Abstract
Introduction
Priapism is a condition involving prolonged penile erection unrelated to sexual interest or desire. The ischemic type, including its recurrent variant, is often associated with both physical and psychological complications. As such, management is of critical importance. Ideal therapies for recurrent priapism should address its underlying pathophysiology.
Aim
To review the available literature on priapism management approaches particularly related to nitrergic mechanisms.
Methods
A literature review of the pathophysiology and management of priapism was performed using PubMed.
Main Outcome Measure
Publications pertaining to mechanisms of the molecular pathophysiology of priapism.
Results
Nitrergic mechanisms are characterized as major players in the molecular pathophysiology of priapism. PDE5 inhibitors represent an available therapeutic option with demonstrated ability in attenuating these underlying nitrergic derangements. Several additional signaling pathways have been found to play a role in the molecular pathophysiology of priapism and have also been associated with these nitrergic mechanisms.
Conclusion
An increasing understanding of the molecular pathophysiology of priapism has led to the discovery of new potential targets. Several mechanism-based therapeutic approaches may become available in the future.
Introduction
Priapism is a pathologic condition involving penile erection persisting beyond or in the absence of sexual arousal or desire [1]. Estimates of the incidence rates of this disorder among the general population have widely ranged between 0.34 and 5.34 per 100,000 men per year, with the higher rates observed in patients aged 40 and older [2–4]. Priapism, specifically the ischemic type, has been observed to disproportionately affect certain populations, notably patients with sickle cell disease (SCD), in whom prevalence rates as high as 40% have been observed [5, 6]. SCD patients are at particular risk of experiencing repeated yet self-limited episodes, termed recurrent ischemic priapism (RIP) or stuttering priapism [1]. Despite the transitory nature of these episodes, which typically last less than 3 hours in duration, RIP may herald major ischemic episodes in 30–50% of cases [1, 5–7].
Ischemic priapism is associated with severe complications such as erectile dysfunction (ED) resulting from erectile tissue ischemic damage, particularly after episodes lasting greater than 36 hours [5, 6, 8–10]. Although shorter in duration, RIP episodes have also been associated with a significant risk of ED, with rates ranging from 29–48% [5–7]. Therefore, the management of recurrent episodes is essential in order to prevent or at least reduce possible cavernosal tissue damage and the risk of progression to major ischemic episodes. Here, we review emerging molecular mechanisms, specifically relating to the nitrergic pathway, which can be expected to guide current and future therapeutic options for the management of recurrent priapism.
Normal Erection Physiology
Penile flaccidity is controlled by vasoconstrictive factors which maintain vascular and smooth muscle tone in the basal state [11, 12]. Inhibition of this contractile state, resulting in erection, can occur with genital stimulation, psychosocial excitement, or rapid-eye movement sleep [13]. During erection, smooth muscle relaxation occurs and permits increased arterial blood inflow and expansion of erectile tissues which decreases venous outflow and sustains penile engorgement [14, 15]. The nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway is now recognized to be the critical component in the complex coordination of vasorelaxant and vasoconstrictive mechanisms involved in normal erection physiology [16, 17].
The NO synthase (NOS) enzyme is the principal mediator of NO synthesis and regulates the vascular and neurogenic pathways involved in penile erection. The initiation and maintenance phases of penile erection are regulated by neuronal NOS (nNOS) and endothelial NOS (eNOS), constitutive enzyme isoforms found in nerve terminals and vascular endothelium, respectively [18]. Phosphorylation of these NOS isoforms results in their activation, whereby NO is generated from the substrate L-arginine [19]. Subsequently, NO locally diffuses into smooth muscle cells and binds to the iron substrate within the heme moiety of guanylate cyclase (GC) [20]. Upon activation, GC converts guanosine-5′-triphosphate (GTP) to cGMP, regulating the downstream activation of cGMP-dependent protein kinase G (PKG) that generates cavernosal smooth muscle relaxation and thus penile erection [20]. Termination of the erectile response occurs through the enzymatic activity of cGMP-specific type 5 phosphodiesterase (PDE5), which hydrolyzes the 3′5′ bonds of cGMP, converting it to its inactive state 5′-GMP [21].
Nitrergic Mechanism of Recurrent Priapism
Over 95% of priapism presentations are caused by the ischemic priapism type and are hallmarked by stagnant cavernous blood flow, corporal rigidity, and pain [1, 10]. The repetitive and self-remitting episodes of RIP, an ischemic variant, typically last less than 3 hours in duration [10, 22]. As RIP is found to be significantly prevalent among patients with hematologic disorders, particularly SCD, erythrocyte sludging and vascular stasis were described classically to constitute the primary etiology [9]. Advances in the field have increasingly uncovered more complex molecular mechanisms underlying RIP. Recent investigations have identified decreased NO bioavailability, common in the setting of hematologic disorders such as SCD [23], to be a key factor in the etiology of priapism [24].
Disruption of the nitric oxide (NO) signal transduction pathway, the main erection mediatory system regulating penile erection, has recently been identified to be the principal mechanism underlying the pathophysiology of priapism [24]. Transcriptionally down-regulated PDE5 expression and activity consequent to basally decreased levels of its regulator, cGMP, is the fundamental determinant; without PDE5 function, erections are uncontrolled [24]. Several possibilities exist for yielding functionally decreased cGMP, which is related to a chronic decrease in upstream production of endothelium-derived NO. Vasculopathic damage associated with SCD may lead to a quantitative loss of eNOS, a source of decreased NO bioavailability [24–27]. Through the release of free hemoglobin, an avid scavenger of intravascular NO, hemolysis may also contribute to reducing NO bioavailability. The release of arginase, which reduces L-arginine (a substrate for NO synthesis), and excess production of reactive oxygen species (ROS) (a chronically present state in SCD) may also interfere with the generation and function of endothelium-derived NO [28–30]. The decrease in cGMP production resulting from reduced NO bioavailability leads to a compensatory decrease in cGMP-dependent expression and activity of PDE5 [24, 31].
Although deficiency of endothelium-derived NO reduces basal levels of cGMP, the neuronal source of NO, nNOS, remains intact. Thus, upon neurologic initiation of penile erection (i.e., occurring during REM sleep or sexual activity), neuronally-derived NO can transiently drive cGMP production and accumulation, producing cavernosal tissue relaxation. Because of decreased basal function of PDE5, normal regulation of the erection does not occur, resulting in priapism.
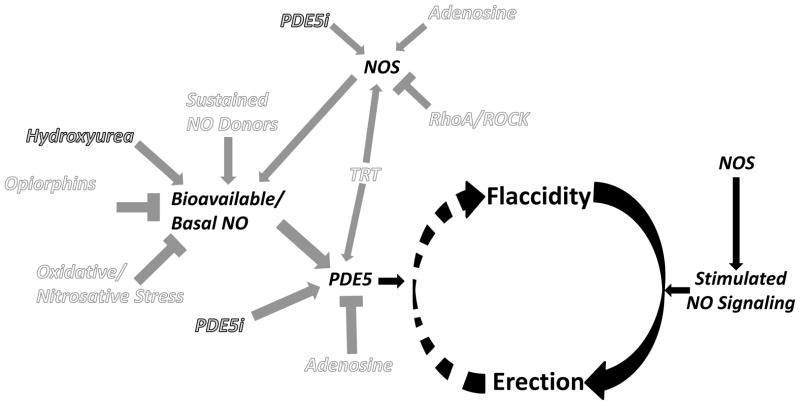
Nitrergic Mechanisms of Management (Figure 1)
Figure 1.
Schematic representation of the nitrergic mechanism underlying penile erection and contributing pathways which may offer targets for management of recurrent ischemic priapism. Decreased basal levels of PDE5 enzyme permits uncontrolled erection (priapism) because of the lack of the normal regulatory control mechanism involved in the return of the penis back to its flaccid state. Gray text represents current therapeutic approaches related to the nitrergic mechanism. White text represents potential pathways for future therapeutic approaches affecting the nitrergic mechanism. Circular arrows signify the pathway between penile erection states. Black arrows relate to the mechanism of penile erection. Gray arrows signify direct or indirect stimulation and gray T-shapes signify direct or indirect inhibition based on evidence suggesting associations of pathways in the setting of priapism. NO = nitric oxide, NOS = nitric oxide synthase, PDE5 = phosphodiesterase type 5, PDE5i = PDE5 inhibitor, TRT = testosterone replacement therapy
The physical and psychosocial complications of recurrent priapism can be devastating. Therefore, episode management is critically important. Although several therapeutic options have been proposed, they typically fail to address the underlying molecular mechanism and often lack sufficient evidence of efficacy [13, 32, 33]. Sympathomimetic agents delivered orally or intracavernosally serve to treat rather than truly prevent RIP episodes. Androgen ablation non-specifically restrains the erectile response, and in addition to adversely affecting the normal sexual response, it carries other potential side effects that render this therapeutic approach problematic [32, 34–42]. In fact, anti-androgen therapy may exacerbate the risk of priapism by suppressing androgen-dependent molecular signaling pathway (i.e., NOS and PDE5) function [43–46]. Therefore, elucidation of the aberrant mechanisms of the nitrergic pathway in the penis provides an opportunity for developing current and future preventative approaches based on the pathophysiologic mechanism of RIP.
Current Therapies
PDE5 Inhibitors
Regimented PDE5 inhibitor therapy represents a mechanistically-sound approach for managing recurrent priapism, particularly in the setting of SCD. A recent investigation demonstrated that in a transgenic SCD mouse model, PDE5 inhibitors delivered continually decreased priapic episodes and restored PDE5 gene activity [47]. Preclinical studies have also suggested that continually delivered PDE5 inhibitors upregulate protein expression of PDE 5, reverse the dysfunctional state of eNOS uncoupling, and enhance activation (phosphorylation) of eNOS [47–49]. Based on the widely publicized erectogenic properties of these agents, this therapeutic effect appears paradoxical. However, the proposed mechanism is a modulatory process involving restoration of the deficient basal levels of cGMP in the penis and feedback upregulation of PDE5 expression and activity towards more normal levels [50]. Therefore, regular use of PDE5 inhibitor therapy may reverse the aberrant NO signaling pathway identified in priapism.
The preventative role of regimented PDE5 inhibitor therapy for RIP was further investigated at a clinical level. In 2 separate observational studies, Burnett et al. reported that 4 of 4 and 6 of 7 patients, respectively, had successful improvement or resolution of their RIP episodes while receiving long-term treatment with daily sildenafil 25 mg with an optional increase to 50 mg or conversion to tadalfil 5 or 10 mg every other day and unassociated with sexual activity [50, 51]. Similar success was observed in individual case reports. A 19 year old man with RIP experienced a significant reduction in episode frequency and maintenance of erectile function following 2 months of PDE5 inhibitor treatment and 6 months after its cessation [52]. A 64 year-old man who poorly tolerated chronic anti-androgen therapy for RIP was successfully transitioned to continual PDE5 inhibitors following ineffective attempts at weaning the anti-androgen therapy [53].
The safety and efficacy of sildenafil for RIP prevention was further investigated in a randomized, controlled trial. Burnett et al. randomized 13 SCD patients with frequent RIP episodes (≥2 per week) to receive sildenafil 50 mg daily or placebo for 8 weeks followed by open-label sildenafil for a subsequent 8 weeks. Although they did not find a 50% reduction in priapism episode frequency as a primary study endpoint during the placebo-controlled phase, they found that over 60% of patients had such a reduction during the open-label phase. No differences were found between the 2 study groups regarding the occurrence of significant adverse effects, which differs from the vaso-occlusive crisis events and hospitalizations that were previously observed among SCD patients treated with sildenafil for pulmonary hypertension during the walk-PHaSST trial [54, 55]. However, the dose and frequency of sildenafil used in the walk-PHaSST trial were significantly higher (20–80 mg, 3 times daily) [55]. While these clinical studies suggest safety and utility in the use of regimented PDE5 inhibitor therapy, further assessments of efficacy are warranted.
Hydroxyurea
Hydroxyurea, the only FDA-approved agent for management of SCD, is a DNA synthesis inhibitor that induces fetal hemoglobin production and reduces hemolysis. Hydroxyurea therapy has demonstrated significant clinical benefits in decreasing vaso-occlusive crises and prolonging life in SCD patients [56, 57]. In addition to these clinical benefits, several case reports have suggested a benefit of hydroxyurea in preventing RIP episodes and preserving erectile function in the setting of SCD [58–60]. The reduction of hemolysis associated with hydroxyurea therapy may correct the underlying reduced NO bioavailability that occurs with severe hemolysis and is common to SCD [61]. This correction in NO bioavailability may also fit with the action of hydroxyurea as an NO donor. Accordingly, by restoring bioavailable NO, hydroxyurea may reverse aberrant downstream nitrergic signaling pathways [62, 63].
Future Directions
Sustained NO Donors
Complications of SCD such as vaso-occlusive crises and priapism are thought to be related to the underlying reduced NO bioavailability chronically present in SCD [23, 64, 65]. As such, restoration of bioavailable NO towards normal levels is believed to attenuate the frequency and severity of these complications. Thus, physiologic NO administration represents an intriguing area of study. The concept of NO donor use for the treatment of pathologic disease states of the penis is not new. Intracavernosal use of donors such as linsidomine chlorhydrate (SIN-1) and sodium nitroprusside has been considered specifically in the treatment of ED [66, 67]. However, this route of delivery to achieve long-term NO maintenance for priapism prevention is impractical.
In a preclinical study, Lagoda et al. demonstrated the capability of a formulated, sustained NO-releasing compound, 1, 5-Bis-(dihexyl-N-nitrosoamino)-2, 4-dinitrobenzene (C6′), in generating NO, increasing cGMP production, reversing PDE5 dysfunction, and correcting the priapism phenotype observed in SCD as well as combined nNOS and eNOS deficient mouse models of RIP [68]. This investigation supports the role of long-term NO maintenance in the management of RIP. Because bioactivation of nitrate is a source of NO (through reduction to nitrite), dietary sources of inorganic nitrate supplementation which sustainably elevate bioactive NO levels, may be worthy of investigation for RIP prevention [69, 70].
Associated Pathways
Although aberrant nitrergic signaling has been identified as a principal mechanism of RIP, several alternate molecular signaling pathways have also been suggested to have pathophysiologic roles in the priapism phenotype. Interestingly, these pathways have been linked to the derangement of the nitrergic mechanism in the penis [43].
Testosterone
Traditional thought holds that elevated testosterone levels facilitate priapism, in line with studies describing androgen ablation as a therapy for priapism [35, 40] and case reports that, conversely, have associated priapism episodes with testosterone replacement therapy (TRT) in hypogonadal males [71–73]. It is noteworthy that the latter reports document the use of intramuscular testosterone esters that result in supra-physiologic levels of testosterone. In an assessment of safety data from 3 clinical trial, no association was found between the occurrence of priapism episodes and TRT in hypogonadal men using the topical TRT agent, Androgel, at eugonadal levels [74]. Patients with SCD, who are at a higher risk of developing RIP, have not been demonstrated to have elevated testosterone levels, and, to the contrary, these patients have been found to have a high prevalence of hypogonadism [75]. Hypogonadism is theorized to further exacerbate the already aberrant molecular signaling of nitrergic mechanisms in SCD-associated RIP [43, 76]. Administration of TRT in these patients has actually been found to reduce, rather than enhance, priapism episode frequency, while improving sexual function [76]. Testosterone administration is reported to reduce priapic activity in transgenic SCD mice with low serum testosterone levels in preliminary investigations [Burnett, unpublished study]. These effects would seem consistent with the molecular actions of androgens, which are known to regulate NOS and PDE5 expression, as their levels are decreased in testosterone deficiency and restored with TRT [45, 46]. Contrary to traditional perceptions, preservation of normative testosterone levels may not cause RIP and rather may equate with an erection homeostatic purpose in the future. It is conceivable that current hormonal modulation involving anti-androgen therapy exerts a non-specific anti-erectile effect in priapism prevention.
Opiorphins
Opiorphin, a pentapeptide that inhibits neutral endopeptidases, is recognized to be a potential effector of cavernosal smooth muscle function, specifically the excessive relaxation characteristic of priapism [77]. Preclinical investigations involving gene transfer of opiorphin homologues have shown priapism-like conditions at high doses in rat models [77–79]. Inhibition of ornithine decarboxylase (ODC), a polyamine synthesis pathway enzyme found to be upregulated in these models of experimental priapism, has been found to prevent this priapic activity, suggesting its role as an effector [79]. Decreased gene expression of eNOS and PDE5 and increased arginase 1 and 2 (polyamine synthesis pathway enzymes) in penile tissues have been identified in these models and confirmed in transgenic SCD mice [79]. The upregulation of ODC and decreased arginase 1 and 2 expression may promote the shunting of L-arginine, necessary for NO synthesis, from the nitrergic pathway to the polyamine synthesis pathway [32]. Therefore, the opiorphin signaling pathway may represent a target for future management, and its therapeutic regulation may restore NO bioavailability.
Oxidative/Nitrosative Stress
Under normal physiologic conditions, homeostatic balance of oxidative/nitrosative stress is maintained through the action of enzymes and scavengers of ROS and reactive nitrogen species (RNS) [30, 80]. However, in states of chronic oxidative/nitrosative stress, such as in SCD, these protective mechanisms fail, and consequently, this equilibrium is lost [30]. Increased sources and markers of oxidative stress have been found in penile tissue of transgenic SCD mice and humans with RIP [81–83], and ROS/RNS generation has been shown to contribute to the reperfusion injury-related cavernosal tissue damage occurring during resolution of ischemic priapism [84]. These reactive species have been found to react with NO, decreasing NO bioavailability. Thus, controlling ROS/RNS may offer an approach for modulating derangement in the nitrergic signaling pathway [30].
Adenosine
Adenosine is a signaling nucleoside that functions through specific G protein-couple receptors to achieve various effects [85]. One such receptor, ADORA2B, is found to induce cAMP and subsequent relaxation, specifically in corporal smooth muscle [86–88]. Recent investigations suggest that excessive adenosine signaling contributes to priapism, as mouse models deficient in adenosine deaminase (ADA), the enzyme responsible for adenosine metabolism, demonstrate a priapism phenotype which is reversed with administration of the deficient enzyme [88, 89]. The role of adenosine as it relates to the nitrergic pathway is complex. Activation of the ADORA2B receptor functions to increase activation of eNOS (thus increase NO production) [90], but it can also down-regulate PDE5 gene expression under hypoxic conditions [91]. Targeting the adenosine receptor may provide another potential approach for RIP prevention.
RhoA/Rho Kinase
Mediation of penile vasoconstriction (flaccidity) involves the RhoA/Rho kinase (ROCK) signal transduction pathway [92, 93]. Dysregulation in Rho signaling is thought to contribute to priapism, as eNOS knockout and transgenic SCD mice, animal models of priapism, demonstrate decreased penile RhoA/ROCK activity [25, 94]. The decreased expression of these signaling molecules has also been confirmed in penes of patients with SCD [82]. Because this signaling pathway is known to regulate eNOS, and thus influence nitrergic mechanisms, it may represent a potential target for developing future therapeutic strategies for RIP [92, 95, 96].
Conclusion
Advances in our understanding of the molecular pathophysiology of RIP have reduced the scientific mystery of this erectile disorder. Consequently, we are better able to consider and develop therapeutic options that are rationally designed with prevention in mind beyond traditional management approaches that serve only to treat priapism episodes. New directions are focused towards addressing derangements in the nitrergic signaling pathway. Regimented PDE5 inhibitor therapy represents a currently available and mechanistically sound approach for RIP prevention. Future therapeutic options may also rest on the correction of bioavailable NO levels in the penis. Finally, we must acknowledge the complex interplay between nitrergic and other pathways contributing to the molecular pathophysiology RIP and consider that they may provide alternative targets for management approaches in the future.
Acknowledgments
This work was supported by NIH/NIDDK grant R01 DK093917 (to A.L.B.).
Footnotes
Conflict of Interest: None.
References
- 1.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Nehra A, Sharlip ID. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.Kulmala RV, Lehtonen TA, Tammela TL. Priapism, its incidence and seasonal distribution in Finland. Scand J Urol Nephrol. 1995;29:93–6. doi: 10.3109/00365599509180545. [DOI] [PubMed] [Google Scholar]
- 3.Eland IA, van der Lei J, Stricker BH, Sturkenboom MJ. Incidence of priapism in the general population. Urology. 2001;57:970–2. doi: 10.1016/s0090-4295(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 4.Roghmann F, Becker A, Sammon JD, Ouerghi M, Sun M, Sukumar S, Djahangirian O, Zorn KC, Ghani KR, Gandaglia G, Menon M, Karakiewicz P, Noldus J, Trinh QD. Incidence of priapism in emergency departments in the United States. J Urol. 2013;190:1275–80. doi: 10.1016/j.juro.2013.03.118. [DOI] [PubMed] [Google Scholar]
- 5.Adeyoju AB, Olujohungbe AB, Morris J, Yardumian A, Bareford D, Akenova A, Akinyanju O, Cinkotai K, O’Reilly PH. Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. BJU Int. 2002;90:898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 6.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140:1434–7. [PubMed] [Google Scholar]
- 7.Anele UA, Burnett AL. Erectile Dysfunction after Sickle Cell Disease-Associated Recurrent Ischemic Priapism: Profile and Risk Factors. J Sex Med. 2015 doi: 10.1111/jsm.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spycher MA, Hauri D. The ultrastructure of the erectile tissue in priapism. J Urol. 1986;135:142–7. doi: 10.1016/s0022-5347(17)45549-2. [DOI] [PubMed] [Google Scholar]
- 9.Hinman F., Jr Priapism; reasons for failure of therapy. J Urol. 1960;83:420–8. doi: 10.1016/S0022-5347(17)65731-8. [DOI] [PubMed] [Google Scholar]
- 10.Broderick GA. Priapism and sickle-cell anemia: diagnosis and nonsurgical therapy. J Sex Med. 2012;9:88–103. doi: 10.1111/j.1743-6109.2011.02317.x. [DOI] [PubMed] [Google Scholar]
- 11.Christ GJ, Richards S, Winkler A. Integrative erectile biology: the role of signal transduction and cell-to-cell communication in coordinating corporal smooth muscle tone and penile erection. Int J Impot Res. 1997;9:69–84. doi: 10.1038/sj.ijir.3900277. [DOI] [PubMed] [Google Scholar]
- 12.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 13.Bivalacqua TJ, Musicki B, Kutlu O, Burnett AL. New insights into the pathophysiology of sickle cell disease-associated priapism. J Sex Med. 2012;9:79–87. doi: 10.1111/j.1743-6109.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Fournier GR, Jr, Juenemann KP, Lue TF, Tanagho EA. Mechanisms of venous occlusion during canine penile erection: an anatomic demonstration. J Urol. 1987;137:163–7. doi: 10.1016/s0022-5347(17)43911-5. [DOI] [PubMed] [Google Scholar]
- 16.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 17.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 18.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99:4061–6. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, Snyder SH, Burnett AL. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2012;109:16624–9. doi: 10.1073/pnas.1213790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignarro LJ. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol. 1990;67:1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 21.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274:13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 22.Morrison BF, Burnett AL. Priapism in hematological and coagulative disorders: an update. Nat Rev Urol. 2011;8:223–30. doi: 10.1038/nrurol.2011.28. [DOI] [PubMed] [Google Scholar]
- 23.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J Cell Physiol. 2010;224:620–5. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- 24.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–40. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bivalacqua TJ, Musicki B, Hsu LL, Gladwin MT, Burnett AL, Champion HC. Establishment of a transgenic sickle-cell mouse model to study the pathophysiology of priapism. J Sex Med. 2009;6:2494–504. doi: 10.1111/j.1743-6109.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–25. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–94. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 29.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–20. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol. 2007;34:926–32. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin CS, Chow S, Lau A, Tu R, Lue TF. Human PDE5A gene encodes three PDE5 isoforms from two alternate promoters. Int J Impot Res. 2002;14:15–24. doi: 10.1038/sj.ijir.3900802. [DOI] [PubMed] [Google Scholar]
- 32.Morrison BF, Burnett AL. Stuttering priapism: insights into pathogenesis and management. Curr Urol Rep. 2012;13:268–76. doi: 10.1007/s11934-012-0258-9. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J Androl. 2008;10:88–101. doi: 10.1111/j.1745-7262.2008.00314.x. [DOI] [PubMed] [Google Scholar]
- 34.Olujohungbe AB, Adeyoju A, Yardumian A, Akinyanju O, Morris J, Westerdale N, Akenova Y, Kehinde MO, Anie K, Howard J, Brooks A, Davis VA, Khoriatry AI. A prospective diary study of stuttering priapism in adolescents and young men with sickle cell anemia: report of an international randomized control trial--the priapism in sickle cell study. J Androl. 2011;32:375–82. doi: 10.2164/jandrol.110.010934. [DOI] [PubMed] [Google Scholar]
- 35.Abern MR, Levine LA. Ketoconazole and prednisone to prevent recurrent ischemic priapism. J Urol. 2009;182:1401–6. doi: 10.1016/j.juro.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Dahm P, Rao DS, Donatucci CF. Antiandrogens in the treatment of priapism. Urology. 2002;59:138. doi: 10.1016/s0090-4295(01)01492-3. [DOI] [PubMed] [Google Scholar]
- 37.Serjeant GR, de Ceulaer K, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle-cell disease. Lancet. 1985;2:1274–6. doi: 10.1016/s0140-6736(85)91555-7. [DOI] [PubMed] [Google Scholar]
- 38.Shamloul R, el Nashaar A. Idiopathic stuttering priapism treated successfully with low-dose ethinyl estradiol: a single case report. J Sex Med. 2005;2:732–4. doi: 10.1111/j.1743-6109.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg J, Eyre RC. Management of recurrent priapism with epinephrine self-injection and gonadotropin-releasing hormone analogue. J Urol. 1995;153:152–3. doi: 10.1097/00005392-199501000-00054. [DOI] [PubMed] [Google Scholar]
- 40.Hoeh MP, Levine LA. Prevention of recurrent ischemic priapism with ketoconazole: evolution of a treatment protocol and patient outcomes. J Sex Med. 2014;11:197–204. doi: 10.1111/jsm.12359. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita N, Hisasue S, Kato R, Masumori N, Takahashi A, Itoh N, Tsukamoto T. Idiopathic stuttering priapism: recovery of detumescence mechanism with temporal use of antiandrogen. Urology. 2004;63:1182–4. doi: 10.1016/j.urology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Goetz T, Burnett AL. Prostate cancer risk after anti-androgen treatment for priapism. Int Urol Nephrol. 2014;46:757–60. doi: 10.1007/s11255-013-0583-z. [DOI] [PubMed] [Google Scholar]
- 43.Anele UA, Morrison BF, Burnett AL. Molecular Pathophysiology of Priapism: Emerging Targets. Curr Drug Targets. 2014 doi: 10.2174/1389450115666141111111842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traish A, Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med. 2005;2:759–70. doi: 10.1111/j.1743-6109.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 45.Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, Orlando C, Vannelli GB, Aversa A, Natali A, Forti G, Giorgi M, Jannini EA, Ledda F, Maggi M. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–63. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 46.Zvara P, Sioufi R, Schipper HM, Begin LR, Brock GB. Nitric oxide mediated erectile activity is a testosterone dependent event: a rat erection model. Int J Impot Res. 1995;7:209–19. [PubMed] [Google Scholar]
- 47.Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz DE, Champion HC, Burnett AL. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–32. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 49.Musicki B, Bivalacqua TJ, Champion HC, Burnett AL. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J Sex Med. 2014;11:424–30. doi: 10.1111/jsm.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043–8. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 51.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077–84. doi: 10.1111/j.1743-6109.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 52.Tzortzis V, Mitrakas L, Gravas S, Mamoulakis C, Meissner A, Kyriakou D, Melekos MD. Oral phosphodiesterase type 5 inhibitors alleviate recurrent priapism complicating thalassemia intermedia: a case report. J Sex Med. 2009;6:2068–71. doi: 10.1111/j.1743-6109.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 53.Pierorazio PM, Bivalacqua TJ, Burnett AL. Daily phosphodiesterase type 5 inhibitor therapy as rescue for recurrent ischemic priapism after failed androgen ablation. J Androl. 2011;32:371–4. doi: 10.2164/jandrol.110.011890. [DOI] [PubMed] [Google Scholar]
- 54.Burnett AL, Anele UA, Trueheart IN, Strouse JJ, Casella JF. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am J Med. 2014;127:664–8. doi: 10.1016/j.amjmed.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT walk PI, Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–64. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–51. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 57.Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, Ataga K, Swerdlow P, Kutlar A, DeCastro L, Waclawiw MA Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell A Follow-Up MSHP. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17. 5 year follow-up. Am J Hematol. 2010;85:403–8. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saad ST, Lajolo C, Gilli S, Marques JF, Junior, Lima CS, Costa FF, Arruda VR. Follow-up of sickle cell disease patients with priapism treated by hydroxyurea. Am J Hematol. 2004;77:45–9. doi: 10.1002/ajh.20142. [DOI] [PubMed] [Google Scholar]
- 59.Al Jam’a AH, Al Dabbous IA. Hydroxyurea in the treatment of sickle cell associated priapism. J Urol. 1998;159:1642. doi: 10.1097/00005392-199805000-00065. [DOI] [PubMed] [Google Scholar]
- 60.Anele UA, Kyle Mack A, Resar LM, Burnett AL. Hydroxyurea therapy for priapism prevention and erectile function recovery in sickle cell disease: a case report and review of the literature. Int Urol Nephrol. 2014;46:1733–6. doi: 10.1007/s11255-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–11. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood. 2006;108:184–91. doi: 10.1182/blood-2005-11-4454. [DOI] [PubMed] [Google Scholar]
- 63.Gladwin MT, Shelhamer JH, Ognibene FP, Pease-Fye ME, Nichols JS, Link B, Patel DB, Jankowski MA, Pannell LK, Schechter AN, Rodgers GP. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br J Haematol. 2002;116:436–44. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- 64.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Lanzkron S, Castro O, Gordeuk VR, Coles WA, Peters-Lawrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassarre J, Casella JF, De NI. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Head CA, Swerdlow P, McDade WA, Joshi RM, Ikuta T, Cooper ML, Eckman JR. Beneficial effects of nitric oxide breathing in adult patients with sickle cell crisis. Am J Hematol. 2010;85:800–2. doi: 10.1002/ajh.21832. [DOI] [PubMed] [Google Scholar]
- 66.Tarhan F, Kuyumcuoglu U, Kolsuz A, Ozgul A, Canguven O. Effect of intracavernosal sodium nitroprusside in impotence. Urol Int. 1996;56:211–4. doi: 10.1159/000282844. [DOI] [PubMed] [Google Scholar]
- 67.Truss MC, Becker AJ, Djamilian MH, Stief CG, Jonas U. Role of the nitric oxide donor linsidomine chlorhydrate (SIN-1) in the diagnosis and treatment of erectile dysfunction. Urology. 1994;44:553–6. doi: 10.1016/s0090-4295(94)80058-8. [DOI] [PubMed] [Google Scholar]
- 68.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 2014;28:76–84. doi: 10.1096/fj.13-228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–81. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 70.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donaldson JF, Davis N, Davies JH, Rees RW, Steinbrecher HA. Priapism in teenage boys following depot testosterone. J Pediatr Endocrinol Metab. 2012;25:1173–6. doi: 10.1515/jpem-2012-0270. [DOI] [PubMed] [Google Scholar]
- 72.Ichioka K, Utsunomiya N, Kohei N, Ueda N, Inoue K, Terai A. Testosterone-induced priapism in Klinefelter syndrome. Urology. 2006;67:622.e17–8. doi: 10.1016/j.urology.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 73.Shergill IS, Pranesh N, Hamid R, Arya M, Anjum I. Testosterone induced priapism in Kallmann’s syndrome. J Urol. 2003;169:1089. doi: 10.1097/01.ju.0000049199.37765.c9. [DOI] [PubMed] [Google Scholar]
- 74.Burnett AL, Kan-Dobrosky N, Miller MG. Testosterone replacement with 1% testosterone gel and priapism: no definite risk relationship. J Sex Med. 2013;10:1151–61. doi: 10.1111/jsm.12059. [DOI] [PubMed] [Google Scholar]
- 75.Morrison BF, Anele UA, Reid ME, Madden WA, Feng Z, Burnett AL. Is testosterone deficiency a possible risk factor for priapism associated with sickle-cell disease? Int Urol Nephrol. 2015;47:47–52. doi: 10.1007/s11255-014-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrison BF, Reid M, Madden W, Burnett AL. Testosterone replacement therapy does not promote priapism in hypogonadal men with sickle cell disease: 12-month safety report. Andrology. 2013;1:576–82. doi: 10.1111/j.2047-2927.2013.00084.x. [DOI] [PubMed] [Google Scholar]
- 77.Fu S, Tar MT, Melman A, Davies KP. Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells. FASEB J. 2014;28:3633–44. doi: 10.1096/fj.13-248708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–5. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanika ND, Tar M, Tong Y, Kuppam DS, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009;297:C916–27. doi: 10.1152/ajpcell.00656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burnett AL, Musicki B, Jin L, Bivalacqua TJ. Nitric oxide/redox-based signalling as a therapeutic target for penile disorders. Expert Opin Ther Targets. 2006;10:445–57. doi: 10.1517/14728222.10.3.445. [DOI] [PubMed] [Google Scholar]
- 81.Musicki B, Liu T, Sezen SF, Burnett AL. Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis. J Sex Med. 2012;9:1980–7. doi: 10.1111/j.1743-6109.2012.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lagoda G, Sezen SF, Cabrini MR, Musicki B, Burnett AL. Molecular analysis of erection regulatory factors in sickle cell disease associated priapism in the human penis. J Urol. 2013;189:762–8. doi: 10.1016/j.juro.2012.08.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanika ND, Melman A, Davies KP. Experimental priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. Int J Impot Res. 2010;22:363–73. doi: 10.1038/ijir.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munarriz R, Park K, Huang YH, Saenz de Tejada I, Moreland RB, Goldstein I, Traish AM. Reperfusion of ischemic corporal tissue: physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760–4. doi: 10.1016/s0090-4295(03)00484-9. [DOI] [PubMed] [Google Scholar]
- 85.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 86.Wen J, Xia Y. Adenosine signaling: good or bad in erectile function? Arterioscler Thromb Vasc Biol. 2012;32:845–50. doi: 10.1161/ATVBAHA.111.226803. [DOI] [PubMed] [Google Scholar]
- 87.Chiang PH, Wu SN, Tsai EM, Wu CC, Shen MR, Huang CH, Chiang CP. Adenosine modulation of neurotransmission in penile erection. Br J Clin Pharmacol. 1994;38:357–62. doi: 10.1111/j.1365-2125.1994.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Kellems RE, Blackburn MR, Xia Y. Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J Sex Med. 2010;7:3011–22. doi: 10.1111/j.1743-6109.2009.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J. 2011;25:2823–30. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ning C, Wen J, Zhang Y, Dai Y, Wang W, Zhang W, Qi L, Grenz A, Eltzschig HK, Blackburn MR, Kellems RE, Xia Y. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1alpha mediated reduction of PDE5 gene expression. FASEB J. 2014;28:2725–35. doi: 10.1096/fj.13-247833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, Mills TM. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–22. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–21. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 94.Bivalacqua TJ, Ross AE, Strong TD, Gebska MA, Musicki B, Champion HC, Burnett AL. Attenuated RhoA/Rho-kinase signaling in penis of transgenic sickle cell mice. Urology. 2010;76:510.e7–12. doi: 10.1016/j.urology.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–6. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl. 2009;30:352–62. doi: 10.2164/jandrol.108.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]