Abstract
Background
Hemorrhagic shock is the leading cause of potentially preventable death after traumatic injury. Hemorrhage and subsequent resuscitation may result in a dysfunctional systemic inflammatory response and multisystem organ failure, leading to delayed mortality. Clinical evidence supports improved survival and reduced morbidity when fresh blood products are used as resuscitation strategies. We hypothesized that the transfusion of fresh whole blood (FWB) attenuates systemic inflammation and reduces organ injury when compared with conventional crystalloid resuscitation after hemorrhagic shock.
Methods
Male mice underwent femoral artery cannulation and hemorrhage to a systolic blood pressure of 25 mm Hg ± 5 mm Hg. After 60 minutes, the mice were resuscitated with either FWB or lactated Ringer’s solution (LR). Mice were decannulated and killed at intervals for tissue histology, serum cytokine analysis, and vascular permeability studies. Separate groups of mice were followed for survival studies.
Results
When compared with FWB, mice resuscitated with LR required increased resuscitation fluid volume to reach goal systolic blood pressure. When compared with sham or FWB-resuscitated mice, LR resuscitation resulted in increased serum cytokine levels of macrophage inflammatory protein-1α as interleukin-6, interleukin-10, macrophage-derived chemokine, KC, and granulocyte macrophage colony stimulating factor as well as increased lung injury and pulmonary capillary permeability. No survival differences were seen between animals resuscitated with LR or FWB.
Conclusions
Resuscitation with LR results in increased systemic inflammation, vascular permeability, and lung injury after hemorrhagic shock. Resuscitation with FWB attenuates the inflammation and lung injury seen with crystalloid resuscitation. These findings suggest that resuscitation strategies using fresh blood products potentially reduce systemic inflammation and organ injury after hemorrhagic shock.
Keywords: Hemorrhagic shock, Fresh whole blood, Crystalloid, Inflammation
Hemorrhagic shock remains a leading cause of mortality after trauma, second only to severe central nervous system injuries,1 and accounts for the majority of preventable deaths after severe trauma. Improved treatment of hemorrhagic shock could alter the outcomes in up to 24,000 potentially preventable deaths annually in the United States.2 Although advancements in technology and patient care have focused on improving survival in this patient population, the optimal resuscitation strategy to treat hemorrhagic shock continues to be a controversial issue. Conventional teaching advocates the use of initial crystalloid infusion followed by packed red blood cells and additional plasma and platelets as indicated.3 New evidence from recent combat casualty care experience supports a different resuscitation strategy, termed “damage control resuscitation,” promoting the transfusion of blood products in a ratio mimicking the composition of whole blood.4
The initial development of blood component therapy was a direct result of the inefficiency and limitations of fresh whole blood (FWB) as a resuscitation strategy.5 Military and civilian demand for a large-scale system for the collection and distribution of blood products led to a strategy of fractionating blood components. This system of component separation allowed for prolonged storage and improved resource utilization.6 The risks inherent with the transfusion of stored blood products, however, have not been adequately studied. Out of necessity, military combat casualty care may use either FWB or crystalloid solutions as alternative resuscitation fluids given a limited supply of blood components. Retrospective reviews have since linked the transfusion of whole blood with improved early and late survival compared with stored component therapy.2,7 This new evidence indicates there may be a role for the administration of FWB in the setting of severe hemorrhagic shock, bringing the field of transfusion medicine full circle.
The goals of resuscitation after hemorrhagic shock are no longer limited to the restoration of circulation and reperfusion of vital organs. In addition to improving early survival, treatment strategies now need to target the events leading to late mortality. Hemorrhagic shock and subsequent resuscitation have been associated with the development of the systemic inflammatory response syndrome, a dysfunctional inflammatory response after injury.8 Excessive neutrophil activation can damage tissues, leading to acute lung injury, multiorgan failure, and delayed mortality.9 Previous research has shown that various resuscitation strategies including crystalloid, colloid, and hypertonic saline can modulate the inflammation seen after traumatic hemorrhage.10–12 Given the potential role for FWB in the treatment of hemorrhage, its impact on systemic inflammation after traumatic hemorrhage warrants investigation, We hypothesized that the transfusion of FWB would attenuate the inflammatory response when compared with conventional crystalloid resuscitation. Mitigation of the inflammatory cascade after hemorrhagic shock with resuscitation strategies incorporating FWB may decrease organ damage and ultimately improve outcomes.
MATERIALS AND METHODS
Animal Model
Male C57/BL6 mice weighing 21 g to 30 g were purchased from Harlan Laboratories (Indianapolis, IN) fed standard laboratory diet and water ad libitum and acclimated for 1 week in a climate controlled room with a 12-hour light-dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Hemorrhage Model
Mice were anesthetized with intraperitoneal pentobarbital (0.1 mg/gram body weight). After clipping and sterile preparation with povidone-iodine solution and alcohol, the femoral vessels were exposed. Femoral artery cannulation was performed using tapered polyethylene catheters connected to pressure transducers for continuous hemodynamic monitoring (Harvard Apparatus, Holliston, MA). Mice were placed on a circulating water blanket maintained at 41°C to preserve body temperature and avoid hypothermia. After an initial 10-minute period of equilibration, blood was withdrawn over 3 minutes until a systolic blood pressure (SBP) of 25 mm Hg was achieved. Mice were maintained at a SBP of 25 mm Hg ± 5 mm Hg for 60 minutes by drawing or administering shed blood volume.
Collection of FWB
Donor male mice were anesthetized with intraperitoneal pentobarbital (0.1 mg/gram body weight). Whole blood was collected via sterile cardiac puncture with needles and syringes coated with 10,000 units/mL of heparin. After gentle mixing. FWB was kept warm and administered as resuscitation volume to hemorrhaged mice.
Resuscitation
Mice were either resuscitated with FWB from donor animals or lactated Ringer’s solution (LR) to a SBP of 80 mm Hg ± 5 mm Hg. To ensure adequate resuscitation, mice were monitored for 15 minutes, decannulated and then killed at intervals. Sham animals underwent identical femoral arterial cannulation and monitoring for 90 minutes, but were neither hemorrhaged nor resuscitated. A separate group of mice were hemorrhaged but not resuscitated to serve as an unresuscitated group.
Survival
Separate groups of sham animals, unresuscitated mice, hemorrhaged animals resuscitated with FWB, and hemorrhaged animals resuscitated with LR were followed for 7-day survival. Sample size for each group was 15 mice.
Histology
To document architectural injury, the left lung was harvested 4 hours after resuscitation and immediately flushed with formalin. After fixation, tissue samples were embedded in paraffin blocks, cut, and stained with hematoxylin and eosin. Slides were read under standard light microscopy.
Cytokine Analysis
Blood was collected via cardiac puncture at intervals after resuscitation. Plasma samples were allowed to clot and were centrifuged at 6,800g to separate the serum from cellular components. Serum macrophage inflammatory protein (MIP-1α), interleukin-6 (IL-6), interleukin-10 (IL-10), macrophage-derived chemokine (MDC), keratinocyte-derived chemokine (KC), and granulocyte macrophage colony stimulating factor (GMCSF) were measured using multiplex enzyme-linked immunosorbent assay (Quansys Biosciences, Logan, UT).
Vascular Permeability
After resuscitation but before decannulation, 20 mg/kg body weight of 4% Evans Blue (Sigma, St. Louis, MO) was injected via the femoral line. After 30 minutes of circulation, animals underwent laparotomy and sternotomy A needle was inserted into the beating right ventricle, and the superior vena cava was divided sharply below the diaphragm. The intravascular space was flushed with 10-mL heparinized phosphate-buffered saline (PBS; 20 units heparin/mL PBS) at a continual rate of 3 mL/min using a perfusion pump. The left lung was harvested, washed in heparinized PBS, and placed in 100 mg/mL of formamide (Sigma, St. Louis, MO). All samples were incubated at 37°C for 7 days. Evans Blue concentration in each sample was determined by measuring the absorbance at 620 nm against a standard curve.
Statistics
Results are reported as the mean ± SEM. Two-tailed Student t test or analysis of variance with Tukey’s test was used where appropriate to determine significance. Statistical analysis was performed using SigmaPlot 10 software (Systat Software, Chicago, IL). p values ≤0.05 were determined to be significant.
RESULTS
We applied two resuscitation strategies, FWB and LR, to a pressure-controlled, murine model of hemorrhagic shock. Mean SBP was not different between animals resuscitated with FWB and LR during initial equilibration, hemorrhagic shock, or resuscitation periods. The SBP of each group of mice undergoing hemorrhage was significantly different from sham animals during the 60 minutes of hemorrhagic shock (Fig. 1). Despite equal volumes of blood loss during hemorrhage, mice treated with LR required nearly twice as much fluid to resuscitate to the target SBP when compared with animals receiving FWB (Fig. 2). No survival differences were seen between sham animals and hemorrhaged animals or between hemorrhaged animals resuscitated with FWB and LR. In contrast, mortality at 7 days was 93% in unresuscitated mice, when compared with no mortality in sham, FWB, and LR resuscitated mice (data not shown).
Figure 1.
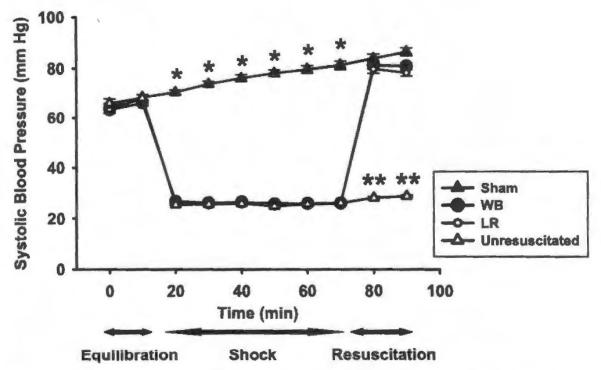
SBP of mice undergoing hemorrhagic shock and resuscitation with FWB or lactated Ringer’s solution (LR) versus sham animals or mice hemorrhaged but not resuscitated (unresuscitated). *p < 0.001 versus other groups, n = 20 mice for each group. **p < 0.001 versus other groups, n = 20.
Figure 2.
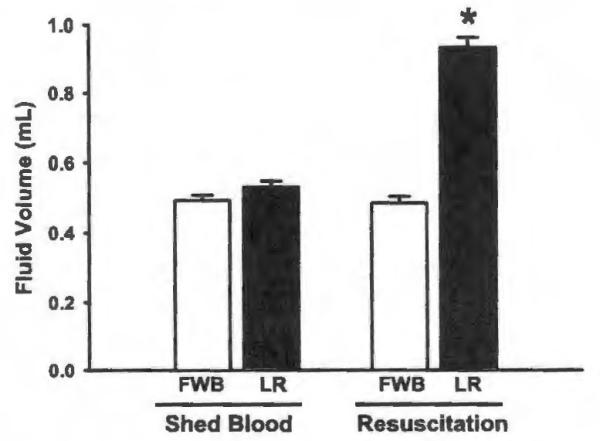
Shed blood volume and resuscitation fluid volume for mice resuscitated with FWB and LR. *p < 0.001 versus FWB, n = 40 for each group.
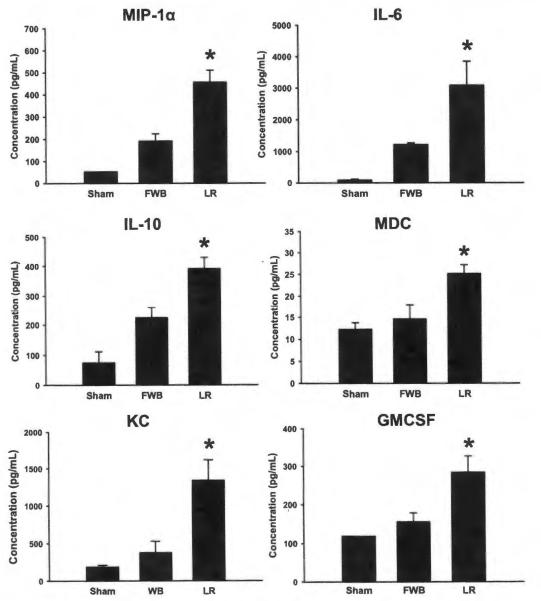
Mice resuscitated with LR exhibited increased serum levels of MIP-1α, IL-6, IL-10, MDC, KC, and GMCSF compared with sham animals and hemorrhaged animals resuscitated with FWB (Fig. 3). Compared with LR mice, mice resuscitated with FWB showed decreased serum levels of each of these cytokines and chemokines which approached the levels found in sham animals.
Figure 3.
Cytokine levels of MIP-1α, IL-6, IL-10, MDC, KC, and GMCSF in the serum of sham animals, animals resuscitated with FWB, and animals resuscitated with LR killed at 30 minutes after resuscitation. *p < 0.05 versus other groups, n = 5 for each group.
When compared with mice resuscitated with LR, unresuscitated mice exhibited decreased levels of MIP-1α (456.7 pg/mL ± 53.9 pg/mL vs. 75.9 pg/mL ± 13.3 pg/mL, LR vs. unresuscitated, p < 0.001), KC (632.2 pg/mL ± 47.3 pg/mL vs. 304.9 pg/mL ± 34.9 pg/mL, LR vs. unresuscitated, p < 0.01), and GMCSF (283.9 pg/mL ± 42.8 pg/mL vs. 136.0 pg/mL ± 17.2 pg/mL, LR vs. unresuscitated, p < 0.05) when killed 30 minutes after resuscitation. Unresuscitated mice exhibited similar levels of MDC when compared with mice resuscitated with LR and killed at 30 minutes.
Lung tissue from mice resuscitated with LR exhibited increased extravasation of Evans Blue dye, compared with both shams and mice resuscitated with FWB, consistent with increased capillary leak in this group (Fig. 4). The extravasation of Evans Blue dye into the lung tissue of animals treated with FWB after hemorrhage was not significantly different from the lung tissue of sham animals. In support of this data, lung histology in sham mice showed normal lung architecture (Fig. 5, A). Mice resuscitated with FWB showed minor thickening of the alveolar walls (Fig. 5, B), whereas mice resuscitated with LR showed marked thickening of the alveolar walls and increased numbers of inflammatory cells (Fig. 5, C).
Figure 4.
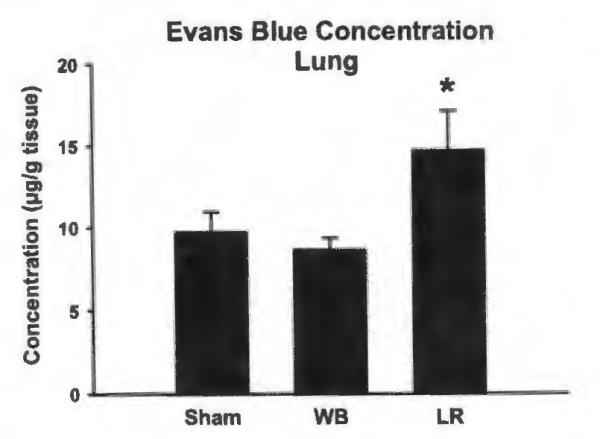
Concentration of Evans Blue in left lung samples of mice after sham procedure or hemorrhage followed by FWB or LR resuscitation. *p < 0.05 versus FWB, n = 9 for each group.
Figure 5.
Representative photomicrographs from lung tissue stained with hematoxylin and eosin and examined with light microscopy. Photomicrograph (A), (B), and (C) represent lung tissue from sham, FWB, and LR mice, respectively. Mice resuscitated with LR exhibited increased alveolar wall thickening and recruitment of inflammatory cells compared with mice resuscitated with FWB or sham animals.
DISCUSSION
In this study, we compared the effect of resuscitation with FWB or lactated Ringer’s solution on the initial inflammatory response to hemorrhagic shock. Hemorrhagic shock with tissue ischemia and subsequent reperfusion injury promotes a systemic inflammatory response, which contributes to the development of late complications. In some patients, this response initiates an influx of inflammatory cells into the lung, manifesting as acute lung injury and acute respiratory distress syndrome (ARDS), each of which confer significant morbidity and late mortality.13 Although each resuscitation strategy used in our model prevented hemorrhage-induced mortality, there were significant differences in systemic inflammation and organ injury between mice resuscitated with crystalloid and FWB. These findings support the concept that conventional fluid resuscitation may prime the inflammatory response for the development of organ injury and late complications including ARDS.
In other animal models, LR has been shown to increase systemic inflammation when compared with experimental fluid resuscitation strategies including hypertonic saline and colloids.14,15 A comparison of the effects on inflammation and lung injury between FWB and crystalloid resuscitation after hemorrhagic shock has not previously been performed, and the relationship between systemic inflammation and lung injury has not been fully evaluated. In our study, we demonstrated that resuscitation with FWB after hemorrhagic shock results in less systemic inflammation and lung injury when compared with resuscitation with crystalloid. This was evident by decreased serum evels of MIP-1α, IL-6, IL-10, MDC, KC, and GMCSF in mice receiving FWB. Each of these mediators seems to be important in pathogenesis of the inflammatory response to hemorrhagic shock.
MIP-1α plays an integral role in neutrophil recruitment in both acute and chronic inflammatory responses in the settings of sepsis, trauma, and hemorrhagic shock.16 In a model of hemorrhage, Chaudry and coworkers17 showed decreased liver and lung injury, neutrophil infiltration, and systemic inflammation in MIP-1α knockout mice compared with wild-type mice. In addition to decreased MIP-1α levels, MIP-1α knockout mice exhibited decreased systemic levels of IL-6 and IL-10, both of which were significantly reduced in mice resuscitated with FWB in our model. Deletion of MIP-1α is also associated with reduced lung inflammation in acute pancreatitis, and inhibition of MIP-1α reduces lung permeability, neutrophil recruitment, and systemic inflammation in a model of sepsis, further supporting its role in neutrophil chemotaxis and infiltration.16,18 Interestingly, in our model, MIP-1α levels were higher in LR resuscitated mice when compared other groups, suggesting an increase in systemic inflammation independent of hemorrhage and reflecting the resuscitation strategy used.
IL-6 is a pleiotropic cytokine that seems to play an important role in the systemic response to inflammation. Tweardy and coworkers19 described an attenuated inflammatory response to hemorrhage with decreased lung myeloperoxidase and organ injury in IL-6-deficient mice. Our data suggest that FWB resuscitation attenuates the systemic release of IL-6 and results in less lung injury when compared with LR resuscitated mice.
The increased expression of the anti-inflammatory cytokine, IL-10, after hemorrhage may indicate a dysfunctional inflammatory state coupled with immunosuppression. There is evidence that IL-10 protects against pulmonary neutrophil infiltration and lung injury after hemorrhage.19 However, other data suggest that elevated IL-10 levels predict worse prognosis.20,21 This may be a result of the immunosuppressive properties of IL-10, which can increase the susceptibility of postinjury infection and increase morbidity.22 The decreased serum levels of MIP-1α, IL-6, and IL-10 after resuscitation with FWB may represent an attenuated inflammatory response and may be responsible for the development of less severe organ damage. Treatment strategies which minimize the systemic levels of these three cytokines, including resuscitation with FWB, may affect clinical outcomes after hemorrhage.
GMCSF and KC (an analog of human IL-8) are important regulators of the inflammatory response through their actions on neutrophils. GMCSF has been shown to prevent neutrophil apoptosis, thereby promoting tissue accumulation of activated cells that contribute injury.23 KC is a potent activator and chemoattractant of neutrophils and functions to promote recruitment of neutrophils to sites of infection or tissue damage.24 In humans, IL-8 has been proven to be a key mediator in the development of ARDS, with increased levels found in bronchoalveolar lavage samples taken from ARDS patients.25 Our study suggests that the decreased expression of GMCSF and KC found in mice resuscitated with FWB correspond to the decreased lung inflammation and injury observed.
In summary, recent clinical data support the potential use of FWB as resuscitation fluid after severe traumatic hemorrhage. The benefits of using FWB compared with crystalloid fluid may lie in its ability to attenuate the dysfunctional inflammation that occurs after resuscitation. Control of the systemic inflammatory response syndrome after injury may ultimately reduce the development of multiorgan failure, ARDS, and late mortality in the setting of hemorrhagic shock.
Acknowledgments
Supported, in part, by awards from the United States Air Force FA8650-05-2-6518 and NIH training grant T32 GM08478.
DISCUSSION
Dr. Mitchell J. Cohen (San Francisco, California): I am incredibly honored for the privilege of serving as a discussant for this outstanding paper from Doctor Makley and colleagues.
In their work Doctor Makley and colleagues address the very interesting and indeed hot topic of whole blood, blood component, and crystalloid therapy in the resuscitation of injured patients.
They used a mouse model of hemorrhagic shock. After shock mice were resuscitated with donor whole blood of Lactated Ringers. Mouse plasma was assayed for inflammatory mediators. Histology was performed and lung vascular permeability measured.
In comparison of two resuscitation strategies LR resuscitated mice exhibited increasing inflammatory mediators. In addition, these mice had increased inflammation on histology and increased lung permeability.
This represents a very well done, very nicely written study and reflects a large amount of experimental animal model work, a huge amount of work I might add.
I commend this group on an ambitious study which I understand from similar work in our lab represents success with a very tedious animal model.
This study adapts the useful mouse model initially developed by Doctors Chaudry—and Ayala and recently utilized by our group to address a very important question.
I have several questions regarding this very nicely done study. What preservative was used to collect the mouse fresh whole blood? Do you have insight as to whether the collection technique or any preservative may affect your results?
Second, why did you target a blood pressure of 25 millimeters of mercury for 60 minutes? How did you choose this metric rather than the more commonly published 40 millimeters of mercury?
In addition, how much modulation was necessary to maintain mice at that 25 millimeter of mercury goal? Did you test other degrees or timing of hemorrhagic shock?
Third, your manuscript maintains that animals were resuscitated with LR or fresh whole blood to a mean arterial pressure of 80 millimeters of mercury which resulted in nearly two-fold volume requirement with LR to maintain an identical pressure.
Can you comment on the volume effect in producing the presented results? Have you given LR or fresh whole blood just to sham mice to see the effect of transfusion or fluid administration volume on animals independent of injury?
Alternatively, is it possible to resuscitate with a mix of fresh whole blood and LR or even aged mouse blood to achieve an independent result?
Lastly, have you added injury to this model with a mid-line laparotomy or femoral fracture to see if the results change?
Overall I compliment you on a very nicely designed and carried out experiment and a very well-written manuscript. I believe that this model holds outstanding promise for the examination of resuscitation after injury and shock.
Dr. Amy T. Makley (Cincinnati, Ohio): Thank you, Doctor Cohen, for reviewing the manuscript as well as for your insightful questions.
With regard to your first question, we collected mouse donor blood into heparinized syringes, kept it warm and then transfused it. We are currently evaluating the effects of more conventional additives and preservatives in red blood cell therapy, including CP2D and AS3. These are used by our institution’s blood bank. We do not believe that our collection technique or the presence of heparin altered our results.
Secondly, we developed a target blood pressure of 25 mmHg given the size of the mice used and their blood volumes. The amount of shed blood volume required to shock these mice to 25 mmHg correlated to a 30 to 40% estimated blood loss, representing a Class 3 or Class 4 hemorrhagic shock. We feel that this target blood pressure corresponds to a very relevant clinical model. In other experiments, we have varied our model to provide a deeper shock and longer shock in order to study a more lethal model of hemorrhage.
Regarding your question concerning the fluid volume required to resuscitate mice with Lactated Ringers solution, we have not yet given warm fresh whole blood or Lactated Ringers to our sham animals. We feel that some of the volume effects indeed may due to the volume required to resuscitate the Lactated Ringers mice, especially regarding the pulmonary capillary leak present in this group.
Finally, we have added injury to this model. We have a variation of our model in which mice undergo a laparotomy and splenectomy in addition to hemorrhage and resuscitation. Interestingly in this model the resuscitation with Lactated Ringers does result in higher mortality. The transfusion of warm, fresh whole blood in our splenectomy model reduces that mortality and confers a survival advantage.
Dr. Joseph Cushieri (Seattle, Washington): Just to expand a little bit on Doctor Cohen’s question as well as what you just mentioned, this was a significant hemorrhagic shock model where you’re reaching Class 3 to Class 4 shock with a 7 percent survival in those that aren’t resuscitated. In normal resuscitation, these patients would not be just resuscitated with crystalloid because of ongoing ischemia and potential conversation of uncompensated shock to compensated shock, but would have blood administration. Is this a potential confounder? Or are you actually looking at ongoing ischemia?
Do you have data to demonstrate that actually in addition to just normalizing blood pressure you actually improve the perfusion state so there was correction of ongoing acidosis, etc? Thanks.
Dr. Martin Schreiber (Portland, Oregon): Two quick questions from Marty Schreiber, Portland, Oregon. I have to admit, I was kind of surprised when you were comparing fresh whole blood to Lactated Ringers.
It’s not really a comparison I would think of doing. Why didn’t you use fresh whole blood and compare it to packed red blood cells or their equivalent in mice?
And, having said that, interestingly it looks like the Lactated Ringers group did preity well to me. All of the changes that you saw were pretty much present at 30 minutes and then rapidly resolved.
What’s the significance of having elevated cytokines at 30 minutes when they’re equivalent at every time point after that? Thanks.
Dr. Phil Spinella (Hartford, Connecticut): To follow up on Doctor Schreiber’s question, I would be more interested in comparing fresh whole blood to a one-to-one ratio with older red cells since that’s the clinical scenario that military physicians are faced with daily. Thank you.
Dr. Amy T. Makley (Cincinnati, Ohio): Thank you very much for those excellent questions. Regarding Dr. Cuschieri’s question, we measured parameters including lactate, ph, and base deficit in these experiments. In mice that were resuscitated with Lactated Ringers or fresh whole blood, we found no differences in these parameters, indicating we adequately able to resuscitate these mice with either resuscitation strategy. This indicated that differences seen between groups did not reflect ongoing ischemia in those mice resuscitated with crystalloid.
In regards to the next question, we currently are looking at component therapy in this hemorrhage model. We have successfully developed methods for blood component separation as well as storage, modeled after techniques used by our own blood bank. Our preliminary data indicates that resuscitation with a 1:1 ratio of fresh packed cells and plasma shows an inflammatory cytokine profile very similar to those mice that received warm fresh whole blood. However, with the transfusion of stored mouse components, as is most clinically relevant, we are finding increased systemic inflammation following hemorrhage, as compared to fresh plasma and packed red cells.
Finally, to address the question of the early inflammatory cytokine changes present at 30 minutes which then rapidly extinguish, we have consistently found that the mouse inflammatory response is very rapid. This systemic inflammatory peak is followed by profound evidence of capillary leak in the lung. Four hours after resuscitation, we found significant evidence of architectural lung injury which may be tied to that initial inflammatory response seen is so early in the mouse, indicating lasting effects.
I would like to thank the association for the privilege of the podium.
Footnotes
Presented at the 68th Annual Meeting of the American Association for the Surgery of Trauma, October 1–3, 2009, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4 Suppl):S69–S76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kortbeek JB, Al Turki SA, Ali J, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64:1638–1650. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 4.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med. 2008;36(7 Suppl):S340–S345. doi: 10.1097/CCM.0b013e31817e2ef9. [DOI] [PubMed] [Google Scholar]
- 6.Hess JR, Thomas MJ. Blood use in war and disaster: lessons from the past century. Transfusion. 2003;43:1622–1633. doi: 10.1046/j.1537-2995.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 7.Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60(6 Suppl):S59–S69. doi: 10.1097/01.ta.0000219013.64168.b2. [DOI] [PubMed] [Google Scholar]
- 8.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 9.Bahrami S, Zimmermann K, Szelenyi Z, et al. Small-volume fluid resuscitation with hypertonic saline prevents inflammation but not mortality in a rat model of hemorrhagic shock. Shock. 2006;25:283–289. doi: 10.1097/01.shk.0000208808.03148.ea. [DOI] [PubMed] [Google Scholar]
- 10.Watters JM, Tieu BH, Todd SR, et al. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61:300–308. doi: 10.1097/01.ta.0000224211.36154.44. discussion 308-309. [DOI] [PubMed] [Google Scholar]
- 11.Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243:47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patient with traumatic hemorrhagic shock. Ann Surg. 2007;245:635–641. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–L15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincenzi R, Cepeda LA, Pirani WM, Sannomyia P, Rocha-E-Silva M, Cruz RJ., Jr Small volume resuscitation with 3% hypertonic saline solution decrease inflammatory response and attenuates end organ damage after controlled hemorrhagic shock. Am J Surg. 2009;198:407–414. doi: 10.1016/j.amjsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Alam HB, Stanton K, Koustova E, Burris D, Rich N, Rhee P. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60:91–99. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Standiford TJ, Kunkel SL, Lukacs NW, et al. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- 17.Hsieh CH, Frink M, Hsieh YC, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–2812. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 18.Gerard C, Frossard JL, Bhatia M, et al. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997;100:2022–2027. doi: 10.1172/JCI119734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Distinct effects of systemic infusinn of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock. Shock. 2000;14:41–48. doi: 10.1097/00024382-200014010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Kobbe P, Schmidt J, Stoffels B, Chanthaphavong RS, Bauer AJ, Pape HC. IL-10 administration attenuates pulmonary neutrophil infiltration and alters pulmonary iNOS activation following hemorrhagic shock. Inflamm Res. 2009;58:170–174. doi: 10.1007/s00011-009-8116-z. [DOI] [PubMed] [Google Scholar]
- 21.Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42:863–870. doi: 10.1097/00005373-199705000-00017. discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 22.Miller AC, Rashid RM, Elamin EM. The “T” in trauma: the helper T-cell response and the role of inununomodulation in trauma and burn patients. J Trauma. 2007;63:1407–1417. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- 23.Fanning NF, Kell MR, Shorten GD, et al. Circulating granulocyte macrophage colony-stimulating factor in plasma of patients with the systemic inflammatory response syndrome delays neutrophil apoptosis through inhibition of spontaneous reactive oxygen species generation. Shock. 1999;11:167–174. doi: 10.1097/00024382-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 25.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]