Abstract
IMPORTANCE
Myopia (nearsightedness) has its onset in childhood and affects about one-third of adults in the United States. Along with its high prevalence, myopia is expensive to correct and is associated with ocular diseases that include glaucoma and retinal detachment.
OBJECTIVE
To determine the best set of predictors for myopia onset in school-aged children.
DESIGN, SETTING, AND PARTICIPANTS
The Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study was an observational cohort study of ocular development and myopia onset conducted at 5 clinical sites from September 1, 1989, through May 22, 2010. Data were collected from 4512 ethnically diverse, nonmyopic school-aged children from grades 1 through 8 (baseline grades 1 through 6) (ages 6 through 13 years [baseline, 6 through 11 years]).
MAIN OUTCOMES AND MEASURES
We evaluated 13 candidate risk factors for their ability to predict the onset of myopia. Myopia onset was defined as −0.75 diopters or more of myopia in each principal meridian in the right eye as measured by cycloplegic autorefraction at any visit after baseline until grade 8 (age 13 years). We evaluated risk factors using odds ratios from discrete time survival analysis, the area under the curve, and cross validation.
RESULTS
A total of 414 children became myopic from grades 2 through 8 (ages 7 through 13 years). Of the 13 factors evaluated, 10 were associated with the risk for myopia onset (P < .05). Of these 10 factors, 8 retained their association in multivariate models: spherical equivalent refractive error at baseline, parental myopia, axial length, corneal power, crystalline lens power, ratio of accommodative convergence to accommodation (AC/A ratio), horizontal/vertical astigmatism magnitude, and visual activity. A less hyperopic/more myopic baseline refractive error was consistently associated with risk of myopia onset in multivariate models (odds ratios from 0.02 to 0.13, P < .001), while near work, time outdoors, and having myopic parents were not. Spherical equivalent refractive error was the single best predictive factor that performed as well as all 8 factors together, with an area under the curve (C statistic) ranging from 0.87 to 0.93 (95% CI, 0.79–0.99).
CONCLUSIONS AND RELEVANCE
Future myopia can be predicted in a nonmyopic child using a simple, single measure of refractive error. Future trials for prevention of myopia should target the child with low hyperopia as the child at risk.
Myopia is a common disorder of the eye that creates blurry distance vision; vision can be corrected with spectacles, contact lenses, or refractive surgery. The eye that becomes myopic elongates out of proportion to its focal length so that light rays from distant objects focus in front of rather than on the retina. Myopia affects 1 in 3 individuals in the United States aged 12 to 54 years,1 and its prevalence appears to be on the rise.2 Its management costs the US health care system almost $4 billion annually.3,4
Juvenile-onset myopia is the most common variety, with its peak incidence during elementary school2,5,6 and progression through the teenaged years. The debate concerning the etiology of myopia has centered on the relative contribution of genetic vs environmental influences.7,8 High heritability for myopia from family and twin studies9 and the identification of more than 40 genetic loci linked to or associated with myopia lend support to a strong genetic contribution.10,11 The classic environmental risk factor for myopia has been near work (eg, reading, study, watching television, and computer use).12–14 Longitudinal studies15,16 have not borne out a major role for near work as the cause of myopia in children.
From this controversy, the paradigm has shifted away from concern about near work toward recognition that time outdoors may be a more important environmental influence. Children who spend more time outdoors have a much lower probability of having or developing myopia. 15,17,18
The treatment of myopia has focused on optical19 or pharmaceutical20,21 interventions to slow eye growth and thus retard progression, but the most desirable treatment would prevent myopia onset altogether. If a preventive treatment were available, the prediction of risk for myopia onset in early childhood would be crucial to the accurate application of any treatment. Previous attempts to predict myopia have included observations that more hyperopia (farsightedness) at school entry protects against myopia development22 and that refractive error in infancy predicts childhood myopia,23 although the latter result is debatable.24 Previous work has described the very good performance of refractive error at ages 8 to 9 years for myopia onset prediction (sensitivity, 87%; specificity, 73%)25 and the poor predictive performance of refractive error and parental myopia at grade 1 (age 6 years) (sensitivity, 62.5%; specificity, 81.9%).26 Factors such as ocular optical and structural variables before myopia onset may be informative.25,27–29 Reduced accommodative accuracy at near and increased convergence of the eyes with accommodation are also potentially predictive factors.30–32 A predictive model developed using data from Asian children33 had high sensitivity and specificity but required difficult measurement of ocular components and a computationally complex equation.
The National Eye Institute–funded Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study was a 5-center cohort study of almost 5000 children examined annually during school grades 1 through 8 (ages 6–13 years). All major ocular variables were measured, and data concerning environmental risk factors were collected. Children were enrolled in grade 1 (age 6 years), when most did not have myopia, and then were followed up each year through myopia onset and subsequent progression. Risk factors before myopia onset in the children with myopia were then compared with those same factors in the children who did not develop myopia. The analyses that framed the question were: given a baseline grade/age, what measurements taken at that moment help to identify children who will develop myopia? We anticipated that different sets of risk factors would be associated with different ages; however, we also expected that a theme would emerge across the risk factor sets. The purpose of this study was to describe an overarching predictive model for onset of juvenile myopia.
Methods
Study Participants
All children enrolled in the CLEERE Study from September 1, 1989, through May 22, 2010, who were nonmyopic at study entry and who had at least 2 annual study visits were included in this analysis. Children with a parent-reported race/ ethnicity designation of missing or other were excluded. The CLEERE Study was approved by the institutional review boards at the University of Alabama, Birmingham; School of Optometry, University of California, Berkeley; College of Optometry, University of Houston, Houston, Texas; Southern California College of Optometry, Fullerton; and the Department of Ophthalmology and Vision Science, University of Arizona, Tucson. Parents and children were provided an explanation of the study. The parents provided written informed consent, whereas the children provided verbal or written assent.
Procedures
Myopia onset was defined as −0.75 diopters (D) or more myopia in each principal meridian in the right eye measured by cycloplegic autorefraction at any visit after baseline. We used autorefraction because of its high reliability.34,35 Measurement methods have been described in detail previously.25
Predictors to be evaluated were selected based on the CLEERE Study protocol and included direct measures and parental reports. Thirteen predictors were evaluated that have been previously reported as possibly associated with the development and/or progression of myopia: (1) the child’s spherical equivalent refractive error at baseline; (2) self-reported parental myopia (0, 1, or 2 myopic parents)36,37; (3) the parents’ report of the child’s visual activity (as an indicator of near work); (4) the parents’ report of the child’s time spent outdoors; (5) axial length; (6) crystalline lens thickness; (7) corneal power; (8) crystalline lens power; (9) ratio of accommodative convergence to accommodation (AC/A ratio); (10) relative peripheral refractive error; (11) accommodative lag; and (12–13) astigmatism magnitude by orientation (horizontal/vertical or oblique).
Statistical Analysis
Using discrete time survival analysis, we analyzed the predictive value of the baseline values of the 13 possible predictor variables (plus age, sex, and race/ethnicity) at each of the school grades 1 through 6 (ages 6–11 years). For each baseline grade/age model, the control variables were entered as main effects, as were the baseline values of the risk factors. For example, the model with the spherical equivalent at grade 3 (age 8 years) that was fitted using the data set from grade 3 (age 8 years) had the following form for individual i:
The term logit h(ti) is a shortcut for the logarithm of the hazard odds. Because the model is discrete time, the hazard at time t is the probability of myopia onset at time t among those who are still at risk for myopia. The terms G4 through G8 are indicators set to 1 when an individual’s data are from the grade and age indicated. The terms allow the probability of myopia onset to take a different value in each grade and age (grades 4–8 [ages 9–13 years]). A parameter αj is the value of the logarithm of the odds in grade j for at-risk individuals in the group that results when categorical predictor values are the reference values and continuous predictors are zero. A parameter βj is the shift in the logarithm of the odds attributed to a predictor. The shift is assumed to be the same across all grades and ages. Race/ ethnicity was treated as a categorical variable with white used as the reference level.
As indicated, discrete time hazard models fit the log odds of the probability of myopia onset at time t among those who are still at risk for myopia. A common metric of effect size when log odds are fitted is an odds ratio, which is the metric we used.
The analysis had the potential to generate more than 8000 multivariate models. To pare down the number of models and to find the best predictors at each age, the following techniques were used and assumptions were made. For each grade (age): (1) The data set was limited to children with complete data at that grade (age) level and at least 1 follow-up visit. Data from the same child could appear in more than 1 data set; however, for each child, only those observations taken at the baseline and subsequent-to-baseline visits were used. (2) Backward stepwise selection was used to identify a sequence of 13 nested models, with each model having 1 less predictor. The selection method began with a model that included all 13 risk factors and the control variables. Each subsequent step removed the risk factor whose absence least harmed model fit as measured by likelihood. The process terminated when a single risk factor remained. (3) Ten-fold cross-validation was used to evaluate model fit. In other words, the data set was randomly divided into 10 subsets, allocating those with and without myopia onset separately to ensure that each subset had a sufficient number of myopia events. Each nested model was fitted using 9 of the 10 subsets, and its likelihood was estimated using the remaining subset, rotating the role of the test set across the 10 subsets. (4) The mean of the likelihood estimates from the 10 test sets was then calculated to produce a likelihood estimate for each model. (5) Steps 3 and 4 were repeated 200 times each, producing 200 likelihood estimates for each model. (6) The model with the largest mean likelihood was identified, and a 95% CI around the true likelihood was estimated using the bootstrap results. (7) The model with the fewest terms and a mean maximum likelihood of no less than the lower limit of the CI was defined as the best model of risk for myopia onset for the given baseline grade (age).
The area under a receiver operating characteristic curve is commonly used to compare the clinical significance of alternative models and is the probability that, in a randomly selected pair of individuals with and without future myopia, the model correctly ranks the predicted risk for myopia onset as higher for the individual with future myopia than for the individual without future myopia. The overall C statistic is a generalization of the area under the curve for survival data.38 The C statistic can be thought of as the mean area under the curve across time-dependent receiver operating characteristic curves, ranging from 0.5 to 1.0. The C statistic provides a measure of model discrimination when data are censored, which is the case for our setting. Because of incomplete follow-up, we did not know the event status of all of our study participants through grade 8 (age 13 years). The C statistic uses every possible pairing of participants to estimate the probability that an individual from the event group has a higher risk for the event than does an individual from the nonevent group. Individuals with incomplete follow-up can inform this estimate until they leave the risk set. We report the overall C statistic when comparing the performance of alternative models using 10-fold cross validation.
Results
We enrolled and collected data from a total of 4927 children. Of those, 48 individuals were excluded based on the missing/ other race/ethnicity designation, and 367 were excluded because they entered the study with myopia, leaving 4512 children available for analysis. Racial/ethnic composition included 36.2% white, 22.2% Hispanic, 16.2% African American, 11.6% Native American, and 13.7% Asian American children. The sample was equally split by sex (49.6% female). At each of grades 1 through 6 (ages 6–11 years), at least 2000 nonmyopic children underwent evaluation at that grade (age) level with atleast 1 subsequent annual visit. Table 1 shows their grade(age) at study entry, mean length of follow-up, and mean number of follow-up visits as a function of baseline age. Of those children, 414 became myopic from grades 2 through 8 (ages 7–13 years). Mean (SD) age in grade 1 was 6.7 (0.5) years, increasing by roughly 1 year per grade to reach 11.8 (0.6) years by grade 6.
Table 1.
Grade and Age at Baseline Entry Into the CLEERE Study
| Baseline Gradea | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| No. of children | 2026 | 2239 | 2589 | 2572 | 2423 | 2330 |
| Age, y | 6.7 (0.4) | 7.8 (0.5) | 8.8 (0.5) | 9.8 (0.5) | 10.8 (0.6) | 11.8 (0.6) |
| Length of follow-up, y | 5.4 (1.8) | 4.7 (1.6) | 4.0 (1.4) | 3.3 (1.1) | 2.6 (0.8) | 1.9 (0.5) |
| No. of follow-up visits | 5.2 (1.9) | 4.5 (1.6) | 3.9 (1.4) | 3.2 (1.1) | 2.5 (0.8) | 1.8 (0.5) |
Abbreviation: CLEERE, Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error.
Unless otherwise indicated, data are expressed as mean (SD).
Most of the 13 candidate predictor variables were consistently related to the risk for future myopia in univariate models (only baseline grades 1, 3, and 6 [ages 6, 8, and 11 years] are shown for brevity) (Table 2). The exceptions were corneal power, near work in diopter-hours, and accommodative lag. We then used the statistical methods described above to generate the 6 best multivariate models for each baseline grade (age). They ranged from the smallest number of terms (3) in the grade 4 model to 5 terms for grade 6 (ages 9 and 11 years, respectively). The multivariate models for baseline grades 1, 3, and 6 (ages 6, 8, and 11 years) are presented in Table 3. Age (as a continuous variable), sex, and the 5 race/ethnicity categories were also terms in each model. Of the 8 terms across baseline grades, only spherical equivalent refractive error and astigmatism magnitude appeared in the best model for every baseline grade (age).
Table 2.
Univariate Analysis of Risk Factors for Developing Myopia
| Variable | Odds Ratio (95% CI)a | ||
|---|---|---|---|
| Grade 1 | Grade 3 | Grade 6 | |
| Age, y | 6 | 8 | 11 |
| AC/A ratio, PD/D | 1.22 (1.16–1.28)b | 1.27 (1.22–1.33)b | 1.30 (1.22–1.38)b |
| Axial length, mm | 1.95 (1.65–2.31)b | 2.50 (2.14–2.91)b | 3.34 (2.68–4.17)b |
| Crystalline lens power, D | 0.91 (0.84–0.97)c | 0.81 (0.76–0.87)b | 0.73 (0.65–0.82)b |
| No. of myopic parents | |||
| 1 | 1.35 (1.03–1.76)d | 1.36 (1.05–1.76)d | 1.77 (1.19–2.62)c |
| 2 | 2.23 (1.63–3.04)b | 2.40 (1.76–3.27)b | 3.51 (2.18–5.64)b |
| Corneal power, D | 1.18 (1.10–1.27)b | 1.07 (0.99–1.14) | 1.07 (0.97–1.19) |
| Visual activity, diopter-hour | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Astigmatism magnitude by orientation | |||
| Horizontal/vertical | 0.43 (0.32–0.58)b | 0.50 (0.38–0.66)b | 0.38 (0.25–0.56)b |
| Oblique | 0.67 (0.46–0.97)d | 0.52 (0.35–0.76)b | 0.36 (0.21–0.62)b |
| Accommodative lag, D | 0.81 (0.69–0.95)c | 0.92 (0.80–1.06) | 0.84 (0.69–1.03) |
| Crystalline lens thickness, mm | 0.55 (0.29–1.06) | 0.31 (0.16–0.58)b | 0.13 (0.05–0.32)b |
| Refractive error, D | |||
| Relative peripheral | 1.36 (1.19–1.57)b | 1.46 (1.30–1.64)b | 1.65 (1.39–1.96)b |
| Spherical equivalent | 0.10 (0.08–0.13)b | 0.06 (0.05–0.08)b | 0.05 (0.03–0.07)b |
| Time spent outdoors, h/wk | 0.98 (0.97–1.00)d | 0.97 (0.95–0.98)b | 0.96 (0.94–0.99)d |
Abbreviations: AC/A, accommodative convergence to accommodation; D, diopter; PD, prism diopter.
Odds ratios describe the association between risk of future myopia in any subsequent grade/age and candidate predictor variables at baseline grades 1, 3, and 6 (ages 6, 8, and 11 years, respectively), adjusted for age, sex, and race/ethnicity.
P < .001; significance of the odds ratio compared with 1.00 associated with a 1-unit increase in each variable within each grade level.
P < .01.
P < .05.
Table 3.
Multivariate Analysis of Risk Factors for Developing Myopiaa
| Variable | Odds Ratio (95% CI)b | ||
|---|---|---|---|
| Grade 1 | Grade 3 | Grade 6 | |
| Age, y | 6 | 8 | 11 |
| AC/A ratio, PD/D | 1.12 (1.06–1.18)c | 1.18 (1.07–1.31)d | |
| Axial length, mm | 2.91 (2.17–3.90)c | ||
| Crystalline lens power, D | 0.85 (0.77–0.94)c | ||
| No. of myopic parents | |||
| 1 | 2.94 (1.51–5.76)d | ||
| 2 | 7.73 (3.43–17.41)c | ||
| Corneal power, D | 1.52 (1.34–1.71)c | ||
| Visual activity, diopter-hour | 0.99 (0.97–1.00)d | ||
| Astigmatism magnitude, horizontal/vertical, D | 0.45 (0.33–0.61)c | 0.26 (0.19–0.36)c | 0.15 (0.09–0.28)c |
| Spherical equivalent, D | 0.13 (0.10–0.16)c | 0.04 (0.03–0.06)c | 0.02 (0.01–0.03)c |
Abbreviations: AC/A, accommodative convergence to accommodation; D, diopter; PD, prism diopter.
Blank cells represent nonsignificant terms that were not included in the multivariate model for that baseline grade.
Odds ratios describe the association between risk of future myopia in any subsequent grade/age and candidate predictor variables at baseline grades 1, 3, and 6 (ages 6, 8, and 11 years, respectively) adjusted for age, sex, and race/ethnicity.
P < .001
P < .01
Applying different prediction models to each grade (age) using variables that are difficult to measure, such as the response AC/A ratio, lens power, or axial length, would not be feasible for the clinician (ie, optometrist or ophthalmologist). An analysis was therefore performed to quantify the loss of predictive information if the number of variables in the model was reduced using the C statistic,38 which represented the mean area under the curve across all prediction intervals for a given baseline grade (age). Model performance using all 8 terms improved from 0.88 to 0.94 as intervals decreased with increasing baseline grade (age). Model performance was nearly unaffected by a reduction in the number of model terms. Regardless of baseline grade (age), the C statistic only decreased by 0.01 or 0.02 as the number of terms was reduced from all 8 to the spherical equivalent refractive error only. Knowing the spherical equivalent improved prediction compared with age, sex, and race/ethnicity data alone; the C statistics using only the demographic data ranged from 0.58 to 0.68. In models that included spherical equivalent, the C statistic ranged from 0.87 to 0.93 (95% CI, 0.79–0.99). Therefore, baseline cycloplegic spherical equivalent appears to fulfill the criterion of effective predictive ability while preserving parsimony and feasibility.
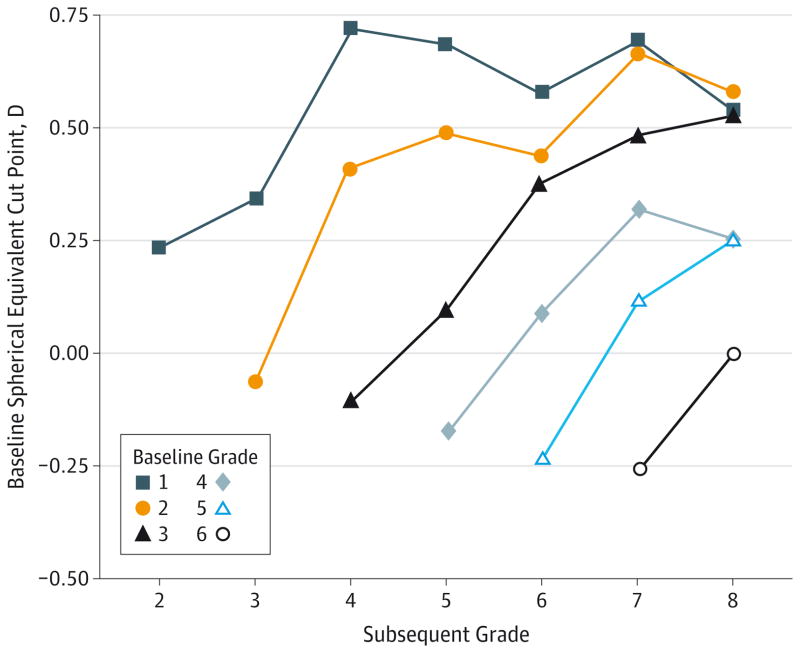
The criterion level of spherical equivalent that predicts the onset of myopia in subsequent grades (ages) with the best combination of sensitivity and specificity is shown in the Figure. The spherical equivalent cut points were estimated using a discrete time hazard model with only spherical equivalent as a predictor. Limiting the model to the spherical equivalent provided a one-to-one correspondence between predicted risk for myopia and the level of spherical equivalent. The best combination of sensitivity and specificity was determined using the Youden index,39 which is the maximum value of sensitivity + specificity - 1. The Youden index identifies the combination of sensitivity and specificity that is associated with the maximum vertical distance between the receiver operating characteristic curve and the diagonal or chance line.
Figure. Cut Points for Predicting Future Myopia.
The cut points for optimal spherical equivalent refractive error for predicting the development of future myopia to grade 8 (age 13 years) are shown. Each baseline grade 1 through 6 (ages 6–11 years) is represented with 2 to 7 possible subsequent grades after baseline.
The upward slopes of the lines indicate that the cut point for prediction of conversion to myopia in 1 year tended to be less hyperopic compared with the cut points that are predictive of conversion several years later. A child with less hyperopia is at greater risk for developing myopia sooner. The downward trend for the placement of the lines in the Figure indicates that later baseline grades (ages) had less hyperopic cut points across subsequent grades (older ages). A given level of hyperopia is more protective as the child becomes older. If preventive treatments were to be applied based on these cut points, one might consider using the following most hyperopic values for the given baseline grade (age): less hyperopic than +0.75 D for grade 1 (age 6 years), +0.50 D or less hyperopic for grades 2 and 3 (ages 7 and 8 years), +0.25 D or less hyperopic for grades 4 and 5 (ages 9 and 10 years), and emmetropic or more myopic for grade 6 (age 11 years).
Discussion
Analyses identified cycloplegic spherical equivalent refractive error as the single best predictor of the onset of myopia. The range of C statistics from 0.87 to 0.93 for spherical equivalent refractive error, sex, and race/ethnicity compares well with the 0.815 reported from a far more complex model.33 The cut point that optimized sensitivity and specificity was also identified for each baseline grade from 1 through 6 (baseline age from 6–11 years) (Figure). Children examined in grade 1 (age 6 years) with less than +0.75 D of hyperopia are at increased risk for developing myopia. This predictive model should enable clinicians and scientists to evaluate the risk for myopia in a child using simple, feasible measures. The model could be used by optometrists and ophthalmologists to plan children’s eye examination schedules across the school years. It could be used by clinical investigators conducting a myopia prevention study so that children at higher risk for myopia could be enrolled preferentially. It could assist in event rate estimation for sample size planning.
This model has temporal flexibility in that refractive error measurement could be at any grade from 1 to 6 or any age from 6 to 11 years. Sensitivity and specificity are the poorest and the CIs are the widest when the prediction is based on early baseline grade (age) data with onset much later.26 Study strengths are the racial/ethnic diversity of the sample, the large sample size and high statistical power, the length of follow-up, the characterization of refractive error under cycloplegia, and the assessment of a large set of predictive factors for myopia onset.
Limitations of the present study include the volunteer nature of the sample. Perhaps myopic parents were more likely to enroll their children who had not yet developed myopia because of the parents’ interest in their own refractive error. The project originally began in 1989 in northern California as the Orinda Longitudinal Study of Myopia with a predominantly white sample.5 Beginning in the late 1990s, a more racially and ethnically diverse sample was added using a recruitment scheme that could limit the generalizability of the results. Specifically, clinical sites were chosen for their ability to recruit a particular racial/ethnic group (eg, Eutaw, Alabama, for African American children; Houston, Texas, for Hispanic children). This scheme may have resulted in less variability among children of a given race/ethnicity because of their geographic similarity. Refractive error and other biometric data were collected from the right eye only to keep the protocol relatively brief and to maintain the other eye for reading during the school day. Behaviors related to risk factors, such as reading and computer use, may have changed during the course of the study as technology evolved. Last, the conduct of a study of this size and scope resulted in losses to follow-up that are not typical of myopia treatment studies.
Conclusions
In the early 1960s, a longitudinal study conducted in Ojai, California,22 first identified the ability of refractive error in grade 1 (age 6 years) to predict later myopia onset. Thirty years later, the first predictive model from the current data set developed predictions based on refractive error in grade 3 (age 8 years) only.25 The present analysis extends these previous findings to include the use of cycloplegia, all baseline grades from 1 through 6 (ages 6 through 11 years), outcomes over time through grade 8 (age 13 years), and the cut point of the spherical equivalent refractive error for optimized prediction. Current spherical equivalent refractive error is the best single predictor of future myopia.
Acknowledgments
Funding/Support: The Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study is supported by grants U10-EY08893 and R21-12273 from the National Eye Institute and the Office of Minority Research/ National Institutes of Health, by the Ohio Lions Eye Research Foundation, and by the E. F. Wildermuth Foundation.
Group Members
The CLEERE Study Group (as of March 2012) includes the following centers and investigators: Sandral Hullett, MD, MPH (principal investigator [PI], 1997–2006), Robert N. Kleinstein, OD, MPH, PhD (coinvestigator, 1997–2006), Janene Sims, OD (optometrist, 1997–2001 and 2004–2006), Raphael Weeks, OD (optometrist, 1999–2006), Sandra Williams (study coordinator, 1999–2006), LeeAndra Calvin (study coordinator, 1997–1999), and Melvin D. Shipp, OD, MPH, DrPH (coinvestigator, 1997–2004) (Franklin Primary Health Center, Inc, Mobile, Alabama); Nina E. Friedman, OD, MS (PI, 1999–2001), Pamela Qualley, MA (study coordinator, 1997–2001), Donald O. Mutti, OD, PhD (PI, 1996–1999), and Karla Zadnik, OD, PhD (optometrist, 1996–2001) (School of Optometry, University of California, Berkeley); Ruth E. Manny, OD, PhD (PI, 1997–2006), Suzanne M. Wickum, OD (optometrist, 1999–2006), Ailene Kim, OD (optometrist, 2003–2006), Bronwen Mathis, OD (optometrist, 2002–2006), Mamie Batres (study coordinator, 2004–2006), Sally Henry (study coordinator, 1997–1998), Janice M. Wensveen, OD, PhD (optometrist, 1997–2001), Connie J. Crossnoe, OD (optometrist, 1997–2003), Stephanie L. Tom, OD (optometrist, 1999–2002), Jennifer A. McLeod (study coordinator, 1998–2004), Julio C. Quiralte (study coordinator, 1998–2005), and Gaby Solis (study coordinator, 2005–2006) (College of Optometry, University of Houston, Houston, Texas); Susan A. Cotter, OD, MS (PI, 2004–2006; optometrist, 1997–2004), Julie A. Yu, OD (PI, 1997–2004; optometrist, 2005–2006), Raymond J. Chu, OD (optometrist, 2001–2006), Carmen N. Barnhardt, OD, MS (optometrist, 2004–2006), Jessica Chang, OD (optometrist, 2005–2006), Kristine Huang, OD (optometrist, 2005–2006), Rebecca Bridgeford (study coordinator, 2005–2006), Connie Chu, OD (optometrist, 2004–2005), Soonsi Kwon, OD (optometrist, 1998–2004), Gen Lee (study coordinator, 1999–2003), John Lee, OD (optometrist, 2000–2003), Robert J. Lee, OD (optometrist, 1997–2001), Raymond Maeda, OD (optometrist, 1999–2003), Rachael Emerson (study coordinator, 1997–1999); and Tracy Leonhardt (study coordinator, 2003–2004) (Southern California College of Optometry, Marshall B. Ketchum University, Fullerton); and J. Daniel Twelker, OD, PhD (PI, 2000–2010), Mabel Crescioni, DrPh (study coordinator, 2009–2010), Dawn Messer, OD, MPH (optometrist, 2000–2010), Denise Flores (study coordinator, 2000–2007), Rita Bhakta, OD (optometrist, 2000–2004), Katie Garvey, OD (optometrist, 2005–2008), and Amanda Mendez Roberts, OD (optometrist, 2008–2010) (Department of Ophthalmology and Vision Science, University of Arizona, Tucson). Resource centers include the chairperson’s office, College of Optometry, The Ohio State University: Karla Zadnik, OD, PhD (chairperson, 1997-present), and Jodi M. Malone Thatcher, RN (study coordinator, 1997–2012); Videophakometry Reading Center, College of Optometry, The Ohio State University: Donald O. Mutti, OD, PhD (director, 1997-present), Huan Sheng, MD, PhD (reader, 2000–2006), Holly Omlor (reader, 2003–2006), Meliha Rahmani, MPH (reader, 2004–2007), Jaclyn Brickman (reader, 2002–2003), Amy Wang (reader, 2002–2003), Philip Arner (reader, 2002–2004), Samuel Taylor (reader, 2002–2003), Myhanh T. Nguyen, OD, MS (reader, 1998–2001), Terry W. Walker, OD, MS (reader, 1997–2001), and Vidhya Subramanian, MS (reader, 2006–2007); Optometry Coordinating Center, College of Optometry, The Ohio State University: Lisa A. Jones-Jordan, PhD (director, 1997-present), Linda Barrett (data entry operator, 1997–2007), John Hayes, PhD (biostatistician, 2001–2007), G. Lynn Mitchell, MAS (biostatistician, 1998-present), Melvin L. Moeschberger, PhD (consultant, 1997-present), Loraine T. Sinnott, PhD (biostatistician, 2005-present), Pamela Wessel (program coordinator, 2000-present), Julie N. Swartzendruber, MA (program coordinator, 1998–2000), and Austen Tanner (data entry operator, 2008–2010); Project Office, National Eye Institute, Rockville, Maryland: Donald F. Everett, MA; and Executive Committee: Karla Zadnik, OD, PhD (chairperson), Lisa A. Jones-Jordan, PhD, Robert N. Kleinstein, OD, MPH, PhD, Ruth E. Manny, OD, PhD, Donald O. Mutti, OD, PhD, J. Daniel Twelker, OD, PhD, and Susan A. Cotter, OD, MS.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Author Contributions: Drs Zadnik and Sinnott had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zadnik, Sinnott, Kleinstein, Mutti.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Zadnik, Sinnott, Mutti.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Sinnott, Jones-Jordan.
Obtained funding: Zadnik, Cotter, Jones-Jordan, Kleinstein, Manny, Twelker, Mutti.
Administrative, technical, or material support: All authors.
Study supervision: Zadnik, Cotter, Jones-Jordan, Kleinstein, Manny, Twelker, Mutti.
Role of the Funder/Sponsor: Donald F. Everett, MA, of the National Eye Institute was involved in the design and conduct of the study. The other funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Vitale S, Ellwein L, Cotch MF, Ferris FL, III, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008;126(8):1111–1119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitale S, Sperduto RD, Ferris FL., III Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 4.Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999–2002. Ophthalmology. 2006;113(12):2163–2170. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Blum HL, Peters HB, Bettman JW. Vision Screening for Elementary Schools: The Orinda Study. Berkeley: University of California Press; 1959. [Google Scholar]
- 6.Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci. 1998;39(1):120–133. [PubMed] [Google Scholar]
- 7.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24(1):1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Mutti DO, Zadnik K, Adams AJ. Myopia: the nature versus nurture debate goes on. Invest Ophthalmol Vis Sci. 1996;37(6):952–957. [PubMed] [Google Scholar]
- 9.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the Twin Eye Study. Invest Ophthalmol Vis Sci. 2001;42(6):1232–1236. [PubMed] [Google Scholar]
- 10.Kiefer AK, Tung JY, Do CB, et al. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9(2):e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Consortium for Refractive Error and Myopia (CREAM); Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group; Wellcome Trust Case Control Consortium 2 (WTCCC2); Fuchs’ Genetics Multi-Center Study Group. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia [published correction in Nat Genet. 2013;45(2):712] Nat Genet. 2013;45(3):314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware J. Observations relative to the near and distant sight of different persons. Philos Trans R Soc Lond B Biol Sci. 1813;103:31–50. [Google Scholar]
- 13.Cohn H. The Hygiene of the Eye in Schools. London, England: Simpkin, Marshall & Co; 1886. [Google Scholar]
- 14.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43(12):3633–3640. [PubMed] [Google Scholar]
- 15.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47(5):1839–1844. doi: 10.1167/iovs.05-1081. [DOI] [PubMed] [Google Scholar]
- 17.Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93(8):997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- 18.Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53(6):2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 20.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD US Pirenzepine Study Group. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122(11):1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 21.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch MJ. Predictability of refraction at age 14 on the basis of testing at age 6: interim report from the Ojai Longitudinal Study of Refraction. Am J Optom Arch Am Acad Optom. 1964;41:567–573. doi: 10.1097/00006324-196410000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vis Sci. 1993;8:337–344. [Google Scholar]
- 24.Mutti DO, Zadnik K. The utility of three predictors of childhood myopia: a Bayesian analysis. Vision Res. 1995;35(9):1345–1352. doi: 10.1016/0042-6989(94)00225-b. [DOI] [PubMed] [Google Scholar]
- 25.Zadnik K, Mutti DO, Friedman NE, et al. Ocular predictors of the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 1999;40(9):1936–1943. [PubMed] [Google Scholar]
- 26.Jones-Jordan LA, Sinnott LT, Manny RE, et al. Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group. . Early childhood refractive error and parental history of myopia as predictors of myopia. Invest Ophthalmol Vis Sci. 2010;51(1):115–121. doi: 10.1167/iovs.08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss DA, Jackson TW. Clinical findings before the onset of myopia in youth, I: ocular optical components. Optom Vis Sci. 1995;72(12):870–878. doi: 10.1097/00006324-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Goss DA, Jackson TW. Clinical findings before the onset of myopia in youth, 4: parental history of myopia. Optom Vis Sci. 1996;73(4):279–282. doi: 10.1097/00006324-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mutti DO, Hayes JR, Mitchell GL, et al. CLEERE Study Group. . Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gwiazda J, Grice K, Thorn F. Response AC/A ratios are elevated in myopic children. Ophthalmic Physiol Opt. 1999;19(2):173–179. doi: 10.1046/j.1475-1313.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 31.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34 (3):690–694. [PubMed] [Google Scholar]
- 32.Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41(9):2469–2478. [PubMed] [Google Scholar]
- 33.Zhang M, Gazzard G, Fu Z, et al. Validating the accuracy of a model to predict the onset of myopia in children. Invest Ophthalmol Vis Sci. 2011;52(8):5836–5841. doi: 10.1167/iovs.10-5592. [DOI] [PubMed] [Google Scholar]
- 34.Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992;33(7):2325–2333. [PubMed] [Google Scholar]
- 35.Davies LN, Mallen EA, Wolffsohn JS, Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003;80(4):320–324. doi: 10.1097/00006324-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Walline JJ, Zadnik K, Mutti DO. Validity of surveys reporting myopia, astigmatism, and presbyopia. Optom Vis Sci. 1996;73(6):376–381. doi: 10.1097/00006324-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children’s eye size. JAMA. 1994;271(17):1323–1327. [PubMed] [Google Scholar]
- 38.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 39.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]