Abstract
Background
The aim of controlling hypertension is to protect against arteriosclerosis. Calcium channel blockers (CCBs) and renin-angiotensin-aldosterone system (RAAS) inhibitors have been reported to have antihypertensive effects, but their effect on the progression of arteriosclerosis is not fully understood. The cardio-ankle vascular index (CAVI) was developed to estimate arterial stiffness, which reflects arteriosclerosis. In this study, we investigated the longer term effects of CCBs and RAAS inhibitors on the progression of arteriosclerosis by monitoring the CAVI.
Methods
Our subjects were 115 consecutive, non-smoking hypertensive patients on oral treatment with a CCB and/or RAAS inhibitor for at least 3 years in whom the CAVI was measured on two occasions approximately 1 year apart during the period from January 2009 to December 2011. Changes in CAVI were evaluated in patients administered a CCB alone (group C), an RAAS inhibitor (group R) alone, or both drugs together (group B). Changes in laboratory findings, blood pressure, and ankle-brachial index were similarly evaluated.
Results
No significant change in laboratory findings, blood pressure, or ankle-brachial index was noted in any of the groups. The CAVI decreased slightly in group R (first recording 8.80±1.03, second recording 8.57±0.97, P=0.517) and increased significantly in group C (first 8.45±0.92, second 8.95±1.04, P=0.038), but showed no significant change in group B (first 9.01±1.26, second 9.05±1.35, P=0.851).
Conclusion
Long-term administration of a CCB alone increased the CAVI, but this effect was offset by the concomitant use of a RAAS inhibitor, indicating that a RAAS inhibitor might protect against arteriosclerosis.
Keywords: cardio-ankle vascular index, renin-angiotensin-aldosterone system inhibitor, calcium channel blocker
Introduction
The cardio-ankle vascular index (CAVI) is a noninvasive measurement in which the stiffness parameter β, which reflects vascular elasticity, is calculated using pulse wave velocity. It is an index of intrinsic vascular stiffness (vascular extensibility) independent of blood pressure.1 Vascular extensibility has been reported to be involved in vascular endothelial dysfunction, which is considered to be an antecedent of arteriosclerosis.2 The CAVI increases when there is a concurrent symptomatic arteriosclerotic disorder such as angina pectoris.3 Therefore, the CAVI is useful for assessment of vascular function, including in hypertensive patients. While the mean blood pressure recorded for the Japanese population has been decreasing after a peak in approximately 1965–1990,4 it is estimated that there are still approximately 40 million Japanese people with hypertension, in whom prevention of subsequent arteriosclerotic complications is important, along with blood pressure control. Calcium channel blockers (CCBs) and renin-angiotensin-aldosterone system (RAAS) inhibitors have been reported to have both antiarteriosclerotic and antihypertensive effects, and are frequently used in Japan. The effects of short-term administration of these drugs on the CAVI have been evaluated,5 but not the effects of long-term administration. Therefore, we investigated the effects of long-term administration of these drugs alone or in combination on the CAVI.
Subjects and methods
Study subjects
The subjects were 115 consecutive, non-smoking hypertensive patients on oral treatment with a CCB and/or a RAAS inhibitor for at least 3 years in whom the CAVI was measured on two occasions approximately 1 year apart during the period from January 2009 to December 2011. Thirty-four of the patients were receiving a CCB (group C), 16 were receiving a RAAS inhibitor (group R), and 65 were receiving both a CCB and a RAAS inhibitor (group B). Their outcomes were evaluated retrospectively. The exclusion criteria were: age under 30 years or over 85 years; being on maintenance hemodialysis; a history of vascular disease, cardiovascular surgery, or vascular catheterization; an ankle-brachial index (ABI) <0.9 or >1.3; and change of medication during the study period. This retrospective study was approved by the ethics committee of our hospital (approval number 25–57). All patients involved in this study provided written informed consent before the study.
General findings
Sixty-nine of the 115 patients were receiving antihypertensive drugs other than a CCB or RAAS inhibitor and drugs to improve lipid and glucose metabolism. These concomitant medications were compared between the groups. Age, sex, and differences in systolic and diastolic blood pressure between the two assessments were also evaluated. Blood pressure was measured on three occasions after resting for at least 5 minutes in a seated position on the same day as the CAVI and ABI measurements, and the mean values were used. We also investigated body weight and recorded the body mass index (BMI, calculated as body weight [kg]/height [cm]).2 Obesity was defined as BMI ≥25 according to the Japan Society for the Study of Obesity.
Laboratory analysis
Parameters for lipid metabolism (total cholesterol, triglycerides, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, and LDL/HDL ratio [L/H]), glucose metabolism (HbA1c), and renal function (blood urea nitrogen and creatinine) were measured, and the changes between the two sets of measurements were compared between the groups. Blood tests were performed within 3 months either side of determination of the CAVI. The blood samples were collected before breakfast after resting for at least 5 minutes. The LDL cholesterol level was calculated using the Friedewald equation
CAVI and ABI
The changes in CAVI and ABI between the first and second measurements were compared between the groups. The CAVI and ABI were measured after 10 minutes resting in a supine position using the VaSera CAVI instrument (VS-1500E, Fukuda Denshi Co, Ltd, Tokyo, Japan). The patients were instructed not to eat or drink for 12 hours prior to the test. The CAVI was expressed as the mean of bilateral values. Patients with an ABI <0.9 were excluded from the study. Sinus rhythm was confirmed in all patients on a 12-lead electrocardiogram.
Statistical analysis
The data are presented as the mean ± standard deviation. The paired t-test was used for comparisons between two groups and one-way analysis of variance and Tukey–Kramer tests were used for comparisons between three groups. We used a Windows computer (Excel, Microsoft XP) and the Stat View statistical package (Stat View 4.0, SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to be statistically significant.
Results
Patient characteristics
Table 1 shows the baseline patient characteristics at the first measurement of the CAVI. There was no significant difference in duration of antihypertensive drug use between the groups. The mean age was 66.7±7.8 (42–81) years in group C, 63.8±8.1 (49–72) years in group R, and 67.6±8.5 (40–82) years in group B, with no significant difference between the groups. Males comprised 38.2%, 50.0%, and 60.0% in groups C, R, and B, respectively, with a significantly higher number in group B than in group C (P=0.039). The intervals between the two CAVI determinations were 503.3±278.3, 426.0±243.2, and 362.5±224.0 days in groups C, R, and B, respectively, being significantly longer in group C than in group B (P=0.023). No significant difference was noted with regard to concomitant medications. An L-type CCB was used in all cases, with the exception of one patient in group B who was receiving a T-type agent. In group B, five patients were receiving an angiotensin converting enzyme inhibitor, and the others were receiving an angiotensin II receptor blocker (ARB). Only four patients in group B received a thiazide during the study period. There were no significant differences in use of other oral medications, including vasoactive agents, between the groups.
Table 1.
Characteristics of study patients
| Baseline characteristics | Group R (n=16) | Group C (n=34) | Group B (n=65) |
|---|---|---|---|
| Duration of hypertension (years) | 5.72±1.74 | 5.81±1.85 | 5.95±2.02 |
| Age (years) | 63.8±8.1 | 66.7±7.8 | 67.6±8.5 |
| Sex, male/female | 8/8 | 13/21 | 39/21 |
| Laboratory intervals (days) | 419.8±215.8 | 471.6±274.4 | 356.2±212.9 |
| CAVI intervals (days) | 426.0±243.2 | 503.3±278.3 | 362.5±224.0* |
| Incidence: n (%) | |||
| Obesity | 7 (43.8) | 15 (44.1) | 26 (40.0) |
| Hyperlipidemia | 9 (56.3) | 18 (53.0) | 28 (43.1) |
| Diabetes mellitus | 3 (18.8) | 4 (11.8) | 10 (15.4) |
| Other BP-lowering drugs: n (%) | |||
| α-Blocker | 1 (6.3) | 1 (2.9) | 5 (7.7) |
| β-Blocker | 0 (0) | 3 (8.8) | 8 (12.3) |
| HMG-CoA reductase inhibitor, n (%) | 7 (43.8) | 14 (41.2) | 16 (24.6) |
| Glucose-lowering drugs, n (%) | 2 (12.5) | 2 (5.9) | 8 (12.3) |
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using one-way analysis of variance and Tukey–Kramer tests.
P<0.05 versus group C.
Abbreviations: CAVI, cardio-ankle vascular index; BP, blood pressure; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A.
Laboratory and general findings in all groups
Systolic and diastolic blood pressures were lower on the second measurement in all groups, but the differences were not significant (Table 2). Blood pressure values were not significantly different between the three groups on the first or second measurement. There was also no statistically significant difference in body weight or BMI between the first and second measurements (Table 2). No significant changes in lipid or glucose metabolism or renal function parameters were noted on blood tests (Tables 3–5).
Table 2.
Changes in general findings in all groups
| First measurement | Second measurement | P-value | |
|---|---|---|---|
| Group R | |||
| Systolic BP (mmHg) | 133.4±21.4 | 131.5±13.6 | NS |
| Diastolic BP (mmHg) | 85.8±11.9 | 82.5±8.9 | NS |
| Height (cm) | 162.7±6.5 | 162.6±6.3 | NS |
| Body weight (kg) | 62.3±9.9 | 62.0±9.7 | NS |
| BMI (kg/cm2) | 23.5±3.2 | 23.4±3.1 | NS |
| Group C | |||
| Systolic BP (mmHg) | 135.3±13.5 | 134.4±15.1 | NS |
| Diastolic BP (mmHg) | 83.6±7.8 | 81.8±10.4 | NS |
| Height (cm) | 160.3±8.5 | 160.2±8.3 | NS |
| Body weight (kg) | 61.7±6.6 | 61.5±6.3 | NS |
| BMI (kg/cm2) | 24.1±2.6 | 24.1±2.5 | NS |
| Group B | |||
| Systolic BP (mmHg) | 134.6±18.4 | 133.0±14.6 | NS |
| Diastolic BP (mmHg) | 82.5±9.7 | 81.7±9.1 | NS |
| Height (cm) | 160.7±7.6 | 161.2±7.5 | NS |
| Body weight (kg) | 62.6±8.8 | 62.5±8.6 | NS |
| BMI (kg/cm2) | 24.2±2.2 | 24.0±2.4 | NS |
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using the paired t-test.
Abbreviations: BP, blood pressure; BMI, body mass index; NS, not statistically significant.
Table 3.
Changes in laboratory findings in group R
| Group R | First measurement | Second measurement | P-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 201.8±23.6 | 200.8±22.0 | NS |
| HDL cholesterol (mg/dL) | 59.3±15.9 | 60.0±15.4 | NS |
| Triglycerides (mg/dL) | 171.0±87.0 | 179.6±105.1 | NS |
| LDL cholesterol (mg/dL) | 122.3±11.7 | 118.3±14.0 | NS |
| L/H | 2.16±0.81 | 2.06±0.68 | NS |
| Blood sugar (mg/dL) | 102.3±16.1 | 126.2±60.2 | NS |
| HbA1c (%) | 6.10±0.77 | 6.43±2.41 | NS |
| BUN (mg/dL) | 13.4±4.3 | 13.5±4.1 | NS |
| Creatinine (mg/dL) | 0.72±0.16 | 0.70±0.18 | NS |
| Estimated GFR | 75.9±15.7 | 79.0±19.7 | NS |
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using the paired t-test.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; L/H, LDL/HDL ratio; HbA1c, glycated hemoglobin; BUN, blood urea nitrogen; GFR, glomerular filtration rate; NS, not statistically significant.
Table 4.
Changes in laboratory findings in group C
| Group C | First measurement | Second measurement | P-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 201.9±29.3 | 197.1±30.9 | NS |
| HDL cholesterol (mg/dL) | 64.6±16.2 | 66.6±21.2 | NS |
| Triglycerides (mg/dL) | 140.5±66.6 | 132.6±74.6 | NS |
| LDL cholesterol (mg/dL) | 118.4±26.4 | 111.5±24.6 | NS |
| L/H | 1.96±0.70 | 1.86±0.72 | NS |
| Blood sugar (mg/dL) | 120.7±39.9 | 124.6±38.9 | NS |
| HbA1c (%) | 5.57±0.63 | 5.62±0.73 | NS |
| BUN (mg/dL) | 14.9±4.3 | 15.4±3.8 | NS |
| Creatinine (mg/dL) | 0.68±0.16 | 0.71±0.18 | NS |
| Estimated GFR | 77.1±15.7 | 73.9±16.5 | NS |
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using the paired t-test.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; L/H, LDL/HDL ratio; HbA1c, glycated hemoglobin; BUN, blood urea nitrogen; GFR, glomerular filtration rate; NS, not statistically significant.
Table 5.
Changes in laboratory findings in group B
| Group B | First measurement | Second measurement | P-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 196.2±30.2 | 198.0±34.7 | NS |
| HDL cholesterol (mg/dL) | 59.6±16.7 | 62.6±17.7 | NS |
| Triglycerides (mg/dL) | 139.7±58.4 | 134.5±56.0 | NS |
| LDL cholesterol (mg/dL) | 116.6±24.0 | 118.9±26.1 | NS |
| L/H | 2.03±0.62 | 1.95±0.62 | NS |
| Blood sugar (mg/dL) | 126.8±43.8 | 123.4±38.5 | NS |
| HbA1c (%) | 5.79±0.65 | 5.72±0.68 | NS |
| BUN (mg/dL) | 15.3±3.6 | 16.0±3.6 | NS |
| Creatinine (mg/dL) | 0.79±0.17 | 0.80±0.17 | NS |
| Estimated GFR | 69.4±14.1 | 68.9±14.8 | NS |
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using the paired t-test.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; L/H, LDL/HDL ratio; HbA1c, glycated hemoglobin; BUN, blood urea nitrogen; GFR, glomerular filtration rate; NS, not statistically significant.
CAVI and ABI in all groups
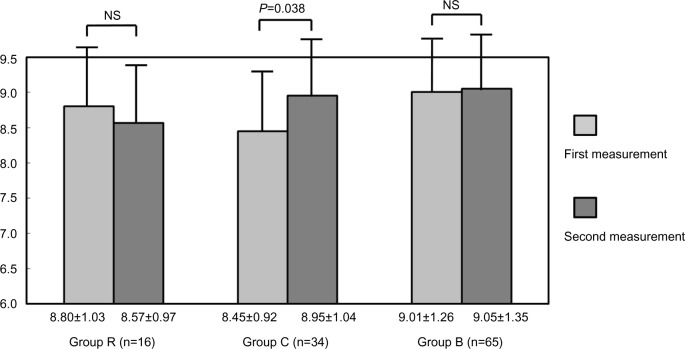
The ABI was ≥0.9 in all subjects during the study period. No significant change in ABI was noted in any group. In group C, the ABI (right) was 1.13±0.07 on the first recording and 1.13±0.08 on the second, and ABI (left) was 1.12±0.07 and 1.12±0.07, respectively; in group R, the ABI (right) was 1.16±0.08 on the first recording and 1.13±0.08 on the second, and ABI (left) was 1.14±0.07 and 1.10±0.07, respectively; and in group B, the ABI (right) was 1.11±0.08 on the first recording and 1.12±0.08 on the second, and ABI (left) was 1.12±0.07 and 1.12±0.07, respectively. The CAVI at the first measurement was not significantly different between the groups; at the second measurement, there was no significant change in CAVI in group R or group B, but there was a significant increase in group C (8.80±1.03 [first] and 8.57±0.97 [second] in group R; 9.01±1.26 and 9.05±1.35, respectively, in group B, and 8.45±0.92 and 8.95±1.04, respectively, in group C; P=0.038), as shown in Figure 1.
Figure 1.
In groups R and B, there were no significant changes in CAVI between the first and second measurements. However, the second CAVI measurement was significantly higher than the first in group C.
Notes: Continuous data are expressed as the mean ± standard deviation. P-values were determined using the paired t-test.
Abbreviations: CAVI, cardio-ankle vascular index; NS, not statistically significant.
Discussion
CAVI and blood pressure
Blood pressure at the initial measurement was in the 130–139 mmHg range in most subjects and fulfilled the criterion of the guidelines for the management of hypertension published by the Japanese Society of Hypertension.7 The LDL cholesterol level was in the range of 100–199 mg/dL, and the L/H ratio was approximately 2, suggesting adequate control based on the guidelines for prevention of atherosclerotic cardiovascular diseases published by the Japan Atherosclerosis Society.8 Also, while some patients were diabetic, HbA1cwas approximately 6, which is deemed to be “good” control according to evidence-based practice guidelines for the treatment of diabetes in Japan.9 Therefore, our study population could be considered to have relatively satisfactory control with regard to lifestyle-related diseases, including hypertension. This state remained unchanged on the second examination, so this study is considered to have evaluated changes in arteriosclerotic parameters in patients with relatively well-controlled lifestyle-related diseases treated with a CCB, RAAS inhibitor, or both over a long period. A previous study of the effects of a CCB and an ARB showed a fall in CAVI due to a decrease in oxidative stress.5 In the present study, however, blood pressure decreased significantly after administration of both drugs, and their effects cannot be excluded. Blood pressure values remained virtually unchanged during treatment with antihypertensive medication, and it is considered important that the effects of blood pressure changes could be eliminated.
CAVI and sympathetic nerve activity
CAVI was the only parameter examined that showed a significant change between the first and second measurements. While the CAVI showed a significant increase in group C, it decreased, albeit not significantly, in group R, and remained nearly unchanged with only a slight elevation in group B. CCBs are antihypertensive drugs that are widely used in Japan because of their consistent antihypertensive effect and reasonable price. However, they have been reported to cause reflex sympathetic hyperactivity induced by a rapid decrease in blood pressure due to vasodilation, and this action is more marked with short-acting CCBs.10 Myocardial β1 receptors are stimulated by noradrenaline released from sympathetic nerve terminals, causing an increase in the force of cardiac contraction and reflex tachycardia. Therefore, short-acting CCBs increase cardiovascular events in patients with ischemic heart disease and cause a dose-dependent increase in total mortality.11,12 Moreover, sympathetic hyperactivity activates the RAAS, and RAAS inhibitors are considered to suppress the enhanced sympathetic activity. Compound drugs of an ARB and a CCB are considered to be useful from this viewpoint.13 The Valsartan Amlodipine Randomized Trial (VART) study indicated the usefulness of ARB/CCB combination therapy and ARBs were shown to suppress myocardial sympathetic activity by 123I-metaiodobenzylguanidine scintigraphy.14 The Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) and OlmeSartan and Calcium Antagonists Randomized (OSCAR) studies also demonstrated the effectiveness of ARB/CCB combination therapy,15,16 and the Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial showed the usefulness of combination therapy comprising an angiotensin-converting enzyme inhibitor and a CCB.17 Since the present study was retrospective, we did not evaluate the effects of the drugs on sympathetic activity, but this difference in sympathetic activity is considered to have induced the change in the CAVI.
Study limitations
This retrospective study has some limitations. The reference value of the CAVI increases with age by approximately 0.05 per year,18 and has also been reported to be approximately 0.5 higher in males than in females.18 Age and sex have slight differences between the three groups, but these differences were not significant. These slight differences are considered to have been eliminated because the present study was longitudinal rather than cross-sectional. However, the interval between the two CAVI measurements was significantly longer in group C than in group B. A difference of approximately 140 days corresponds to a difference of approximately 0.02 in the CAVI. Since the difference observed in group C was approximately 0.5, this effect is considered negligible, but is still a study limitation. Also, some CCBs used clinically today have been reported not to enhance sympathetic activity.19,20 Differences in duration of action of CCBs have also been reported.21 In the present study, the effect of any difference in duration of action could not be compared between the drugs due to the limited size of the patient population. However, many of the CCBs administered in the present study were L-type, and there may have been differences in the effect of L-type compared with N-type or T-type CCBs. A further limitation of our present study was its small sample size, with only 16 patients in group R. Therefore, in order to clarify the effect of long-term administration of RAAS inhibitors and CCBs, large-scale clinical prospective studies are required.
Conclusion
This small-scale study using the CAVI demonstrated that concomitant use of RAAS inhibitors suppresses the progression of arteriosclerosis on long-term administration of CCBs. Sympathetic activity is considered to be involved in the mechanism of this suppression, but large-scale clinical studies are necessary to confirm this.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13:101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 2.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72:598–604. doi: 10.1253/circj.72.598. [DOI] [PubMed] [Google Scholar]
- 4.Ueshima H. Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb. 2007;14:278–286. doi: 10.5551/jat.e529. [DOI] [PubMed] [Google Scholar]
- 5.Miyashita Y, Saiki A, Endo K, et al. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on cardio-ankle vascular index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb. 2009;16:621–626. doi: 10.5551/jat.497. [DOI] [PubMed] [Google Scholar]
- 6.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 7.Ogihara T, Kikuchi K, Matsuoka H, et al. Japanese Society of Hypertension Committee The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 8.Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases. J Atheroscler Thromb. 2014;21(4):296–298. 2012. [PubMed] [Google Scholar]
- 9.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 11.Alderman MH, Cohen H, Roqué R, Madhavan S. Effect of long-acting and short-acting calcium antagonists on cardiovascular outcomes in hypertensive patients. Lancet. 1997;349:594–598. doi: 10.1016/S0140-6736(96)08359-6. [DOI] [PubMed] [Google Scholar]
- 12.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 13.Sever PS, Messerli FH. Hypertension management 2011: optimal combination therapy. Eur Heart J. 2011;32:2499–2506. doi: 10.1093/eurheartj/ehr177. [DOI] [PubMed] [Google Scholar]
- 14.Narumi H, Takano H, Shindo S, et al. Effects of valsartan and amlodipine on cardiorenal protection in Japanese hypertensive patients: the Valsartan Amlodipine Randomized Trial. Hypertens Res. 2011;34:62–69. doi: 10.1038/hr.2010.186. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa H, Kim-Mitsuyama S, Jinnouchi T, Matsui K, Arakawa K. Rationale, design and patient baseline characteristics of OlmeSartan and calcium antagonists randomized (OSCAR) study: a study comparing the incidence of cardiovascular events between high-dose angiotensin II receptor blocker (ARB) monotherapy and combination therapy of ARB with calcium channel blocker in Japanese elderly high-risk hypertensive patients (ClinicalTrials. gov no. NCT00134160) Hypertens Res. 2009;32:575–580. doi: 10.1038/hr.2009.60. [DOI] [PubMed] [Google Scholar]
- 16.Ogihara T, Matsuzaki M, Umemoto S, et al. Combination Therapy of Hypertension to Prevent Cardiovascular Events Trial Group Combination therapy for hypertension in the elderly: a sub-analysis of the Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) Trial. Hypertens Res. 2012;35:441–448. doi: 10.1038/hr.2011.216. [DOI] [PubMed] [Google Scholar]
- 17.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Ishizuka N, Miyashita H, Shirai K. For standardization of the cardio-ankle vascular index (CAVI) as a noninvasive, blood pressure-independent test of atherosclerosis epidemiological study on reference values and their validity. Niigata J Med Technol. 2008;48:2–9. Japanese. [Google Scholar]
- 19.Takano Y, Ueyama T, Ishikura F. Azelnidipine, unique calcium channel blocker could prevent stress-induced cardiac dysfunction like α · β blocker. J Cardiol. 2012;60:18–22. doi: 10.1016/j.jjcc.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Kishi T, Hirooka Y, Konno S, Sunagawa K. Cilnidipine inhibits the sympathetic nerve activity and improves baroreflex sensitivity in patients with hypertension. Clin Exp Hypertens. 2009;31:241–249. doi: 10.1080/10641960902822492. [DOI] [PubMed] [Google Scholar]
- 21.Kuramoto K, Ichikawa S, Hirai A, Kanada S, Nakachi T, Ogihara T. Azelnidipine and amlodipine: a comparison of their pharmacokinetics and effects on ambulatory blood pressure. Hypertens Res. 2003;26:201–208. doi: 10.1291/hypres.26.201. [DOI] [PubMed] [Google Scholar]