Abstract
Objective
To examine whether comprehensive chromosome screening (CCS) for preimplantation genetic screening (PGS) has an effect on improving in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) outcomes compared to traditional morphological methods.
Methods
A literature search was conducted in PubMed, EMBASE, CNKI and ClinicalTrials.gov up to May 2015. Two reviewers independently evaluated titles and abstracts, extracted data and assessed quality. We included studies that compared the IVF/ICSI outcomes of CCS-based embryo selection with those of the traditional morphological method. Relative risk (RR) values with corresponding 95% confidence intervals (CIs) were calculated in RevMan 5.3, and subgroup analysis and Begg’s test were used to assess heterogeneity and potential publication bias, respectively.
Results
Four RCTs and seven cohort studies were included. A meta-analysis of the outcomes showed that compared to morphological criteria, euploid embryos identified by CCS were more likely to be successfully implanted (RCT RR 1.32, 95% CI 1.18–1.47; cohort study RR 1.74, 95% CI 1.35–2.24). CCS-based PGS was also related to an increased clinical pregnancy rate (RCT RR 1.26, 95% CI 0.83–1.93; cohort study RR 1.48, 95% CI 1.20–1.83), an increased ongoing pregnancy rate (RCT RR 1.31, 95% CI 0.64–2.66; cohort study RR 1.61, 95% CI 1.30–2.00), and an increased live birth rate (RCT RR 1.26, 95% CI 1.05–1.50; cohort study RR 1.35, 95% CI 0.85–2.13) as well as a decreased miscarriage rate (RCT RR 0.53, 95% CI 0.24–1.15; cohort study RR 0.31, 95% CI 0.21–0.46) and a decreased multiple pregnancy rate (RCT RR 0.02, 95% CI 0.00–0.26; cohort study RR 0.19, 95% CI 0.07–0.51). The results of the subgroup analysis also showed a significantly increased implantation rate in the CCS group.
Conclusions
The effectiveness of CCS-based PGS is comparable to that of traditional morphological methods, with better outcomes for women receiving IVF/ICSI technology. The transfer of both trophectoderm-biopsied and blastomere-biopsied CCS-euploid embryos can improve the implantation rate.
Introduction
It has been thirty-seven years since the first IVF (in vitro fertilization) baby was born in 1978 [1]. In spite of recent advances, the majority of IVF cycles fail to achieve a live birth. One of the main causes of the depressing clinical outcomes has been proven to be embryo aneuploidy [2–4]. Aneuploidy is a very common abnormality in human embryos generated by IVF, particularly for women with advanced maternal age (AMA) [5, 6]. By the age of 40, it is not unusual for the proportion of aneuploid embryos to exceed 50% [7]. A high percentage of aneuploidy has also been found in the embryos of women with repeated implantation failure (RIF) [8, 9], repeated pregnancy loss (RPL) [10] and a partner with low sperm quality [11, 12]. Even for younger women (<35 years) with good prognosis, the aneuploidy rate remains high [13–15]. An aneuploid embryo is scarcely able to form a viable pregnancy. The high frequency of aneuploidy and its likely deleterious effects on embryo viability has led to the suggestion that embryos should be tested for chromosomal abnormalities before determining which ones to transfer to patients. Because traditional embryo selection methods based on morphology are incapable of detecting chromosomal abnormalities [16, 17], preimplantation genetic screening (PGS) was developed.
Fluorescence in situ hybridization (FISH) testing of a panel of chromosomes was previously the most widely applied method for aneuploidy screening. However, because previous data from random controlled trials with FISH-based PGS (PGS#1) showed no beneficial effects on live birth rates after IVF and even lower live birth rates for women with AMA [18, 19], the utilization of PGS#1 in attempts to improve IVF outcome has declined worldwide [20, 21]. The inefficiency of PGS#1 occurs for many reasons. One of the main limitations of FISH is that it can only test a restricted number of chromosomes in PGS. For embryos that are aneuploidy for untested chromosomes, FISH-based PGS cannot make an accurate evaluation. However, the objective of comprehensive chromosome screening (CCS) is to assess the entire chromosome complement (24 chromosomes).
Several studies have been conducted to assess the effect of CCS-based PGS on IVF/intracytoplasmic sperm injection (ICSI) outcomes, and 2 systematic reviews were published recently [22, 23]. However, these 2 systematic reviews did not conduct pooled analyses of the included studies. Therefore, we conducted a meta-analysis with more eligible studies to provide a more precise and comprehensive estimation of CCS-based PGS.
Materials and Methods
Literature search
We conducted electronic searches in the databases PubMed, EMBASE, CNKI (China National Knowledge Infrastructure) and ClinicalTrials.gov up to May 20, 2015 with no study design limitations and no language restrictions. The following search terms were used: ‘preimplantation genetic diagnosis’ or ‘PGD’ or ‘preimplantation genetic screening’ or ‘preimplantation test’ or ‘screening for aneuploidies’ or ‘embryo selection’ or ‘embryo screening’ and ‘comprehensive chromosomal screening’ or ‘CCS’ or ‘array comparative genomic hybridization’ or ‘array CGH’ or ‘aCGH’ or ‘single nucleotide polymorphism’ or ‘SNP’ or ‘quantitative real-time PCR’ or ‘qPCR’ or ‘next-generation sequencing’ or ‘NGS’. Moreover, a manual search of published articles was conducted to identify additional relevant studies.
Study selection and data extraction
After duplicate publications were removed, two authors (CMH and WSY) independently examined the potentially relevant trials by checking the titles, abstracts and full-texts, and any problems of disagreement were resolved through group discussion. We adapted the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow-chart to depict the study selection process (see S1 File. PRISMA checklist. ) [24]. Published clinical trials were eligible for inclusion if they compared CCS-based PGS using blastocyst biopsy/trophectoderm biopsy to traditional morphological methods in genetically normal couples undergoing IVF and/or ICSI. Because polar body (PB) aneuploidy screening can only detect chromosomal abnormalities of meiotic origin and is limited to predicting subsequent embryo ploidy, we excluded studies associated with polar body biopsy to prevent selection bias. Then, two authors (CMH and WSY) independently extracted related information pertaining to the first author’s name, study design, year of publication, study period, geographic region, sample sizes of the groups using CCS versus morphological methods, type of CCS, patient characteristics, indication for PGS, day of biopsy, day of transfer, and fresh or frozen cycles. The primary outcome was the implantation rate per ET, and the secondary outcomes were the clinical pregnancy rate per cycle, ongoing pregnancy rate per cycle, live birth rate per cycle, miscarriage rate and multipregnancy rate. In all of the studies, the participants who underwent embryo biopsy for CCS-based PGS were defined as the CCS group, and the participants who used traditional morphological methods were defined as the control group.
Quality assessment
Two reviewers (CMH and WSY) independently used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included observational studies [25]. The NOS includes selection, comparability and outcome for cohort studies: 4 scores are assigned for the selection part, 2 scores for comparability and 3 scores for the outcome part. Studies with scores of 0 to 3, 4 to 6 and 7 to 9 were considered as low, moderate and high quality, respectively. The Cochrane Collaboration’s Handbook was used to assess the quality of the RCTs according to the following criteria: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias [26]. A judgment of ‘Yes’ indicates a low risk of bias, ‘No’ indicates a high risk of bias, and ‘Unclear’ indicates an unclear or unknown risk of bias.
Statistical analysis
The effect of CCS-based PGS was assessed for the RCTs and cohort studies separately. We calculated the relative risk (RR) with corresponding 95% confidence intervals (CIs) for all of the outcomes reported in each study. A meta-analysis of the outcomes was performed when there were available data that could be combined and meta-analytical pooling was feasible. We assessed heterogeneity among the studies by conducting a standard Cochrane’s Q test with a significance level of α = 0.10. The I2 statistical test was performed to further examine heterogeneity. I2≥50% was considered to indicate substantial heterogeneity [27]. When heterogeneity existed, we attempted to identify potential sources of heterogeneity by examining individual studies and conducting subgroup analyses. Fixed-effect models were used to pool outcomes when heterogeneity among studies was considered to be statistically insignificant. Otherwise, a random-effect model was used to combine the results. Moreover, subgroup analyses were conducted according to study design, location, age of the participants, indication for PGS, stage of biopsy, platform for CCS and number of embryos transferred. Publication bias was estimated using Begg’s test [28]. A value of “Pr>|z|” greater than 0.05 for Begg’s funnel plots was considered to indicate negative publication bias. A one-way sensitivity analysis was performed to explore certain factors that would influence the effects. All of the statistics were two-tailed, and P<0.05 was considered statistically significant. RevMan 5.3 was used to perform the meta-analysis.
Results
Study selection and characteristics
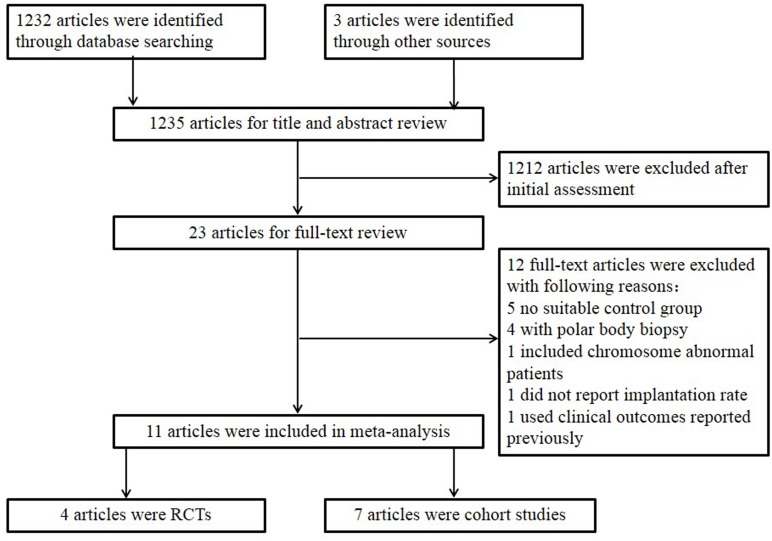
A total of 1235 unduplicated titles and abstracts were identified in the initial search, and 23 articles were selected to undergo full-text assessment. Twelve studies did not fulfill the inclusion criteria, of these, five studies had no suitable control group, one study included chromosome abnornal patients, one study used clinical outcome data reported previously in another study we included in our meta-analysis, one study did not report implantation rate, four studies associated with polar body biopsy. Finally, 4 RCTs and 7 cohort studies that assessed the outcomes of CCS-based PGS versus traditional morphological-based selection in women undergoing IVF/ICSI met our inclusion criteria and were included in the meta-analysis [29–39]. A flow chart of the trials included in the meta-analysis is shown in Fig 1.
Fig 1. Flow chart of search and selection strategy.
Overall, the 11 included studies accounted for 2425 ART cycles (RCTs: 247 for the CCS group, 255 for the control group; cohort studies: 729 for the CCS group, 1194 for the control group) in 2338 women, with ages ranging from 23 to 43 years. Of these studies, 8 were performed in North America, 2 in Europe [36, 37] and 1 in Asia [29]. For 3 RCTs, CCS-based PGS was performed for the women with good prognosis, and for 1 RCT [39] and all the cohort studies, CCS-based PGS was indicated in AMA, RIF, RPL, or for other reasons. The platform for CCS was CGH for 5 studies, qPCR for 3 studies, SNP for 1 study, and NGS for 1 study. Moreover, 3 studies performed embryo biopsy in the cleavage stage [29, 31, 37], and the others in the blastocyst stage. Single embryo transfer was performed in 3 studies [30, 32, 34], and 8 studies performed more than one embryo transfer. The main characteristics and quality features of the 4 RCTs and 7 cohort studies are shown in Tables 1 and 2.
Table 1. Characteristics of included RCTs.
| Study and years | Patients (PGS/control) | Cycles (PGS/control) | Study design | Platform for PGS | Inclusion criteria | Exclusion criteria | ART and embryo transfer | Biopsy | Outcomes | Quality features |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang 2012 | Total 112 (56/56) | Total 103 (56/56) | Prospective randomized single-blind controlled trial | aCGH | <35 years; a history of regular ovulation; etiology of infertility was tubal factor or male factor (or both); no prior IVF treatment; no prior miscarriage; normal intrauterine contour; both ovaries intact; basal serum FSH<10 IU/l, basal estradiol<60 pg/ml. | Treatment incorporated donor gametes or frozen/thawed embryos. | ICSI; SET for both groups; all cycles involved day 6 blastocyst transfer; all cycles were fresh cycles. | Laser-assisted hatching for all 6–8 cleavage stage embryos on day 3; 3–5 herniated TE cells removed; biopsy on blastocysts on day 5. | Clinical pregnancy rate (same as implantation rate for SET); on-going pregnancy rate (more than 20 weeks GA); missed abortion rate. | Randomization by random number table; concealment of allocation not reported; single-blind; 2 centers; full paper; power calculation not reported; no intention to treat. |
| Schoolcraft 2012 | Total 60 (30/30) | Total 60 (30/30) | IRB approved randomized control trial | SNP micro-array | >35 years | Exclusion criteria not reported. | ART method not reported; number of embryos transferred not reported; all cycles involved blastocyst transfer; frozen cycles for PGS group, fresh cycles for control group. | Biopsy method not reported; removed TE cells for biopsy. | Implantation rate; first trimester pregnancy lose rate. | Randomization by computer; concealment of allocation not reported; blinding not reported; one centre; only abstract; power calculation not reported; intention to treat. |
| Forman 2013 | Total 175 (89/86) | Total 175 (89/86) | Randomized open label noninferiority trial | Real-time, polymerase chain reaction-based CCS. | <42 years; at most one prior failed IVF cycle; normal endometrial cavity; normal ovarian reverse (AMH>1.2 ng/mL, day 3 FSH<12 IU/L); at least 2 expanded blastocysts suitable for transfer or cryopreservation by day 6 of embryo development. | Severe male infertility; anovulatory polycystic ovarian syndrome; BMI>30 kg/m2. | ICSI; SET for CCS group, 2 blastocysts for control group. Fresh cycles involved day6 blastocyst transfer; frozen frozen cycles involved blastocyst transfer 5 days after starting P. Both groups involved fresh and frozen cycles. | Laser-assisted hatching for all cleaved embryos on day 3; number of TE cells removed not reported; biopsy on blastocysts on day 5. | Ongoing pregnancy rate; clinical miscarriage rate; sustained implantation rate; multiple pregnancy rate | Randomization by computer; concealment of allocation achieved by sequentially numbered, opaque, sealed envelopes; not blinded; 1 center; full paper; power calculation; intention to treat; block randomization used for fresh and frozen cycles. |
| Scott 2013 | Total 155 (72/83) | Total 155 (72/83) | Prospective randomized controlled trial | Rapid quantitative real-time polymerase chain reaction (qPCR)-based comprehensive chromosome screening | 21–42 years; no more than one prior failed IVF retrieval; normal endometrial cavity; basal FSH level<15IU/L; basal follicle cunt >8; available ejaculate sperm; willingness to limit transfer order to a maximum 2 embryos; 2 or more blastocysts by the afternoon of day 5. | Less than 2 blastocysts by day 5. | ICSI; maximum 2 embryos for each patients. CCS group involved blastocysts transfer on day 6; control group involved blastocysts on day 5; all cycles were fresh cycles. | Laser-assisted hatching for all embryos on day 3; number of TE cells removed not reported; biopsy on blastocysts on day 5. | Implantation rate; pregnancy rate; delivery rate. | Randomization by computer; concealment of allocation not reported; blind not reported; 1 center; full paper; power calculation; intention to treat; block randomization used for different age groups. |
Table 2. Characteristics of included cohort studies.
| Study and years | Patients (PGS/control) | Cycles (PGS/control) | Study design | Type of CCS used | Characteristics of CCS group patients | ART and embryo transfer | Biopsy | Outcomes | Confounders adjusted for | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Schoolcraft 2010 | Total 119; (45/113) | Total 119; (45/113) | Prospective matched cohort study | Comparative genomic hybridization. | maternal age >35 years and/or with a history of unsuccessful IVF treatment or previous spontaneous abortion. | ICSI; maximum 4 for transfer in the CCS group; all cycles involved blastocyst transfer; all cycles were frozen cycles. | Laser; 3–10 TE cells(mean 5) removed; biopsy on expanding or expanded blastocysts on day 5 or day 6. | bHCG positive rate pre cycle; implantation rate (fetal sac); ongoing implantation rate (a fetus with heartbeat); Live birth rate | Year of treatment; one center; maternal age; day3 FSH level; day of transfer; number of previous unsuccessful IVF attempts. | 9 |
| Schoolcraft 2013 | Total 737 (347/390) | Total 737 (347/390) | Prospective unmatched cohort study | CCS (method for CCS not reported). | The majority of female infertility presented with normal ovarian reserve (based on day 3 FSH, E2, antimullerian hormone, and antral follicle count). The majority of male infertility patients showed no indications of male-factor infertility (based on sperm concentration, motility, and strict Kruger morphology). | ART method not reported; SET for all groups; all cycles involved blastocyst transfer; only frozen cycles for PGS group; both frozen and fresh cycles for control group. | Biopsy method not reported; biopsy on blastocysts. | Implantation rate; missed abortion rate; ongoing pregnancy rate | One center. | 7 |
| Greco 2014 | Total 121 (88/33) | Total 121 (88/33) | Prospective matched cohort study | Array CGH | <36 years; without a history of recurrent miscarriages; without abnormal karyotype, uterine abnormalities, autoimmune conditions, thrombophilia, severe endometriosis and reduced ovarian reserve; male patients without severe infertility (<500.000 motile sperm/mL after preparation) or high sperm DNA fragmentation; 43 couples with a history of 3–9 implantation failures, 45 couples underwent the first IVF attempt (good prognosis). | ICSI; SET for PGS group; 1–2 embryos for transfer in the control group; all cycles involved blastocyst transfer; included both frozen and fresh cycles in both groups. | Laser; 5–10 TE cells removed; biopsy on blastocysts on day 5 or day 6. | bHCG positive rate; implantation rate; clinical pregnancy rate; biochemical pregnancy rate; anembryonic pregnancy rate; tubal pregnancy rate; spontaneous abortion rate | Maternal age; day 3 FSH; day 3 AMH; antral follicle count; sperm count; sperm motility; sperm morphology; day of transfer; PGS patients divided into 2 subgroups (RIF PGS group and NO RIF PGS group) | 8 |
| Keltz 2013 | Total 346 (35/311) | Total 433 (39/394) | Retrospective unmatched cohort study | Array CGH | At least of 5 embryos six or more cells on day 3; indication for PGS included advanced maternal age, RIF, RPL. | ICSI; generally maximum 1 embryo for transfer for patients <35 years, maximum 2 for patients >35years; PGS group involved only blastocyst transfer; all cycles were fresh cycles. | Laser; single blastomere removed; biopsy on cleavage-stage embryos on day 3. | Implantation rate; clinical pregnancy rate; ongoing pregnancy rate; multiple-pregnancy rate; miscarriage rate (prior to 20 gestational weeks) | Year of treatment; one center; number of healthy-appearing embryos on day 3; sub-analysis for maternal age (<35years or >35years). | 9 |
| Wang 2014 | Total 54 (25/29) | Total 54 (25/29) | Prospective unmatched cohort study | Array CGH | 2 or more spontaneous abortions; without abnormal karyotype, uterine abnormalities, autoimmune conditions, severe endometriosis and reduced ovarian reserve; no indications of male-factor infertility. | ICSI; maximum 2 embryos for transfer in PGS group, maximum 3 in the control group; all cycles involved blastocyst transfer; all cycles were frozen cycles. | Laser; 1–2 blastomeres removed; biopsy on cleavage-stage embryos on day 3. | Implantation rate; clinical pregnancy rate; first trimester abortion rate. | Year of treatment; one center. | 7 |
| Forman 2012 | Total 322 (140/182) | Total 322 (140/182) | Retrospective matched cohort study | Quantitative real-time PCR (qPCR). | Had four or more mature follicles (>14mm) on the day of hCG administration. Indication for PGS: advanced maternal age (>35 years); had a previous failed IVF cycle; had a history of recurrent pregnancy loss; wanted to optimize outcomes with SET. | ICSI for PGS group; SET for both groups; all cycles involved blastocyst transfer; included both frozen and fresh cycles in both groups. | Laser; about 5 TE cells removed; biopsy on blastocysts on day 5. | Chemical preganncy rate; ongoing pregnancy rate; clinical pregnancy rate; monozygotic twin rate; gestational age at delivery; birthweight. | Maximal Day 3 FSH; prior deliveries; prior COH/IUI cycles; prior FETs. | 7 |
| Lukaszuk 2014 | Total 98 (45/53) | Total 98 (45/53) | Prospective matched cohort study | Semiconductor—based next-generation sequencing (NGS) | Repeated implantation failures (more than 2 previous unsuccessful failures). | ICSI; minimum 1 embryo for transfer in both groups; all cycles involved blastocyst transfer; all cycles were fresh cycles. | Laser; single blastomere removed; biopsy on cleavage-stage embryos on day 3. | Clinical pregnancy rate (per cycle; per ET); implantation rate; multiple pregnancy rate; ectopic pregnancy rate; OHSS rate; biochemical pregnancy rate; spontaneous abortion rate; ongoing pregnancy rate; live birth rate. | Year of treatment; one center; infertility etiology; number of failed cycles; duration of infertility; maternal age; BMI; antral follicle count; range of hormonal and other prognostic markers (AMH, inhibin B, basal FSH, basal LH, basal E2, DHEAS, testosterone, SHBG). | 9 |
Quality assessment
Study design and methodological quality varied among the 4 RCTs. One study used a random number table to generate a randomized sequence [32]; 1 study used computer-generated randomization [35]; randomization was stratified by age group in 1 study [33] and the remaining study did not explicitly describe sequence generation [39]. Adequate measures of allocation concealment were used and explicitly described in only one study [35]. Single blinding was performed in one study [32], 1 study was not blinded [35], and the remaining 2 studies did not describe the method of blinding [33, 39].
For the 7 cohort studies that were included, the NOS scores ranged from 7 to 9, with a mean score of 8. All of the studies provided information on the populations in the CCS group and the control group. However, only 4 studies were well matched between the CCS group and the control group [38, 36, 31, 37], and the other 3 studies were unmatched [29, 30, 34]; thus, comparability bias might exist in the 3 studies because important factors that could influence the results were not controlled for. The follow-up period for outcome was adequate for all of the studies, and the outcome measurement was objective.
Main outcomes
Implantation rate
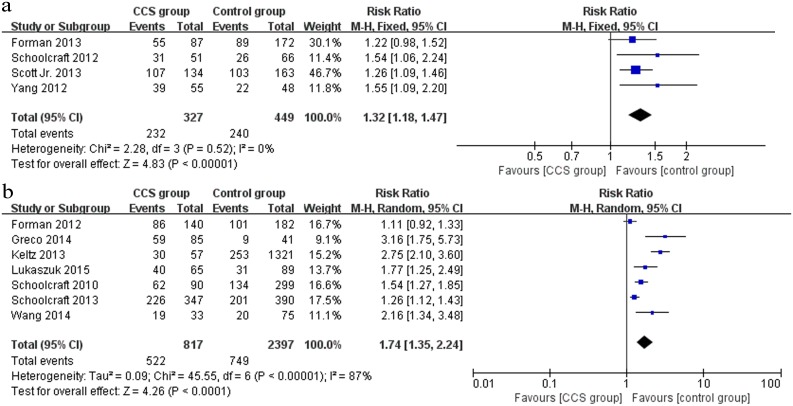
All of the included studies (4 RCTs and 7 cohort studies) provided data on the implantation rate. Within the 4 RCTs (n = 776) that compared CCS-based PGS and traditional morphological-based selection, the CCS group showed a higher implantation rate than the control group (RR 1.32, 95% CI 1.18–1.47) (Fig 2a). The same effects were observed within the 7 cohort studies (n = 3214) (RR 1.74, 95% CI 1.35–2.24) (Fig 2b).
Fig 2. Forest plots showing the results of meta-analysis on implantation comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on implantation of RCTs; (b) Forest plot of pooled RR on implantation of cohort studies.
Clinical pregnancy
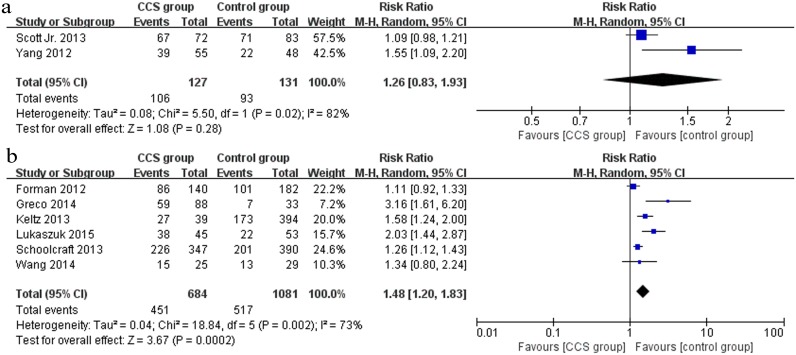
Eight studies (2 RCTs and 6 cohort studies) reported clinical pregnancy outcomes. The outcome from the pooled analysis of 2 RCTs [33, 32] (n = 258) showed a non-significant effect between the CCS group and the control group (RR 1.26, 95% CI 0.83–1.93) (Fig 3a), whereas a statistically significant increase in clinical pregnancy rate because of CCS-based PGS was observed in 6 cohort studies [29, 36, 30, 31, 37, 34] (n = 1765) (RR 1.48, 95% CI 1.20–1.83) (Fig 3b).
Fig 3. Forest plots showing the results of meta-analysis on clinical pregnancy comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on clinical pregnancy of RCTs; (b) Forest plot of pooled RR on clinical pregnancy of cohort studies.
Ongoing pregnancy
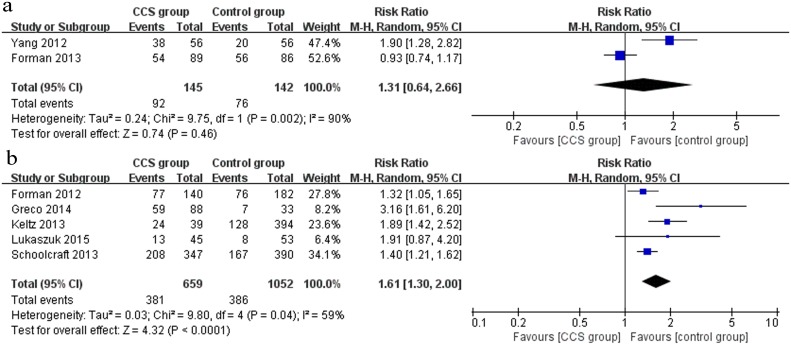
Seven studies (2 RCTs and 5 cohort studies) presented data regarding ongoing pregnancy. The pooled ongoing pregnancy rate appeared to be higher in the CCS group than in the control group in 2 RCTs [35, 32] (n = 287), but there was no significant difference between the two groups (RR 1.31, 95% CI 0.64–2.66) (Fig 4a). However, the pooled outcome of 5 cohort studies [36, 30, 31, 37, 34] (n = 1711) showed that CCS significantly improved the ongoing pregnancy rate (RR 1.61, 95% CI 1.30–2.00) (Fig 4b).
Fig 4. Forest plots showing the results of meta-analysis on ongoing pregnancy comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on ongoing pregnancy of RCTs; (b) Forest plot of pooled RR on ongoing pregnancy of cohort studies.
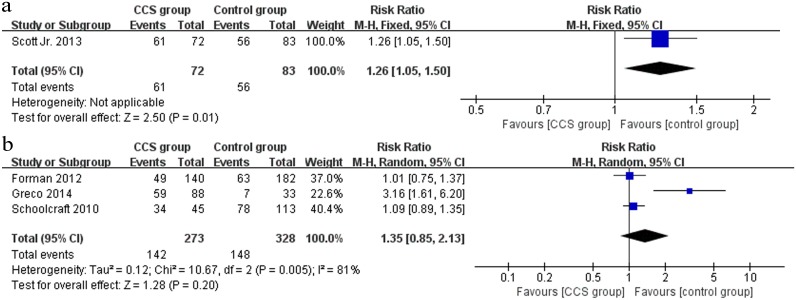
Live birth
A total of 4 studies (1 RCT and 3 cohort studies) investigated the outcome of live birth. In the RCT [33], there was a statistically significantly higher live birth rate in the CCS group (61/72) compared to the control group (56/83) (RR 1.26, 95% CI 1.05–1.50) (Fig 5a). However, when the outcome was pooled for the 3 cohort studies [38, 36, 34] (n = 601), no significant difference in live birth rate was observed between the CCS group and the control group (RR 1.35, 95% CI 0.85–2.13) (Fig 5b).
Fig 5. Forest plots showing the results of meta-analysis on live birth comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on live birth of RCTs; (b) Forest plot of pooled RR on live birth of cohort studies.
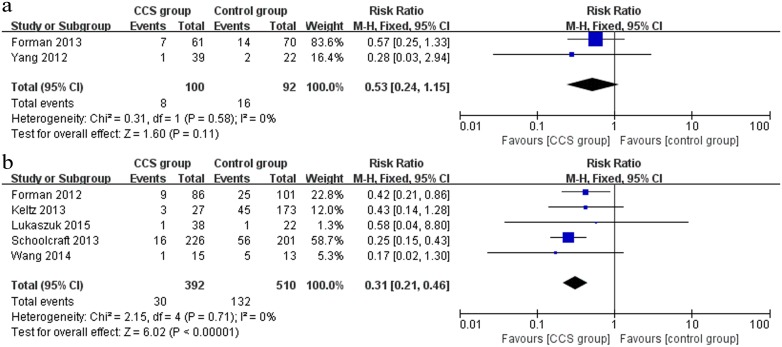
Miscarriage rate
Two RCTs [35, 32] and 5 cohort [29–31, 37, 34] studies evaluated the outcome of miscarriage between the CCS group and the control group. The pooled outcome from 2 RCTs (n = 192) showed a decreased miscarriage rate in the CCS group, but there was no significant difference between the two groups (RR 0.53, 95% CI 0.24–1.15) (Fig 6a). Nevertheless, a pooled analysis of outcome including the 5 cohort studies (n = 902) showed that the miscarriage rate was significantly lower in the CCS group (RR 0.31, 95% CI 0.21–0.46) (Fig 6b).
Fig 6. Forest plots showing the results of meta-analysis on miscarriage comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on miscarriage of RCTs; (b) Forest plot of pooled RR on miscarriage of cohort studies.
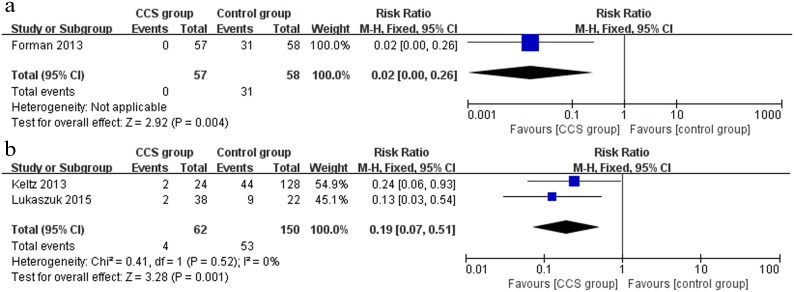
Multiple pregnancy
Only 3 studies (1 RCT and 2 cohort studies) reported multiple pregnancy rate data. In the RCT [35], the multiple pregnancy rate was significantly lower in the CCS group (0/57) than in the control group (31/58) (RR 0.02, 95% CI 0.00–0.26) (Fig 7a). Moreover, the same effect was observed in the pooled outcome of the 2 cohort studies [31, 37] (RR 0.19, 95% CI 0.07–0.51) (Fig 7b).
Fig 7. Forest plots showing the results of meta-analysis on multiple pregnancy comparing the effect of CCS-based PGS and traditional morphological method after IVF/ICSI.
(a) Forest plot of pooled RR on multiple pregnancy of RCTs; (b) Forest plot of pooled RR on multiple pregnancy of cohort studies.
A summary of the results of the meta-analysis comparing CCS with no CCS outcomes in the included RCTs and cohort studies is presented in Table 3.
Table 3. Summary of results of meta-analysis of CCS compared with no CCS outcomes in included RCTs and cohort studies.
| Outcome | No. of participants or cycles (trials) | CCS group | Control group | Pooled effct RR (95% CI) | Analysis model | Heterogeneity (I2) | |
|---|---|---|---|---|---|---|---|
| Implantation rate | RCTs | 776 (4) | 232/327 | 240/449 | 1.32 (1.18, 1.47) | Fixed | 0% |
| Cohort studies | 3214 (7) | 522/817 | 749/2397 | 1.74 (1.35, 2.24) | Random | 87% | |
| Clinical pregnancy | RCTs | 258 (2) | 106/127 | 93/131 | 1.26 (0.83, 1.93) | Random | 82% |
| Cohort studies | 1756 (6) | 451/684 | 517/1081 | 1.48 (1.20, 1.83) | Random | 73% | |
| Ongoing pregnancy | RCTs | 287 (2) | 92/145 | 76/142 | 1.31 (0.64, 2.66) | Random | 90% |
| Cohort studies | 1711 (5) | 381/659 | 386/1052 | 1.61 (1.30, 2.00) | Random | 59% | |
| Live birth | RCTs | 155 (1) | 61/72 | 56/83 | 1.26 (1.05, 1.50) | Fixed | NA |
| Cohort studies | 601 (3) | 142/273 | 148/328 | 1.35 (0.85, 2.13) | Random | 81% | |
| Miscarriage rate | RCTs | 192 (2) | 8/100 | 16/92 | 0.53 (0.24, 1.15) | Fixed | 0% |
| Cohort studies | 902 (5) | 30/392 | 132/510 | 0.31 (0.21, 0.46) | Fixed | 0% | |
| Multiple pregnancy | RCTs | 115 (1) | 0/57 | 31/58 | 0.02 (0.00, 0.26) | Fixed | NA |
| Cohort studies | 212 (2) | 4/62 | 53/150 | 0.19 (0.07, 0.51) | Fixed | 0% | |
Heterogeneity and subgroup analysis
The heterogeneity in the pooled risk estimates of our outcomes ranged from an I2 test result of 0 to 90% for both the RCTs and the cohort studies. Therefore, we performed a subgroup analysis for both the RCTs and the cohort studies addressing factors that might result in heterogeneity: age, location of the study, indication for CCS, day of biopsy, platform for CCS, number of embryos transferred and study design. We did not perform the subgroup analysis for outcomes other than the implantation rate because of the low number of related reports. As shown in Table 4, the implantation rate was higher in the CCS group than in the control group in any individual subgroup, and except for the pooled effect of the 2 retrospective cohort studies showing no significant differences between the CCS group and the control group, there were significant differences in the implantation rate in all of the other pooled subgroup analyses. The subgroup analysis results showed that the heterogeneity values were not substantially changed by the factors mentioned above.
Table 4. Subgroup analysis outcomes.
| Subgroup | Outcome | No. of studies | CCS group Implantated/Transferred embryos | Control group Implantated/Transferred embryos | Pooled effct RR (95% CI) | Analysis model | Heterogeneity (I2) | |
|---|---|---|---|---|---|---|---|---|
| RCTs | Age | <35y | 3 | 201/276 | 214/383 | 1.29 (1.15, 1.45) | Fixed | 0% |
| >35y | 1 | 31/51 | 26/66 | 1.45 (1.06, 2.24) | Fixed | NA | ||
| Indications for CCS | Good prognosis | 3 | 201/276 | 214/383 | 1.29 (1.15, 1.45) | Fixed | 0% | |
| AMA | 1 | 31/51 | 26/66 | 1.45 (1.06, 2.24) | Fixed | NA | ||
| Platform for PGS | aCGH | 1 | 39/55 | 22/48 | 1.55 (1.09, 2.20) | Fixed | NA | |
| qPCR | 2 | 162/221 | 192/335 | 1.25 (1.10, 1.41) | Fixed | 0% | ||
| SNP | 1 | 31/51 | 36/66 | 1.54 (1.06, 2.24) | Fixed | NA | ||
| Cohort studies | Study design | Prospective studies | 5 | 406/620 | 395/894 | 1.70 (1.33, 2.17) | Random | 75% |
| Retrospective studies | 2 | 116/197 | 354/1503 | 1.73 (0.71, 4.24 | Random | 97% | ||
| Age | <35y | 2 | 99/150 | 40/130 | 2.25 (1.25, 4.06) | Random | 67% | |
| >35y | 4 | 404/634 | 689/2192 | 1.53 (1.13, 2.08) | Random | 91% | ||
| Location | North America | 4 | 404/634 | 689/2192 | 1.52 (1.13, 2.08) | Random | 91% | |
| Europe | 2 | 99/150 | 40/130 | 2.25 (1.25, 4.06) | Random | 67% | ||
| Asia | 1 | 19/33 | 20/75 | 2.16 (1.34, 3.48) | Random | NA | ||
| Indications for CCS | AMA, RIF, RPL or other | 4 | 404/634 | 689/2192 | 1.52 (1.13, 2.08) | Random | 91% | |
| RIF | 2 | 68/106 | 40/130 | 2.21 (1.27, 3.84) | Random | 61% | ||
| RPL | 1 | 19/33 | 20/75 | 2.16 (1.34, 3.48) | Random | NA | ||
| Stage of biopsy | Blastocyst stage biopsy | 4 | 433/662 | 445/912 | 1.42 (1.12, 1.79) | Random | 80% | |
| Cleavage stage biopsy | 3 | 89/155 | 304/1485 | 2.23 (1.66, 2.99) | Random | 52% | ||
| Platform for PGS | aCGH | 4 | 170/265 | 416/1736 | 2.23 (1.53, 3.27) | Random | 81% | |
| NGS | 1 | 40/65 | 31/89 | 1.77 (1.25, 2.49) | Random | NA | ||
| qPCR | 1 | 86/140 | 101/182 | 1.11 (0.92, 1.33) | Random | NA | ||
| Embryo transfer | Single embryo transfer | 2 | 312/487 | 302/572 | 1.20 (1.06, 1.36) | Random | 27% | |
| More than one embryo transfer | 5 | 210/330 | 447/1825 | 2.11 (1.57, 2.83) | Random | 74% |
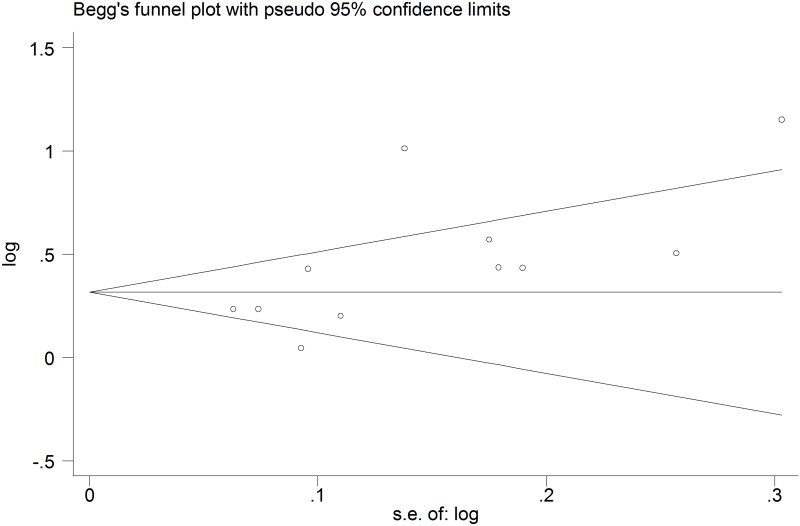
Sensitivity analysis and publication bias
The pooled effect results remained practically unchanged when we performed a one-way sensitivity analysis. Begg’s test did not show significant small-study bias (p = 0.062) (Fig 8).
Fig 8. Begg’s funnel plot for assessment of publication bias, suggesting no significant small-study bias.
Discussion
To review the current literature regarding the use of CCS technology for PGS and embryo selection and to assess the effect of CCS on the clinical outcomes of IVF, we included 11 studies (4 RCTs and 7 cohort studies) accounting for 2425 ART cycles in 2338 women to conduct a meta-analysis. Our results showed that CCS-based PGS was statistically significantly associated with an increased implantation rate (RCT-based RR 1.32, 95% CI 1.18–1.47; cohort study RR 1.74, 95% CI 1.35–2.24). The RR value demonstrated that there is great potential benefit from using CCS-based PGS over traditional morphological methods. And CCS-based PGS was also related to increases in the clinical pregnancy rate, ongoing pregnancy rate, and live birth rate, and decreases in the miscarriage and multipregnancy rates. To our knowledge, two systematic reviews evaluating the clinical effectiveness of CCS-based PGS have been published recently [23, 22]. Dahdouh’s study [23], which included only 3 RCTs on good prognosis patients, found that when the same number of embryos were transferred, CCS-based PGS was associated with a higher implantation rate and clinical pregnancy rate. The literature search in Lee’s systematic review [22] included the same 3 RCTs and 16 observational studies, and in addition to the trials assessing trophectoderm biopsy and blastomere biopsy, their study also included trials using PB biopsy for CCS. Our results were mainly in line with those of the systematic reviews, but this is the first meta-analysis to compare CCS-based embryo selection to traditional morphological methods, and we evaluated more outcomes including the live birth rate and multipregnancy rate.
In our review, all of the included trials were considered to be at low to moderate risk of bias. For the 4 RCTs included, one was presented as an abstract. Two studies provided information about random sequence generation. Most of the trials did not provide detailed information on allocation concealment or the method of blinding; however, blinding is not likely to influence outcome judgment or measurement. Three RCTs reported no loss to follow-up, and intention-to-treat analysis was performed for these studies. In one study, 9 patients were missing for various reasons, but intention-to-treat analysis was conducted. For the 7 included cohort studies (5 prospective studies and 2 retrospective studies), selection bias was not thought to play a major role, but comparability bias might exist among these studies.
The effect of CCS-based PGS on the implantation rate for patients with different indications for PGS and from different regions was also analyzed. The results of Dahdouh’s systematic review [23] revealed that the use of CCS-based PGS improved the implantation rate in good prognosis patients, and the results of our meta-analysis revealed that CCS-based PGS can also benefit patients with AMA, RIF and RPL. Because most of the patients involved in our study presented with normal ovarian reserves and no male factor infertility, whether CCS-based PGS can also benefit these patients, particularly for women who do not have an abundance of embryos to evaluate, remains to be determined. No statistically significant difference was found in the subgroup analysis conducted by patient location.
There are 3 possible sources of material for PGS testing: the first and second polar bodies, 1 or 2 blastomere biopsy from cleavage-stage embryos and 5–10 trophoblast cell biopsy from blastocysts. The accuracy of PB testing is significantly lower than cleavage stage blastomere testing or the trophoblast cell analysis, primarily because of the inability of this method to comprehensively assess all origins of embryonic aneuploidy [40, 41]. Regarding the advantages of cleavage stage biopsy versus blastocyst biopsy, recent studies found that a day 3 biopsy decreased the blastocyst rate and the implantation rate whereas a blastocyst biopsy showed no effect on embryonic developmental competence [35, 31, 42, 43]. Another advantage of blastocyst biopsy is the ability to test 5–10 trophectoderm cells, resulting in greater efficiency and a lower no-result rate [44]. Based on data procured from observational studies included in our meta-analysis, cleavage stage PGS was also feasible for CCS, and it showed an even better implantation outcome than blastocyst stage PGS (RR 2.23, 95% CI 1.66–2.99 vs. RR 1.42, 95% CI 1.12–1.79). However, further high quality evidence derived from randomized control trials is still required to determine the best time for CCS biopsy.
CCS-based PGS can be performed through a wide variety of methods. For DNA amplification, the available methods include multiple displacement amplification (MDA) [45–49], PCR (polymerase chain reaction) [50–52], and targeted multiplex PCR [53, 54]. For evaluating the amplified DNA, methods include CGH (comparative genetic hybridization) arrays [55–57, 36, 31, 38, 29, 32], SNP (single nucleotide polymorphism) arrays [48, 49, 51, 52, 58], NGS (next-generation sequencing)-based CCS [59, 60, 37] and qPCR-based CCS [61, 62, 54, 33, 34]. Among these methods, array CGH was the first technology to be widely used and has been validated by testing cells of a known genotype [63]. Comparing to other methods, NGS may offer more potential advantages including lower cost, reduced time and higher chromosomal analysis resolution [56, 64]. Moreover, the equivalence of NGS-based CCS to array CGH in the detection of chromosomal aneuploidy has been demonstrated [65, 66]. Most of the studies included in our analysis used aCGH and qPCR for karyotype screening. The only cohort study that used qPCR showed no significant effect on the implantation rate between the CCS group and the control group, and pooled data from the other separate PGS platform subgroup analyses all showed improvements in the implantation outcome.
One of the main targets of CCS-based PGS is to increase the number of singleton deliveries, which are considered the ideal outcome of IVF. Two RCTs and 2 cohort studies included in our meta-analysis transferred single embryos for both groups, and the other 2 RCTs and 5 cohort studies transferred significantly more embryos for the control group than the CCS group. According to our results, CCS improved the implantation rate even when fewer embryos were transferred.
Generally, the selection of embryos for transfer is based on traditional morphological assessment alone. Nonetheless, this method is not efficient enough because on average, only 1 in 4 treatments results in successful implantation. The development of alternative methods for diagnosing embryo viability preimplantation brings hope for improving the time to pregnancy and facilitating eSET. Other technologies for evaluating embryos include PGS, time-lapse microscopy, embryo proteomics and metabolomics. Time-lapse systems can take digital images at frequent time intervals without removing the embryos from the incubator. In recent years, algorithms based on morphokinetic parameters obtained through time-lapse methods have been created to predict the competence of embryo development, but until now, evidence of significant differences in clinical outcomes has been insufficient to choose between time-lapse systems and conventional incubation [67–69]. Some recent studies have tried to correlate time-lapse morphokinetic parameters with aneuploidy, but the results of these studies indicated that the selection of embryos by time-lapse cannot yet replace PGS [70–72]. Proteomics and metabolomics are typically able to characterize thousands of proteins and metabolites reflected the physiological status of embryos. Although studies have reported positive results regarding correlations between metabolic status and embryo developmental competence, the available clinical data on IVF outcomes still lack support for the use of proteomics or metabolomics [73–77].
The traditional morphological method prevents damage caused by the biopsy procedure and cuts the cost of genetic testing while having the potential to provide good cumulative pregnancy and live birth rates. However, the transfer of aneuploid embryos may result in miscarriage, and repeated transfer cycles not only cause emotional stress but also result in extra costs, including the costs of repeated endometrial preparation, monitoring scans and working days loss; such costs can exceed the euploidy testing cost. Although not all euploid embryos detected by CCS-based PGS can lead to successful implantation, but at present, CCS-based PGS appears to be the most reliable method for diagnosing preimplantation embryo viability. With the development of NGS, the cost of PGS may decline significantly, allowing greater access for more patients. Better designed randomized controlled trials are required to provide sufficient evidence regarding the efficiency of CCS-based PGS and to compare this technology to other methods used to evaluate preimplantation embryo viability.
Supporting Information
(DOC)
Data Availability
All data are available from published studies in PubMed, Embase, CNKI, and ClinicalTrials.gov databases. These studies are referenced in the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366 10.1016/S0140-6736(78)92957-4 [DOI] [PubMed] [Google Scholar]
- 2. Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertility and sterility. 1999;71(4):715–8. 10.1016/S0015-0282(98)00525-1 [DOI] [PubMed] [Google Scholar]
- 3. Bettio D, Venci A, Levi Setti PE. Chromosomal abnormalities in miscarriages after different assisted reproduction procedures. Placenta. 2008;29 Suppl B:126–8. 10.1016/j.placenta.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 4. Fragouli E, Wells D, Delhanty JD. Chromosome abnormalities in the human oocyte. Cytogenetic and genome research. 2011;133(2–4):107–18. 10.1159/000323801 [DOI] [PubMed] [Google Scholar]
- 5. Mantzouratou A, Mania A, Fragouli E, Xanthopoulou L, Tashkandi S, Fordham K, et al. Variable aneuploidy mechanisms in embryos from couples with poor reproductive histories undergoing preimplantation genetic screening. Hum Reprod. 2007;22(7):1844–53. 10.1093/humrep/dem102 [DOI] [PubMed] [Google Scholar]
- 6. Scott RT Jr, Franasiak JM, Forman EJ. Comprehensive chromosome screening with synchronous blastocyst transfer: time for a paradigm shift. Fertility and sterility. 2014;102(3):660–1. 10.1016/j.fertnstert.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 7. Gianaroli L, Magli MC, Ferraretti AP. The in vivo and in vitro efficiency and efficacy of PGD for aneuploidy. Molecular and cellular endocrinology. 2001;183 Suppl 1:S13–8. 10.1016/S0303-7207(01)00570-6 [DOI] [PubMed] [Google Scholar]
- 8. Voullaire L, Wilton L, McBain J, Callaghan T, Williamson R. Chromosome abnormalities identified by comparative genomic hybridization in embryos from women with repeated implantation failure. Molecular human reproduction. 2002;8(11):1035–41. 10.1093/molehr/8.11.1035 [DOI] [PubMed] [Google Scholar]
- 9. Fragouli E, Katz-Jaffe M, Alfarawati S, Stevens J, Colls P, Goodall NN, et al. Comprehensive chromosome screening of polar bodies and blastocysts from couples experiencing repeated implantation failure. Fertility and sterility. 2010;94(3):875–87. 10.1016/j.fertnstert.2009.04.053 [DOI] [PubMed] [Google Scholar]
- 10. Kiss A, Rosa RF, Dibi RP, Zen PR, Pfeil JN, Graziadio C, et al. [Chromosomal abnormalities in couples with history of recurrent abortion]. Revista brasileira de ginecologia e obstetricia: revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2009;31(2):68–74. 10.1590/S0100-72032009000200004 [DOI] [PubMed] [Google Scholar]
- 11. Baruch S, Kaufman DJ, Hudson KL. Preimplantation genetic screening: a survey of in vitro fertilization clinics. Genetics in medicine: official journal of the American College of Medical Genetics. 2008;10(9):685–90. [DOI] [PubMed] [Google Scholar]
- 12. Kahraman S, Sertyel S, Findikli N, Kumtepe Y, Oncu N, Melil S, et al. Effect of PGD on implantation and ongoing pregnancy rates in cases with predominantly macrocephalic spermatozoa. Reproductive biomedicine online. 2004;9(1):79–85. 10.1016/S1472-6483(10)62114-1 [DOI] [PubMed] [Google Scholar]
- 13. Munne S, Sandalinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenatal diagnosis. 2004;24(8):638–43. 10.1002/pd.957 [DOI] [PubMed] [Google Scholar]
- 14. Munne S, Ary J, Zouves C, Escudero T, Barnes F, Cinioglu C, et al. Wide range of chromosome abnormalities in the embryos of young egg donors. Reproductive biomedicine online. 2006;12(3):340–6. 10.1016/S1472-6483(10)61007-3 [DOI] [PubMed] [Google Scholar]
- 15. Reis Soares S, Rubio C, Rodrigo L, Simon C, Remohi J, Pellicer A. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertility and sterility. 2003;80(3):656–7. 10.1016/S0015-0282(03)00787-8 [DOI] [PubMed] [Google Scholar]
- 16. Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Human Reproduction. 2008;23(11):2596–608. 10.1093/humrep/den287 [DOI] [PubMed] [Google Scholar]
- 17. Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertility and sterility. 2011;95(2):520–4. 10.1016/j.fertnstert.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 18. Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. The New England journal of medicine. 2007;357(1):9–17. 10.1056/NEJMoa067744 [DOI] [PubMed] [Google Scholar]
- 19. Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Human reproduction update. 2011;17(4):454–66. 10.1093/humupd/dmr003 [DOI] [PubMed] [Google Scholar]
- 20. Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, SenGupta SB, et al. The ESHRE PGD Consortium: 10 years of data collection. Human reproduction update. 2012;18(3):234–47. 10.1093/humupd/dmr052 [DOI] [PubMed] [Google Scholar]
- 21. Ginsburg ES, Baker VL, Racowsky C, Wantman E, Goldfarb J, Stern JE. Use of preimplantation genetic diagnosis and preimplantation genetic screening in the United States: a Society for Assisted Reproductive Technology Writing Group paper. Fertility and sterility. 2011;96(4):865–8. 10.1016/j.fertnstert.2011.07.1139 [DOI] [PubMed] [Google Scholar]
- 22. Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30(2):473–83. 10.1093/humrep/deu303 [DOI] [PubMed] [Google Scholar]
- 23. Dahdouh EM, Balayla J, Garcia-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reproductive biomedicine online. 2015;30(3):281–9. 10.1016/j.rbmo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 26.Higgins JP GS. Conchrane Handbook for Systematic Reviews of Interventions 5.1.0[updated March 2011] The Cochrane Collaboration., Available: www.cochrane-handbook.org.
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Zhang J, Huang J, Li L, Ma Y, Zhang H, et al. Applied reseach of array comperative genomic hybridization (aCGH) based PGS on repeated miscarriage women. Reroduction and Contraception. 2014(02):121–5. [Google Scholar]
- 30. Schoolcraft WB, Katz-Jaffe MG. Comprehensive chromosome screening of trophectoderm with vitrification facilitates elective single-embryo transfer for infertile women with advanced maternal age. Fertility and sterility. 2013;100(3):615–9. 10.1016/j.fertnstert.2013.07.1972 [DOI] [PubMed] [Google Scholar]
- 31. Keltz MD, Vega M, Sirota I, Lederman M, Moshier EL, Gonzales E, et al. Preimplantation genetic screening (PGS) with Comparative genomic hybridization (CGH) following day 3 single cell blastomere biopsy markedly improves IVF outcomes while lowering multiple pregnancies and miscarriages. Journal of assisted reproduction and genetics. 2013;30(10):1333–9. 10.1007/s10815-013-0070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Molecular cytogenetics. 2012;5(1):24 10.1186/1755-8166-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertility and sterility. 2013;100(3):697–703. 10.1016/j.fertnstert.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 34. Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod. 2012;27(4):1217–22. 10.1093/humrep/des020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertility and sterility. 2013;100(1):100–7 e1 10.1016/j.fertnstert.2013.02.056 [DOI] [PubMed] [Google Scholar]
- 36. Greco E, Bono S, Ruberti A, Lobascio AM, Greco P, Biricik A, et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. BioMed research international. 2014;2014:457913 10.1155/2014/457913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lukaszuk K, Pukszta S, Wells D, Cybulska C, Liss J, Plociennik L, et al. Routine use of next-generation sequencing for preimplantation genetic diagnosis of blastomeres obtained from embryos on day 3 in fresh in vitro fertilization cycles. Fertility and sterility. 2015;103(4):1031–6. 10.1016/j.fertnstert.2014.12.123 [DOI] [PubMed] [Google Scholar]
- 38. Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertility and sterility. 2010;94(5):1700–6. 10.1016/j.fertnstert.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 39. Schoolcraft WB, Surrey E, Minjarez D, Gustofson RL, Scott RT Jr, Katz-Jaffe MG. Comprehensive chromosome screening (CCS) with vitrification results in improved clinical outcome in women >35 years: a randomized control trial. Fertility and sterility. 2012;98(3, Supplement):S1 10.1016/j.fertnstert.2012.07.002 23084567 [DOI] [Google Scholar]
- 40. Capalbo A, Bono S, Spizzichino L, Biricik A, Baldi M, Colamaria S, et al. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum Reprod. 2013;28(2):509–18. 10.1093/humrep/des394 [DOI] [PubMed] [Google Scholar]
- 41. Scriven PN, Ogilvie CM, Khalaf Y. Embryo selection in IVF: is polar body array comparative genomic hybridization accurate enough? Human reproduction (Oxford, England). 2012;27(4):951–3. 10.1093/humrep/des017 [DOI] [PubMed] [Google Scholar]
- 42. Rubio C, Rodrigo L, Mir P, Mateu E, Peinado V, Milan M, et al. Use of array comparative genomic hybridization (array-CGH) for embryo assessment: clinical results. Fertility and sterility. 2013;99(4):1044–8. 10.1016/j.fertnstert.2013.01.094 [DOI] [PubMed] [Google Scholar]
- 43. Scott RT Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertility and sterility. 2013;100(3):624–30. 10.1016/j.fertnstert.2013.04.039 [DOI] [PubMed] [Google Scholar]
- 44. Adler A, Lee HL, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reproductive biomedicine online. 2014;28(4):485–91. 10.1016/j.rbmo.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 45. Renwick PJ, Lewis CM, Abbs S, Ogilvie CM. Determination of the genetic status of cleavage-stage human embryos by microsatellite marker analysis following multiple displacement amplification. Prenatal diagnosis. 2007;27(3):206–15. 10.1002/pd.1638 [DOI] [PubMed] [Google Scholar]
- 46. Zhang YF, Luo HN, Li XP, Zhang YS. [Applications and prospect of multiple displacement amplification in preimplantation genetic diagnosis]. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2012;29(4):431–4. [DOI] [PubMed] [Google Scholar]
- 47. Treff NR, Su J, Tao X, Northrop LE, Scott RT Jr. Single-cell whole-genome amplification technique impacts the accuracy of SNP microarray-based genotyping and copy number analyses. Molecular human reproduction. 2011;17(6):335–43. 10.1093/molehr/gaq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, Shaw MA, et al. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. Journal of medical genetics. 2010;47(10):651–8. 10.1136/jmg.2009.069971 [DOI] [PubMed] [Google Scholar]
- 49. Johnson DS, Cinnioglu C, Ross R, Filby A, Gemelos G, Hill M, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Molecular human reproduction. 2010;16(12):944–9. 10.1093/molehr/gaq062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harper JC, Harton G. The use of arrays in preimplantation genetic diagnosis and screening. Fertility and sterility. 2010;94(4):1173–7. 10.1016/j.fertnstert.2010.04.064 [DOI] [PubMed] [Google Scholar]
- 51. Treff NR, Su J, Tao X, Levy B, Scott RT Jr. Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertility and sterility. 2010;94(6):2017–21. 10.1016/j.fertnstert.2010.01.052 [DOI] [PubMed] [Google Scholar]
- 52. Konings P, Vanneste E, Jackmaert S, Ampe M, Verbeke G, Moreau Y, et al. Microarray analysis of copy number variation in single cells. Nature protocols. 2012;7(2):281–310. 10.1038/nprot.2011.426 [DOI] [PubMed] [Google Scholar]
- 53. Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT Jr. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertility and sterility. 2012;97(4):819–24. 10.1016/j.fertnstert.2012.01.115 [DOI] [PubMed] [Google Scholar]
- 54. Treff NR, Fedick A, Tao X, Devkota B, Taylor D, Scott RT Jr. Evaluation of targeted next-generation sequencing-based preimplantation genetic diagnosis of monogenic disease. Fertility and sterility. 2013;99(5):1377–84 e6 10.1016/j.fertnstert.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 55. Simpson JL. Preimplantation genetic diagnosis to improve pregnancy outcomes in subfertility. Best practice & research Clinical obstetrics & gynaecology. 2012;26(6):805–15. 10.1016/j.bpobgyn.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 56. Handyside AH. 24-chromosome copy number analysis: a comparison of available technologies. Fertility and sterility. 2013;100(3):595–602. 10.1016/j.fertnstert.2013.07.1965 [DOI] [PubMed] [Google Scholar]
- 57. Mir P, Rodrigo L, Mercader A, Buendia P, Mateu E, Milan-Sanchez M, et al. False positive rate of an arrayCGH platform for single-cell preimplantation genetic screening and subsequent clinical application on day-3. Journal of assisted reproduction and genetics. 2013;30(1):143–9. 10.1007/s10815-012-9918-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan YQ, Tan K, Zhang SP, Gong F, Cheng DH, Xiong B, et al. Single-nucleotide polymorphism microarray-based preimplantation genetic diagnosis is likely to improve the clinical outcome for translocation carriers. Hum Reprod. 2013;28(9):2581–92. 10.1093/humrep/det271 [DOI] [PubMed] [Google Scholar]
- 59. Wang L, Wang XH, Zhang JG, Song Z, Wang SF, Gao Y, et al. Detection of Chromosomal Aneuploidy in Human Preimplantation Embryos by Next-Generation Sequencing. Biol Reprod. 2014;90(5):6 10.1095/biolreprod.113.116459 [DOI] [PubMed] [Google Scholar]
- 60. Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, et al. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertility and sterility. 2014;101(5):1375–U41. 10.1016/j.fertnstert.2014.01.051 [DOI] [PubMed] [Google Scholar]
- 61. Treff NR. Genome-wide analysis of human preimplantation aneuploidy. Seminars in reproductive medicine. 2012;30(4):283–8. 10.1055/s-0032-1313907 [DOI] [PubMed] [Google Scholar]
- 62. Treff NR, Forman EJ, Katz-Jaffe MG, Schoolcraft WB, Levy B, Scott RT Jr. Incidental identification of balanced translocation carrier patients through comprehensive chromosome screening of IVF-derived blastocysts. Journal of assisted reproduction and genetics. 2013;30(6):787–91. 10.1007/s10815-013-0008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Molecular human reproduction. 2008;14(12):703–10. 10.1093/molehr/gan062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yin X, Tan K, Vajta G, Jiang H, Tan Y, Zhang C, et al. Massively parallel sequencing for chromosomal abnormality testing in trophectoderm cells of human blastocysts. Biol Reprod. 2013;88(3):69 10.1095/biolreprod.112.106211 [DOI] [PubMed] [Google Scholar]
- 65. Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, et al. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertility and sterility. 2014;101(5):1375–82. 10.1016/j.fertnstert.2014.01.051 [DOI] [PubMed] [Google Scholar]
- 66. Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014;29(12):2802–13. 10.1093/humrep/deu277 [DOI] [PubMed] [Google Scholar]
- 67. Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. The Cochrane database of systematic reviews. 2015;2:CD011320 10.1002/14651858.CD011320.pub2 [DOI] [PubMed] [Google Scholar]
- 68. Polanski LT, Coelho Neto MA, Nastri CO, Navarro PA, Ferriani RA, Raine-Fenning N, et al. Time-lapse embryo imaging for improving reproductive outcomes: systematic review and meta-analysis. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;44(4):394–401. 10.1002/uog.13428 [DOI] [PubMed] [Google Scholar]
- 69. Siristatidis C, Komitopoulou MA, Makris A, Sialakouma A, Botzaki M, Mastorakos G, et al. Morphokinetic parameters of early embryo development via time lapse monitoring and their effect on embryo selection and ICSI outcomes: a prospective cohort study. Journal of assisted reproduction and genetics. 2015;32(4):563–70. 10.1007/s10815-015-0436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swain JE. Could time-lapse embryo imaging reduce the need for biopsy and PGS? Journal of assisted reproduction and genetics. 2013;30(8):1081–90. 10.1007/s10815-013-0048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Campbell A, Fishel S, Laegdsmand M. Aneuploidy is a key causal factor of delays in blastulation: author response to 'A cautionary note against aneuploidy risk assessment using time-lapse imaging'. Reproductive biomedicine online. 2014;28(3):279–83. 10.1016/j.rbmo.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 72. Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J, et al. Assessing morphokinetic parameters via time lapse microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? Journal of assisted reproduction and genetics. 2014;31(9):1231–42. 10.1007/s10815-014-0285-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vergouw CG, Kieslinger DC, Kostelijk EH, Botros LL, Schats R, Hompes PG, et al. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27(8):2304–11. 10.1093/humrep/des175 [DOI] [PubMed] [Google Scholar]
- 74. Hardarson T, Ahlstrom A, Rogberg L, Botros L, Hillensjo T, Westlander G, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27(1):89–96. 10.1093/humrep/der373 [DOI] [PubMed] [Google Scholar]
- 75. Nagy ZP, Sakkas D, Behr B. Symposium: innovative techniques in human embryo viability assessment. Non-invasive assessment of embryo viability by metabolomic profiling of culture media ('metabolomics'). Reproductive biomedicine online 2008;17(4):502–7. 10.1016/S1472-6483(10)60236-2 [DOI] [PubMed] [Google Scholar]
- 76. Kotze DJ, Hansen P, Keskintepe L, Snowden E, Sher G, Kruger T. Embryo selection criteria based on morphology VERSUS the expression of a biochemical marker (sHLA-G) and a graduated embryo score: prediction of pregnancy outcome. Journal of assisted reproduction and genetics. 2010;27(6):309–16. 10.1007/s10815-010-9403-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Conaghan J, Hardy K, Handyside AH, Winston RM, Leese HJ. Selection criteria for human embryo transfer: a comparison of pyruvate uptake and morphology. Journal of assisted reproduction and genetics. 1993;10(1):21–30. 10.1007/BF01204436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All data are available from published studies in PubMed, Embase, CNKI, and ClinicalTrials.gov databases. These studies are referenced in the paper.