Abstract
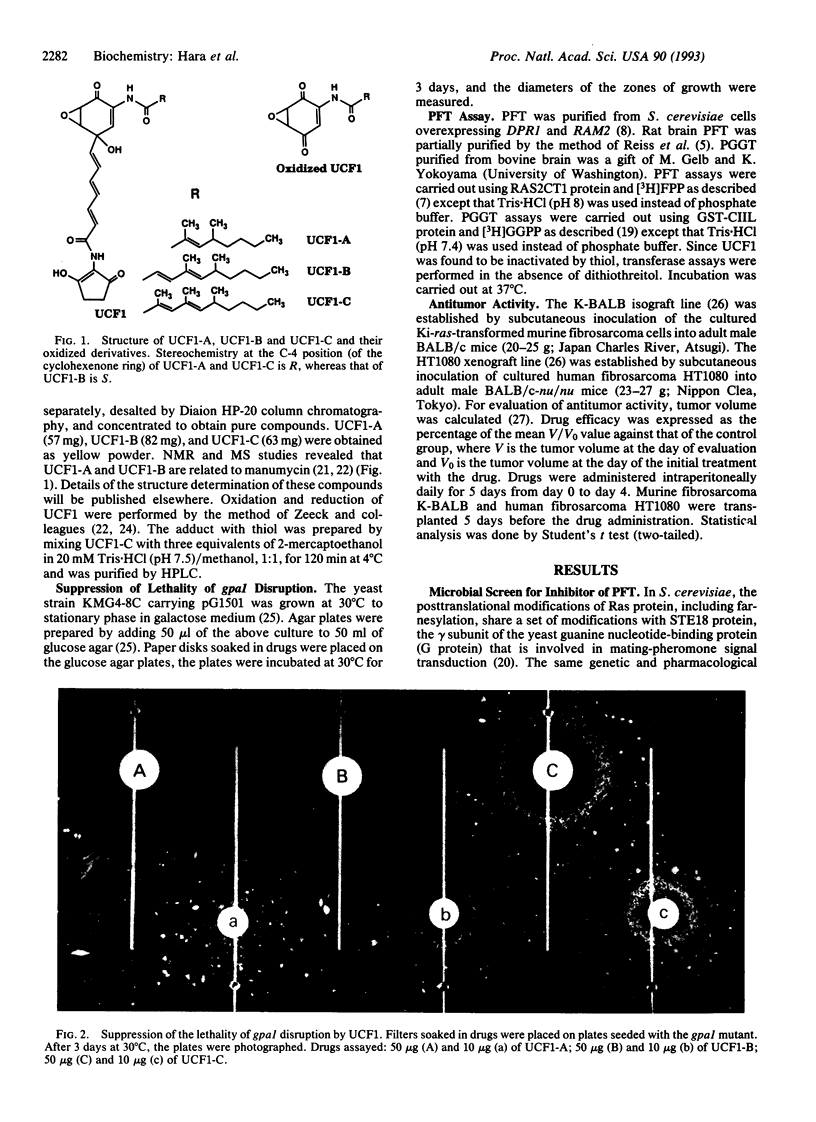
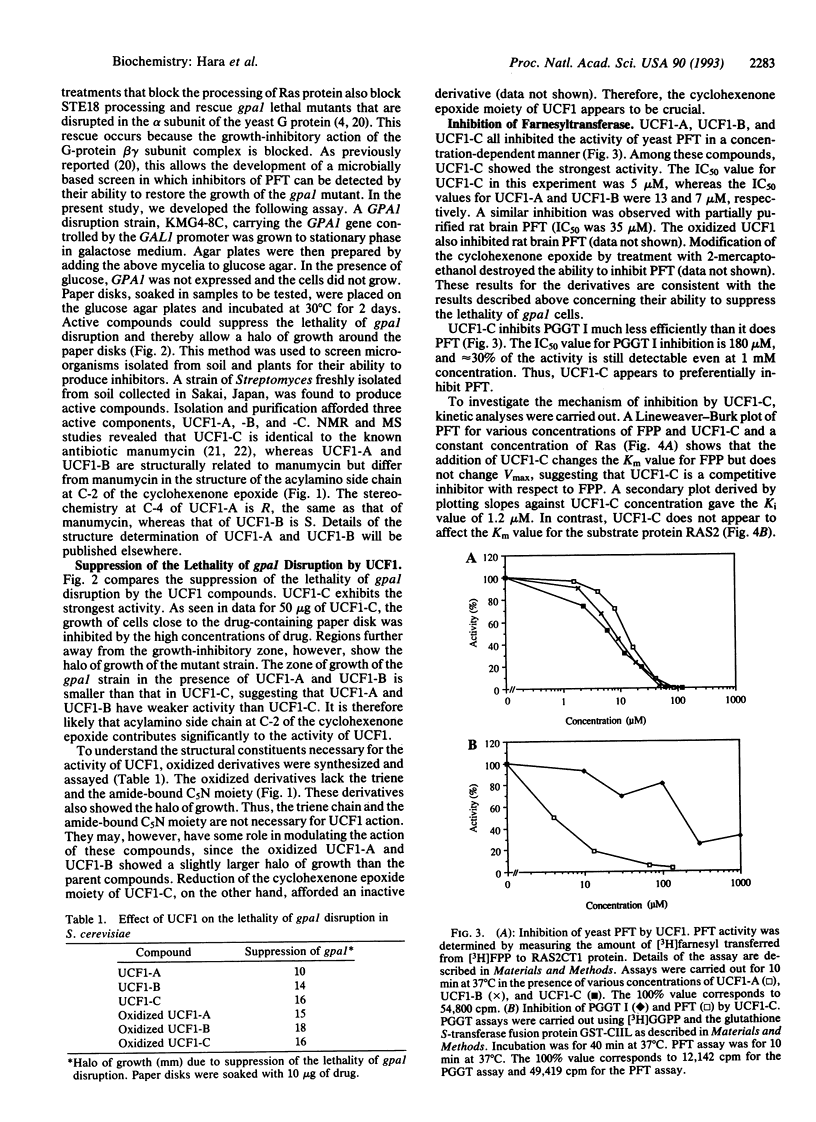
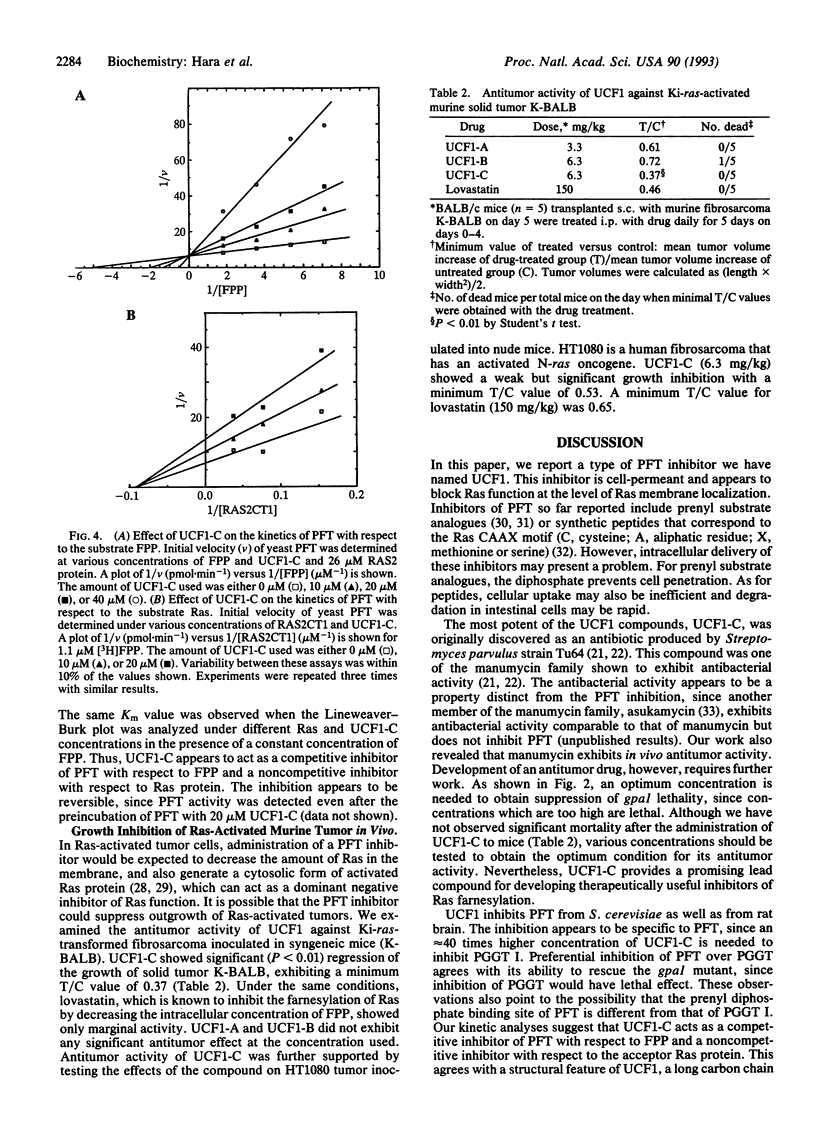
A microbial screen using a yeast strain with conditional deficiency in the GPA1 gene was carried out to search for inhibitors of protein farnesyltransferase (PFT). A strain of Streptomyces was found to produce active compounds named UCF1-A, UCF1-B, and UCF1-C. Structural determination of these compounds revealed that UCF1-C is identical to the known antibiotic, manumycin, whereas UCF1-A and UCF1-B are structurally related to manumycin. All three UCF1 compounds suppress the lethality of gpa1 disruption, with UCF1-C exhibiting the strongest activity. UCF1 inhibits yeast as well as rat brain PFT. Fifty percent inhibition of yeast PFT activity is observed with 5 microM UCF1-C. Kinetic analyses of the inhibition suggest that UCF1-C acts as a competitive inhibitor of PFT with respect to farnesyl pyrophosphate, exhibiting a Ki of 1.2 microM, whereas the same compound appears to act as a noncompetitive inhibitor of PFT with respect to the farnesyl acceptor, the Ras protein. UCF1-C shows significant activity to inhibit the growth of Ki-ras-transformed fibrosarcoma, raising the possibility of its use as an antitumor drug.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akinaga S., Gomi K., Morimoto M., Tamaoki T., Okabe M. Antitumor activity of UCN-01, a selective inhibitor of protein kinase C, in murine and human tumor models. Cancer Res. 1991 Sep 15;51(18):4888–4892. [PubMed] [Google Scholar]
- BUZZETTI F., GAEUMANN E., HUETTER R., KELLER-SCHIERLEIN W., NEIPP L., PRELOG V., ZAEHNER H. STOFFWECHSELPRODUKTE VON MIKROORGANISMEN. 41. MANUMYCIN. Pharm Acta Helv. 1963 Dec;38:871–874. [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Andres D. A., Goldstein J. L., Russell D. W., Brown M. S. cDNA cloning and expression of the peptide-binding beta subunit of rat p21ras farnesyltransferase, the counterpart of yeast DPR1/RAM1. Cell. 1991 Jul 26;66(2):327–334. doi: 10.1016/0092-8674(91)90622-6. [DOI] [PubMed] [Google Scholar]
- Das N. P., Allen C. M. Inhibition of farnesyl transferases from malignant and non-malignant cultured human lymphocytes by prenyl substrate analogues. Biochem Biophys Res Commun. 1991 Dec 16;181(2):729–735. doi: 10.1016/0006-291x(91)91251-7. [DOI] [PubMed] [Google Scholar]
- Der C. J., Cox A. D. Isoprenoid modification and plasma membrane association: critical factors for ras oncogenicity. Cancer Cells. 1991 Sep;3(9):331–340. [PubMed] [Google Scholar]
- Finegold A. A., Johnson D. I., Farnsworth C. C., Gelb M. H., Judd S. R., Glomset J. A., Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4448–4452. doi: 10.1073/pnas.88.10.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold A. A., Schafer W. R., Rine J., Whiteway M., Tamanoi F. Common modifications of trimeric G proteins and ras protein: involvement of polyisoprenylation. Science. 1990 Jul 13;249(4965):165–169. doi: 10.1126/science.1695391. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Schofield T. L., Scolnick E. M., Sigal I. S. Xenopus oocyte germinal-vesicle breakdown induced by [Val12]Ras is inhibited by a cytosol-localized Ras mutant. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6630–6634. doi: 10.1073/pnas.86.17.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Stradley S. J., Reiss Y., Gierasch L. M. Nonfarnesylated tetrapeptide inhibitors of protein farnesyltransferase. J Biol Chem. 1991 Aug 25;266(24):15575–15578. [PubMed] [Google Scholar]
- Gomez R., Goodman L. E., Tripathy S. K., O'Rourke E., Manne V., Tamanoi F. Purified yeast protein farnesyltransferase is structurally and functionally similar to its mammalian counterpart. Biochem J. 1993 Jan 1;289(Pt 1):25–31. doi: 10.1042/bj2890025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. E., Judd S. R., Farnsworth C. C., Powers S., Gelb M. H., Glomset J. A., Tamanoi F. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9665–9669. doi: 10.1073/pnas.87.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Chen P., Chen S. Y., Vancura K. L., Michaelis S., Powers S. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11373–11377. doi: 10.1073/pnas.88.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Diehl R. E., Schaber M. D., Rands E., Soderman D. D., He B., Moores S. L., Pompliano D. L., Ferro-Novick S., Powers S. Structural homology among mammalian and Saccharomyces cerevisiae isoprenyl-protein transferases. J Biol Chem. 1991 Oct 5;266(28):18884–18888. [PubMed] [Google Scholar]
- Michaeli T., Field J., Ballester R., O'Neill K., Wigler M. Mutants of H-ras that interfere with RAS effector function in Saccharomyces cerevisiae. EMBO J. 1989 Oct;8(10):3039–3044. doi: 10.1002/j.1460-2075.1989.tb08454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima I., Nakafuku M., Nakayama N., Brenner C., Miyajima A., Kaibuchi K., Arai K., Kaziro Y., Matsumoto K. GPA1, a haploid-specific essential gene, encodes a yeast homolog of mammalian G protein which may be involved in mating factor signal transduction. Cell. 1987 Sep 25;50(7):1011–1019. doi: 10.1016/0092-8674(87)90167-x. [DOI] [PubMed] [Google Scholar]
- Moomaw J. F., Casey P. J. Mammalian protein geranylgeranyltransferase. Subunit composition and metal requirements. J Biol Chem. 1992 Aug 25;267(24):17438–17443. [PubMed] [Google Scholar]
- Moores S. L., Schaber M. D., Mosser S. D., Rands E., O'Hara M. B., Garsky V. M., Marshall M. S., Pompliano D. L., Gibbs J. B. Sequence dependence of protein isoprenylation. J Biol Chem. 1991 Aug 5;266(22):14603–14610. [PubMed] [Google Scholar]
- Ohya Y., Goebl M., Goodman L. E., Petersen-Bjørn S., Friesen J. D., Tamanoi F., Anraku Y. Yeast CAL1 is a structural and functional homologue to the DPR1 (RAM) gene involved in ras processing. J Biol Chem. 1991 Jul 5;266(19):12356–12360. [PubMed] [Google Scholar]
- Omura S., Kitao C., Tanaka H., Oiwa R., Takahashi Y. A new antibiotic,, asukamycin, produced by Streptomyces. J Antibiot (Tokyo) 1976 Sep;29(9):876–881. doi: 10.7164/antibiotics.29.876. [DOI] [PubMed] [Google Scholar]
- Pompliano D. L., Rands E., Schaber M. D., Mosser S. D., Anthony N. J., Gibbs J. B. Steady-state kinetic mechanism of Ras farnesyl:protein transferase. Biochemistry. 1992 Apr 21;31(15):3800–3807. doi: 10.1021/bi00130a010. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Seabra M. C., Armstrong S. A., Slaughter C. A., Goldstein J. L., Brown M. S. Nonidentical subunits of p21H-ras farnesyltransferase. Peptide binding and farnesyl pyrophosphate carrier functions. J Biol Chem. 1991 Jun 5;266(16):10672–10677. [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Trueblood C. E., Yang C. C., Mayer M. P., Rosenberg S., Poulter C. D., Kim S. H., Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science. 1990 Sep 7;249(4973):1133–1139. doi: 10.1126/science.2204115. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Goldstein J. L., Südhof T. C., Brown M. S. Rab geranylgeranyl transferase. A multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J Biol Chem. 1992 Jul 15;267(20):14497–14503. [PubMed] [Google Scholar]
- Seabra M. C., Reiss Y., Casey P. J., Brown M. S., Goldstein J. L. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991 May 3;65(3):429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Goodwin G. W., Ghomashchi F., Glomset J. A., Gelb M. H. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeck A., Frobel K., Heusel C., Schröder K., Thiericke R. The structure of manumycin. II. Derivatives. J Antibiot (Tokyo) 1987 Nov;40(11):1541–1548. doi: 10.7164/antibiotics.40.1541. [DOI] [PubMed] [Google Scholar]
- Zeeck A., Schröder K., Frobel K., Grote R., Thiericke R. The structure of manumycin. I. Characterization, structure elucidation and biological activity. J Antibiot (Tokyo) 1987 Nov;40(11):1530–1540. doi: 10.7164/antibiotics.40.1530. [DOI] [PubMed] [Google Scholar]




