Abstract
OBJECTIVE
To assess the association between number of embryos transferred and a measure of assisted reproductive technology success that emphasizes good perinatal outcome.
METHODS
We analyzed assisted reproductive technology cycles initiated in 2011 that progressed to fresh embryo transfer among women using autologous oocytes and reported to the U.S. National Assisted Reproductive Technology Surveillance System (n=82,508). Percentages of good perinatal outcome (live birth of a term [at or after 37 weeks of gestation], normal birth weight [2,500 g or greater] singleton) were stratified by prognosis (favorable, average, less favorable), age, embryo stage (day 3, day 5), and number of embryos transferred. Differences in the percentages by number of embryos transferred were evaluated using Fisher’s exact test with Bonferroni correction.
RESULTS
Among patients younger than 35 years with a favorable prognosis, chances of a good perinatal outcome were higher with transferring a single (compared with double) day 5 (43% compared with 27%) or day 3 embryo (36% compared with 30%). Likewise, a higher chance of a good perinatal outcome was observed with transferring a single day 5 embryo in patients 35–37 years old with a favorable prognosis (39% compared with 28%) or patients younger than 35 years old with an average prognosis (35% compared with 26%). A higher chance of good perinatal outcome was associated with transferring two (compared with one) day 3 embryos among patients aged 40 years or younger with an average prognosis or patients younger than 35 years old with a less favorable prognosis.
CONCLUSION
The association between number of embryos transferred and the birth of a term, normal birth weight singleton is described. Among patients younger than 35 years of age undergoing in vitro fertilization with a favorable prognosis, the highest chance of good perinatal outcome is associated with a single embryo transfer.
Since the birth of the first neonate conceived with in vitro fertilization (IVF) in 1978, IVF has allowed millions of patients worldwide to overcome infertility. However, the IVF success story has been accompanied by a significant increase in multiple births as a result of transfer of multiple embryos.1 In the United States, almost half (47%) of IVF neonates are born in multiple gestations,2 which carry increased risks for both mother (gestational hypertension, preeclampsia, gestational diabetes, cesarean delivery, and maternal hospital admission) and neonate (low birth weight, prematurity, neonatal intensive care unit admission, disability, birth defects, and increased morbidity and mortality).3–6
Although it is widely recognized that single embryo transfer is the best way to avoid multiple birth, several barriers prevent its widespread implementation in the United States.7 Patients and clinicians may be unwilling to accept or offer single embryo transfer as a result of the expected lower likelihood of pregnancy or live birth rates, which are traditional measures of IVF success. Shifting the focus from pregnancy and live birth to optimal IVF outcome–healthy singleton birth7 requires adaptation of a new measure of success that accounts for perinatal risks.8
Our objective was to assess the association between number of embryos transferred and a measure of IVF success that emphasizes good perinatal outcome while accounting for both effectiveness (live birth rates) and risks (multiple birth, prematurity, and low birth weight) to inform development of guidelines on the number of embryos to transfer and provide information that can be used during patient counseling.
PATIENTS AND METHODS
Data were obtained from the National Assisted Reproductive Technology Surveillance System maintained by the Division of Reproductive Health, Centers for Disease Control and Prevention (CDC). Fertility clinics in the United States are required to report data on each assisted reproductive technology (ART) cycle to the CDC by the Fertility Clinic Success Rates and Certification Act (Public Law No. 102-493, October 24, 1992). The National ART Surveillance System covers estimated 97% of all cycles performed in the United States.9 Data abstracted from medical records include patients’ demographics, obstetric history, detailed information on the cycle, and information on cycle outcome. The latter is obtained by active follow-up by the fertility clinic and includes information about whether the cycle resulted in pregnancy, pregnancy outcome, and information about each resultant neonate (gestational age, birth weight, and sex). The data from each clinic are validated by the clinics’ medical director before submission. In addition, approximately 7–10% of reporting clinics are randomly selected each year for validation by the CDC, during which data reported by the clinics are compared with information recorded in medical records and discrepancy rates are calculated.10 In 2011, 451 clinics reported more than 151,000 ART cycles. Cycles initiated in 2011 in the United States that progressed to the embryo transfer stage among patients using fresh embryos from autologous oocytes and reported to the National ART Surveillance System (n=82,508) were used in this retrospective population-based study.
For the purposes of data collection and the current analyses, ART is defined as a procedure in which oocytes and sperm are handled outside the patient’s body such as IVF.9 In vitro fertilization involves retrieval of a woman’s eggs, fertilizing the eggs in the laboratory, and then transferring the resulting embryo(s) into the woman’s uterus through the cervix. Fresh autologous cycles are cycles in which newly fertilized (fresh) embryos derived from patient’s own oocytes are used. Good perinatal outcome was defined as a live birth of a term (at or after 37 weeks of gestation), normal birth weight (2,500 g or greater) singleton. Gestational age was calculated by subtracting the date of oocyte retrieval from the date of birth and adding 14 days to adjust for theoretical last menstrual period. Intracytoplasmic sperm injection is a procedure in which a single sperm is injected into the oocyte. Embryo stage at transfer was calculated by subtracting the oocyte retrieval date from the embryo transfer date. Typically, embryos transferred on day 3 correspond to the cleavage stage of development, whereas embryos transferred on day 5 correspond to the blastocyst stage. Patients with a favorable prognosis were defined as those who underwent their first IVF cycle and had extra embryo(s) cryopreserved. Patients with a less favorable prognosis were defined as those who had previous IVF cycle(s), no previous live births, and no extra embryos cryopreserved. The remaining patients who could not be classified in either favorable prognosis or less favorable prognosis group were classified as patients with an average prognosis and included those who 1) underwent their first IVF cycle and had no extra embryo cryopreserved; 2) had previous IVF cycle(s) and no previous live birth(s) but had extra embryo(s) cryopreserved; or 3) had previous IVF cycle(s) and previous live birth(s) (the last subgroup was classified as average prognosis because we were not able to determine whether previous live birth(s) resulted from IVF or natural conception). Elective single embryo transfer is the transfer of a single embryo when at least one additional embryo was cryopreserved.
All statistical analyses were performed using SAS 9.2 statistical software. We first calculated percentage distribution of selected patient and treatment factors for all ART cycles among patients who used fresh embryos from autologous oocytes by age group. We then calculated percentage of transfers resulting in good perinatal outcome by number of embryos transferred stratified by patient prognosis, age, and embryo stage at transfer. As a result of the small number of cycles in some subgroups, we limited our analysis to women aged 40 years or younger for patients with a favorable prognosis and to women aged 42 years or younger for patients with an average and less favorable prognosis. Because more than one ART transfer can be reported for one patient during the reporting year, we limited analyses to the first embryo transfer that satisfied criteria for each prognosis group. The selection of stratification variables and groups was based on their ability to independently predict good perinatal outcome and is similar to factors used in the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology Guidelines on the number of embryos to transfer.11 In supplemental analysis, we calculated percentage of transfers resulting in good perinatal outcome redefined as a singleton or twin term live birth with normal birth weight neonates (redefined good perinatal outcome).
Differences in the percentages of transfers resulting in good perinatal outcome by number of embryos transferred were evaluated using Fisher’s exact test with Bonferroni correction for multiple comparisons. In each group of patient prognosis, patient age, and embryo stage, we compared percentages of transfers resulting in good perinatal outcome for two adjacent numbers of embryos transferred (ie, one compared with two embryos transferred, two compared with three embryos transferred).
The study was approved by the CDC’s institutional review board.
RESULTS
Overall, 82,508 ART cycles that progressed to embryo transfer stage were performed in the United States in 2011 among patients who used fresh embryos derived from autologous oocytes. Almost half (44.2%) of cycles were performed among women aged younger than 35 years (Table 1). Patient and treatment characteristics varied by women’s age. The proportion of cycles among patients who had ART in the past ranged from 34.0% among women aged younger than 35 years to 58.7% among women aged older than 40 years. The proportion of cycles performed among women with previous births ranged from 21.7% in the youngest age group to 36.5% in the oldest. Cycles with intracytoplasmic sperm injection ranged from 76.2% among women aged younger than 35 years to 73.1% among women older than 40 years of age. Embryos were transferred at the blastocyst stage (day 5) in more than half (51.0%) of ART procedures among women aged younger than 35 years and only in 19.1% of procedures among women older than 40 years of age. In the majority of cycles, more than one embryo was transferred. A single embryo (which could be the only embryo available) was transferred in 18.3%, 15.6%, 13.4%, and 16.8% of cycles among patients aged younger than 35, 35–37, 38–40, and older than 40 years, respectively. Availability of extra embryos for cryopreservation ranged from 52.5% of cycles in the youngest group to 10.4% of cycles in the oldest group.
Table 1.
Percentage Distribution of Selected Patient and Treatment Factors for Assisted Reproductive Technology Procedures Among Patients Who Used Fresh Embryos From Autologous Oocytes by Age Group, United States 2011
| Patient and Treatment Factors | Patient’s Age (y)
|
|||
|---|---|---|---|---|
| Younger Than 35 (n=36,476, 44.2%) | 35–37 (n=17,395, 21.1%) | 38–40 (n=16,616, 20.1%) | Older Than 40 (n=12,021, 14.6%) | |
| Patient diagnosis | ||||
| Tubal factor | 8.0 | 9.2 | 7.6 | 4.5 |
| Ovulation disorders | 12.4 | 7.4 | 4.4 | 2.0 |
| Diminished ovarian reserve | 8.5 | 18.0 | 34.1 | 58.5 |
| Endometriosis | 11.4 | 9.4 | 6.7 | 3.1 |
| Uterine factor | 1.0 | 1.7 | 1.7 | 1.0 |
| Male factor | 37.2 | 29.4 | 21.4 | 12.1 |
| Other causes | 7.2 | 7.8 | 8.6 | 9.0 |
| Unexplained cause | 14.3 | 17.1 | 15.4 | 9.9 |
| No. of previous ART procedures | ||||
| 0 | 66.0 | 55.8 | 49.3 | 41.3 |
| 1 or more | 34.0 | 44.2 | 50.7 | 58.7 |
| No. of previous births | ||||
| 0 | 78.3 | 66.8 | 64.8 | 63.5 |
| 1 or more | 21.7 | 33.2 | 35.2 | 36.5 |
| Method of embryo fertilization and transfer | ||||
| IVF without intracytoplasmic sperm injection | 23.8 | 25.3 | 25.3 | 26.9 |
| IVF with intracytoplasmic sperm injection | 76.2 | 74.7 | 74.7 | 73.1 |
| Embryo stage at transfer | ||||
| Cleavage (day 3) | 40.3 | 49.5 | 57.3 | 66.9 |
| Blastocyst (day 5) | 51.0 | 40.5 | 30.8 | 19.1 |
| No. of embryos transferred | ||||
| 1 | 18.3 | 15.6 | 13.4 | 16.8 |
| 2 | 69.4 | 58.0 | 37.9 | 23.8 |
| 3 | 11.0 | 22.6 | 33.5 | 25.9 |
| 4 | 1.1 | 3.2 | 12.5 | 18.4 |
| 5 | 0.2 | 0.5 | 2.2 | 10.9 |
| 6 or more | 0.1 | 0.1 | 0.4 | 4.2 |
| Extra embryos available and cryopreserved | ||||
| No | 47.5 | 59.4 | 74.2 | 89.6 |
| Yes | 52.5 | 40.6 | 25.8 | 10.4 |
| Prognosis group | ||||
| Favorable* | 37.4 | 25.0 | 14.1 | 5.1 |
| Average† | 49.1 | 57.6 | 62.5 | 63.1 |
| Less favorable‡ | 13.4 | 17.4 | 23.4 | 31.8 |
ART, assisted reproductive technology; IVF, in vitro fertilization.
Patients with a favorable prognosis include those who underwent first ART cycle and had extra embryo(s) cryopreserved.
Patients with an average prognosis include those who 1) underwent their first IVF cycle and had no extra embryo cryopreserved; 2) had previous IVF cycle(s), no previous live birth(s), but had extra embryo(s) cryopreserved; or 3) had previous IVF cycle(s) and previous live birth(s).
Patients with a less favorable prognosis include those who had previous ART cycle(s), no previous live birth, and no extra embryos cryopreserved.
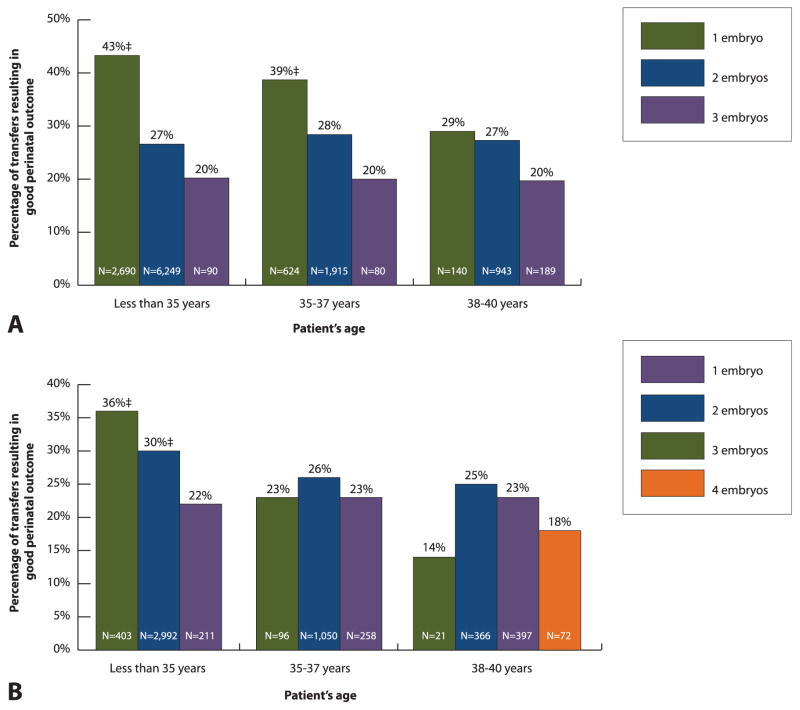
Among patients with a favorable prognosis who were younger than 35 years, single (compared with double) embryo transfer was associated with a higher chance of good perinatal outcome regardless of embryo stage at transfer (Fig. 1). In addition, single (compared with double) embryo transfer was associated with a higher chance of good perinatal outcome in patients with a favorable prognosis who were 35–37 years of age if blastocyst stage embryos were available. In other subgroups of patients with a favorable prognosis, the differences in percentages of good perinatal outcome by number of embryos transferred were not statistically significant.
Fig. 1.
Patients with a favorable prognosis*: percentage of transfers resulting in good perinatal outcome† among patients who used fresh embryos from autologous oocytes for A. blastocyst stage (day 5) and B. cleavage stage (day 3) embryos by patient’s age and number of embryos transferred, United States, 2011. *Patients with a favorable prognosis include those who underwent a first assisted reproductive technology cycle and had extra embryo(s) cryopreserved; transfers among patients older than 40 years and transfers that involved higher number of embryos are not shown as a result of the small numbers. †Good perinatal outcome is defined as live birth of a term (at least 37 weeks of gestation), normal birth weight (at least 2,500 g) singleton. ‡P<.05; comparison with the proportion associated with transfer of additional embryo; if not marked, P≥.05.
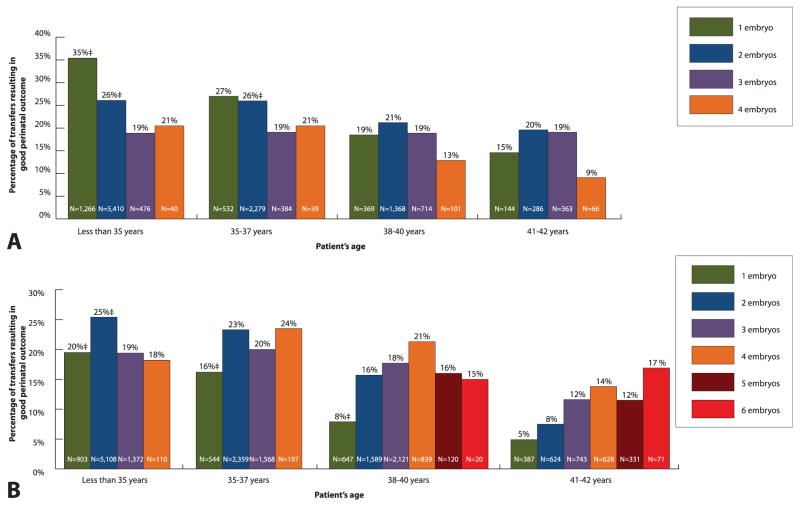
Among patients with an average prognosis who were aged younger than 35 years with available blastocysts, a higher percentage of good perinatal outcome was associated with single (compared with double) blastocyst transfer (Fig. 2). When cleavage stage embryos were available in the average prognosis group, transferring two embryos (compared with one) was associated with a higher chance of good perinatal outcome among patients aged younger than 35 years, 35–37 years, and 38–40 years.
Fig. 2.
Patients with an average prognosis*: percentage of transfers resulting in good perinatal outcome† among patients who used fresh embryos from autologous oocytes for A. blastocyst stage (day 5) and B. cleavage stage (day 3) embryos by patient’s age and number of embryos transferred, United States, 2011. *Patients with an average prognosis include those who: 1) underwent their first in vitro fertilization (IVF) cycle and had no extra embryo(s) cryopreserved; 2) had previous IVF cycle(s), no previous live birth(s), but had extra embryo(s) cryopreserved; or 3) had previous IVF cycle(s) and previous live birth(s); transfers among patients older than 42 years of age and transfers that involved higher number of embryos are not shown as a result of the small numbers. †Good perinatal outcome is defined as live birth of a term (at least 37 weeks of gestation), normal birth weight (at least 2,500 g) singleton. ‡P<.05; comparison with the proportion associated with transfer of additional embryo; if not marked, P≥.05.
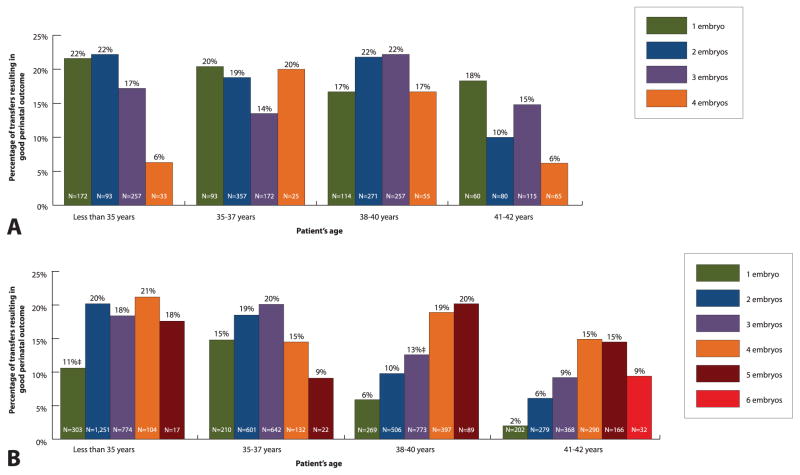
Among patients with a less favorable prognosis, most differences in percentages of good perinatal outcome by number of embryos transferred were not statistically significant (Fig. 3). Among patients with a less favorable prognosis who were aged younger than 35 years with cleavage stage embryos, a higher chance of good perinatal outcome was associated with transferring two embryos (compared with one).
Fig. 3.
Patients with a less favorable prognosis*: percentage of transfers resulting in good perinatal outcome† among patients who used fresh embryos from autologous oocytes for A. blastocyst stage (day 5) and B. cleavage stage (day 3) embryos by patient’s age and number of embryos transferred, United States, 2011. *Patients with a less favorable prognosis include those who had previous assisted reproductive technology (ART) cycle(s), no previous live birth(s), and no extra embryo(s) cryopreserved; transfers among patients older than 42 years and transfers that involved higher number of embryos are not shown as a result of the small numbers. †Good perinatal outcome is defined as live birth of a term (at least 37 weeks of gestation), normal birth weight (at least 2,500 g) singleton. ‡P<.05; comparison with the proportion associated with transfer of additional embryo; if not marked, P≥.05.
When “good perinatal outcome” was redefined as a singleton or twin term live birth with normal birth weight neonates, the percentages of “redefined good perinatal outcome” were 0–5% points higher, but comparisons by number of embryos transferred showed similar results. When blastocyst stage embryos were available, the chance of “redefined good perinatal outcome” was higher with transferring a single (compared with double) embryo among patients with a favorable prognosis who were younger than 35 years (44% compared with 33%) or 35–37 years (39% compared with 33%) and among patients with an average prognosis who were younger than 35 years (36% compared with 32%). When cleavage stage embryos were available among patients with a favorable prognosis who were younger than 35 years, the chance of “redefined good perinatal outcome” with transferring two embryos (33%) was not significantly different from that with one embryo (36%) but was significantly higher than that with three embryos (25%). A higher chance of a “redefined good perinatal outcome” was associated with transferring two (compared with one) day 3 embryos among patients with an average prognosis who were younger than 35 years (28% compared with 20%), 35–37 years (26% compared with 16%), or 38–40 years (17% compared with 8%) and among patients with a less favorable prognosis who were younger than 35 years (22% compared with 11%).
DISCUSSION
Our study describes the association between number of embryos transferred and good perinatal outcome by age, embryo stage, and prognosis. According to this analysis of population-based surveillance data, a higher chance of a good perinatal outcome in patients with a favorable prognosis who were aged younger than 35 years is associated with single embryo transfer.
This measure of IVF success, which emphasizes good perinatal outcome, balances success and risks of the procedure and provides a better summary measure than traditional measures—pregnancy and live birth rates—that focus solely on effectiveness. We deliberately chose transfer instead of cycle as a denominator in the current analysis to allow for stratification by embryo stage at transfer and number of embryos transferred. However, presenting percentage of cycles resulting in good perinatal outcome, as proposed in Birth Emphasizing a Successful Singleton at Term,8 is an appropriate measure of IVF success that takes into account the burden of treatment for all initiated cycles, including those cancelled before embryo transfer. Other proposed measures of success that reflect perinatal risk of the procedure are also useful but have some limitations: percentage of cycles or transfers resulting in singleton live births12,13 does not take into account the risk of low birth weight among IVF singletons5 and percentage of cycles resulting in the delivery of term neonates (singleton or twins)14 does not take into account the risk of term twin births.15 Nevertheless, it is likely that the use of any measure of IVF success that takes into account perinatal risks of the procedure will demonstrate that transferring a single embryo in the patients with a most favorable prognosis will result in the best perinatal outcome.
Using percentage of transfers resulting in good perinatal outcome when making decisions about the number of embryos to transfer may result in fewer neonates born preterm or with low birth weight. In patients with a favorable prognosis who were younger than 35 years, transferring a single embryo results in the higher chance of the good perinatal outcomes and may result in substantial savings in health care costs. Using 2011 National ART Surveillance System data, single embryo transfer in all patients with a favorable prognosis who are 35 years could result in 77.7% reduction of neonates born preterm in that group: from 37.2% (3,698) neonates who were born preterm after 2011 ART cycles to 12.2% (823) neonates who would have been born preterm if a single embryo was transferred. Assuming previously published estimate of societal economic burden of $61,718 (2013 U.S. dollars) per preterm neonate,16,17 universal single blastocyst transfer only in patients with a favorable prognosis who are younger than 35 years will result in saving of $177,439,250 (2013 U.S. dollars) annually. Although this strategy is projected to decrease live birth rates by 10.4% from observed 55.0% (7,453 births) to estimated 49.3% (6,676 births) based on 2011 National ART Surveillance System data, a high chance of pregnancy can be achieved in a subsequent cycle using a cryopreserved embryo18 at a cost of approximately $3,517 (2013 U.S. dollars) per frozen or thawed cycle17,19 or $2,732,709 (2013 U.S. dollars). One large multicenter trial comparing effectiveness of elective single embryo transfer (with subsequent transfer of a single thawed embryo if the first cycle was unsuccessful) with double embryo transfer among patients with a good prognosis showed that cumulative live birth rates were not significantly different among the two groups.20
In the United States, the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology Practice Guidelines play a major role in reducing the number of multiple births by specifying maximum number of embryos to transfer after IVF.21 These guidelines, first published in 1998, have repeatedly lowered the maximum number of embryos recommended for transfer, which led to the substantial reduction of multiple births, especially higher-order multiple births.7,22 Our findings can inform the development of future guidelines by providing a measure of success that accounts for both effectiveness and safety by focusing on the stated goal of the treatment—a healthy singleton neonate.7 Our results show that moving closer to that goal requires lowering the number of embryos transferred, especially among patients with a favorable prognosis. However, experience from countries that were successful in reducing IVF-related multiple births suggest that strict limits on the number of embryos to transfer need to be accompanied by mandated insurance coverage of ART.23–25
Several limitations common to observational study designs apply to our population-based analysis. Individual circumstances that guided the decisions on the number of embryos to transfer such as embryo quality considerations were unknown. Although the results were stratified by the most important patient and treatment characteristics that were available, it is possible that residual confounders remained. Although we lacked the data on embryo quality—the most likely unmeasured confounder—we used cryopreservation of supernumerary embryos, which has been shown to be a good predictor of embryo quality despite variable cryopreservation practices.26,27 Because we used U.S. National ART Surveillance System data for the current analysis, study results can only be generalized to cycles performed in the United States.
The United States has one of the highest rates of preterm births in the world with an annual societal economic burden of at least $26.2 billion.16,28 Although it is difficult to address an array of socioeconomic, biological, and environmental contributors of preterm births, prevention of IVF-related preterm births can be achieved by limiting the number of embryos transferred in the appropriate group of patients. American Society for Reproductive Medicine and Society for Assisted Reproductive Technology Guidelines on the number of embryos transferred and numerous publications on the topic7,11,29 have helped to significantly reduce higher-order multiple births in the United States. It is now time to focus on reducing the rate of twin births, which continues to grow in part as a result of the frequent transfer of two embryos to patients with a good prognosis.7,30 The percentage of embryo transfers resulting in good perinatal outcome is the measure of IVF success that balances its effectiveness and risks and should be considered in developing future guidelines and in counseling patients on the number of embryos to transfer as well as in clinic-specific outcome reporting.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Disclosure
The authors did not report any potential conflicts of interest.
LEVEL OF EVIDENCE: II
References
- 1.Reynolds MA, Schieve LA, Martin JA, Jeng G, Macaluso M. Trends in multiple births conceived using assisted reproductive technology, United States, 1997–2000. Pediatrics. 2003;111:1159–62. [PubMed] [Google Scholar]
- 2.Sunderam S, Kissin DM, Flowers L, Anderson JE, Folger SG, Jamieson DJ, et al. Assisted reproductive technology surveillance—United States, 2009. MMWR Surveill Summ. 2012;61:1–23. [PubMed] [Google Scholar]
- 3.MacKay AP, Berg CJ, King JC, Duran C, Chang J. Pregnancy-related mortality among women with multifetal pregnancies. Obstet Gynecol. 2006;107:563–8. doi: 10.1097/01.AOG.0000200045.91015.c6. [DOI] [PubMed] [Google Scholar]
- 4.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–77. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 5.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe AG, Derom C. Follow-up of twins: health, behaviour, speech, language outcomes and implications for parents. Early Hum Dev. 2006;82:379–86. doi: 10.1016/j.earlhumdev.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine. Elective single-embryo transfer. Fertil Steril. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Breheny SA, MacLachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Hum Reprod. 2004;19:3–7. doi: 10.1093/humrep/deh028. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2011 assisted reproductive technology fertility clinic success rates report. Atlanta (GA): U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 10.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2010 assisted reproductive technology national summary report. Atlanta (GA): U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 11.Practice Committee of American Society for Reproductive Medicine; Practice Committee of Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2013;99:44–6. doi: 10.1016/j.fertnstert.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Land JA, Evers JL. What is the most relevant standard of success in assisted reproduction? Defining outcome in ART: a Gordian knot of safety, efficacy and quality. Hum Reprod. 2004;19:1046–8. doi: 10.1093/humrep/deh215. [DOI] [PubMed] [Google Scholar]
- 13.Evers JL. Female subfertility. Lancet. 2002;360:151–9. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 14.Buckett W, Tan SL. What is the most relevant standard of success in assisted reproduction? The importance of informed choice. Hum Reprod. 2004;19:1043–5. doi: 10.1093/humrep/deh206. [DOI] [PubMed] [Google Scholar]
- 15.Boulet SL, Schieve LA, Nannini A, Ferre C, Devine O, Cohen B, et al. Perinatal outcomes of twin births conceived using assisted reproduction technology: a population-based study. Hum Reprod. 2008;23:1941–8. doi: 10.1093/humrep/den169. [DOI] [PubMed] [Google Scholar]
- 16.Behrman RE, Butler AS, editors. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm birth: causes, consequences, and prevention. societal costs of preterm birth. Washington (DC): National Academies Press; 2007. p. 12. [PubMed] [Google Scholar]
- 17.United States Department of Labor, Bureau of Labor Statistics. Consumer price index inflation calculator. Available at: http://www.bls.gov/data/inflation_calculator.htm. Retrieved September 12, 2013.
- 18.Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–62. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91:2281–94. doi: 10.1016/j.fertnstert.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 21.Jungheim ES, Ryan GL, Levens ED, Cunningham AF, Macones GA, Carson KR, et al. Embryo transfer practices in the United States: a survey of clinics registered with the Society for Assisted Reproductive Technology. Fertil Steril. 2010;94:1432–6. doi: 10.1016/j.fertnstert.2009.07.987. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg ML, Boulet S, Kissin D, Warner L, Jamieson DJ. Elective single embryo transfer trends and predictors of a good perinatal outcome—United States, 1999 to 2010. Fertil Steril. 2013;99:1937–43. doi: 10.1016/j.fertnstert.2013.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissonnette F, Phillips SJ, Gunby J, Holzer H, Mahutte N, St-Michel P, et al. Working to eliminate multiple pregnancies: a success story in Quebec. Reprod Biomed Online. 2011;23:500–4. doi: 10.1016/j.rbmo.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Gordts S, Campo R, Puttemans P, Brosens I, Valkenburg M, Norre J, et al. Belgian legislation and the effect of elective single embryo transfer on IVF outcome. Reprod Biomed Online. 2005;10:436–41. doi: 10.1016/s1472-6483(10)60818-8. [DOI] [PubMed] [Google Scholar]
- 25.Karlström PO, Bergh C. Reducing the number of embryos transferred in Sweden-impact on delivery and multiple birth rates. Hum Reprod. 2007;22:2202–7. doi: 10.1093/humrep/dem120. [DOI] [PubMed] [Google Scholar]
- 26.Wang JG, Douglas NC, Dicken C, Nakhuda GS, Guarnaccia MM, Sauer MV. Cryopreservation of supernumerary high quality embryos predicts favorable outcomes for patients undergoing repeated cycles of in vitro fertilization. Fertil Steril. 2008;89:368–74. doi: 10.1016/j.fertnstert.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Stern JE, Lieberman ES, Macaluso M, Racowsky C. Is cryopreservation of embryos a legitimate surrogate marker of embryo quality in studies of assisted reproductive technology conducted using national databases? Fertil Steril. 2012;97:890–3. doi: 10.1016/j.fertnstert.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 28.March of Dimes, PMNCH, Save the Children, WHO. Chapter 2: 15 million preterm births, priorities for action based on national, regional and global estimates. In: Howson CP, Kinney MV, Lawn JE, editors. Born too soon: the global action report on preterm birth. Geneva (Switzerland): World Health Organization; 2012. [Google Scholar]
- 29.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980–2009. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]