Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most widespread and dangerous pathogens in healthcare settings. We carried out this case-control-control study at a tertiary care hospital in Guangzhou, China, to examine the antimicrobial susceptibility patterns, risk factors and clinical outcomes of MRSA infections.
Methods
A total of 57 MRSA patients, 116 methicillin-susceptible Staphylococcus aureus (MSSA) patients and 102 S. aureus negative patients were included in this study. We applied the disk diffusion method to compare the antimicrobial susceptibilities of 18 antibiotics between MRSA and MSSA isolates. Risk factors of MRSA infections were evaluated using univariate and multivariate logistic regression models. We used Cox proportional hazards models and logistic regression analysis to assess the hospital stay duration and fatality for patients with MRSA infections.
Results
The MRSA group had significantly higher resistance rates for most drugs tested compared with the MSSA group. Using MSSA patients as controls, the following independent risk factors of MRSA infections were identified: 3 or more prior hospitalizations (OR 2.8, 95% CI 1.3–5.8, P = 0.007), chronic obstructive pulmonary disease (OR 5.9, 95% CI 1.7–20.7, P = 0.006), and use of a respirator (OR 3.6, 95% CI 1.0–12.9, P = 0.046). With the S. aureus negative patients as controls, use of a respirator (OR 3.8, 95% CI 1.0–13.9, P = 0.047) and tracheal intubation (OR 8.2, 95% CI 1.5–45.1, P = 0.016) were significant risk factors for MRSA infections. MRSA patients had a longer hospital stay duration and higher fatality in comparison with those in the two control groups.
Conclusions
MRSA infections substantially increase hospital stay duration and fatality. Thus, MRSA infections are serious issues in this healthcare setting and should receive more attention from clinicians.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has spread throughout the world and has become one of the most frequent bacteria among healthcare-associated infections since it was first identified in 1961 [1–3]. MRSA can cause a number of life-threatening infections, such as septic shock, endocarditis, and severe pneumonia [4]. MRSA infections are closely linked to increased mortality, extra length of hospital stay and excess costs [5–8].
The spread of MRSA is also a serious issue in China. The proportion of MRSA among all S. aureus isolates has reached 50–70% based on former studies [9–11]. Due to the terrible prognosis of MRSA infections [7, 8], epidemiological information is urgently needed to help prevent and control these infections. However, recent systematic epidemiological surveys of hospital-acquired MRSA are lacking in Guangzhou, one of the largest cities in Southern China. Thus, we launched the current study to elucidate the antimicrobial susceptibility patterns, risk factors and clinical outcomes of MRSA infections in a tertiary care hospital in Guangzhou, China.
Materials and Methods
Setting and study design
The present study was carried out in a 1000-bed tertiary care hospital in Guangzhou, and patients admitted from January 2013 to December 2013 were included.
The enrolled S. aureus culture-positive patients were those for whom an S. aureus strain was first obtained from clinical samples at least 48 hours after admission. S. aureus patients were categorized into the MRSA group if their S. aureus isolates were positive for the mecA gene and resistant to oxacillin; otherwise, they were classified as methicillin-susceptible Staphylococcus aureus (MSSA) patients. Patients who were negative for S. aureus infections throughout hospitalization formed the S. aureus negative group. Patients were excluded from our study if they were discharged from the hospital within 48 hours.
Our study was designed to include three separate investigations. One compared the antimicrobial susceptibility profiles between the MRSA group and MSSA group. We also illustrated the risk factors of MRSA infections using two separate case-control studies: MRSA versus MSSA and MRSA versus S. aureus negative. Finally, we explored the impact of MRSA infections on the patients’ hospital stay duration and fatality.
Data were extracted from patients’ electronic medical records and included age, gender, ward, type of specimen, history of hospitalization, surgery, intensive care unit (ICU) admission, underlying illness, use of immunosuppressive drugs and antibiotics as well as treatment with invasive procedures during hospitalization, death versus survival, and length of hospital stay
Our research was conducted according to the Declaration of Helsinki. Our research was retrospective and only involved data obtained from electronic medical records and isolated strains. The doctors in the hospital anonymized the patients' identifying information, and the information was inaccessible to the authors throughout the data collection and data analysis process. Formal ethics approval from the Ethics Committee at Guangdong Pharmaceutical University and written/oral consent from the patients were not obtained.
Laboratory methods
S. aureus isolates were identified using the Vitek 32 microbial identification system (bioMerieux, France). Antimicrobial susceptibility testing was conducted using the Kirby-Bauer disc diffusion and E-test methods following the guidelines of the Clinical and Laboratory Standard Institute [12]. In total, S. aureus strains were tested for susceptibility to 18 antibiotics, including gentamicin, imipenem, ciprofloxacin, levofloxacin, penicillin, vancomycin, linezolid, amoxicillin, ampicillin, oxacillin, sulfamethoxazole/trimethoprim, clindamycin, quinupristin/dalfopristin, rifampicin, chloramphenicol, tetracycline, cefazolin, and erythromycin. The E-test method was applied for vancomycin and oxacillin, and the disc diffusion method was used for the other agents.
Statistical analysis
The sensitivities of the isolates to single drug agents were compared between the MRSA and MSSA groups using the chi-square test. Univariate and multivariate logistic regression models were used to explore risk factors for MRSA healthcare-associated infections. Variables with P values < 0.1 in the univariate logistic regression were introduced into a stepwise forward multivariate logistic regression model. Odds ratios (ORs) along with 95% confidential intervals (CIs) were calculated to assess the strengths of all associations. The Wald test was used to systematically evaluate all pairwise interactions. The effect of MRSA infections on hospital stay duration was assessed with Cox proportional hazards models with adjustment for independent risk factors of MRSA. The impact of MRSA infections on fatality was evaluated by logistic regression analysis (MRSA versus MSSA) and exact logistic regression analysis (MRSA versus non-S. aureus), with adjustment for variables that had P values < 0.1 in the univariate MRSA predictor analysis. A two-tailed P value below 0.05 was considered statistically significant. All statistical analyses were performed using Stata software, version 13.0 (College Station, Texas).
Results
Patients
A total of 57 patients with MRSA isolates, 116 patients with MSSA isolates and 102 patients without S. aureus isolates were included in this study. The most common S. aureus specimen was sputum (97/173, 56.1%), followed by abscess (45/173, 26.0%). The neurology department ranked first in proportion among the wards that the patients hospitalized in (41/173, 23.7%), followed by the dermatology department (35/173, 20.2%).
Antimicrobial susceptibility testing
Of the S. aureus strains evaluated, 92% (160/173) displayed resistance to penicillin, 90% (156/173) to ampicillin, 78% (134/173) to levofloxacin, 54% (94/173) to erythromycin, 45% (78/173) to tetracycline, 39% (67/173) to clindamycin, 35% (61/173) to ciprofloxacin, 33% (57/173) to oxacillin, 27% (47/173) to amoxicillin, imipenem and cefazolin, 27% (46/173) to gentamicin, 13% (23/173) to chloramphenicol, 9% (15/173) to sulfamethoxazole/trimethoprim, 3% (6/173) to rifampicin, 2% (4/173) to quinupristin/dalfopristinand, and 0% (0/173) to linezolid and vancomycin. According to chi-square test, the resistance rates for individual agents were significantly higher in the MRSA group than in the MSSA group, with the exception of linezolid, vancomycin, levofloxacin and quinupristin/dalfopristin (Table 1).
Table 1. Comparison of antimicrobial susceptibility between MRSA and MSSA.
| Drug agent | MRSA (n = 57) | MSSA (n = 116) | P |
|---|---|---|---|
| No. (%) resistant isolates | No. (%) resistant isolates | ||
| GEN | 38 (66.67) | 8 (6.90) | <0.001 |
| IMI | 47 (82.46) | 0 (0.00) | <0.001 |
| CIP | 44 (77.19) | 17 (14.66) | <0.001 |
| LEV | 49 (85.96) | 85 (73.28) | 0.060 |
| PEN | 57 (100.00) | 103 (88.79) | 0.001 |
| VAN | 0 (0.00) | 0 (0.00) | NA* |
| LIN | 0 (0.00) | 0 (0.00) | NA* |
| AMO | 47 (82.46) | 0 (0.00) | <0.001 |
| AMP | 56 (98.25) | 100 (86.21) | 0.012 |
| OXA | 57 (100.00) | 0 (0.00) | <0.001 |
| S/T | 15 (26.32) | 0 (0.00) | <0.001 |
| CLI | 42 (73.68) | 25 (21.55) | <0.001 |
| Q/D | 3 (5.26) | 1 (0.86) | 0.081 |
| RIF | 5 (8.77) | 1 (0.86) | 0.009 |
| CHL | 14 (24.56) | 9 (7.76) | 0.002 |
| TET | 45 (78.95) | 33 (28.45) | <0.001 |
| CEF | 47 (82.46) | 0 (0.00) | <0.001 |
| ERY | 53 (92.98) | 41 (35.34) | <0.001 |
* NA: Not Available.
GEN: gentamicin; IMI: imipenem; CIP: ciprofloxacin; LEV: levofloxacin; PEN: penicillin; VAN: vancomycin; LIN: linezolid; AMO: amoxicillin; AMP: ampicillin; OXA: oxacillin; S/T: sulfamethoxazole/trimethoprim; CLI: clindamycin; Q/D: quinupristin/dalfopristin; RIF: rifampicin; CHL: chloramphenicol; TET: tetracycline; CEF: cefazolin; ERY: erythromycin.
Risk factors
Gender, age, underlying conditions, invasive procedures, ICU stay, surgery, and history of hospitalization and antibiotics usage were included as potential influencing factors for MRSA acquisition. The results of the multivariable logistic analyses of risk factors for MRSA infections are shown in Table 2. Three or more prior hospitalizations, chronic obstructive pulmonary disease, and use of a respirator were independently and statistically associated with MRSA infections in comparison to MSSA infections (pseudo R2 = 0.16, area under ROC curve = 0.72). Use of a respirator and tracheal intubation were identified as risk factors for MRSA infections in comparison to S. aureus negative patients (pseudo R2 = 0.09, area under ROC curve = 0.62). No statistically significant interactions between any risk factors were found.
Table 2. Multivariate logistic regression analysis of risk factors for MRSA infections.
| Risk factor | MRSA versus MSSA | MRSA versus S. aureus (-) | ||
|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | |
| Prior hospitalizations≥3 | 2.8 (1.3–5.8) | 0.007 | ||
| COPD | 5.9 (1.7–20.7) | 0.006 | ||
| Respirator | 3.6 (1.0–12.9) | 0.046 | 3.8 (1.0–13.9) | 0.047 |
| Tracheal intubation | 8.2 (1.5–45.1) | 0.016 | ||
aOR: adjusted Odds Ratio; COPD: Chronic Obstructive Pulmonary Disease.
Clinical outcomes
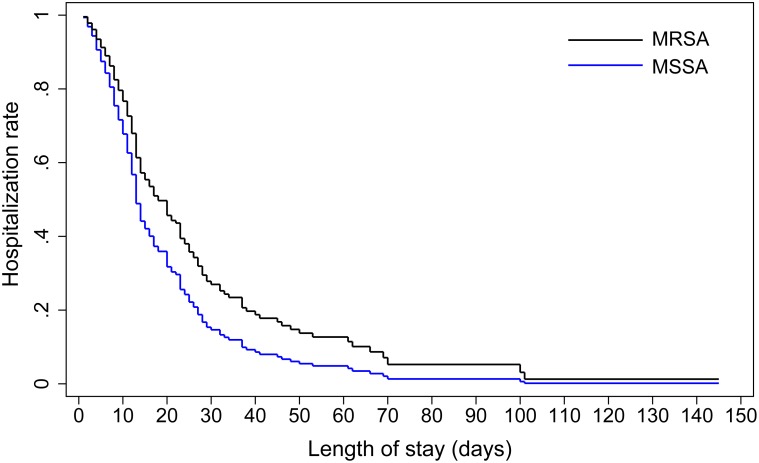
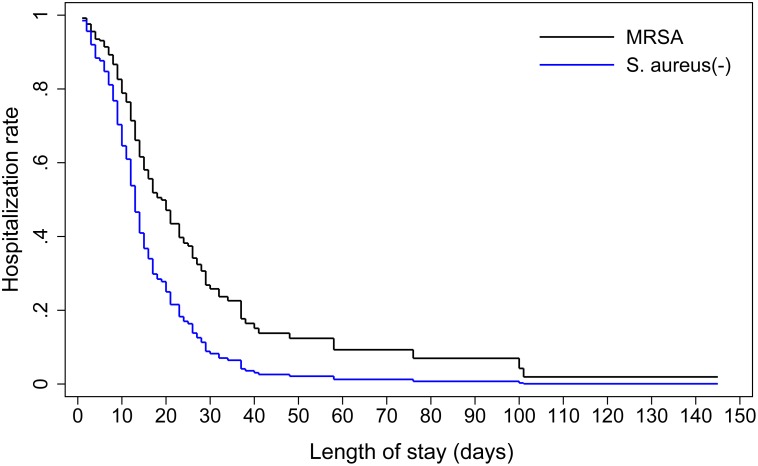
Cox proportional hazards regression models were introduced to examine the possible relationships between MRSA infections and length of hospital stay. The patients who died during hospitalization were censored in the model. MRSA infections were associated with a longer duration of hospitalization, and this association was still significant after adjusting for other covariates (MRSA versus MSSA: Table 3 and Fig 1; MRSA versus non-S. aureus: Table 4 and Fig 2). The fatality rates of the MRSA, MSSA and S. aureus negative groups were 21% (12/57), 8% (9/116) and 0% (0/112), respectively. MRSA infections were associated with increased risk of fatality (MRSA versus MSSA: adjusted OR 2.7, 95% CI 1.0–7.0, pseudo R2 = 0.08, area under ROC curve = 0.71; MRSA versus S. aureus negative, adjusted OR 31.6, 95% CI 4.4-inf, model score = 26.41).
Table 3. Cox proportional hazards analysis of the association between MRSA infections and length of hospital stay (compared with MSSA).
| Variable | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| MRSA | 1.8 (1.2–2.5) | 0.002 | 1.5 (1.0–2.2) | 0.046 |
| Prior hospitalizations≥3 | 1.5 (1.1–2.1) | 0.025 | 1.3 (0.9–1.9) | 0.156 |
| COPD | 1.2 (0.7–2.1) | 0.468 | 0.9 (0.5–1.7) | 0.840 |
| Respirator | 2.2 (1.2–3.9) | 0.010 | 1.7 (0.9–3.2) | 0.090 |
HR: Hazard Ratio; COPD: Chronic Obstructive Pulmonary Disease.
Fig 1. Comparison of hospitalization rates for patients with MRSA infections and those with MSSA infections.
Adjusted for three or more prior hospitalizations, COPD and respirator use.
Table 4. Cox proportional hazards analysis of the association between MRSA infections and length of hospital stay (compared with non-S. aureus).
| Variable | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| MRSA | 2.2 (1.5–3.2) | <0.001 | 1.8 (1.2–2.7) | 0.002 |
| Respirator | 2.2 (1.2–3.9) | 0.007 | 1.4 (0.7–2.6) | 0.310 |
| Tracheal intubation | 2.7 (1.3–5.5) | 0.007 | 1.8 (0.8–3.8) | 0.143 |
HR: Hazard Ratio.
Fig 2. Comparison of hospitalization rates for patients with MRSA infections and those without S. aureus infections.
Adjusted for respirator use and tracheal intubation.
Discussion
S. aureus is among one of the six most infamous ESKAPE pathogens that easily develop antibiotic resistance (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter species, Pseudomonas aeruginosa and Enterobacter species) [13]. MRSA infections were recognized as a major threat to healthcare settings in China [14, 15]. To the best of our knowledge, this study is the first to provide comprehensive data on the antimicrobial resistance of clinical MRSA isolates, the risk factors for MRSA infections, and the hospital stay duration and fatality of MRSA infected individuals in a tertiary care hospital in Guangzhou, China.
S. aureus isolates displayed remarkably high rates of resistance to penicillin and ampicillin relative to the other antibiotics tested, which is in accordance with previous reports [16, 17]. The possible complex molecular mechanism of resistance to these drugs involves circumvention of the mechanism of action of penicillin, which is its binding protein 2a (PBP2a), a gene product of the mecA gene that is involved in cell wall biosynthesis [18]. A recent study also demonstrated that some isolates have evolved from MSSA to MRSA due to antibiotic exposure, which highlights the significance of appropriate antibiotics usage in clinical settings [19]. We did not find any strains resistant to vancomycin and linezolid, indicating those antibiotics are still effective for treating S. aureus healthcare-associated infections. The resistance rates of most agents were higher in the MRSA group than in the MSSA group, so it is necessary to implement strategies to reduce the ratio of MRSA infections to MSSA infections in healthcare settings.
The multivariate analysis indicated that chronic obstructive pulmonary disease is an independent risk factor for MRSA infections. An association between underlying illness and MRSA infections has also been suggested by recent studies [20–22]. Use of a respirator and tracheal intubation were also confirmed as independent risk factors for MRSA acquisition. This was not surprising, given that previous reports have obtained similar results regarding the role of invasive procedures in the drug resistance of S. aureus [23, 24]. In addition, we found that a history of multiple hospitalizations was statistically associated with MRSA infections, which is similar to the findings of another recent report [25]. Therefore, more intensive infection control measures should be implemented for patients with these risk factors.
We quantitatively evaluated the impact of MRSA infections on clinical outcomes and found that it resulted in a longer hospital stay and higher fatality in comparison to the two control groups. The results agree with those of some previous meta-analyses conducted in various countries and regions [6, 26, 27]; those studies detected a positive relationship between MRSA infections and poor prognosis.
Our research has certain strengths. First, we employed a case-control-control study design, and this two parallel case-control design greatly reduces the selection bias caused by the use of a single control group and improves the accuracy of our results. Furthermore, we selected all cases and controls from one tertiary care hospital so that differences between institutions, which are potential confounders, were avoided. Moreover, our analyses were mainly based on the electronic medical records review instead of self-report questionnaires, which reduces recall bias to ensure the accuracy of our data.
As with any retrospective study, our study also bears some limitations. First, we could not follow patients after discharge, because our data sources were electronic medical records, so we could not rule out the possibility that some discharged patients may have had a reoccurrence of the healthcare-associated infections or even died as a result of them. Furthermore, some typos may have been present in the electronic medical records, and it is difficult to observe and correct those errors through retrospective inspection of the records. In addition, the sample size was small, which may have reduced the statistical power of our results and therefore negatively impacted the value of our findings.
In summary, MRSA had greater resistance rates for the majority of antibiotics tested when compared to MSSA. Three or more prior hospitalizations, chronic obstructive pulmonary disease, use of a respirator and tracheal intubation were identified as independent risk factors for MRSA infections. The acquisition of MRSA infections resulted in a longer hospital stay and increased fatality.
Acknowledgments
The authors are grateful to the staff of the hospital for their assistance with collecting the S. aureus strains and medical records.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by Natural Science Foundation of Guangdong Education Department (2013KJCX0112). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. ERIKSEN KR. "Celbenin"-resistant staphylococci. Ugeskr Laeger. 1961; 123: 384–386. [PubMed] [Google Scholar]
- 2. Rasmussen G, Monecke S, Brus O, Ehricht R, Soderquist B. Long term epidemiology of methicillin-resistant Staplylococcus aureus bacteria isolates in Sweden. PLoS One. 2014; 9: e114276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghirardi B, Pietrasanta C, Ciuffini F, Manca MF, Uccella S, Lavizzari A, et al. Management of outbreaks of nosocomial pathogens in neonatal intensive care unit. Pediatr Med Chir. 2013; 35: 263–268. [DOI] [PubMed] [Google Scholar]
- 4. Xiao M, Wang H, Zhao Y, Mao LL, Brown M, Yu YS, et al. National surveillance of methicillin-resistant Staphylococcus aureus in China highlights a still-evolving epidemiology with 15 novel emerging multilocus sequence types. J Clin Microbiol. 2013; 51: 3638–3644. 10.1128/JCM.01375-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006; 42: S82–S89. [DOI] [PubMed] [Google Scholar]
- 6. Park DA, Lee SM, Peck KR, Joo EJ, Oh EG. Impact of Methicillin-Resistance on Mortality in Children and Neonates with Staphylococcus aureus Bacteremia: A Meta-analysis. Infect Chemother. 2013; 45: 202–210. 10.3947/ic.2013.45.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005; 52: 113–122. [DOI] [PubMed] [Google Scholar]
- 8. Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005; 26: 166–174. [DOI] [PubMed] [Google Scholar]
- 9. Zhao C, Sun H, Wang H, Liu Y, Hu B, Yu Y, et al. Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in China: results from the Gram-Positive Cocci Resistance Surveillance program (2005–2010). Diagn Microbiol Infect Dis. 2012; 73: 174–181. 10.1016/j.diagmicrobio.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 10. Xiao YH, Giske CG, Wei ZQ, Shen P, Heddini A, Li LJ. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist Updat. 2011; 14: 236–250. 10.1016/j.drup.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 11. Li T, Song Y, Zhu Y, Du X, Li M. Current status of Staphylococcus aureus infection in a central teaching hospital in Shanghai, China. BMC Microbiol. 2013; 13: 153 10.1186/1471-2180-13-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 13. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48: 1–12. 10.1086/595011 [DOI] [PubMed] [Google Scholar]
- 14. Jones RN, Castanheira M, Hu B, Ni Y, Lin SS, Mendes RE, et al. Update of contemporary antimicrobial resistance rates across China: reference testing results for 12 medical centers (2011). Diagn Microbiol Infect Dis. 2013; 77: 258–266. 10.1016/j.diagmicrobio.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 15. Sun DD, Ma XX, Hu J, Tian Y, Pang L, Shang H, et al. Epidemiological and molecular characterization of community and hospital acquired Staphylococcus aureus strains prevailing in Shenyang, Northeastern China. Braz J Infect Dis. 2013; 17: 682–690. 10.1016/j.bjid.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rongpharpi SR, Hazarika NK, Kalita H. The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in assam with special reference to MRSA. J Clin Diagn Res. 2013; 7: 257–260. 10.7860/JCDR/2013/4320.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahsay A, Mihret A, Abebe T, Andualem T. Isolation and antimicrobial susceptibility pattern of Staphylococcus aureus in patients with surgical site infection at Debre Markos Referral Hospital, Amhara Region, Ethiopia. Arch Public Health. 2014; 72: 16 10.1186/2049-3258-72-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuda C, Suvorov M, Vakulenko SB, Mobashery S. The basis for resistance to beta-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J Biol Chem. 2004; 279: 40802–40806. [DOI] [PubMed] [Google Scholar]
- 19. van Hal SJ, Steen JA, Espedido BA, Grimmond SM, Cooper MA, Holden MT, et al. In vivo evoluation of antimicrobial resistance in a series of Staphylococcus aureus patients isolates: the entire picture or a cautionary tale? J Antimicrob Chemother. 2014; 69: 363–367. 10.1093/jac/dkt354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fascia DT, Singanayagam A, Keating JF. Methicillin-resistant Staphylococcus aureus in orthopaedic trauma: identification of risk factors as a strategy for control of infection. J Bone Joint Surg Br. 2009; 91: 249–252. 10.1302/0301-620X.91B2.21339 [DOI] [PubMed] [Google Scholar]
- 21. March A, Aschbacher R, Dhanji H, Livermore DM, Bottcher A, Sleghel F, et al. Colonization of residents and staff of a long-term-care facility and adjacent acute-care hospital geriatric unit by multiresistant bacteria. Clin Microbiol Infect. 2010; 16: 934–944. 10.1111/j.1469-0691.2009.03024.x [DOI] [PubMed] [Google Scholar]
- 22. Chen CC, Pass SE. Risk factors for and impact of methicillin-resistant Staphylococcus aureus nasal colonization in patients in a medical intensive care unit. Am J Infect Control. 2013; 41:1100–1101. 10.1016/j.ajic.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 23. Abe Y, Shigemura K, Yoshida H, Fujisawa M, Arakawa S. Risk factors for anti-MRSA drug resistance. Int J Antimicrob Agents. 2012; 40: 423–426. 10.1016/j.ijantimicag.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 24. Porto JP, Santos RO, Gontijo FP, Ribas RM. Active surveillance to determine the impact of methicillin resistance on mortality in patients with bacteremia and influences of the use of antibiotics on the development of MRSA infection. Rev Soc Bras Med Trop. 2013; 46: 713–718. 10.1590/0037-8682-0199-2013 [DOI] [PubMed] [Google Scholar]
- 25. Harinstein L, Schafer J, D'Amico F. Risk factors associated with the conversion of methicillin-resistant Staphylococcus aureus colonization to healthcare-associated infection. J Hosp Infect. 2011; 79: 194–197. 10.1016/j.jhin.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 26. Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust. 2001; 175: 264–267. [DOI] [PubMed] [Google Scholar]
- 27. Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Hellmich M, Hopkins S, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five perspective, observational studies. J Infect. 2014; 68: 242–251. 10.1016/j.jinf.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.