell survival and promoting B10 cells activity and immunosuppression in CLL.
MATERIALS & METHODS
Patients
Patients were recruited from the Alfred Hospital and the Peter MacCallum Cancer Centre. All patients were over 18 years of age and were diagnosed with progressive CLL in accordance with the National Cancer Institute (NCI) Working Group criteria for diagnosis and staging. All samples were taken from patients with advanced disease who had not yet received treatment and would require treatment in the near future, according to the NCI clinical guidelines (2). The Australasian Leukaemia and Lymphoma Group (ALLG) Tissue Bank provided cryosamples of CLL lymphocytes. The Australian Red Cross Blood Service collected and provided age-matched healthy donor (HD) whole blood samples. All patients involved in this study provided written informed consent. Patients undergoing therapy for CLL or other pathologies were excluded from this study. This study was approved by the ALLG scientific and management committees, and the ethics review boards of The Alfred Hospital and Monash University.
Mice
BAFF-Tg, BAFF−/−, TACI−/− and BAFF-R−/− mice were on a C57BL/6 background and have been described previously (22). C57BL/6 mice were used as wild-type (WT) controls. EμTCL1-Tg mice have previously been described (9), were maintained on a B6C3 background and used at 12 months (-mo) of age. B6C3 mice were used as WT controls for EμTCL1-Tg mice. All mice were age-matched and housed in a high barrier pathogen-free facility. The relevant institutional Animal Ethics Committee approved all experimental procedures.
Lymphocyte isolation
PBS (Invitrogen, Carlsbad, CA, USA) and separated by Ficoll-Paque (GE Healthcare, Piscataway, NJ, USA) centrifugation. Mouse splenocytes were isolated by disaggregation and 70μm filtration (BD, San Diego, CA, USA) to form a single cell suspension. Cells were enumerated using a Z™ Series Coulter Counter (Beckman Coulter Inc., NSW, Australia).
Antibodies, flow cytometry and analysis
For flow cytometric analysis, single-cell suspensions were stained as described (25). Intracellular staining was conducted using a Fixation/Permeabilization kit (eBioscience, San Diego, CA, USA) following incubation of cells with Leukocyte Activation Cocktail (BD, San Diego, CA, USA) with Brefeldin A or Monensin (eBioscience). Fragment crystallizable (Fc) receptors were blocked using purified CD16/32 monoclonal antibody (mAb) (BD). Viable lymphocytes were assessed using Live/Dead Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA, USA). Matching isotype mAbs were used to control for background staining.
Anti-mouse CD5 (53-7.3), B220 (RA3-6B2), CD3 (17A2) and CD39 (24DMS1) were purchased from eBioscience. Anti-mouse CD19 (6D5) was from Biolegend (San Diego, CA, USA). Anti-mouse CD4 (RM4-5), CD25 (PC61) and Foxp3 (MF23) were purchased from BD.
Human cells were stained with CD20 APC-Cy7 (L27) and CD5 PE-Cy7 (L17F12), (BD, San Diego, CA, USA). TLR9 PE (26C593.2) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). CD19 PE (HIB19) was purchased from eBioscience. A BD LSRII flow cytometer was used for assessment of cell suspensions (San Jose, CA, USA). Analysis was conducted using FlowJo software (Treestar, Ashland, OR, USA).
Cell cultures
RPMI and DMEM media (Invitrogen, Carlsbad, CA, USA) were used for all mouse and human cultures, respectively. Media were supplemented with 10% FCS, L-glutamine, 2ME, HEPES and Pen/Strep; all from Invitrogen (Carlsbad, CA, USA). Stimulatory factors were used at the following concentrations: LPS, 30ug/ml; mouse BAFF recombinant protein, 50ng/ml; human BAFF recombinant protein, 50ng/ml; mouse APRIL recombinant protein, 50 ng/ml; human APRIL protein, 50ng/ml; all from R&D Systems (Minneapolis, MN, USA). CpG B ODN 1826, 1μM; CpG B ODN 2006, 1μM; all purchased from Invivogen (San Diego, CA, USA). CpG stimulations were conducted from 5-48 hrs. In vitro neutralization of protein ligands BAFF and IL-10 was achieved with purified anti-human BAFF (AF124), anti-mouse BAFF (AF2106), anti-human IL-10 (AF-217-NA) and anti-mouse IL-10 (AF-417-NA) all from R&D Systems (Minneapolis, MN, USA) and used at 50μg/ml. In vitro neutralization of BAFF receptors TACI, BAFF-R and BCMA was achieved with purified anti-mouse TACI (AF1041), anti-mouse BAFF-R (AF1357), anti-mouse BCMA (AF593) and anti-human TACI (AF174), anti-human BAFF-R (AF1162) and anti-human BCMA (AF193) all from R&D Systems (Minneapolis, MN, USA) and used at 1μg/ml. Cells were cultured in 96-, 48-, or 24-wells cell culture plates (BD, San Diego, CA, USA).
Suppression Assay
In vitro suppression assays were carried out in RPMI supplemented with 10% FCS in 96-well V-bottom plates (Costar, Corning, NY). 2.5×104 autologous CD4+CD25− responder T cells were FACS-sorted from spleens of WT or EμTCL1-Tg mice and stained with AlexaFluor cytotracker proliferation dye (eBioscience, San Diego, CA, USA). FACS-sorted CD4+CD25+CD39+ Tregs were cultured with IL-10 for 12 hrs, then FACS-sorted and co-cultured as ratios of 1:8, 1:4 and 1:2 relative to responder cells. Stimulation was carried out with CD3/CD28 beads (Thermofisher Scientific, Waltham, MA, USA) at 1:100 per well, with or without exogenous IL-10 (R&D Systems, Minneapolis, MN, USA) at 50ng/ml for 72 hours at 37°C. Proliferation was assessed by FACS and expressed as frequency of detectable AlexaFluor stained events.
Serum and secreted cytokine quantification
Human and mouse IL-10 and BAFF concentrations in serum and supernatant were quantified using human and mouse ELISA Duo Sets (R&D Systems, Minneapolis, MN, USA), as per manufacturer protocol.
Statistical analysis
Data are shown as means (± SEM). The student’s t-test was used to determine significant differences between means. All statistical analyses were performed with GraphPad software (Prism Version 6.0d, 2013, San Diego, CA, USA). A P value ≤ 0.05 was considered statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001.
RESULTS
Correlation between serum BAFF and IL-10 concentrations in EμTCL1-Tg mice
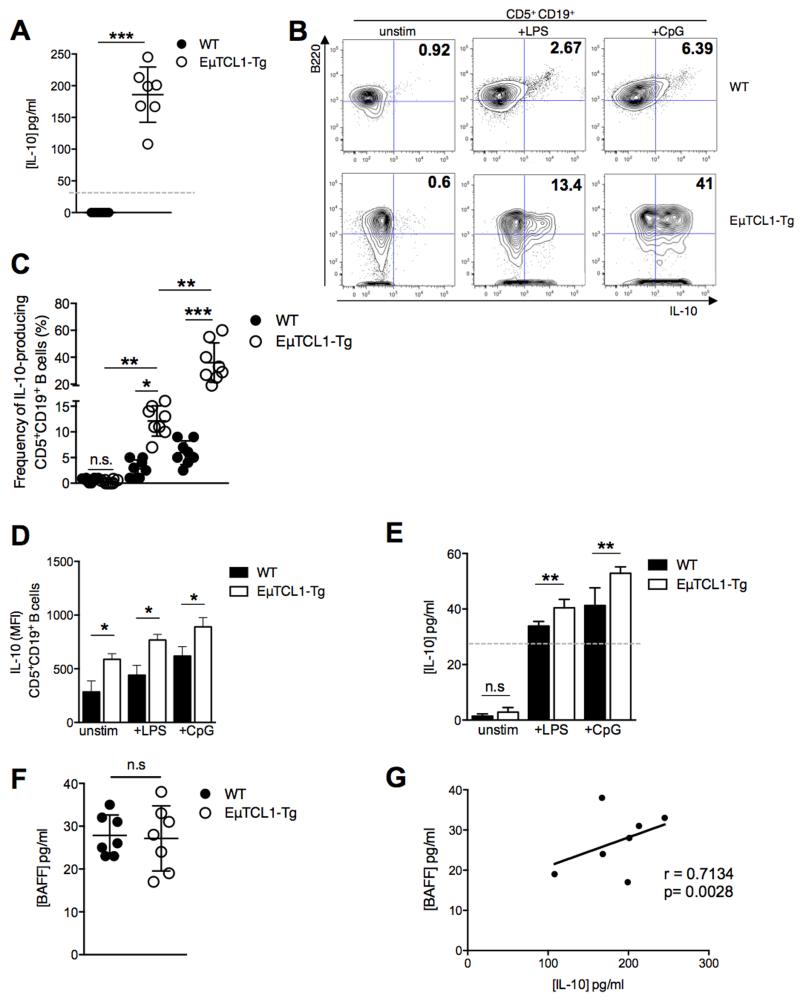
The role of BAFF in CLL pathogenesis has been described in part, and IL-10 has also been recently implicated in CLL pathogenesis (20, 26). We sought to investigate associations between concentrations of IL-10 and BAFF in the serum of mice with CLL-like disease. We observed that EμTCL1-Tg mice at 12-mo of age had significantly increased serum IL-10 concentrations compared to age-matched WT mice (Figure 1A). Splenic B cells from EμTCL1-Tg mice, cultured with PMA/ionomycin, and LPS (a TLR4 ligand) or CpG (a TLR9 ligand), displayed significantly increased frequency of IL-10-expressing cells and increased intracellular IL-10 expression (MFI) compared to similarly treated WT B cells (Figure 1B-1D). Furthermore, stimulated splenic B cells from EμTCL1-Tg mice secreted more IL-10 compared to similarly treated WT splenic B cells (Figure 1E). BAFF concentrations are reportedly not elevated in the sera of patients with CLL (27, 28). Consistent with this observation, no difference in serum BAFF concentrations was detected in EμTCL1-Tg mice relative to WT controls (Figure 1F). These data support our model and previous studies reporting that measureable levels of BAFF in the sera of CLL patients appear to decrease with advanced disease (28, 29). This is expected and explained by growing numbers of BAFF receptor-expressing CLL cells emerging and “mopping” BAFF away from the circulation (28). Interestingly, while circulating BAFF levels appeared normal in EμTCL1 Tg mice, we noted that serum IL-10 concentrations correlated significantly with detectable serum BAFF concentrations (Figure 1G).
Figure 1. Analysis of BAFF and IL-10 production by splenic CD5+CD19+ B cells in WT and EμTCL1-Tg mice.
(A) Serum IL-10 levels for WT and Eμ-TCL1-Tg mice at 12-mo (n=7 per group) were measured by ELISA. (B) Murine splenic B10 cells were gated by flow cytometry as B220+ CD5+ CD19+ IL-10+ as indicated in representative dot plots. Percentages of gated IL-10-expressing cells are indicated in the upper right quadrant of each plot (C) Percentages of gated IL-10-expressing B cells in WT mice and EμTCL1-Tg mice at 12-mo of age (n=8 per group) (D) Splenic B cells taken from WT mice and EμTCL1-Tg mice at 12-mo of age were stimulated with LPS or CpG and assessed for intracellular IL-10 expression by flow cytometry (n=8 per group) and (E) IL-10 secretion by ELISA. (F) Serum BAFF levels for WT and Eμ-TCL1-Tg mice at 12-mo (n=7 per group) were measured by ELISA. (G) Correlation between serum levels of BAFF and IL-10 in EμTCL1-Tg mice at 12-mo of age (n=7). In A-F, horizontal bars indicate the mean. In A-G, data are representative of at least two independent experiments.
Correlation between serum BAFF and IL-10 concentrations in patients with CLL
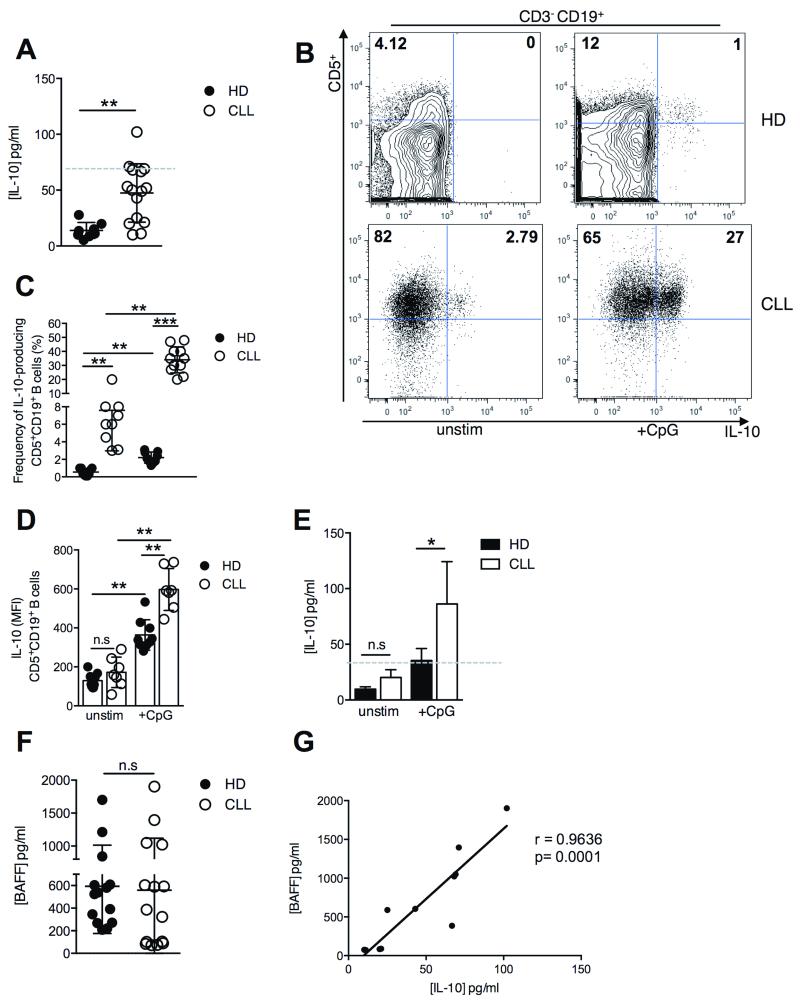
We next investigated whether a similar pattern of BAFF and IL-10 production could be observed in the sera of patients with progressive CLL. Indeed, we detected significantly higher IL-10 concentrations in the sera of patients with progressive CLL relative to healthy donor (HD) sera (Figure 2A). Recent studies have described the increase in serum IL-10 levels, over time, in progressive CLL (20). We observed a similar pattern in the sera of patients with progressive CLL assessed at multiple time points (Supplementary Figure 1). Previous investigations have noted that the inverse relationship of serum BAFF with CLL progression reflects increased numbers of circulating B cells consuming soluble BAFF (28). Furthermore, both the frequency of IL-10-expressing cells and the levels of intracellular expression of IL-10 in the cells (MFI) were significantly increased in CpG-stimulated cultures of CD5+CD19+ B cells from patients with progressive CLL compared to similarly treated B cells from HD (Figure 2B-2D). Likewise, in vitro CpG stimulation of B cells from CLL patients resulted in significantly increased levels of IL-10 in the supernatant, compared to similarly treated B cells from HD (Figure 2E). Variable serum BAFF concentrations were detected in both HD and patients with progressive CLL, yet no significant difference in serum BAFF concentrations was observed between CLL patients and HD (Figure 2F). Again this confirmed previous reports that increased BAFF production by CLL cells is masked by large numbers of BAFF receptor-expressing CLL cells, which mop available BAFF protein in the circulation (29). Importantly, levels of circulating IL-10 and BAFF were positively correlated in serum samples of CLL patients (Figure 2G), recapitulating the relationship observed in EμTCL1-Tg mice (Figure 1F). While BAFF levels are generally reduced in the sera of patients with CLL due to increased numbers of BAFF-binding CLL B cells (28), these levels vary. Similar observations were made in EμTCL1-Tg mice. Importantly, the highest levels of BAFF strongly correlated with the highest IL-10 levels in both CLL patients and EμTCL1-Tg mice, suggesting a possible relationship between BAFF production and IL-10-mediated immunosuppression.
Figure 2. Analysis of BAFF and IL-10 production by CD5+CD19+ B cells from the peripheral blood of HD and CLL patients.
(A) Serum IL-10 levels for HD (n=8) and patients with progressive CLL (n=15) were measured by ELISA. (B) B10 cells from peripheral blood were gated by flow cytometry as CD3− CD5+ CD19+ CD20+ IL-10+ as indicated in representative dot plots. (C) Percentages of gated IL-10-expressing B cells in HD (n=9) and CLL (n=11) (D) CD5+CD19+ B cells isolated from the peripheral blood of HD (n= 8) and patients with progressive CLL (n=7) were stimulated with CpG and assessed for intracellular IL-10 expression by flow cytometry and (D) IL-10 secretion by ELISA (n=8 and n=15, respectively). (E) Serum BAFF levels for HD (n=14) and CLL patients (n=15) were measured by ELISA. (F) Correlation between serum levels of BAFF and IL-10 in CLL patients (n=11). In A-E, horizontal bars indicate the mean. In A-F, data are representative of at least two independent experiments.
Exogenous BAFF enhances IL-10 production by B cells from EμTCL1-Tg mice and CLL patients
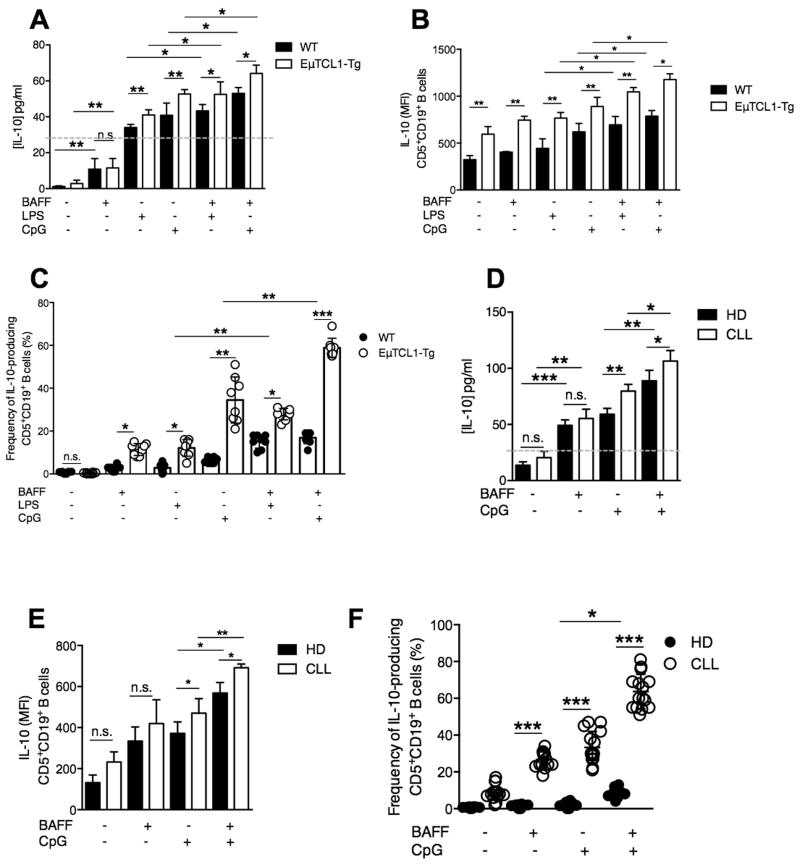
The positive correlation between serum IL-10 and BAFF concentrations suggested a potential causal relationship between the two cytokines in EμTCL1-Tg mice and patients with CLL. We investigated whether in vitro stimulation with exogenous BAFF could enhance IL-10 secretion by CD5+CD19+ splenic B cells from WT and EμTCL1-Tg mice. Indeed, CD5+CD19+ B cells from WT and EμTCL1-Tg mice secreted significantly more IL-10 when stimulated with BAFF compared to unstimulated control cultures (Figure 3A). Moreover, treatment with CpG alone also induced detectable levels of IL-10 production compared to unstimulated control cultures (Figure 3A). Furthermore, treatment with BAFF in combination with LPS or CpG induced a further significant increase in detectable IL-10 production compared to BAFF or CpG alone (Figure 3A). Importantly, CD5+CD19+ B cells from EμTCL1-Tg mice secreted significantly more IL-10 in response to combined BAFF and LPS or CpG stimulation compared to B cells from WT mice (Figure 3A). We observed significant increases in both intracellular IL-10 expression and frequency of IL-10-producing B cells from WT and EμTCL1-Tg mice following co-stimulation with BAFF and LPS or CpG, with a greater effect observed with EμTCL1-Tg B cells (Figure 3B and 3C).
Figure 3. Effects of BAFF and TLR co-stimulation on IL-10 expression by B10 cells from EμTCL1-Tg mice and CLL patients.
(A) Splenic B cells isolated from WT mice and EμTCL1-Tg mice at 12-mo of age were stimulated with LPS or CpG in combination with BAFF and assessed for IL-10 secretion by supernatant ELISA and (B) intracellular IL-10 expression by flow cytometry (n=6 per group) as in Figure 1. (C) Percentages of gated IL-10-expressing B cells from WT mice and EμTCL1-Tg mice stimulated with LPS or CpG in combination with BAFF (n=8 per group) (D) CD5+ CD19+ CD20+ B cells isolated from the peripheral blood of HD (n=8) and patients with progressive CLL (n=15) were stimulated with CpG in combination with BAFF, and assessed for intracellular IL-10 secretion by ELISA (n = 8 and n=15, respectively) and (E) IL-10 expression by flow cytometry as in Figure 2. (F) Percentages of gated IL-10-expressing B cells in HD (n=8) and CLL (n=15). In A-F, horizontal bars indicate the mean. In A-F, data are representative of at least two independent experiments.
We next examined the response of peripheral B cells from HD and CLL patients, when stimulated with BAFF and CpG. Combined BAFF and CpG stimulation of CD5+CD19+ B cells from CLL patients and HD resulted in significantly increased IL-10 secretion compared to CpG stimulation alone (Figure 3D), and this effect was more pronounced in CLL B cells compared to B cells from HD (Figure 3D). Intracellular IL-10 expression and frequency of IL-10-producing B cells was significantly increased in both HD and CLL patient groups when cultured with BAFF and CpG, and this effect was most striking in CLL B cells (Figure 3E and 3F).
Collectively, these results suggest that IL-10 production by CD5+CD19+ B cells in EμTCL1-Tg and CLL patients is significantly enhanced by BAFF stimulation, and can act synergistically with LPS or CpG stimulation. BAFF also appears to play a dual role in CLL, stimulating B cell survival and also contributing to IL-10 production by CLL cells, an aspect likely to contribute to immunosuppression. Moreover, while levels of circulating BAFF appear similar between controls and mouse or human CLL samples, the highest levels of IL-10 detected in CLL reflect the fact that in CLL the vast majority of cells mopping circulating BAFF behave like B10 cells (20), whereas in healthy controls IL-10-producing B cells account for a very small proportion of all B cells, hence explaining why IL-10 levels are not elevated in healthy controls.
Attenuation of IL-10 secretion following inhibition of TACI signaling in B cells from EμTCL1-Tg mice and CLL patients
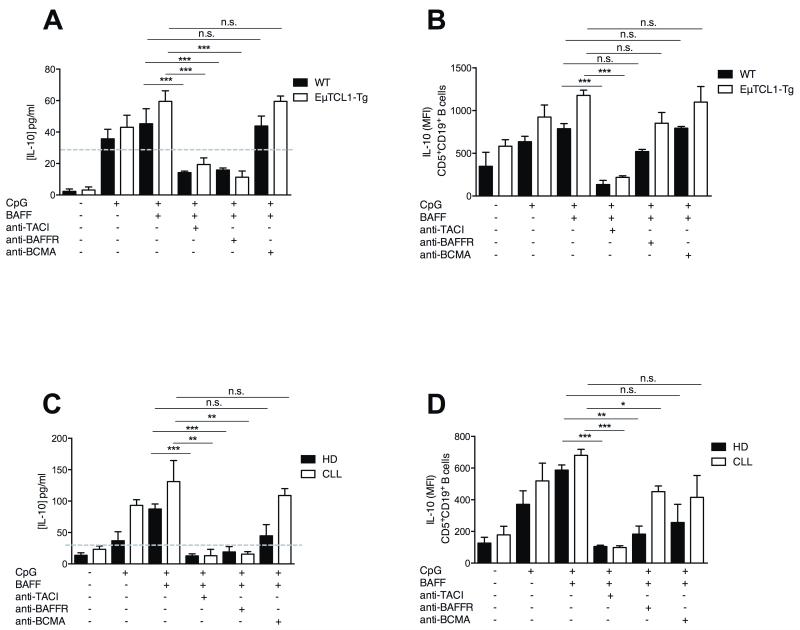
The three known BAFF receptors have distinct functions in B cell homeostasis (reviewed in (22)). The well-characterised pro-survival activity of BAFF is mediated via BAFF-R and is considered a central pathogenic axis driving tumor persistence in CLL (23). We sought to determine which receptors contributed to the observed BAFF-induced IL-10 production by B cells from EμTCL1-Tg mice and CLL patients (Figure 3). Splenic CD5+CD19+ B cells isolated from 12-mo WT and EμTCL1-Tg mice were cultured with CpG and BAFF, with or without blocking antibodies specific for TACI, BAFF-R or BCMA. Inhibition of TACI or BAFF-R resulted in a significant reduction of IL-10 secretion from both EμTCL1-Tg mice and WT splenic B cells (Figure 4A). BAFF-R is required for BAFF-driven pro-survival signals to support B cell maintenance (30). Reduced IL-10 levels in the cultures supplemented with a BAFF-R inhibitor reflected an expected impact on B cell survival (Figure 4A), which has been observed previously (30). Indeed, BAFF-R blockade did not affect the level of cytoplasmic IL-10 in the cells (Figure 4B), further supporting a role for BAFF-R in B cell viability rather than a direct effect on IL-10 expression. In contrast, both IL-10 secretion and intracellular IL-10 production by CD5+CD19+ B cells co-stimulated with CpG and BAFF, were significantly decreased in response to TACI inhibition which does not affect B cell viability (Figure 4A and 4B).
Figure 4. Reduced IL-10 production following BAFF receptor inhibition of B cells from EμTCL1-Tg mice and CLL patients.
(A) CD5+CD19+ B cells isolated from 12-mo old WT and EμTCL1-Tg mice (n=6 per group) were stimulated with BAFF +/− CpG, in combination with inhibitors for TACI, BAFF-R or BCMA. IL-10 production measured by ELISA and (B) intracellular staining as in Figure 1. (C) CD5+CD19+ B cells isolated from HD (n=8) and CLL patients (n=15) were stimulated with BAFF +/− CpG, in combination with inhibitors for TACI, BAFF-R or BCMA. IL-10 production measured by ELISA and (D) intracellular staining as in Figure 2. In A-D, horizontal bars indicate the mean. In A-D, data are representative of at least two independent experiments.
Similarly, CD5+ CD19+ B cells from CLL patients and HD were stimulated with CpG and BAFF in vitro, with or without inhibition of BAFF receptors. Recapitulating the observations in our mouse model, TACI inhibition resulted in attenuated IL-10 secretion and intracellular expression by CD5+ CD19+ B cells from both CLL patients and HD (Figure 4C and 4D). Similar to mouse B cells, inhibition of BAFF-R resulted in attenuation of IL-10 secretion but a less pronounced reduction in intracellular IL-10 expression by co-stimulated CD5+CD19+ B cells from CLL patients and HD (Figure 4C and 4D). These data are reflecting in vitro inhibition of B cell survival in culture (30). Taken together, our data suggest that inhibition of TACI signaling results in abrogation of BAFF-induced IL-10 production and indicates that BAFF acts directly through TACI to induce IL-10 production by CD5+CD19+ B cells from HD and CLL patients.
Reduced IL-10 production by TACI−/− splenic CD5+CD19+ B cells
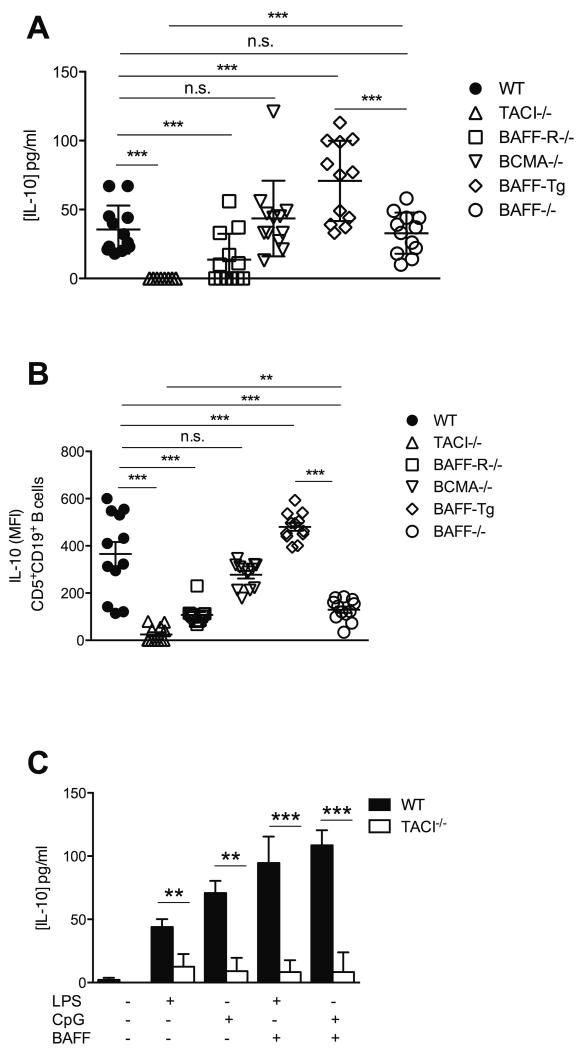
To further interrogate the contribution of the BAFF system to IL-10 production by splenic CD5+CD19+ B cells in WT and EμTCL1-Tg mice, we measured serum IL-10 concentrations in mice overexpressing BAFF (BAFF-Tg) and mice deficient in BAFF (BAFF−/−) or one of three BAFF receptors, namely TACI−/−, BAFF-R−/− and BCMA−/− mice. Total serum IL-10 levels were severely impaired in TACI−/− mice compared to WT controls (Figure 5A). Conversely, serum IL-10 levels in BAFF-Tg mice were significantly elevated compared WT mice, confirming the positive induction effect of BAFF on IL-10 production (Figure 5A). Interestingly, loss of BAFF-R led to slightly reduced serum IL-10 levels, however, this effect was not as striking as in TACI−/− mice and reflected the impaired number of mature B cells in these animals (30) (Figure 5A). Furthermore, BAFF−/− mice displayed a non-significant reduction in total serum IL-10. This difference suggested that residual APRIL signaling via TACI might have contributed to some of the observed IL-10 production in BAFF−/− or BAFFR−/− mice. Loss of BCMA did not have an impact on IL-10 production, consistent with its expression on B cells that do not normally produce IL-10 (31).
Figure 5. Abnormal IL-10 production by B cells from BAFF receptor deficient mice.
(A) Serum IL-10 levels for C57BL/6, BAFF-Tg, BAFF−/−, TACI−/−, BAFF-R−/− and BCMA−/− mice at 12-mo of age were measured by ELISA (n=12 per group). (B) CD5+CD19+ splenic B cells isolated from of 12-mo old C57BL/6, BAFF-Tg, BAFF−/−, TACI−/−, BAFF-R−/− and BCMA−/− mice were assessed for intracellular IL-10 levels by flow cytometry. (C) CD5+CD19+ splenic B cells isolated from C57BL/6 and TACI−/− mice (n=6 per group) were cultured 12-h with or without LPS or CpG, and supernatants assessed for IL-10 levels by ELISA. In A-C, horizontal bars indicate the mean. In A-C, data are representative of at least three independent experiments.
Assessment of cytoplasmic IL-10 levels showed that splenic CD5+CD19+ B cells from BAFF-Tg mice expressed more IL-10 compared to WT mice (Figure 5B). Conversely, CD5+CD19+ B cells from TACI−/− mice displayed significantly diminished cytoplasmic IL-10 levels relative to WT controls (Figure 5B). Similarly, remnant splenic CD5+CD19+ B cells in BAFF−/− mice displayed significantly reduced intracellular IL-10 expression but higher IL-10 levels than TACI−/− counterparts reflecting the greater proportion of immature B cells and the paucity in mature B cells more likely to normally express IL-10 (Figure 5B). Furthermore, in vitro stimulation of TACI−/− splenic B cells with LPS or CpG in combination with BAFF failed to induce detectable concentrations of IL-10 in culture supernatants (Figure 5C). Collectively, these data strongly suggest that TACI signaling is a dominant controller of IL-10 production by normal and leukemic B cells in both mice and humans. It remains difficult to clarify whether the intermediate effect seen in BAFF−/− or BAFFR−/− mice reflects a direct role of this axis on IL-10 production or if it is an indirect consequence of B cell loss. The little impact on intracellular IL-10 expression suggests the latter. Indeed, in the absence of BAFF survival signals, very few TACI+ B cells would persist, including potential B10 precursors. In any case, both TACI and BAFF emerged as two essential elements that are critical for IL-10 production by B cells.
IL-10 enhancement of regulatory T cell function in CLL
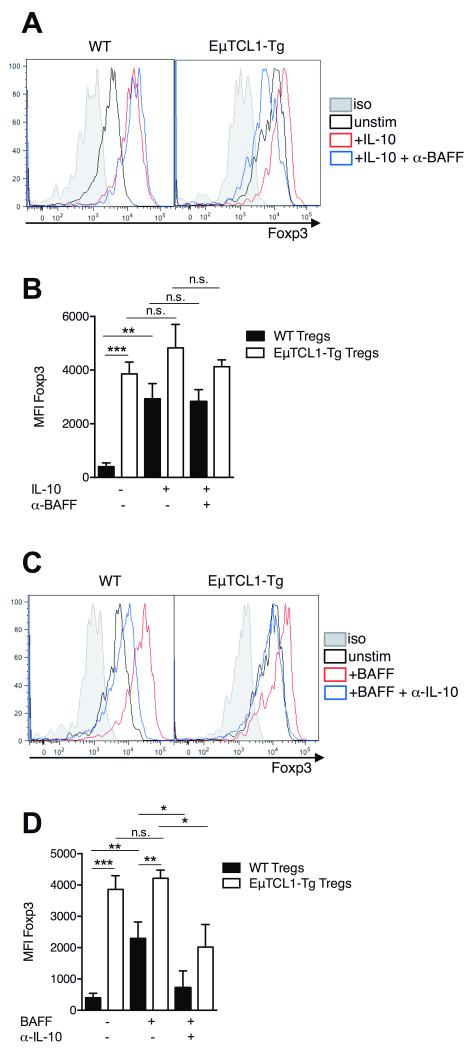
We next examined the relationship between IL-10 secretion by CD5+CD19+ B cells and its impact on the emergence of regulatory T cells (Tregs), which are known contributors to immunosuppression in CLL (10, 11). CD4+CD25+CD39+ T cells have been shown to give rise to Tregs with increased Foxp3 expression and suppressive activity compared to CD4+CD25+CD39− T cells (32). Splenic Tregs isolated from 12-mo WT mice displayed significantly increased intracellular Foxp3 expression following IL-10 stimulation compared to untreated Tregs (Figure 6A and 6B). Interestingly, we observed that IL-10 stimulation resulted in the increased frequency and absolute number of Tregs (Supplementary Figure 3). Foxp3 expression was significantly higher in unstimulated Tregs from EμTCL1-Tg mice and was not significantly increased following IL-10 stimulation compared to untreated Tregs (Figure 6A and 6B). This is likely due to in vivo exposure to high levels of circulating IL-10 (20, 33) (and Figure 1A) Furthermore, blocking BAFF in these IL-10 stimulated Treg cultures did not result in significantly reduced Foxp3 expression by Tregs from WT or EμTCL1-Tg mice (Figure 6A and 6B). These data supported the notion that BAFF first stimulates IL-10 production, which then increases Foxp3 expression in Tregs.
Figure 6. IL-10 modulation of Foxp3 expression in Tregs from WT and EμTCL1-Tg mice.
(A) CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice were cultured for 12-hr +/− IL-10 with or without anti-BAFF blocking antibody and assessed for intracellular Foxp3 expression by flow cytometry. (B) MFI levels of intracellular Foxp3 in Tregs from WT and EμTCL1-Tg mice cultured for 12-hr +/−IL-10 with or without anti-BAFF blocking antibody. (C) CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice were cultured for 12-hr +/−BAFF and anti-IL-10 blocking antibody and assessed for intracellular Foxp3 expression by flow cytometry. (B) MFI levels of intracellular Foxp3 in Tregs from WT and EμTCL1-Tg mice cultured for 12-hr +/− BAFF and anti-IL-10 blocking antibody. In B and D, horizontal bars indicate the mean. In A-D, data are representative of at least two independent experiments.
In reciprocal Treg cultures stimulated with exogenous BAFF, we observed that Foxp3 expression was increased in Tregs from WT mice (Figure 6C and 6D). This effect was also observed in BAFF-stimulated Tregs from EμTCL1-Tg mice (Figure 6C and 6D). Moreover, blocking IL-10 in BAFF stimulated Treg cultures resulted in significantly reduced Foxp3 expression by Tregs from both WT and EμTCL1-Tg mice (Figure 6C and 6D). These data suggest that the Foxp3 upregulation in response to BAFF is driven primarily via production of IL-10, and that BAFF did not exert a direct effect of Foxp3 expression (Figure 6C and 6D). Furthermore, in vitro T cell suppression assays demonstrated that IL-10-stimulated Tregs from WT or EμTCL1-Tg mice were significantly more suppressive of T cell activation than untreated Tregs (Supplementary Figure 4), although unstimulated EμTCL1-Tg Tregs were more potent suppressors than unstimulated WT Tregs, in keeping with their intrinsic higher Foxp3 expression compared to WT controls (Figure 6D). Therefore, BAFF-driven IL-10 production by CLL B cells appears to have secondary immunosuppressive effects including the generation of Tregs with greater suppressive activity, driven by higher Foxp3 expression (reviewed in (34)).
Collectively, this study demonstrates that the contribution of BAFF to CLL pathogenesis extends beyond survival of the tumor cells, and is also characterised by the induction of IL-10 producing B cells in a TACI-dependent manner, and the secondary induction of Tregs to establish a multifocal immunosuppressive microenvironment.
DISCUSSION
Immunosuppression is a significant complicating factor in the development of effective CLL therapies and, ultimately, the leading cause of severe and often fatal infections (35). Novel strategies that stimulate immune reactivation are needed to improve the effectiveness of CLL therapies and enhance CLL patients’ survival and quality of life (36). The mechanisms driving the loss of natural immunity in CLL have not been fully addressed, however, we have recently described a central mechanism of plasmacytoid dendritic cell (pDC)-associated immunodeficiency (37). Similarly, IL-10 production by LPS or CpG stimulated CLL B cells has been recently reported (20). Importantly, these innate ligands are well-known inducers of local BAFF production from macrophages, dendritic cells (DCs) and monocytes (reviewed in (38)).
In this study, we demonstrated that BAFF plays a key role via its receptor TACI in driving B10 activity in healthy individuals and patients with progressive CLL. These findings describe a novel role of BAFF in the context of malignancy, where IL-10 production is a significant contributor to immunosuppression. These findings were conserved across species, indicating the importance of the mechanism. Moreover, these data provide a rationale for a renewed focus on the BAFF system, particularly TACI, as a therapeutic target in CLL.
Our study confirmed that increased IL-10 production was not a reflection of a BAFF-driven increase in B cell numbers. Instead, our work has shown a role for BAFF in directly stimulating increased IL-10 production by B cells. These observations have a parallel in studies using clinical samples from patients with Systemic Lupus Erythematosus (SLE). Patients with SLE present with B cell lymphopenia (39) despite high levels of BAFF in their serum (40). Interestingly, these same patients display increased IL-10 production by circulating B cells (41, 42) and increased frequency of B10 cells in the blood (43). This is likely due to high TACI expression on circulating B cells from SLE patients (our unpublished data). We have observed similarly high levels of TACI expression on splenic B cells from BAFF-Tg mice with lupus-like disease (44). TLR4 and TLR9 stimulation upregulates TACI expression on the surface of MZ B cells in the spleen (44, 45), sensitizing cells to BAFF-TACI signaling and this may explain the IL-10 induction effect of LPS and CpG in B cells (20).
CLL B cells express high levels of TLR9 (46) and activation of TLR9 in mouse and human B cells strongly up-regulates TACI expression in these cells (47). Therefore, it is reasonable that increased TLR9 expression in CLL B cells facilitates TACI expression and TACI-mediated IL-10 production. TACI-TLR9 crosstalk likely potentiates the CpG-induced IL-10 production we observed. Indeed, TLR9-activated CLL cells display significantly upregulated TACI expression compared to normal B cells (23). Furthermore, CpG stimulation of B cells has been shown to induce local BAFF production, further amplifying the signals driving IL-10 production (48). Our findings are further reinforced by recent reports that calcium related signalling events, of which TACI is a key regulator, are critical components of IL-10 production by CLL B cells (49).
The requirement of TACI signaling for IL-10 production by CLL B cells suggests that strategies aimed at inhibiting TACI signaling may be useful in treating CLL. Indeed, Phase 1b trials of the TACI-Ig therapeutic, atacicept, demonstrated disease stabilization in CLL patients with progressive CLL (50) but further trials have yet to be conducted, alone or in combination with other agents.
Many reports have posited a role for B10 cells in the control of autoimmune inflammation such as multiple sclerosis (MS) (51, 52), and as pivotal inducers of Treg activity (52, 53). In fact, BAFF and IL-10 are implicated in expanding the Treg compartment and Treg suppressive activity, respectively (54, 55), and our findings suggest that this axis may contribute to immunosuppression in CLL. Indeed, Treg numbers are elevated in CLL patients (13, 56) and BAFF-Tg mice (54).
Our observation that TACI mediates increased B10 activity is further reflected by the adverse effect of atacicept in a Phase 2 MS clinical trial, in which patients develop an exacerbated disease in the absence of BAFF- and APRIL-mediated signaling (57). Indeed recent work has provided strong evidence linking B10 cell function with protection in Experimental Autoimmune Encephalitis (EAE) (58). Whether the protective role of B10 cells in EAE is linked to our observation that BAFF-dependent IL-10 production induces Foxp3 expression in Tregs remains to be determined, as Tregs are also key protective cellular elements in MS (59). Our data suggest that administering atacicept to MS patients inhibited both BAFF and APRIL binding to TACI on B cells and, as a result, abrogated essential B10 regulatory activity, leading to exacerbated disease. This clinical outcome strongly supports our notion that TACI-mediated IL-10 production by B cells is an important regulatory mechanism in CLL and extends to other clinical disease settings.
These data emerge at a time when regulatory B cells are finally receiving attention for their previously underappreciated role in malignancy, clearly refocusing our understanding of BAFF in CLL, revealing a dual function, which extends beyond a classical role in B cell survival. Our work also confirmed a role for BAFF in promoting the production of IL-10 by B cells in the healthy state (20) and further clarified this aspect by identifying TACI as the receptor driving this effect. Targeting TACI to disable B10 cells may ameliorate immunosuppression in CLL, restore immune surveillance, and improve patient survival and performance during chemotherapy.
Supplementary Material
Supplementary Figure 1. Analysis of Serum IL-10 over time in HD and CLL patients. IL-10 levels in the serum of HD (n=7) or patients with progressive CLL (n=7) were measured by ELISA using CLL samples collected the day of diagnosis and, at a 3- and 6-month follow up visit thereafter.
Supplementary Figure 2. CD5 isotype staining. B cells from peripheral blood were gated by flow cytometry as CD3− CD19+ and stained with CD5+ (left panel) or isotype control (right panel) as indicated on the Y-axis.
Supplementary Figure 3. In vitro stimulation of Tregs with exogenous IL-10. (A) CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice were cultured for 12-hr +/− IL-10 and assessed by flow cytometry. Proportion of CD4+CD25+CD39+ regulatory T cells is indicated. (B) Absolute numbers of CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice following 12-hr culture with IL-10 or not.
Supplementary Figure 4. Suppressive activity of IL-10 stimulated Tregs. FACS-sorted splenic CD4+CD25+CD39+ T cells from (A) WT and (B) EμTCL1-Tg mice were treated with or without exogenous IL-10 for 12-hr, then purified by FACS and co-cultured with AlexaFluor cytotracker-labelled autologous splenic CD4+CD25− responder T cells stimulated with CD3/CD28 beads. Proliferation of responders was assessed by flow cytometry gating on dilution of AlexaFluor staining compared to non-dividing AlexaFluor-labelled T cell control. Inhibition of proliferation is expressed as a percentage of responders detectable post-culture; the ratio of suppressor and responder cells is indicated.
Key Points.
BAFF signals via TACI to enhance IL-10 production by both regulatory B cells and CLL B cells.
TACI inhibition may be beneficial in ameliorating immunosuppression in CLL patients.
Acknowledgments
We thank Professor Stephen Jane for critical appraisal of the manuscript, the AMREP flow cytometry team for technical assistance, the AMREP Animal Service staff for colony health management, and the ALLG Tissue Bank for provision of PBL samples. We especially thank all patients involved in this study. SBT is supported by an NHMRC RD Wright Career Development Fellowship. This study was funded by the Worldwide Cancer Research (formerly Association for International Cancer Research (AICR)) UK, and the National Health and Medical Research Council (NHMRC) of Australia.
ABBREVIATIONS
- ALL
acute lymphocytic leukemia
- ALLG
Australasian Leukaemia and Lymphoma Group
- AMREP
Alfred Medical Research and education Precinct
- BAFF
B cell activating factor from the TNF family
- CLL
Chronic lymphocytic leukemia
- DCs
dendritic cells
- Fc
Fragment crystallizable
- HD
healthy donors
- Ig
immunoglobulin
- IGVH
immunoglobulin variable heavy chain
- Lin−
lineage negative
- mAb
monoclonal antibody
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- TCL1
T cell leukemia gene 1
- Tg
transgenic
- TLR
toll-like receptor
- TNF
tumour necrosis factor
- Treg
regulatory T cells
- WT
wild-type
Footnotes
Supplementary information is available at Leukemia’s website.
Conflict of Interest Disclosures
The authors have no conflict of interest to declare.
REFERENCES
- 1.Woyach JA, Lozanski G, Ruppert AS, Lozanski A, Blum KA, Jones JA, et al. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26(6):1442–4. doi: 10.1038/leu.2011.375. Epub 2012/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews Immunology. 2011;11(1):34–46. doi: 10.1038/nri2901. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 4.Huttmann A, Klein-Hitpass L, Thomale J, Deenen R, Carpinteiro A, Nuckel H, et al. Gene expression signatures separate B-cell chronic lymphocytic leukaemia prognostic subgroups defined by ZAP-70 and CD38 expression status. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20(10):1774–82. doi: 10.1038/sj.leu.2404363. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 5.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. Epub 1999/09/09. [PubMed] [Google Scholar]
- 6.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. Epub 2008/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. The Journal of clinical investigation. 2005;115(7):1797–805. doi: 10.1172/JCI24176. Epub 2005/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herling M, Patel KA, Weit N, Lilienthal N, Hallek M, Keating MJ, et al. High TCL1 levels are a marker of B-cell receptor pathway responsiveness and adverse outcome in chronic lymphocytic leukemia. Blood. 2009;114(21):4675–86. doi: 10.1182/blood-2009-03-208256. Epub 2009/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):6955–60. doi: 10.1073/pnas.102181599. Epub 2002/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofbauer JP, Heyder C, Denk U, Kocher T, Holler C, Trapin D, et al. Development of CLL in the TCL1 transgenic mouse model is associated with severe skewing of the T-cell compartment homologous to human CLL. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(9):1452–8. doi: 10.1038/leu.2011.111. Epub 2011/05/25. [DOI] [PubMed] [Google Scholar]
- 11.Gorgun G, Ramsay AG, Holderried TA, Zahrieh D, Le Dieu R, Liu F, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6250–5. doi: 10.1073/pnas.0901166106. Epub 2009/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jak M, Mous R, Remmerswaal EB, Spijker R, Jaspers A, Yague A, et al. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leukemia & lymphoma. 2009;50(5):788–801. doi: 10.1080/10428190902803677. Epub 2009/05/20. [DOI] [PubMed] [Google Scholar]
- 13.Giannopoulos K, Schmitt M, Kowal M, Wlasiuk P, Bojarska-Junak A, Chen J, et al. Characterization of regulatory T cells in patients with B-cell chronic lymphocytic leukemia. Oncology reports. 2008;20(3):677–82. Epub 2008/08/13. [PubMed] [Google Scholar]
- 14.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. The Journal of clinical investigation. 2008;118(7):2427–37. doi: 10.1172/JCI35017. Epub 2008/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porakishvili N, Kardava L, Jewell AP, Yong K, Glennie MJ, Akbar A, et al. Cytotoxic CD4+ T cells in patients with B cell chronic lymphocytic leukemia kill via a perforin-mediated pathway. Haematologica. 2004;89(4):435–43. Epub 2004/04/13. [PubMed] [Google Scholar]
- 16.Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78(6):374–7. Epub 1993/11/01. [PubMed] [Google Scholar]
- 17.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. doi: 10.4049/jimmunol.180.9.5771. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 18.Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 19.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends in immunology. 2013;34(4):169–73. doi: 10.1016/j.it.2012.10.007. Epub 2012/12/05. [DOI] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2013;27(1):170–82. doi: 10.1038/leu.2012.165. Epub 2012/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine & growth factor reviews. 2013 doi: 10.1016/j.cytogfr.2013.04.003. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay F, Schneider P. Cracking the BAFF code. Nature reviews Immunology. 2009;9(7):491–502. doi: 10.1038/nri2572. Epub 2009/06/13. [DOI] [PubMed] [Google Scholar]
- 23.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B595 lymphocyte stimulator by B-chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100(8):2973–9. doi: 10.1182/blood-2002-02-0558. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184(7):3321–5. doi: 10.4049/jimmunol.0902551. Epub 2010/03/09. [DOI] [PubMed] [Google Scholar]
- 25.Zhou LJ, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Molecular and cellular biology. 1994;14(6):3884–94. doi: 10.1128/mcb.14.6.3884. Epub 1994/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lech-Maranda E, Mlynarski W, Grzybowska-Izydorczyk O, Borowiec M, Pastorczak A, Cebula-Obrzut B, et al. Polymorphisms of TNF and IL-10 genes and clinical outcome of patients with chronic lymphocytic leukemia. Genes, chromosomes & cancer. 2013;52(3):287–96. doi: 10.1002/gcc.22028. Epub 2012/12/12. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer G, Hodgson K, Pereira A, Juan M, Elena M, Colomer D, et al. Combined analysis of levels of serum B-cell activating factor and a proliferation-inducing ligand as predictor of disease progression in patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2011;52(11):2064–8. doi: 10.3109/10428194.2011.591008. Epub 2011/06/29. [DOI] [PubMed] [Google Scholar]
- 28.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188(1):497–503. doi: 10.4049/jimmunol.1102321. Epub 2011/11/30. [DOI] [PubMed] [Google Scholar]
- 29.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–88. doi: 10.1182/blood-2003-02-0540. Epub 2003/09/25. [DOI] [PubMed] [Google Scholar]
- 30.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, et al. BAFF mediates survival of peripheral immature B lymphocytes. The Journal of experimental medicine. 2000;192(10):1453–66. doi: 10.1084/jem.192.10.1453. Epub 2000/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. The Journal of experimental medicine. 2004;199(1):91–8. doi: 10.1084/jem.20031330. Epub 2004/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–32. doi: 10.1182/blood-2006-12-064527. Epub 2007/04/24. [DOI] [PubMed] [Google Scholar]
- 33.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10(11):1178–84. doi: 10.1038/ni.1791. Epub 2009/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4(11):889–99. doi: 10.1038/nri1488. Epub 2004/11/02. [DOI] [PubMed] [Google Scholar]
- 35.Sylvan SE, Rossmann E, Mozaffari F, Porwit A, Norin S, Karlsson C, et al. Phase I study of lenalidomide and alemtuzumab in refractory chronic lymphocytic leukaemia: maintaining immune functions during therapy-induced immunosuppression. British journal of haematology. 2012;159(5):608–12. doi: 10.1111/bjh.12077. Epub 2012/10/10. [DOI] [PubMed] [Google Scholar]
- 36.Giannopoulos K, Mertens D, Stilgenbauer S. Treating chronic lymphocytic leukemia with thalidomide and lenalidomide. Expert opinion on pharmacotherapy. 2011;12(18):2857–64. doi: 10.1517/14656566.2011.635644. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 37.Saulep-Easton D, Vincent FB, Le Page M, Wei A, Ting SB, Croce CM, et al. Cytokine-driven loss of plasmacytoid dendritic cell function in chronic lymphocytic leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2014 doi: 10.1038/leu.2014.105. Epub 2014/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Molecular immunology. 2005;42(7):763–72. doi: 10.1016/j.molimm.2004.06.041. Epub 2005/04/15. [DOI] [PubMed] [Google Scholar]
- 39.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165(10):5970–9. doi: 10.4049/jimmunol.165.10.5970. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 40.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus. 2013;22(9):873–84. doi: 10.1177/0961203313496302. Epub 2013/07/13. [DOI] [PubMed] [Google Scholar]
- 41.Amel Kashipaz MR, Huggins ML, Lanyon P, Robins A, Powell RJ, Todd I. Assessment of Be1 and Be2 cells in systemic lupus erythematosus indicates elevated interleukin-10 producing CD5+ B cells. Lupus. 2003;12(5):356–63. doi: 10.1191/0961203303lu338oa. Epub 2003/05/27. [DOI] [PubMed] [Google Scholar]
- 42.Llorente L, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Maillot MC, Durand-Gasselin I, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. European cytokine network. 1993;4(6):421–7. Epub 1993/11/01. [PubMed] [Google Scholar]
- 43.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–41. doi: 10.1182/blood-2010-07-294249. Epub 2010/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. The Journal of experimental medicine. 2007;204(8):1959–71. doi: 10.1084/jem.20062567. Epub 2007/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acosta-Rodriguez EV, Craxton A, Hendricks DW, Merino MC, Montes CL, Clark EA, et al. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. European journal of immunology. 2007;37(4):990–1000. doi: 10.1002/eji.200636698. Epub 2007/03/16. [DOI] [PubMed] [Google Scholar]
- 46.Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, et al. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115(24):5041–52. doi: 10.1182/blood-2009-03-213363. Epub 2010/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178(12):7531–9. doi: 10.4049/jimmunol.178.12.7531. Epub 2007/06/06. [DOI] [PubMed] [Google Scholar]
- 48.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179(9):5947–57. doi: 10.4049/jimmunol.179.9.5947. Epub 2007/10/20. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler S, Gartner K, Scheuermann U, Zoeller T, Hantzschmann J, Over B, et al. Ca(2+) -related signaling events influence TLR9-induced IL-10 secretion in human B cells. European journal of immunology. 2014;44(5):1285–98. doi: 10.1002/eji.201343994. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 50.Kofler DM, Gawlik BB, Elter T, Gianella-Borradori A, Wendtner CM, Hallek M. Phase 1b trial of atacicept, a recombinant protein binding BLyS and APRIL, in patients with chronic lymphocytic leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26(4):841–4. doi: 10.1038/leu.2011.286. Epub 2011/10/08. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation. 2008;118(10):3420–30. doi: 10.1172/JCI36030. Epub 2008/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–52. doi: 10.4049/jimmunol.1001307. Epub 2010/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–79. doi: 10.4049/jimmunol.1100284. Epub 2011/04/06. [DOI] [PubMed] [Google Scholar]
- 54.Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182(2):793–801. doi: 10.4049/jimmunol.182.2.793. Epub 2009/01/07. [DOI] [PubMed] [Google Scholar]
- 55.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614. Epub 1997/10/24 21:29. [DOI] [PubMed] [Google Scholar]
- 56.D’Arena G, Laurenti L, Minervini MM, Deaglio S, Bonello L, De Martino L, et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leukemia research. 2011;35(3):363–8. doi: 10.1016/j.leukres.2010.08.010. Epub 2010/10/01. [DOI] [PubMed] [Google Scholar]
- 57.Hartung HP, Kieseier BC. Atacicept: targeting B cells in multiple sclerosis. Therapeutic advances in neurological disorders. 2010;3(4):205–16. doi: 10.1177/1756285610371146. Epub 2010/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. doi: 10.1038/nature11501. Epub 2012/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183(11):7602–10. doi: 10.4049/jimmunol.0901881. Epub 2009/11/18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Analysis of Serum IL-10 over time in HD and CLL patients. IL-10 levels in the serum of HD (n=7) or patients with progressive CLL (n=7) were measured by ELISA using CLL samples collected the day of diagnosis and, at a 3- and 6-month follow up visit thereafter.
Supplementary Figure 2. CD5 isotype staining. B cells from peripheral blood were gated by flow cytometry as CD3− CD19+ and stained with CD5+ (left panel) or isotype control (right panel) as indicated on the Y-axis.
Supplementary Figure 3. In vitro stimulation of Tregs with exogenous IL-10. (A) CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice were cultured for 12-hr +/− IL-10 and assessed by flow cytometry. Proportion of CD4+CD25+CD39+ regulatory T cells is indicated. (B) Absolute numbers of CD4+CD25+CD39+ regulatory T cells isolated from WT and EμTCL1-Tg mice following 12-hr culture with IL-10 or not.
Supplementary Figure 4. Suppressive activity of IL-10 stimulated Tregs. FACS-sorted splenic CD4+CD25+CD39+ T cells from (A) WT and (B) EμTCL1-Tg mice were treated with or without exogenous IL-10 for 12-hr, then purified by FACS and co-cultured with AlexaFluor cytotracker-labelled autologous splenic CD4+CD25− responder T cells stimulated with CD3/CD28 beads. Proliferation of responders was assessed by flow cytometry gating on dilution of AlexaFluor staining compared to non-dividing AlexaFluor-labelled T cell control. Inhibition of proliferation is expressed as a percentage of responders detectable post-culture; the ratio of suppressor and responder cells is indicated.