Abstract
Recent genome-wide association studies have revealed that variations near the gene locus encoding the transcription factor Krüppel-like factor 14 (KLF14) are strongly associated with HDL cholesterol (HDL-C) levels, metabolic syndrome, and coronary heart disease. However, the precise mechanisms by which KLF14 regulates lipid metabolism and affects atherosclerosis remain largely unexplored. Here, we report that KLF14 is dysregulated in the liver of 2 dyslipidemia mouse models. We evaluated the effects of both KLF14 overexpression and genetic inactivation and determined that KLF14 regulates plasma HDL-C levels and cholesterol efflux capacity by modulating hepatic ApoA-I production. Hepatic-specific Klf14 deletion in mice resulted in decreased circulating HDL-C levels. In an attempt to pharmacologically target KLF14 as an experimental therapeutic approach, we identified perhexiline, an approved therapeutic small molecule presently in clinical use to treat angina and heart failure, as a KLF14 activator. Indeed, in WT mice, treatment with perhexiline increased HDL-C levels and cholesterol efflux capacity via KLF14-mediated upregulation of ApoA-I expression. Moreover, perhexiline administration reduced atherosclerotic lesion development in apolipoprotein E–deficient mice. Together, these results provide comprehensive insight into the KLF14-dependent regulation of HDL-C and subsequent atherosclerosis and indicate that interventions that target the KLF14 pathway should be further explored for the treatment of atherosclerosis.
Introduction
Atherosclerosis-related cardiovascular disease (CVD), including coronary heart disease (CHD), ischemic stroke, and peripheral arterial disease, is the most common cause of death and disability worldwide. Epidemiologic studies and experimental observations have consistently shown that decreased levels of HDL cholesterol (HDL-C) and apolipoprotein A-I (ApoA-I), the major protein component of HDL-C, are powerful, independent predictors of CHD risk (1–4). High levels of HDL-C and ApoA-I are strongly associated with a reduced cardiovascular risk, even among statin-treated patients achieving LDL cholesterol (LDL-C) levels of less than 50 mg/dl (5). Indeed, both HDL and ApoA-I have cardiovascular protective effects, including reverse cholesterol transport (RCT) and antiinflammatory and antioxidative effects. HDL-C efflux capacity, a new biomarker that characterizes a key step in RCT, is a strong inverse predictor of coronary disease events (6, 7). Recently, a population-based cohort study has demonstrated that a high HDL-C efflux capacity is associated with a decreased risk of coronary disease, even after adjustment for traditional cardiovascular risk factors, including HDL particle numbers and size (8).
As HDL particles and ApoA-I are the key acceptors of cholesterol efflux, it may be necessary to develop therapeutic strategies to raise functional HDL and/or ApoA-I levels and enhance their antiatherogenic functions. Most of the oral administration of HDL-raising agents, such as niacin, cholesteryl ester transfer protein (CETP) inhibitors, and fibrates, has yielded convincing results in increasing HDL-C levels, but the effects on reducing cardiovascular risk and enhancing RCT need to be further investigated (9–11). Turning on endogenous production of ApoA-I to facilitate new HDL particle formation is becoming one of the most attractive approaches, which is strongly supported by results from human ApoA-I transgenic mice and virus-mediated overexpression of ApoA-I in a mouse model of experimental atherosclerosis (11, 12). RVX-208, a bromodomain and extraterminal domain inhibitor, is an orally active small molecule that upregulates ApoA-I production (12–14). However, in patients with CHD, administration of RVX-208 did not statistically reduce cardiovascular events and the percentage of coronary atheroma volume due to its small effect on HDL-C levels and significant side effects in the ASSURE study (15, 16). Therefore, the identification of novel molecules that regulate ApoA-I production is essential for increasing ApoA-I and HDL production and conferring protection against atherosclerosis.
Large-scale GWAS have identified that a genetic variant on chromosome 7 is strongly associated with both HDL trait and CHD (17–21). This variant lies in a noncoding region in the vicinity of KLF14 and TSGA13, which encode Krüppel-like factor 14 and testis-specific gene A13, respectively. KLF14 is a member of a large family of zinc-finger transcription factors that have been widely studied in embryogenesis and cell proliferation, differentiation, and development. The 18 KLFs described in mammals possess highly conserved cysteine and histamine zinc fingers, critical for recognition and binding to CACCC or CGCCC DNA motifs (22). KLF14, a maternally expressed imprinted gene without introns, is robustly associated with HDL-C levels, CHD, type 2 diabetes, obesity, and cancer (17, 18, 20, 21, 23–28). In fact, KLF14 has been recently proposed as a master trans-regulator of multiple genes that are associated with metabolic phenotypes in adipose tissue (26), T regulatory cell differentiation (29), and lipid-mediated signaling through a distinct epigenetic mechanism (20), though its role in lipid metabolism and CVD remains to be determined. Fortunately, through a combination of animal models, genetic tools, and pharmacological screening, we have identified a mechanistic role, reported here, for KLF14 in regulating HDL metabolism and cholesterol efflux capacity by modulation of ApoA-I production. Moreover, we identify a small drug, perhexiline, as a KLF14 activator, administration of which reduces atherosclerosis development in apolipoprotein E–deficient (Apoe–/–) mice. Collectively, these findings demonstrate molecular mechanisms by which the KLF14 pathway could serve as a promising therapeutic target for the treatment of atherosclerosis.
Results
Hepatic KLF14 expression is reduced in dyslipidemia mouse models.
In an attempt to identify genes that may play a critical role in regulation of lipid metabolism using real-time polymerase chain reaction (PCR) analysis, we first examined the expression profiles of 43 candidate genes that are associated with the HDL-C trait and CHD, as indicated by previous GWAS (17–19, 21), in the liver from 2 dyslipidemia mouse models. We found that Klf14 mRNA expression was significantly decreased, by approximately 70%, in the liver of C57BL/6 mice in response to a high-fat diet (HFD) (Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/JCI79048DS1). We next assessed Klf14 mRNA expression in the liver of the leptin-deficient (ob/ob) mouse, a well-accepted model characterized by obesity, insulin resistance, and dyslipidemia, and found that the levels of this gene were reduced by 52% (Supplemental Figure 1C). Among the tissues of healthy adult C57BL/6 mice examined, KLF14 protein expression was detected in liver and kidney, while heart showed the highest level of KLF14 (Supplemental Figure 2). Consistent with mRNA expression, KLF14 protein levels were decreased in the livers from mice fed HFD and ob/ob mice compared with control animals (Supplemental Figure 1, D and E).
Sterol-response element–binding proteins (SREBPs), transcription factors that regulate the expression of genes involved in the synthesis of cholesterol, fatty acids, and triglycerides (TG) in mammalian cells, are upregulated in the liver of both the dyslipidemia mouse models (30, 31). We also observed a significant upregulation of SREBP1 in the liver from C57BL/6 mice fed a HFD (Supplemental Figure 3A). Next, to test the effects of SREBPs on KLF14 expression, we performed luciferase reporter gene assays using human KLF14 promoter (KLF14-luc, spanning –1567 to +65, relative to the transcription start site) construct. Fitting with the effects observed for endogenous Klf14 expression, SREBP1c and SREBP2 significantly repressed the transcription at the human KLF14 promoter (Supplemental Figure 3B). Since KLF14 expression is reduced in the liver of dyslipidemia models, we postulated that hepatic KLF14 might play an important role in lipid metabolism and performed additional experimentations to test the validity of this idea.
Overexpression of KLF14 increases HDL-C levels and cholesterol efflux capacity.
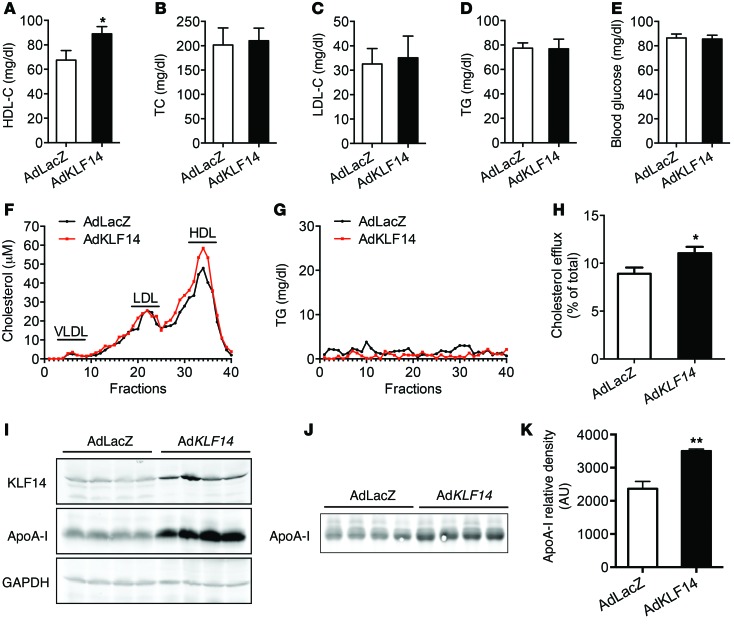
To investigate whether KLF14 contributes to lipid metabolism, as inferred from genotype-to-phenotype correlations previously made in humans, C57BL/6 mice were fed a HFD for 12 weeks and recombinant adenoviruses encoding human KLF14 (AdKLF14) or β-galactosidase (AdLacZ) were injected via tail vein. After 6 days, AdKLF14-treated animals showed a 29% increase in HDL-C compared with the AdLacZ-treated group (Figure 1A), whereas total cholesterol (TC), LDL-C levels, TG, and fasting blood glucose (Figure 1, B–E) were not affected. Fast protein liquid chromatography (FPLC) analysis of pooled sera from each experimental group confirmed that AdKLF14-treated mice had increased circulating levels of HDL-C (Figure 1F), but not TG (Figure 1G). We also observed an increase in ApoA-I protein levels in both the liver and serum of AdKLF14-treated mice (Figure 1, I–K). We detected that KLF14 induced marked mRNA increases in Klf14 and Apoa1 in livers from mice treated with AdKLF14 (Supplemental Figure 4, A and B). Although we observed a slight upregulation of ApoC3 mRNA (Supplemental Figure 4C), which is linked in a genetic cluster with Apoa1 (30), the circulation ApoC3 levels did not increase dramatically, as measured by ELISA (Supplemental Figure 4H). As expected, overexpression of human KLF14 did not affect mouse endogenous Klf14 expression (Supplemental Figure 4E). The expression of genes related to cholesterol metabolism, including ApoA-II, apoB, and 3-hydroxy-3-methylglutaryl-CoA reductase, did not change in livers from AdKLF14-treated mice (Supplemental Figure 4, D, F, and G). We also did tail vein injection with adenovirus containing human KLF11, which is a member of the same family of metabolic regulator KLF proteins, and found that treatment with AdKLF11 did not affect both HDL-C and LDL-C levels in C57BL/6 mice fed a HFD (Supplemental Figure 5). Conversely, efficient in vivo shRNA-based knockdown of KLF14 in the liver dramatically decreased plasma HDL-C levels, but had no effect on TG (Supplemental Figure 6, A, D, and E). We also observed a reduced circulating level of ApoA-I by Western blot (Supplemental Figure 6, B and C).
Figure 1. Overexpression of KLF14 increases both HDL-C and ApoA-I levels and cholesterol efflux capacity.
Adenoviral vectors containing LacZ (AdLacZ) or human KLF14 (AdKLF14) (5 × 108 pfu per mouse) were administered via tail vein injection to C57BL/6 mice fed HFD for 12 weeks (n = 10 per group). Serum samples were collected at day 6 and subjected individually to analytical chemistry to measure HDL-C (A), TC (B), LDL-C (C), TG (D), and fasting blood glucose (E) or to determine cholesterol and TG levels from pooled samples by FPLC (fractions 1 to 40) (F and G). *P < 0.05, Student’s t test. (H) The ABCA1-mediated cholesterol efflux capacity of serum from AdKLF14- or AdLacZ-treated mice is expressed as the percentage of cholesterol efflux of total cell cholesterol (n = 10 per group). *P < 0.05, Student’s t test. Representative Western blot results show that AdKLF14-treated mice exhibited increased expression of ApoA-I levels in the liver (I) and serum (J). (K) Quantifications of ApoA-I levels in the serum from AdLacZ and AdKLF14-treated mice by Western blot (n = 10 per group). Values represent mean ± SEM. **P < 0.01, Student’s t test.
ApoA-I and HDL particles play critical roles in the process of RCT, in which cholesterol from nonhepatic peripheral tissues is transferred to HDL particles and returns to the liver for biliary excretion (6, 32). HDL functionality is critical for the assessment of HDL-mediated atheroprotective effects. Thus, subsequently, we quantified the ATP-binding cassette transporter ABCA1-mediated cholesterol efflux capacity of serum from AdKLF14- or AdLacZ-treated mice. We found that, concomitant with increased HDL-C and ApoA-I levels, cholesterol efflux capacity increased significantly in the KLF14-treated group (Figure 1H). Therefore, collectively, these data demonstrate that KLF14 regulates lipid metabolism and establish that KLF14 expression directly modulates the levels of ApoA-I and HDL-C in vivo.
KLF14 is a regulator of ApoA-I expression.
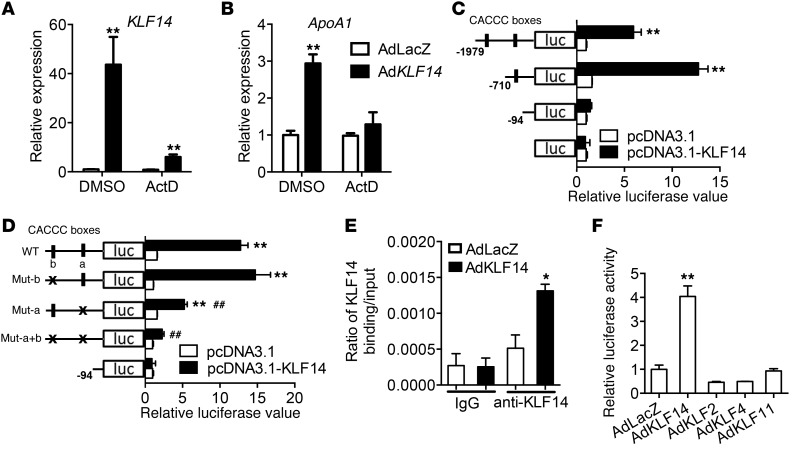
In accordance with our in vivo observations, overexpression of KLF14 in HepG2 cells resulted in increased APOA1 transcription, and this KLF14-induced APOA1 upregulation was blocked by actinomycin D (Figure 2, A and B), a transcriptional inhibitor, suggesting that KLF14 regulates ApoA-I at the transcriptional level. These results led us to investigate whether KLF14 functions as a transcriptional regulator of this protein. Indeed, initial evidence derived from analysis of the 5′ flanking regions of human APOA1 identified a sequence (CACCC box) similar to the recently described functional KLF14-binding site (20). As shown in Figure 2C, the region from the APOA1 promoter containing these sites (located at nt –491/–486 and –1943/–1938) displayed increased reporter activity relative to control vector. Interestingly, mutation of the nt –491/–486 site greatly reduced promoter activity, while a similar change in the nt 1943/–1938 site had no significant change (Figure 2D). ChIP assay revealed that KLF14 binds the promoter region that harbors the proximal CACCC box (–491/–486) (Figure 2E), demonstrating that this is a functional KLF14-binding site in the human APOA1 promoter. Similarly, adenovirus-mediated overexpression of KLF14 significantly upregulated Apoa1 mRNA expression and ChIP assay revealed that KLF14 was bound to the promoter region that harbors the CACCC boxes (–499/–494 and –451/–446) in mouse primary hepatocytes (Supplemental Figure 7, A and B). Given the similar DNA-binding preferences of KLF family members (22), we considered whether other KLF transcription factors could regulate ApoA-I expression. While AdKLF14 cotransfection upregulated APOA1 promoter activity in HepG2 cells, adenoviral vectors containing KLF2, KLF4, or KLF11 failed to increase APOA1 promoter reporter activity (Figure 2F), indicating that these effects are specific for KLF14.
Figure 2. KLF14 is a regulator of APOA1 expression.
HepG2 cells were infected with AdLacZ or AdKLF14 for 24 hours and then incubated in medium containing ActD (5 μg/ml) or DMSO for another 24 hours (n = 3). KLF14 (A) and APOA1 (B) mRNA levels were determined by real-time qPCR. **P < 0.01, 2-way ANOVA and multiple comparisons. (C) The structure of human APOA1 promoter used in the luciferase assays indicating 2 putative CACCC-box KLF-binding sites. Expression of KLF14 with human APOA1 promoter assay demonstrated that KLF14 significantly increased ApoA-I luciferase activity (n = 3). **P < 0.01, compared with control vector, 2-way ANOVA and multiple comparisons. (D) Mutations of the 2 putative KLF-binding sites demonstrated ApoA-I expression is dependent on KLF14 and CACCC-box binding sites (n = 3). **P < 0.01, compared with pGL4-basic vector; ##P < 0.01, compared with APOA1 promoter WT, 2-way ANOVA and multiple comparisons. (E) ChIP assay revealed significant enrichment of KLF14 protein on the human APOA1 promoter in HepG2 cells (n = 3). *P < 0.05, 2-way ANOVA and multiple comparisons. (F) Luciferase activity assay demonstrated that KLF14, not KLF2, KLF4, or KLF11, led to an increase in APOA1 promoter activity in HepG2 cells (n = 3). **P < 0.01, 2-way ANOVA and multiple comparisons. Representative of at least 3 experiments.
Loss of hepatic KLF14 induces decreased HDL-C levels.
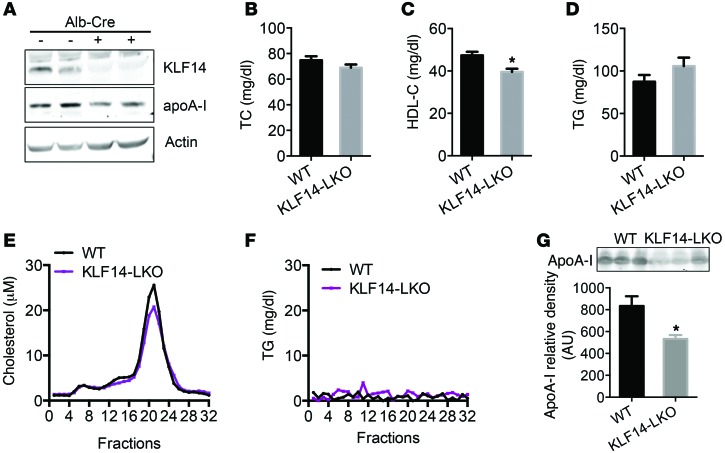
To study the role of hepatic KLF14 in lipid metabolism, we generated liver-specific Klf14-KO (LKO) mice using the Cre-loxP strategy (Supplemental Figure 8A). We generated mice harboring floxed Klf14 alleles in which only 1 exon of Klf14 was flanked by loxP sites (Klf14fl/fl). To ablate Klf14 in the liver, we crossed the Klf14fl/fl mice with mice harboring a Cre transgene under the control of the promoter for the albumin (Alb) gene (Alb-Cre mice). Deletion of Klf14 in the liver was confirmed at the genomic DNA (Supplemental Figure 8B), mRNA (Supplemental Figure 8C), and protein levels (Figure 3A). We found that deletion of Klf14 was specific to the liver, as its mRNA levels in other tissues were comparable to those in the control mice (Supplemental Figure 8C). As shown in Figure 3, TC and TG levels were comparable between WT and Klf14fl/fl (herein referred to as KLF14-LKO) mice at 8 weeks of age (Figure 3, B, D, and F). However, deletion of Klf14 in the liver resulted in an approximately 14.9% decrease in HDL-C levels compared with those of Klf14fl/fl littermates (Figure 3C). In addition, we also detected a decrease in the levels of ApoA-I in both the liver and the circulation of the KLF14-LKO mice (Figure 3, A and G). These results reveal that hepatic KLF14 contributes to HDL metabolism.
Figure 3. Liver-specific deletion of Klf14 showed decreased HDL-C levels.
(A) Western blot of KLF14 and ApoA-I expression in the livers of KLF14-LKO and littermate control (WT) mice. TC (B), HDL-C (C), and TG (D) levels were determined in KLF14-LKO and littermate control mice fasted overnight (n = 5–7 for each genotype). *P < 0.05, Student’s t test. (E and F) Pooled serum samples from KLF14-LKO and WT mice were assayed by HPLC, and cholesterol and TG levels (fractions 1 to 32) were determined. (G) Representative Western blot and quantifications of ApoA-I levels by Western blot analysis in 1 μl of serum samples from WT and KLF14-LKO mice. Values represent mean ± SEM (5–7 for each genotype). *P < 0.05, Student’s t test.
Drug screening identifies perhexiline as an inducer of KLF14 expression.
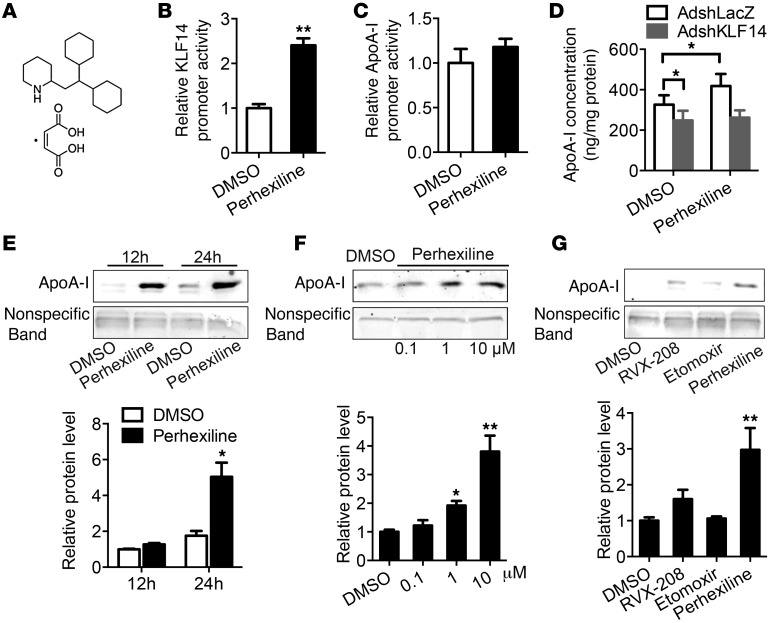
The preceding data demonstrate that upregulation of KLF14 expression results in increased ApoA-I and HDL-C levels, underscoring a fundamental role for KLF14 in maintaining the homeostasis of lipid metabolism. Therefore, we initiated efforts toward identifying pharmacological interventions that can activate endogenous Klf14 and thereby increase ApoA-I and HDL-C levels. For this purpose, we designed a high-throughput screening using a human KLF14 promoter–driven luciferase reporter, KLF14-luc. From the primary screening of a chemical library of the NIH/JDRF Custom Collection including 1,040 compounds, 8 compounds were identified as activating KLF14-luc activity 2-fold or more (Supplemental Table 1). It is noteworthy that we confirmed that perhexiline significantly increased KLF14 promoter activity, but not APOA1 promoter activity, after incubation for 12 hours (Figure 4, A–C). To investigate the effect of perhexiline on ApoA-I production, we detected the ApoA-I levels in the medium by ELISA and Western blot. Following treatment of HepG2 cells with perhexiline (10 μM) for 24 hours, the production of ApoA-I in the medium was increased by 28% compared with DMSO-treated cells (Supplemental Figure 9A). Efficient knockdown of KLF14 significantly decreased the production of ApoA-I induced by perhexiline, suggesting that this effect was largely dependent on the KLF14 in hepatocytes (Figure 4D and Supplemental Figure 9, B and C). Next, our experiments demonstrated that perhexiline induced ApoA-I production in time-dependent and dose-dependent manners (Figure 4, E and F). Previous pharmacological studies indicated that perhexiline is a potent inhibitor of mitochondrial carnitine palmitoyltransferase I (CPT-1). To identify whether the effect of perhexiline on ApoA-I production is dependent on the inhibition of the CPT-1 pathway, we used another well-established CPT-1 inhibitor, etomoxir (10 μM), which did not induce ApoA-I production (Figure 4G). RVX-208 was used as a positive control because this drug upregulates ApoA-I production in hepatocytes (refs. 12–14 and Figure 4G). As ApoA-I is synthesized mainly in the liver and small intestine of mammals (33), we also found that perhexiline activated KLF14 and ApoA-I expression in Caco2 cells, a human enterocytic cell line (Supplemental Figure 10, A and B), suggesting that perhexiline may stimulate ApoA-I production both in liver and intestine, which shows an additional potential mechanistic and pharmacological importance.
Figure 4. Drug screening identifies perhexiline as an activator of KLF14.
(A) Diagram of the chemical structure of the perhexiline maleate salt. (B and C) Luciferase activity of reporters was analyzed in HepG2 cells transfected with pGL4-KLF-luc or pGL4–ApoA-I–Luc constructs after 12 hours treatment with 10 μM perhexiline or DMSO. **P < 0.01, Student’s t test. Values represent mean ± SEM; n = 3. (D) HepG2 cells were infected with AdshLacZ or AdshKLF14 for 48 hours and then incubated with 10 μM perhexiline for 24 hours in DMEM containing 0.2% BSA. The ApoA-I concentrations in the medium were detected by ELISA. *P < 0.05, 2-way ANOVA and multiple comparisons. Values represent mean ± SEM; n = 6. (E) HepG2 cells were treated with DMSO or perhexiline at 10 μM for indicated time points in DMEM containing 0.2% BSA, and ApoA-I production was detected by Western blot. (F) HepG2 cells were treated with DMSO or perhexiline at indicated dosage for 24 hours in DMEM containing 0.2% BSA, and ApoA-I production was detected by Western blot. (G) HepG2 cells were treated with DMSO, perhexiline, RVX-208, or etomoxir at 10 μM for 24 hours in DMEM containing 0.2% BSA, and ApoA-I production was detected by Western blot. Quantifications from 3 independent experiments are shown in E–G, and values represent mean ± SEM. *P < 0.05; **P < 0.01, 2-way ANOVA and multiple comparisons.
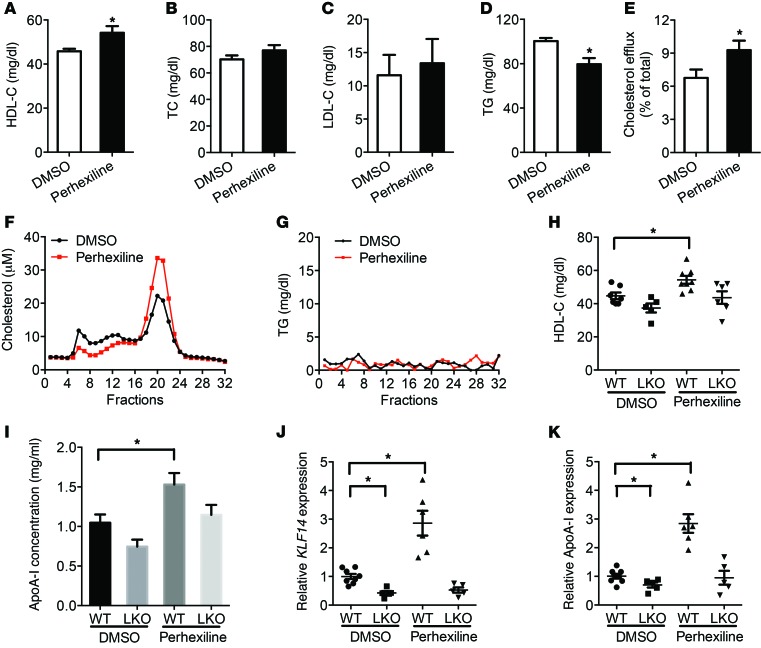
Administration of perhexiline increases KLF14, HDL, and ApoA-I levels in vivo.
To examine the potential role of perhexiline on upregulation of ApoA-I in vivo, we treated C57BL/6 mice with perhexiline at 10 mg/kg/d via gavage administration for 5 consecutive days. At day 7, perhexiline-treated animals showed an 18.3% increase in HDL-C levels and slightly decreased TG levels compared with the DMSO-treated group, whereas the TC, LDL-C, and TG levels did not significantly change (Figure 5, A–D). We also confirmed an increase in HDL-C levels, but not TG, by HPLC, using pooled serum from DMSO- or perhexiline-treated mice (Figure 5, F and G). These effects were associated with an approximately 4.1-fold upregulation of Klf14 and a 2-fold upregulation of Apoa1 in the liver by real-time quantitative PCR (qPCR) compared with mice treated with vehicle only (Supplemental Figure 11, A and B). As expected, we quantified the ABCA1-mediated cholesterol efflux capacity of serum from DMSO- or perhexiline-treated mice and found that cholesterol efflux capacity markedly increased in the perhexiline-treated group (Figure 5E). Thus, perhexiline behaves as the first KLF14 activator having a beneficial impact on the regulation of ApoA-I and HDL-C levels and function. To determine whether hepatic KLF14 deficiency interferes with perhexiline-induced increase in HDL-C levels, KLF14-LKO and littermate control mice were administrated with perhexiline or DMSO as control via gavage for 5 days. We found a significant increase in HDL-C and ApoA-I levels in perhexiline-treated control mice, though not in the perhexiline-treated KLF14-LKO counterparts (Figure 5, H and I). Subsequently, using real-time qPCR, we measured Klf14 and Apoa1 mRNA levels in livers obtained from mice belonging to each of these groups. Perhexiline-treated control mice showed a significant upregulation of Klf14 and Apoa1 expression in the liver as compared with KLF14-LKO mice (Figure 5, J and K). Thus, the systemic administration of perhexiline increases HDL-C levels in a manner that is largely dependent on hepatic KLF14.
Figure 5. Administration of perhexiline increased HDL-C levels in vivo.
C57BL/6J mice placed on a HFD for 12 weeks were treated with DMSO or perhexiline maleate salt (10 mg/kg/d) for 5 consecutive days by gavage administration, and plasma samples were collected at day 7 (n = 10 per group). HDL-C (A), TC (B), LDL-C (C), and TG (D) levels were measured. *P < 0.05, Student’s t test. (E) The ABCA1-mediated cholesterol efflux capacity of serum from DMSO- or perhexiline-treated mice is expressed as the percentage of cholesterol efflux of total cell cholesterol (n = 10 per group). *P < 0.05, Student’s t test. Pooled serum samples from DMSO- or perhexiline-treated mice were assayed by HPLC, and cholesterol (F) and TG (G) levels (fractions 1 to 32) were determined. (H–K) KLF14-LKO and littermate control mice were treated with DMSO or perhexiline maleate salt (10 mg/Kg/d) for 5 consecutive days by gavage administration, and plasma samples were collected at day 7 (n = 5–8 for each genotype). HDL-C levels were determined (H) and ApoA-I levels were quantified by Western blot analysis (I) (n = 5–8 for each genotype). (J and K) qRT-PCR analysis showing the expression levels of Klf14 and Apoa1 in indicated groups. Data are expressed relative to 18S RNA (n = 5–8 for each genotype). Values represent mean ± SEM. *P < 0.05; **P < 0.01, 2-way ANOVA and multiple comparisons.
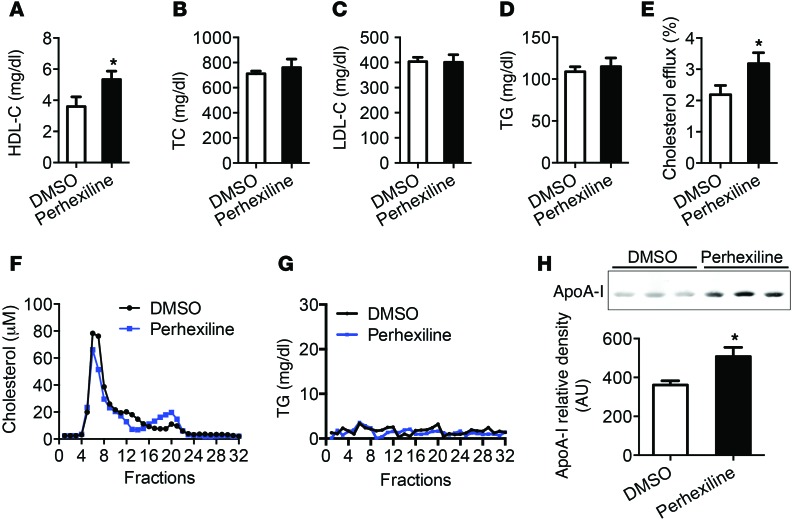
Administration of perhexiline reduces atherosclerosis development in Apoe–/– mice.
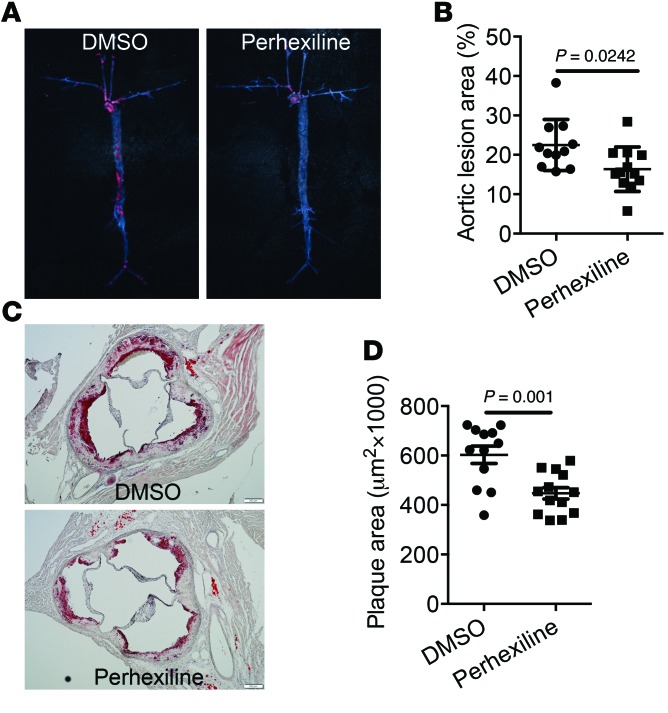
We tested the effect of perhexiline-mediated activation of the KLF14 pathway on atherosclerosis in Apoe–/– mice. After 10-week challenge of a high-cholesterol diet (HCD), Apoe–/– mice were treated 3 times a week (Monday, Wednesday, and Friday) with either perhexiline (10 mg/kg) or DMSO for 6 weeks via gavage administration. The circulating HDL-C levels were significantly increased in perhexiline-treated mice, but no significant differences were found in TC, TG, and LDL-C levels relative to control animals (Figure 6, A–D). Next, we quantified the ABCA1-mediated cholesterol efflux capacity and found that this process was markedly increased in the perhexiline-treated group (Figure 6E). HPLC analysis of pooled sera from each experimental group confirmed that perhexiline-treated mice had increased circulating HDL-C levels, but no changes in TG (Figure 6, F and G). We also detected an increase in ApoA-I protein levels in plasma from perhexiline-treated mice (Figure 6H). Most importantly, perhexiline treatment significantly inhibited atherosclerotic lesion formation, by 27.3%, as measured by the fraction of the surface area in en face aorta trees stained by oil red O (Figure 7, A and B). The cross-sectional plaque area in the aortic sinus was also attenuated by 30.2% in perhexiline-treated Apoe–/– mice compared with DMSO-treated animals (Figure 7, C and D). These findings indicate that perhexiline, the KLF14 activator, inhibited atherosclerosis development.
Figure 6. Administration of perhexiline increased HDL-C and ApoA-I levels and enhanced serum cholesterol efflux capacity in Apoe–/– mice.
Apoe–/– mice were placed on a HFD for 12 weeks and then were treated with DMSO or perhexiline at 10 mg/kg for 6 weeks (3 times a week) via gavage administration with continuous HFD (n = 15 per group). Plasma samples were collected and subjected individually to analytical chemistry to measure HDL-C (A), TC (B), LDL-C (C), and TG (D). *P < 0.05, Student’s t test. (E) The ABCA1-mediated cholesterol efflux capacity of serum from DMSO- or perhexiline-treated mice is expressed as the percentage of cholesterol efflux of total cell cholesterol (n = 15 per group). *P < 0.05, Student’s t test. The pooled serum from DMSO- or perhexiline-treated mice was analyzed by HPLC (fractions 1 to 32) and the cholesterol (F) and TG (G) levels in each fraction were measured. (H) Representative Western blot and quantifications of ApoA-I levels by Western blot analysis in 3 μl of plasma samples from mice treated with DMSO or perhexiline (n = 14 per group). *P < 0.05, Student’s t test. Values represent mean ± SEM.
Figure 7. Administration of perhexiline reduces atherosclerosis development in Apoe–/– mice.
Apoe–/– mice were placed on a HCD for 12 weeks and then were treated with DMSO or perhexiline at 10 mg/kg for 6 weeks (3 times a week) via gavage administration with continuous HCD. Perhexiline-treated mice exhibited decreased oil red O–stained lesions in the whole aorta (A) as well as reduced cross-sectional plaque area in the aortic sinus (C). Scale bars: 100 μm. Quantified en face (B) and histology (D) data are shown. Data represent mean ± SEM (n = 11–12). Student’s t test.
Discussion
Numerous epidemiological studies have demonstrated that HDL-C and ApoA-I levels are strong, independent, inverse predictors for CVD risk (1–5). In large cohort studies including patients hospitalized with CHD, a low HDL-C is the most prevalent lipoprotein abnormality, with more than half of patients having admission HDL-C levels of less than 40 mg/dl (34, 35). HDL-C efflux capacity, which is a marker of HDL function that measures RCT, has a strong inverse association with coronary disease events (6, 7). In this study, we provide evidence that KLF14 and its activator contribute to lipid metabolism in a manner that contributes to the suppression of atherosclerosis. Using gain- and loss-of-function approaches, we demonstrate that KLF14 increases both HDL-C levels and cholesterol efflux capacity by the regulation of ApoA-I production. A screening for compounds that can activate the expression of KLF14 led us to identify perhexiline as a KLF14 activator that, when administered to animals, increases ApoA-I and HDL-C levels and enhances cholesterol efflux capacity in vivo. Notably, the treatment of ApoE-deficient mice with perhexiline attenuates atherosclerosis development.
HDL has been reported to exhibit many antiatherogenic properties, including its role in cholesterol efflux, inducing nitric oxide production, and antioxidant, antiinflammatory, and antiapoptotic effects. Recently, large cross-sectional studies have demonstrated that HDL-C cholesterol efflux capacity is decreased in CHD patients, which is independent of plasma HDL-C levels (6), suggesting that enhancing HDL functions may be the clinically relevant therapeutic target. Recent evidence has supported the concept that serum cholesterol efflux capacity is a strong predictor of cardiovascular risk, as cholesterol efflux capacity is an integrated measurement of HDL quantity and quality and also reflects the important function of HDL particles (6–8). Both HDL particles and ApoA-I can serve as acceptors of cholesterol from peripheral cells to the liver. Recombinant HDL (ApoA-I Milano) or reconstituted HDL particle infusion causes significant reduction in aortic cholesterol content and plaque size in small clinical trials and in rabbits (36–41). Raising the level of ApoA-I also results in a quick and significant plaque regression in mice (42–44). Consequently, approaches aimed at turning on endogenous production of ApoA-I are becoming critical strategies for increasing the number of circulating HDL particles and thus improving HDL functionality (13, 15, 45). Our findings demonstrate that the activation of KLF14 enhanced ABCA1-mediated cholesterol efflux capacity of serum by increasing circulation ApoA-I and HDL-C levels. The effect of KLF14 was determined to be specific for the upregulation of ApoA-I and HDL-C levels, as KLF2, KLF4, and KLF11 induced neither ApoA-I expression in vitro nor HDL-C levels in vivo. Recently, KLF11 was found to play a critical role in regulating hepatic TG metabolism, but not in cholesterol metabolism (46), suggesting that, although belonging to the same subfamily of metabolic regulator KLF proteins, these 2 highly related members have distinct functions. It was shown previously that KLF14 is a master trans-regulator of multiple genes that are associated with metabolic phenotypes in adipose tissue (26). These findings suggest that the role of KLF14 could be a central regulator of lipid metabolism in health and disease.
Unbiased human genetic studies can identify new pathways relevant to complex diseases, and the newly confirmed pathways could become novel therapeutic targets for CVDs. GWAS play a major role in the identification of lipid-associated loci with substantial effect on CHD risk. Although HDL-C levels inversely correlate with CHD, both genetic and pharmacological studies have not definitively confirmed a causative role of HDL-C in CHD. Notably, genetic studies performed during the past decade have tested the causal relationship between HDL-C and CHD using gene variants associated with elevated HDL levels, such as CETP, lecithin-cholesterol acyltransferase (LCAT), ABCA1, and hepatic TG lipase (LIPC) (47–49). These investigations, however, have largely failed to support a strong causal association between genetically raised plasma HDL-C levels and CHD risk. One important limitation of these studies, for instance, is that the functional characteristics of the HDL particles were not determined. On the other hand, recently, population-based cohort studies have demonstrated that a high HDL-C efflux capacity is associated with a decreased risk of coronary disease (8). Nonetheless, the causal relationship of the functional role of HDL, such as cholesterol efflux capacity and antiinflammatory effects, with the risk of CHD remains to be further investigated. Among those genes identified by GWAS, the T allele of rs4731702, located near the KLF14 gene, is significantly associated with HDL-C levels and a decreased risk of CHD and type 2 diabetes (17, 18, 50). Functional studies demonstrate that the T allele of rs4731702 is associated with increased expression of KLF14 in adipose tissue (50). In addition, the T allele carriers have higher ApoA-I levels in the Mulao population in China (21). We observed that KLF14 is downregulated in dyslipidemia mouse models and found that overexpression of KLF14 increases HDL-C levels by upregulating hepatic ApoA-I levels. To determine the contribution of hepatic KLF14 to both ApoA-I and HDL-C production, we generated LKO mice and found that they displayed a decrease in HDL-C levels. Thus, the combined results of experiments using a wide variety of complementary genetic approaches firmly establish a regulatory role for KLF14 in HDL functionality and atherosclerosis.
In aggregate, our present study has uncovered the central role of KLF14 in lipid metabolism and atherosclerosis. This conclusion is based on several findings, including the following: (a) KLF14 binds to the human APOA1 promoter to regulate the transcription of this gene, (b) KLF14 and its activator, perhexiline, increase ApoA-I and HDL-C levels in vivo and improve HDL function, and (c) administration of perhexiline inhibits atherosclerosis development in ApoE-deficient mice. We also show that perhexiline stimulates ApoA-I production in both hepatocytes and enterocytic cells, which may be an important basis for antiatherosclerotic effects. These data raise the interesting possibility that activation of the KLF14 pathway may be a therapeutic target for atherosclerosis treatment.
Currently, perhexiline is clinically used to treat angina and heart failure primarily in Australia, New Zealand, and the United Kingdom (51–55), with beneficial effects attributed to inhibition of mitochondrial CPT1. In the present study, we did not observe an obvious change of ApoA-I production in the medium from hepatocytes treated with etomoxir, another member of the CPT1 inhibitor group (56), suggesting the effect of perhexiline on ApoA-I expression is not likely the result of CPT1 inhibition. To examine whether upregulation of ApoA-I by perhexiline required hepatic KLF14, we treated KLF14-LKO mice with this drug and looked at the levels of this lipoprotein. We found that perhexiline administration increased HDL-C levels in WT mice, but this effect was attenuated in KLF14-LKO mice, indicating that the effect of this drug in increasing HDL-C is largely dependent on hepatic KLF14. Because the liver and intestine are two well-characterized tissues responsible for the biosynthesis of ApoA-I and HDL-C, it is difficult to strictly rule out the possible contributions of intestinal cells to lipid metabolism in this systemic treatment model. ApoC-III is a component of remnant particles, and loss-of-function mutations in this apolipoprotein have been associated with markedly reduced levels of TG as well as with the risk of CHD (57). Although we did not observe an increase of ApoC-III levels in the circulation of untreated mice, the mRNA encoding this protein was mildly upregulated by perhexiline. Thus, future studies will focus on investigating whether this phenomenon constitutes a side effect of this drug. In summary, the results of the current study demonstrate that KLF14 directly regulates ApoA-I transcription and increases circulating ApoA-I and HDL levels. Furthermore, we show that perhexiline, which acts as a pharmacological activator of KLF14 expression, attenuates atherosclerosis development. Although the mechanism or mechanisms of how perhexiline regulates KLF14 expression and lipid metabolism require further investigation, our study reveals an important role for KLF14-dependent regulation of HDL-C metabolism. These findings suggest that activation of KLF14 could be a potential target for prevention and treatment of atherosclerotic CVD.
Methods
Conditional disruption of Klf14 in mice.
The only exon of the Klf14 gene was flanked by loxP sites. Germline transmission of the loxP-flanked allele and Flp recombinase–mediated removal of the frt-flanked selection marker in vivo yielded mice (C57BL/C) harboring a Klf14 allele with 1 frt and 2 loxP sites (Klf14fl/fl). Klf14fl/fl mice were crossed with Alb-Cre transgenic mice (stock number: 003574) purchased from the Jackson Laboratory. Two-month-old male mice were used for experiments. Genomic DNA was extracted from mouse tails and was used for genotyping. Genotyping of KLF14-LKO mice was performed using 2 sets of primers. The first primer set was designed to amplify the Alb-Cre construct (forward, 5′-GAAGCAGAAGCTTAGGAAGATGG-3′; reverse, 5′-TTGGCCCCTTACCATAACTG-3′). Genotyping of Klf14fl/fl mice was performed by PCR amplification (forward, 5′-TAGTGAGGAAAGGAAGAGCAGGTAGGA-3′; reverse, 5′-TCACATGAGGAAACAGACAAGCAAAAG-3′).
Animals and diets.
C57BL/6, ob/ob (leptin-deficient mice), Apoe–/–, and Alb-Cre transgenic mice were purchased from the Jackson Laboratory and were housed at 22 ± 1°C on a 12-hour light/12-hour dark cycle. C57BL/6 mice had free access to water and rodent chow before switching to adjusted kcal HFD (44% from fat, Harlan, T.D. 06415). For hepatic overexpression of LacZ or KLF14, mice were administered AdKLF14 or AdLacZ at a dose of 5 × 108 plaque-forming units via tail vein injection after 12 weeks of HFD feeding. For knockdown of Klf14 in liver, mice were administered AdshKlf14 or AdshLacZ at a dose of 1 × 109 plaque-forming units via tail vein injection after 12 weeks of HFD feeding. Six days after the adenoviral infection, the animals were fasted for 12 hours and then sacrificed. Collected serum and liver tissues were stored at –80°C until processed. A mouse atherosclerosis model was generated by feeding 8-week-old male Apoe–/– mice an atherogenic diet (HCD, 21% fat, 34% sucrose, and 0.2% cholesterol, Harlan, T.D. 88137) for 10 weeks. Then the mice were treated 3 times a week (Monday, Wednesday, and Friday) with perhexiline (10 mg/kg) or DMSO for 6 weeks via gavage administration with continuous HCD.
Blood biochemical tests.
Direct LDL-C, direct HDL-C, and enzymatic-colorimetric assays used to determine serum TC and TG were carried out at the Chemistry Laboratory of the Michigan Diabetes Research and Training Center (University of Michigan). Blood glucose was measured using an ACCU-CHEK glucometer and glucose strips.
Lipoprotein separation by FPLC or HPLC.
Plasma lipoprotein profiles were determined by fast-performance liquid chromatography (FPLC) or by HPLC. For FPLC assay, 180 μl of serum pooled from mice was loaded and eluted at a constant flow rate of 0.50 ml/minute. The 40 fractions per sample were collected after running 36 minutes. For HPLC assay, 50 μl of serum pooled from mice was loaded and eluted at a constant flow rate of 1.0 ml/minute. The 32 fractions per sample were collected after running 5 minutes. Sample elution was monitored spectrophotometrically at an optical density of 280 nm. The cholesterol and TG contents in each fraction were measured with a fluorometric enzymatic assay (Cayman) and TG colorimetric assay in a GloMax-Multi+ plate reader (Promega).
Cells.
The cell lines 293AD, HepG2, J774.1, and Caco2 were obtained from ATCC and cultured according to ATCC protocols. Adenovirus-mediated gene transfer was performed by exposing 70% confluent HepG2 cells to the adenoviruses at a multiplicity of infection of 20 for 2 hours. Primary hepatocytes were isolated from 6- to 10-week-old mice as described previously (58). In brief, mice were anesthetized and the liver was exposed. The liver was perfused with liver perfusion medium and liver digestion medium (Invitrogen), and hepatocytes were washed and separated from other types of cells with Percoll (Sigma-Aldrich). Hepatocytes were seeded on rat tail type I collagen–coated plates or dishes in Williams’ E medium supplemented with 10% FBS for 3 hours, followed by a change to fresh DMEM containing 10% FBS.
Preparation of adenoviral vectors.
The full-length human KLF14 cDNA encoding KLF14 was subcloned into the pCR8⁄GW⁄TOPO entry vector (Invitrogen). After sequencing, the LR recombination reaction was carried out between the entry clone pCR8⁄GW⁄TOPO/KLF14 and the destination vector pAd/CMV/V5-DEST according to the manufacturer’s protocol (Invitrogen). For knockdown experiments, a siRNA oligo, which targets a region 100% conserved between human and mouse, was purchased from Invitrogen. To prepare adenovirus-containing shRNA for KLF14, synthesized oligos were annealed and inserted into BLOCK-iT U6 entry vector. The U6 promoter and shRNA were cloned into the adenoviral plasmid pAd/BLOCK-iT-DEST according to the manufacturer’s instructions. The sequences for shRNA were as follows: shKlf14, 5′-CACCGGCATCCAAGCGACATCAGTCGAAACTGATGTCGCTTGGATGC-3′, 5′-AAAAGCATCCAAGCGACATCAGTTTCGACTGATGTCGCTTGGATGCC-3′; shLacZ, 5′-CACCGCTACACAAATCAGCGATTTCGAAAAATCGCTGATTTGTGTAG-3′, 5′-AAAACTACACAAATCAGCGATTTTTCGAAATCGCTGATTTGTGTAGC-3′.
The 293AD cells were transfected with PacI linearized recombinant adenoviruses. After propagation, the recombinant adenoviruses were purified by CsCl2 density gradient ultracentrifugation. Adenovirus titration was performed using the Adeno-X qPCR Titration Kit (Clontech).
RNA isolation and RT-PCR.
Total RNA from tissues and cells was purified using QIAGEN’s RNeasy kits (QIAGEN). cDNA was synthesized using SuperScript III (Invitrogen), and qPCR was performed using SYBR green reagents (Bio-Rad). Primer pairs for reverse-transcriptase PCR (RT-PCR) are shown in Supplemental Table 2. Gene expression was presented as fold increase compared with RNA isolated from the control group by the comparative CT (2−ΔΔCT) method with 18S RNA as the reference gene.
Cholesterol efflux capacity assays.
The sera from AdKLF14- or AdLacZ-treated mice and DMSO- or perhexiline-treated mice were used for cholesterol efflux studies (6). J774.1 murine macrophages were labeled with 2 μCi/ml 3H-cholesterol for 24 hours in the presence of ACAT inhibitor (Sando 58-035) and equilibrated overnight with 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP in the present of ACAT inhibitor. apoB-depleted serum was obtained by PEG precipitation. 2.8% v/v apoB-depleted serum from mice was used as an efflux acceptor for 4 hours. Efflux was quantified by liquid scintillation and expressed as a percentage of total cell 3H-cholesterol content. All assays were performed in duplicate.
Immunoblotting.
Protein was extracted from the cells or liver tissues with lysis buffer (Thermo Scientific) supplemented with protease inhibitor cocktail (Roche Applied Science). The lysates were resolved by 4% to 12% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with the indicated antibodies. Antibodies used in this study were obtained from the following sources: ApoA-I (Sigma-Aldrich, SAB3500270, 1:2000 working dilution; Santa Cruz Biotechnology Inc., sc-30089, 1:1000 working dilution), KLF14 antibody (Santa Cruz Biotechnology Inc., sc-104345, 1:1000 working dilution was used to detect the overexpression samples; Thermo Scientific, PA5-23784, 1:1000 working dilution was used to detect mouse samples), SREBP1 antibody (Santa Cruz Biotechnology Inc., sc-366, 1:1000 working dilution), and actin and GAPDH antibody (Santa Cruz Biotechnology Inc., sc-1616 and sc-25778, 1:2000 working dilution). IRDye 680RD and 800CW secondary antibodies (LI-COR Biotechnology, 926-32212, 926-32213, 926-32214, 926-68074, 1:10000 working dilution) were used as second antibodies. Western blots were visualized and quantified using an Odyssey Infrared Imaging System (LI-COR Biosciences, version 2.1). Full uncut gels are shown in the supplemental material. The ApoA-I concentrations and ApoC-III in the serum were quantitated by ELISA according to the manufacturer’s protocol. ApoA-I ELISA kits were from Abcam (ab108804) and MyBioSource (MBS702111); the ApoC-III ELISA kit was from Abnova (KA1030).
Plasmids and transient transfection assays.
The genomic fragments harboring the putative KLF-binding sites in human APOA1 promoter were cloned by PCR from the human genomic DNA. The amplified products of 2.1 and 0.7 kb upstream of the translation start site of human APOA1 gene were ligated into the pGL4-luciferase reporter vector (Promega) to generate pGL4-1979/+163-luc, pGL4-710/+163-luc, and pGL4-94/+163-luc plasmids. Promoter activity was further validated by mutation of the 2 putative KLF14-binding sites on the promoter at –1943/–1938 or –491/–486 by replacing CACCC with CAtaC using the QuikChange Site-Directed Mutagenesis Kit (Stratagen). The numbers indicate the distance in nucleotides from the transcription start site (+1) of the human APOA1 gene. To prepare human KLF14 promoter–driven luciferase reporter, the amplified product of 1.6 kb upstream of the translation start site of the human KLF14 gene (–1567 to +65) was ligated into the pGL4-luciferase reporter vector to generate KLF14-luc plasmid. All PCR-generated constructs were verified by sequencing the DNA. Luciferase activity was measured as described before (58). In brief, HepG2 cells were transfected with pGL4-luciferase reporter plasmids and pRenilla-null as internal control (Promega) using Lipofectamine 2000 (Life Technologies). Cells were cultured for 24 hours after transfection, and cell lysates were measured using the Dual Luciferase Reporter Assay System Kit (Promega). For the screen to identify compounds activating KLF14, HepG2 cells were cultured for 24 hours after transfection with KLF14-luc and stimulated with compounds for another 24 hours. Luciferase activity was measured.
ChIP.
ChIP assays were performed according to the manufacturer’s protocol, with minor modifications using the EZ ChIP kit (Millipore) (58). In brief, HepG2 cells or mouse primary hepatocytes were infected with AdKLF14 or AdLacZ for 24 hours and then crosslinked with 1% formaldehyde and quenched prior to harvest and sonication. The sheared chromatin was immunoprecipitated with anti-KLF14 antibody (or control immunoglobulin G) conjugated to protein A/G sepharose beads. The eluted immunoprecipitates were digested with proteinase K, and DNA was extracted and underwent PCR with primers (Supplemental Table 2) flanking the putative KLF14-binding site within APOA1. The supernatant of the control group was used as an input control.
Analysis of atherosclerotic lesions (59).
Two quantitative methods were used in this study. First, en face analysis of atheromatous plaques was used: after staining with oil red O (Sigma-Aldrich) and removal of the adventitia of the whole aorta, aortas were opened longitudinally and pinned flat onto a black-wax plate. The percentage of the plaque area stained by oil red O with respect to the total luminal surface area was quantified with ImageJ analysis software (http://imagej.nih.gov/ij/). Second, the extent of the atherosclerotic lesions in the aortic root was determined: the atherosclerotic lesions in the aortic sinus region were examined at 5 locations, each separated by 80 μm, with the most proximal site starting after the appearance of at least 2 aortic valve leaflets. The largest plaques of the 3 valve leaflets were adopted for morphological analysis. All morphometric analyses were performed in a double-blinded manner.
Statistics.
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, Inc). Statistical comparisons and analyses between 2 groups were performed by 2-tailed, unpaired Student’s t test. Comparisons among 3 groups or more were analyzed with 1-way ANOVA followed by a Newman-Keuls test or 2-way ANOVA and multiple comparisons. P < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
Study approval.
All animal studies were performed according to protocols approved by the University Committee on Use and Care of Animals (UCUCA) of the University of Michigan.
Supplementary Material
Acknowledgments
This work was supported in whole or in part by NIH grants HL068878, HL105114, and HL088391 (to Y.E. Chen), DK89503 and DK097153 (to S. Pennathur), DK52913 (to R. Urrutia), and CA178627 (to G.A. Lomberk) and by American Heart Association National Scientist Development grants 15SDG24470155 (to Y. Guo) and 14SDG19880014 (to Y. Fan).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(10):3819–3830. doi:10.1172/JCI79048.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, et al. Bangdiwala S, and Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.CIR.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.McQueen MJ, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Boekholdt SM, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128(14):1504–1512. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafiane A, Jabor B, Ruel I, Ling J, Genest J. High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am J Cardiol. 2014;113(2):249–255. doi: 10.1016/j.amjcard.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Rohatgi A, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digby JE, Ruparelia N, Choudhury RP. Niacin in cardiovascular disease: recent preclinical and clinical developments. Arterioscler Thromb Vasc Biol. 2012;32(3):582–588. doi: 10.1161/ATVBAHA.111.236315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappelle PJ, van Tol A, Wolffenbuttel BH, Dullaart RP. Cholesteryl ester transfer protein inhibition in cardiovascular risk management: ongoing trials will end the confusion. Cardiovasc Ther. 2011;29(6):e89–e99. doi: 10.1111/j.1755-5922.2010.00201.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg I, Goldbourt U, Boyko V, Behar S, Reicher-Reiss H, BIP Study Group Relation between on-treatment increments in serum high-density lipoprotein cholesterol levels and cardiac mortality in patients with coronary heart disease (from the Bezafibrate Infarction Prevention trial) Am J Cardiol. 2006;97(4):466–471. doi: 10.1016/j.amjcard.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 12.Jahagirdar R, et al. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis. 2014;236(1):91–100. doi: 10.1016/j.atherosclerosis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 13.McLure KG, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8(12): doi: 10.1371/journal.pone.0083190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picaud S, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci U S A. 2013;110(49):19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.[No authors listed] Rvx 208. Drugs R D. 2011;11(2):207–213. doi: 10.2165/11595140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholls SJ, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57(9):1111–1119. doi: 10.1016/j.jacc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, et al. Genome-wide association study validation identifies novel loci for atherosclerotic cardiovascular disease. J Thromb Haemost. 2012;10(8):1508–1514. doi: 10.1111/j.1538-7836.2012.04815.x. [DOI] [PubMed] [Google Scholar]
- 19.Chasman DI, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5(11): doi: 10.1371/journal.pgen.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Assuncao TM, et al. New role for Kruppel-like factor 14 as a transcriptional activator involved in the generation of signaling lipids. J Biol Chem. 2014;289(22):15798–15809. doi: 10.1074/jbc.M113.544346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P, et al. Association of the KLF14 rs4731702 SNP and serum lipid levels in the Guangxi Mulao and Han populations. Biomed Res Int. 2013;2013: doi: 10.1155/2013/231515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, et al. Genome-wide association study identifies novel loci association with fasting insulin and insulin resistance in African Americans. Hum Mol Genet. 2012;21(20):4530–4536. doi: 10.1093/hmg/dds282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohshige T, et al. Association of new loci identified in European genome-wide association studies with susceptibility to type 2 diabetes in the Japanese. PLoS One. 2011;6(10): doi: 10.1371/journal.pone.0026911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees SD, et al. Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia. 2011;54(6):1368–1374. doi: 10.1007/s00125-011-2063-2. [DOI] [PubMed] [Google Scholar]
- 26.Small KS, et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat Genet. 2011;43(6):561–564. doi: 10.1038/ng.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacey SN, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. 2009;41(8):909–914. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steegenga WT, et al. Genome-wide age-related changes in DNA methylation and gene expression in human PBMCs. Age (Dordr) 2014;36(3): doi: 10.1007/s11357-014-9648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarmento OF, et al. A novel role for KLF14 in T regulatory cell differentiation. Cell Mol Gastroenterol Hepatol. 2015;1(2):188–202 e4. doi: 10.1016/j.jcmgh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274(42):30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 31.Mei M, et al. Inflammatory stress exacerbates ectopic lipid deposition in C57BL/6J mice. Lipids Health Dis. 2011;10: doi: 10.1186/1476-511X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellerstein M, Turner S. Reverse cholesterol transport fluxes. Curr Opin Lipidol. 2014;25(1):40–47. doi: 10.1097/MOL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 33.Glickman RM, Green PH, Lees RS, Tall A. Apoprotein A-I synthesis in normal intestinal mucosa and in Tangier disease. N Engl J Med. 1978;299(26):1424–1427. doi: 10.1056/NEJM197812282992602. [DOI] [PubMed] [Google Scholar]
- 34.Acharjee S, Roe MT, Amsterdam EA, Holmes DN, Boden WE. Relation of admission high-density lipoprotein cholesterol level and in-hospital mortality in patients with acute non-ST segment elevation myocardial infarction (from the National Cardiovascular Data Registry) Am J Cardiol. 2013;112(8):1057–1062. doi: 10.1016/j.amjcard.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 35.Sachdeva A, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111–117. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Zhao XQ, Brown BG. ApoA-I(Milano)/phospholipid complex: clinical implications of dose-response studies in rabbit atherosclerosis with intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51(11):1110–1111. doi: 10.1016/j.jacc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Nissen SE, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 38.Tardif JC, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297(15):1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 39.Diditchenko S, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33(9):2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 40.Gille A, Easton R, D’Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
- 41.Waksman R, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55(24):2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 42.Parolini C, et al. Apolipoprotein A-I and the molecular variant apoA-I(Milano): evaluation of the antiatherogenic effects in knock-in mouse model. Atherosclerosis. 2005;183(2):222–229. doi: 10.1016/j.atherosclerosis.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100(17):1816–1822. doi: 10.1161/01.CIR.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 44.Benoit P, et al. Somatic gene transfer of human ApoA-I inhibits atherosclerosis progression in mouse models. Circulation. 1999;99(1):105–110. doi: 10.1161/01.CIR.99.1.105. [DOI] [PubMed] [Google Scholar]
- 45.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, et al. Mouse KLF11 regulates hepatic lipid metabolism. J Hepatol. 2013;58(4):763–770. doi: 10.1016/j.jhep.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, Pare G, Parker AN, Zee RY, Miletich JP, Chasman DI. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18 245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2(1):26–33. doi: 10.1161/CIRCGENETICS.108.817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johannsen TH, et al. Hepatic lipase, genetically elevated high-density lipoprotein, and risk of ischemic cardiovascular disease. J Clin Endocrinol Metab. 2009;94(4):1264–1273. doi: 10.1210/jc.2008-1342. [DOI] [PubMed] [Google Scholar]
- 49.Frikke-Schmidt R, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 50.Kong A, et al. Parental origin of sequence variants associated with complex diseases. Nature. 2009;462(7275):868–874. doi: 10.1038/nature08625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikram H, Maslowski AH. Unstable angina and perhexilene maleate. N Z Med J. 1981;94(692):236–237. [PubMed] [Google Scholar]
- 52.Ashrafian H, Horowitz JD, Frenneaux MP. Perhexiline. Cardiovasc Drug Rev. 2007;25(1):76–97. doi: 10.1111/j.1527-3466.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 53.Phan TT, et al. Multi-centre experience on the use of perhexiline in chronic heart failure and refractory angina: old drug, new hope. Eur J Heart Fail. 2009;11(9):881–886. doi: 10.1093/eurjhf/hfp106. [DOI] [PubMed] [Google Scholar]
- 54.Bansal M, Chan J, Leano R, Pillans P, Horowitz J, Marwick TH. Effects of perhexiline on myocardial deformation in patients with ischaemic left ventricular dysfunction. Int J Cardiol. 2010;139(2):107–112. doi: 10.1016/j.ijcard.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Drury NE, et al. Relationship between plasma, atrial and ventricular perhexiline concentrations in humans: insights into factors affecting myocardial uptake. Br J Clin Pharmacol. 2014;77(5):789–795. doi: 10.1111/bcp.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zarain-Herzberg A, Rupp H. Therapeutic potential of CPT I inhibitors: cardiac gene transcription as a target. Expert Opin Investig Drugs. 2002;11(3):345–356. doi: 10.1517/13543784.11.3.345. [DOI] [PubMed] [Google Scholar]
- 57.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 58.Fan Y, Guo Y, Hamblin M, Chang L, Zhang J, Chen YE. Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor alpha. J Biol Chem. 2011;286(47):40584–40594. doi: 10.1074/jbc.M111.232918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang L, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.