Abstract
Immune-suppressive cell populations, including Tregs and suppressor monocytes, have been implicated in long-term survival of allografts in both human transplant recipients and animal models. The factors that drive differentiation and function of these cell populations are not completely understood. In this issue, Bézie and colleagues identify IL-34 as an important mediator of allograft tolerance in a rat model of heart transplantation. Their data support a model in which IL-34 production by Tregs promotes a population of suppressive macrophages that in turn promote Treg differentiation. The results of this study support further exploration of the immunosuppressive properties of IL-34.
Tregs and immune tolerance
Tolerance to self- and foreign antigens is created by a combination of the deletion of antigen-reactive T cells and control of the remaining cells by a specialized population of T cells known as Tregs. The best-characterized lineage of Tregs is defined by expression of the X-linked transcription factor FOXP3, and this population is essential for the maintenance of self-tolerance (1). The importance of these FOXP3+ Tregs in the preservation of self-tolerance is perhaps best exemplified by the development of multiorgan autoimmunity in patients with IPEX syndrome and in scurfy mice, both of which harbor loss-of-function mutations in the FOXP3-encoding gene.
Tregs also play a critical role in organ and tissue transplantation, and abundant evidence from patients and animal models implicates these cells in long-term allograft survival and tolerance. As the frequency of T cell precursors that are capable of responding to alloantigens is extremely high (~1%–10%; ref. 2), tolerance is likely established by deletion of large numbers of these alloreactive cells (3). However, deletion of alloreactive T cells is incomplete, and new alloreactive T cells are continuously generated in the thymus; therefore, maintaining tolerance to the allograft requires continual monitoring of alloreactive cells in the periphery by Tregs. Indeed, Tregs have been implicated to be essential for graft acceptance in the vast majority of experimental transplant tolerance models.
Like other T cell populations, Tregs mature in the thymus as part of normal lymphoid development. These so-called “natural” or “thymic” Tregs express TCRs (T cell receptors for antigen) that are preferentially attuned to recognize self-antigen. Moreover, thymic-derived Tregs appear to prevent the spontaneous autoimmunity that otherwise would result when self-reactive lymphoid cells escape thymic deletion. A second population of Tregs arises from the conversion of FOXP3– cells into FOXP3+ cells in the periphery and are termed “induced” or “peripheral” Tregs. Induced Tregs are important for materno-fetal tolerance (1, 4). This observation, along with other experimental models (5), suggests that these cells can arise when antigen is encountered in a noninflammatory context. While thymic-derived and induced Tregs have different origins, both subpopulations require specific antigen activation to induce their regulatory function and appear to suppress in an antigen-nonspecific manner once activated (1, 6).
Several antigen-presenting cells, including DCs, macrophages, and B cells, have been shown to suppress inflammation and facilitate the conversion of FOXP3– T cells into FOXP3+ Tregs through reduced expression of T cell costimulatory molecules and secretion of cytokines, such as IL-10 and TGF-β (7–9). Macrophages, which survey the extracellular space by engulfing antigen and activating patrolling T cells, play a role in tolerance induction and maintenance as well. Moreover, suppressive macrophages can induce Treg differentiation (10), though the mechanism has not been completely elucidated.
IL-34: a new player in immune tolerance
In this issue, Bézie et al. demonstrate a new connection between macrophages and Tregs that mediates the immunosuppressive properties of these cells (11). IL-34 shares several characteristics with macrophage CSF (M-CSF or CSF1), which is necessary for the differentiation of monocytes, macrophages, and some DCs. While there is little sequence homology between IL-34 and M-CSF, both cytokines promote monocyte lineage survival through binding their shared receptor CSF1R (also known as CD115) (12). IL-34 is largely considered to promote macrophage, monocyte, and DC survival following its release from various tissues in response to inflammation. Bézie and colleagues now provide evidence that IL-34 is also expressed by rodent and human Tregs and promotes the suppressive properties of macrophages, which in turn promote the upregulation of FOXP3 in Tregs (Figure 1).
Figure 1. Suppressor monocytes secrete a number of molecules including inducible NOS and arginase I to promote allograft survival and suppress alloreactive T cells.
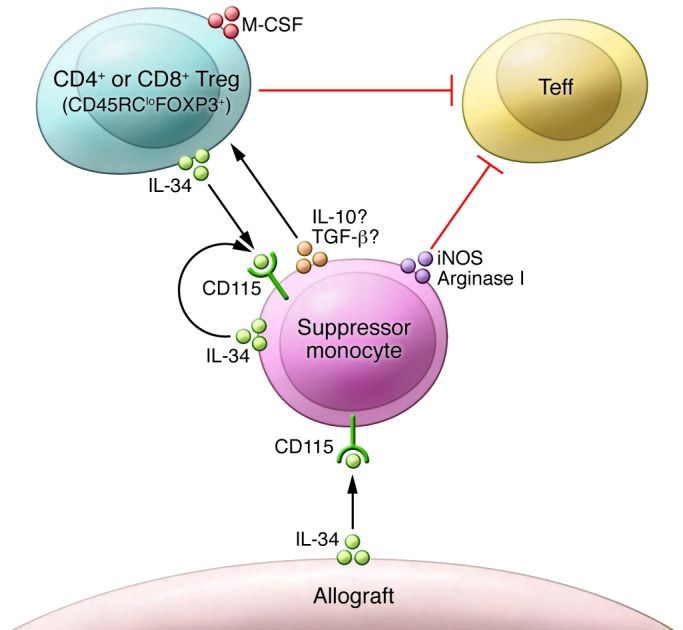
IL-34 is produced by monocytes and cells from a number of organs including the heart and binds to its receptor on monocytes. IL-34 is also expressed by CD45RClo CD4+ and CD8+ Tregs. IL-34 treatment of mixed lymphocyte cultures improves Treg-dependent suppression of effector T cell proliferation. The IL-34 receptor CD115 is not known to be expressed on Tregs; therefore, suppression of the alloresponse and FOXP3 upregulation may be mediated through a suppressor monocyte intermediate. iNOS, inducible NOS; Teff, T effector cell.
Previously, Guillonneau et al. demonstrated that using adenovirus-delivered CD40Ig to block the CD40/CD154 T cell costimulatory pathway promotes transplant tolerance in rats. Moreover, this study revealed that a population of CD8+CD45RClo Tregs mediates tolerance in these animals (13), although it was not clear how this CD45RClo cell population prevented allograft rejection. Bézie et al. extend these findings and show that IL-34 levels in the spleen are substantially increased in CD40Ig-tolerized heart transplant recipients but decreased in animals rejecting their grafts (11). Importantly, adenovirus-mediated overexpression of IL-34 in rats prior to transplantation prolonged graft survival and, in combination with a subtherapeutic dose of the mTOR inhibitor rapamycin, dramatically improved graft tolerance. Moreover, allograft tolerance was also achieved in naive, sublethally irradiated animals following adoptive transfer of Tregs from tolerant animals, confirming a role for Tregs in mediating IL-34–associated tolerance.
Remaining questions
The results of Bézie and colleagues establish that IL-34 mediates transplant tolerance and that Tregs are involved in the process (11). IL-34 is expressed by an extremely wide variety of cell types (7, 12, 14, 15). While Bézie et al. argue that Tregs are the relevant cellular source of IL-34 in their system, it is equally plausible that other cell types are involved, perhaps even macrophages themselves, which are also implicated in tolerance in their model. Future studies using IL-34–deficient Tregs and/or IL-34–deficient macrophages will be needed to address this issue; however, such experiments would entail establishing a different model (mouse vs. rat).
While it remains uncertain precisely how IL-34 inhibits graft rejection, there is strong evidence that macrophages are involved. The IL-34–related cytokine M-CSF has immunosuppressive properties, and exogenous M-CSF decreases T cell proliferation when added to mixed lymphocyte reactions in a dose-dependent manner (16). Moreover, the ability of M-CSF to suppress T cell proliferation in these mixed reactions was lost upon macrophage depletion, suggesting that M-CSF promotes tolerogenic functions of macrophages. The results of Bézie et al. suggest similar features for IL-34 (11). Indeed, these authors demonstrated that Treg-dependent IL-34–mediated graft survival is also macrophage-dependent, as macrophage depletion in their models resulted in rapid rejection of heart allografts, a finding that is consistent with a previous study showing that IL-34 can induce human suppressive macrophages in vitro (17). Together, these results indicate that, similar to M-CSF, interactions between effector T cells, macrophages, and Tregs are required for this suppression to occur.
Macrophages are differentiated by stimuli and cytokines in the microenvironment and are divided into a growing list of subpopulations. For example, exposure to IFN-γ or activation of TLR signaling induces an M1 proinflammatory phenotype characterized by elevated expression of IL-12 and low expression of IL-10 (IL-12hiIL-10lo). In contrast, tumor-associated macrophages have an M2 profile (IL-12loIL-10hi). Treatment of macrophages with IL-4, IL-13, or IL-34, as now demonstrated by Bézie and colleagues (11), induces their differentiation into IL-12loIL-10hi cells, which recruit Tregs and induce nonresponsiveness upon interaction with naive T cells.
What is the relationship of these IL-34–treated macrophages to myeloid-derived suppressor cells, which also promote transplant tolerance (18)? Like IL-34–treated macrophages, this population of cells promotes the development of Tregs in vivo (19). In a murine heart transplant model in which CD40/CD154 blockade plus donor splenocyte transfusion promotes long-term survival, myeloid-derived suppressor cells are required for tolerance and Treg induction (18, 20). Do the IL-34–treated macrophages described by Bézie et al. (11) and the myeloid-derived suppressor cells represent overlapping suppressor cell populations, and do myeloid-derived suppressor cells induce IL-34–expressing Tregs?
Conclusions
The demonstration that IL-34 has immunosuppressive properties is an important and exciting finding, yet many questions remain. For example, is the generation of these IL-34–mediated Treg and suppressive macrophage populations limited to allograft tolerance created by CD40/CD154 blockade or a general feature of immunoregulation in a noninflammatory environment? It is also not clear how IL-34 is acting to induce tolerance. Is there a macrophage-generated intermediate signal that promotes generation of CD45RClo Tregs (see Figure 1)? Is IL-34 secreted along with the known Treg inducers TGF-β and IL-10, and do these cytokines work together to upregulate FOXP3? Are the immunosuppressive properties of IL-34 described by Bézie et al. (11) related to the phenotype of IL-34–deficient mice, which mount defective immune responses in the skin and CNS? Like many provocative studies, the work of Bézie and colleagues raises a plethora of important questions for future investigation. It is clear, however, that IL-34 is now on the map as a potential key player in transplant tolerance.
Acknowledgments
Supported in part by the AST/Novartis Basic Science Faculty Development Grant (to J.I. Kim) and AI-37691 (to L.A. Turka).
Footnotes
Conflict of interest: Laurence A. Turka has received grant funding from Pfizer and serves as a consultant for Solid Biosciences and Neon Therapeutics. A member of his family is an employee of Third Rock Ventures and serves as a consultant for Warp Drive Bio and Sanofi, and another member of his family is an employee of Novartis.
Reference information:J Clin Invest. 2015;125(10):3751–3753. doi:10.1172/JCI84010.
See the related article beginning on page 3952.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suchin E, Wells AD, Turka LA. Quantifying the frequency of alloreactive cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 3.Wells AD, et al. Requirement for T cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5(11):1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 4.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23(6):424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17(6):638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur J Immunol. 2014;44(6):1575–1581. doi: 10.1002/eji.201344365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter NA, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 9.Soroosh P, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage ND, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFβ-1. J Immunol. 2008;181(3):2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 11.Bézie S, et al. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest. 125(10):3952–3964. doi: 10.1172/JCI81227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320(5877):807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 13.Guillonneau C, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-γ, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(4):1096–1106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13(8):753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88(3):495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai T, Yamada M, Simamura S, Motoyoshi K. Recombinant human macrophage-colony stimulating factor suppresses the mouse mixed lymphocyte reaction. Cell Immunol. 1996;171(1):87–94. doi: 10.1006/cimm.1996.0177. [DOI] [PubMed] [Google Scholar]
- 17.Foucher ED, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. PLoS One. 2013;8(2): doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia MR, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120(7):2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 20.Conde P, et al. DC-SIGN(+) macrophages control the induction of transplantation tolerance. Immunity. 2015;42(6):1143–1158. doi: 10.1016/j.immuni.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


