Abstract
The aging population is growing exponentially worldwide. Associated with this greater life expectancy is the increased burden of chronic health conditions, many of which are exacerbated by the continued rise in obesity. In the US, the prevalence of obesity in adults aged 60 years and older increased from 23.6% to 37% in 2010.
Objectives:
This review examines bariatric surgery as a treatment option for obese adults > 60 years old. The most common types of weight-loss surgery are laparoscopic adjustable gastric banding, vertical sleeve gastrectomy, Roux-en-Y gastric bypass, and the duodenal switch.
Methods:
A comprehensive literature search found 349 articles that referred to bariatric surgery in older adults. Of these, 70 relevant articles on bariatric surgery for older adults were utilized for this article.
Results:
Weight-loss surgery procedures were found to be equally safe for both older adults and their younger counterparts. Pre-surgical psychological assessment is critical for positive outcomes for older adults. Benefits of bariatric surgery include a decrease in comorbidities, chronic disease risk, and medication use coupled with improved mobility and quality of life outcomes. Side effects include surgical failure, changes in psychological status, and increased physical and mental stress.
Conclusions:
Bariatric surgery can offer patients an effective and long-lasting treatment for obesity and related diseases. There does not appear to be any one bariatric procedure that is recommended for older adults, so individual needs should be taken into consideration when exploring options. Costs range from US$17,000 for laparoscopic procedures to US$26,000 for open gastric surgeries. Estimated savings start accruing within 3 months of surgery making bariatric surgery a serious cost saving consideration.
Keywords: Older adults, bariatric surgery, outcomes
Introduction
This review examines bariatric surgery as a treatment option for obese adults ≥60 years old. The prevalence of obesity in older adults is rapidly expanding due to the aging of the baby boomer generation. Estimates of obesity in older Americans increased from 9.9 million (24%) in 1990 to 14.6 million (32%) in 2000 to 22.2 million (37%) in 2010.1 Since the baby boomers, those born between 1946 and 1964, are reaching their sixth decade heavier than previous generations, there is a public health concern that these overweight adults will become obese leading to greater risk of chronic disease.2 The baby boomers weighed more and became obese at younger ages than previous generations.3 When the silent generation members, those born between 1926 and 1945, were aged 35–44 years, 14%−18% were obese, but when the baby boomers were that age, those percentages doubled to 28%−32%.3
Given the increased obesity rates for older adults, increased life expectancy does not necessarily mean an increase in healthy years.4 Instead, obese elderly may be facing additional years of discomfort, lack of mobility, and chronic ill health.5,6 The most common obesity-related chronic diseases are type II diabetes, hypertension, heart disease, stroke, certain types of cancers, metabolic syndrome, respiratory disease, sleep apnea, fatty liver disease, osteoarthritis, gall bladder disease, pulmonary embolism, gastro-esophageal reflux disease (GERD), urinary incontinence, chronic renal failure, gout, and depression.7
A literature search was conducted to retrieve relevant articles on bariatric surgery for older adults using EBSCO, MEDLINE, ProQuest, and Web of Science with the forward option. A total of 349 articles were evaluated, and 70 were deemed relevant and utilized for this review. Relevant articles described various types of weight-loss surgeries with the benefits and side effects, postoperative changes in comorbidities and quality-of-life outcomes, and costs of surgery with related cost savings after surgery (Table 1).
Table 1.
Bariatric key findings for patients aged 60 years and above.
| Author/year | Number of participants >60 years | Key findings |
|---|---|---|
| Adams et al.8 | 7925 surgery group compared 7925 control group, 3 age ranges 33–44, 45–54, and >55+ years | No significant difference in age groups. Indicated that patients who have RYGB had decreased long-term mortality from any causes and from disease specific causes but have increased mortality from non-disease causes as compared with control subjects. |
| Dorman et al.9 | N = 43,378 with 1994 >65 years | Patients >65 years did not experience major complications for either open or laparoscopic procedures but likely to have a longer length of hospital stay for either procedure. |
| Dunkle-Blatter et al.10 | 76 >60 years and 989 <60 years | Same length of stay of 2.9 days in hospital. Significant improvement for diabetes and hypertension after RYGB. Weight loss was less but greater reductions in medications. |
| Hallowell et al.11 | 46 >60 years; 31 Medicare | No difference was found in the occurrence of complications in Medicare patients and patients >60 years. Results indicate that bariatric surgery should not be denied based on age or Medicare status. |
| O’Keefe et al.12 | 197 >65 years | Weight-loss surgery is effective in patients >65 years of age, producing EWL, reduction in daily medication use and morbidities. |
| Perry et al.13 | 476 >65 years | Bariatric surgery appears to increase survival even in high-risk Medicare population. Diagnosed prevalence of weight-related comorbid conditions declined after bariatric surgery. |
| Quebbemann et al.14 | 27 >65–73 years | Bariatric surgery can be performed safely in patients >65 years. RYGB procedure is significantly more effective than LAGB. |
| Sugerman et al.15 | 83 >60 years | Bariatric surgery was effective for older patients with low morbidity and mortality. Older patients had more pre- and post-operative comorbidities and lost less weight than younger patients. But weight loss and improvement in comorbidities in older patients were clinically significant. |
| Van Rutte et al.16 | 73 in the range of 55–59 years, 50 in 60–64 years; 12 in 65 years or older | LSG as a primary treatment for older morbidly obese is an effective and relatively safe procedures in terms of weight loss and remission of comorbidities with an acceptable low complication rate. |
| Varela et al.17 | 1339 >60 years | Older adults had longer lengths of stays in hospitals, but bariatric surgery is considered as safe as other gastrointestinal procedure. Mortality is better than expected. |
| Wool et al.18 | 47males >50–59 years; 13 >60 years males | Despite a higher early morbidity rate, obese males >60 years perform as well as male patients aged 50–59 years with respect to excess weight loss, mortality. Length of hospital stay and improvement of diabetes at 1 year postoperatively. |
| Yuan et al.19 | 27 males >65 years | Weight loss and mortality is similar to younger males. Older males had slightly better resolution of both hypertension and diabetes. |
LSG: laparoscopic sleeve gastrectomy; EWL: excess weight loss; RYGB: Roux-en-Y gastric bypass; LAGB: laparoscopic adjustable gastric banding.
Types of bariatric surgery
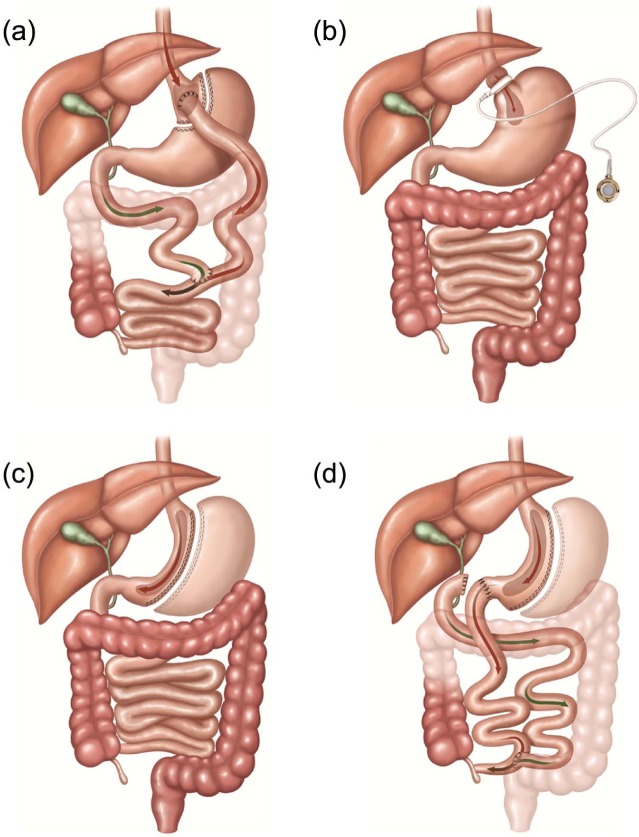
Even though obesity is very difficult to treat with lifestyle changes, the medical community continues to encourage people to lose weight by diet and exercise.7 As a result of these recommendations, overweight and obese people attempt multiple diets, medications, and exercise regiments resulting in limited success over the long term.7,9,20 Increasingly, a viable option for obese patients has been surgery as a means to aid weight loss.3–7 Bariatric surgery that either restricts caloric intake or absorption has been found as the most effective method to lose weight and maintain a healthy lifestyle.3,6–8 There are various bariatric procedures available,20 and generally, the more complex the procedure, greater the weight loss. But more complex/extensive surgery also has greater complications with higher morbidity and mortality rates.9 In the order of frequency performed are the Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding (LAGB), and vertical sleeve gastrectomy (VSG) (Figure 1). Biliopancreatic diversion, with a duodenal switch (BPD-DS) is least commonly performed but is often a consideration for extremely obese individuals20 (Figure 1).
Figure 1.
Most common types of bariatric surgery. (a) Roux-en-Y gastric bypass (RYGB), (b) laparoscopic adjustable banding (LAGB), (c) vertical sleeve gastrectomy (VSG), and (d) biliopancreatic diversion with duodenal switch (BPD-DS).
Source: Neff et al.20
RYGB
RYGB is the most common type of weight-loss surgery worldwide and is considered permanent.21 The stomach is divided into two sections, the upper part is a small pouch which holds about 1–2 ounces of food initially.21 The pouch is connected to the jejunum using a Y-shaped limb of the small intestine bypassing the duodenum.22 Gastric and pancreatic secretions as well as bile mix with chime at the juncture of the jejunum and the duodenum.22 This procedure permanently changes how food is digesting resulting in fewer calories and nutrients being absorbed.13 Weight loss is swift and dramatic, usually 50% of excess weight loss (EWL) in the first 6 months post-surgery, but may continue for up to 2 years.23 Long-term RYGB results show many patients keep weight off for 10 years or longer.24 As an added benefit of the rapid weight loss, health conditions affected by obesity such as diabetes, high blood pressure, high cholesterol, arthritis, sleep apnea, heartburn, and other conditions often improve quickly.13,25–27
Nevertheless, all is not necessarily without challenges. RYGB impairs the body’s ability to absorb calories, dramatically increasing the risk for nutrient deficiencies.28 The most common nutrient deficiencies after RYGB include the vitamins thiamin, vitamin B12 and D, and the minerals iron, copper, and calcium.29 Deficiencies of these nutrients can lead to anemia, fatigue, and osteoporosis; therefore, RYGB patients should plan to take vitamin and mineral supplements the rest of their lives.26,30 Thiamin deficiency has occurred after all bariatric procedures and can be present within weeks or years after surgery.30 Wernicke’s encephalopathy is related to severe thiamin deficiency resulting in cognitive dysfunction and eye disorders such as nystagmus and ocular palsies.30 Vitamin B-12 deficiency results from an intolerance to animal protein after RYGB and lack of intrinsic factor; therefore, monthly B-12 injections are recommended.30 Vitamin D deficiency is a problem for 25%−75% of bariatric patients with many having this deficiency preoperatively. Calcium absorption appears to be diminished possibly due to bypassing the duodenum and lack of Vitamin D.30 Resolving iron deficiency after RYGB can be a complicated issue because oral supplements are not absorbed; therefore, iron must be given intravenously.30 Copper deficiency is also present after RYGB, secondary to iron deficiency; copper status should be investigated as a source of anemia unresponsiveness to other nutrients.30
RYGB is associated with other side effects such as an increased risk of dumping syndrome in which food is “dumped” from the stomach into the small intestine too quickly before it has been adequately digested.29,31,32 Typically, this occurs when eating too many simple carbohydrates which can lead to diarrhea.33 Another long-term complication after RYGB is bone loss which has been greater than with nonsurgical methods of weight loss.31,34 Other potential risks include an increased incidence of hernias and gallstone formations, which can develop post-surgery and require surgical intervention to repair.29 Finally, there is a 1% mortality rate with the RYGB surgery.35
LAGB
LAGB is considered the least invasive and safer than the gastric bypass.36 Benefits of using LAGB are that recovery is quicker, hospital stays are shorter, and the surgery can be reversed.20,25,36,37 LAGB uses an inflatable silicone elastic band to squeeze the stomach into two sections: a smaller upper pouch and a larger lower section. The two sections are still connected, however, the channel or “bottleneck” between them is very small which slows down the emptying of the upper pouch.37 Because LAGB physically restricts the amount of food consumed, it is a successful method in losing weight. However, weight loss is less dramatic and furthermore, weight is more likely regained over time.38 A common side effect of LAGB is vomiting, which is often a result of eating too much too quickly.25 If the band is too tight, acid reflux can result.23,39 Complications with the band itself include slipping out of place, becoming loose, or leaking.38 Sometimes further laparoscopic surgeries are necessary to re-position the band or repair a band leak. Follow-up visits are also required in order to “tighten or loosen” the band to fine-tune how fast food empties from the upper pouch into the residual stomach.40 The process can result in weight fluctuations. LAGB has a 50% failure rate in the long term, as defined by poor weight loss and percentage of band removal.39 Reasons for band removal ranged from inadequate weight loss (76%), gastric pouch dilation (64%), intolerance (21%), and band slippage (12%).39
VSG
About 75% of the stomach is surgically removed in the irreversible VSG procedure (8), usually laprascopically.23 What remains of the stomach is a narrow tube or sleeve which provides for the normal process of stomach emptying and the pyloric valve remains intact. Not only is the appetite reduced, but also consuming very small amounts of food generate early and lasting satiety.41 Because the small intestine is unchanged (not shortened), VSG does not usually affect the absorption of food, so nutritional deficiencies are less of a problem compared to RYGB or BPD-DS.23 In people with very high body mass index (BMI), VSG as a stand-alone procedure results in an average weight loss of greater than 50% of EWL. In patients with very high BMI, the VSG may be followed by a modified RYGB or BPD-DS if significant weight loss is still needed. Since many adults who are morbidly obese have multiple comorbidities, RYGB or BPD-DS may be too risky as an initial procedure.41 Usually, 12–18 months post-VSG, once the patient’s health has improved and some weight has been lost, a second surgery may be undertaken.16,41,42
Since VSG is a relatively new procedure, long-term benefits and risks are still being evaluated. Typical surgical risks such as infection and blood clots apply; moreover, the sleeve itself can have leakage.16 Leakage occurs in less than 2.4% of patients for all sleeve procedures which can be successfully treated by operative or percutaneous drainage and endoscopic stenting.42 Sleeve leakage symptoms include fever, pain in chest/shoulder area, heart palpitations, dizziness, nausea and vomiting.42 VSG is associated with greater weight loss than LAGB, but subsequent weight gain has been seen in nearly all studies where follow-up exceeds 5 years.42,43
BPD-DS
BPD-DS or more commonly referred to as the “duodenal switch” is a more complicated, invasive version of the RYGB and is performed less frequently.44,45 Even though as much as 70% of the stomach is removed during the BPD-DS, the remaining pouch is still larger than those formed during RYGB or LAGB.45,46 As a result, larger meals can be consumed in a sitting; however, there is also a greater, more serious risk of nutritional deficiencies.47,48 Since much of the small intestine is bypassed, digestive enzymes cannot mix with food until it reaches the distal ileum.46,49 The need for dietary supplements is higher among patients with BPD-DS compared to RYGB.44,48
This surgery both restricts intake and reduces absorption area thereby resulting in rapid weight loss with an average long-term EWL of 70%−80%.44,45,47,49 In addition to the rapid weight loss, remission rate for type II diabetes is impressive at 100% within 1 year of surgery.50 Comorbidities such as hypertension, hyperlipidemia, and cardiovascular disease were also significantly reduced.47,49,50 Even with all these positive outcomes, BPD-DS is not without risks. BPD-DS poses many of the same risks as RYGB, including dumping syndrome, GERD, gallstone formation, and hernias.49 Comparing BPD-DS with RYGB, post-surgery BPD-DS required longer hospital stays and more frequent early reoperation than RYGB.44 Nevertheless, overall, there were no differences in late reoperation rates between the two groups.44,46 Despite the complications associated with BPD-DS, one study reported better weight and comorbidity control than RYGB, with even more pronounced benefits among super-obese patients.44,49,51 Because of infection, sepsis, and nutrition deficiencies, mortality rates are greater, ranging from 2.5% to 5% regardless of age.44,49
Bariatric surgery candidate selection
The medical community recommends that surgical treatment of obesity should only be considered after all nonsurgical methods are exhausted.52 Potential bariatric patients are required to have attempted and failed several traditional diet methods.53 In 1991, the National Institutes of Health (NIH) first established bariatric patient selection guidelines which included a BMI of 40 or more or a BMI of 35–39 with one or more obesity-related comorbidities for people aged 18–50 years.52 Age restrictions were initially in place because it was believed that the health risks of bariatric surgeries surpassed beneficial outcomes for aging patients.14,54 In 2006, the NIH recommendations changed, and Medicare reversed their policy to deny bariatric requests based solely on age and age restrictions were eliminated.19,55
Outcomes of bariatric surgery for older persons
Bariatric surgery often results in effective and enduring weight loss with complete resolution or significant improvement in obesity-related comorbidities (Wool et al. 2009).18 After the Medicare-authorized approval of bariatric surgery for older adults in 2006, 2.7% of all bariatric operations were performed on patients older than 60 years old in that year.17 Younger patients may have a greater EWL and have a more complete resolution of their comorbid conditions, but older people reduced the number of prescribed medications that they took.12,29 Age did not influence the rate of occurrence of postoperative complications and outcomes between older and younger patients.11,15,24,57
Physical outcomes of bariatric surgery have steadily improved during the past decade.58 The most common obesity-related chronic diseases are type II diabetes, hypertension, heart disease, stroke, certain types of cancers, metabolic syndrome, respiratory disease, sleep apnea, fatty liver disease, osteoarthritis, gall bladder disease, pulmonary embolism, GERD, urinary incontinence, chronic renal failure, gout, and depression, which can all be improved by weight loss from bariatric surgery.27 Bariatric surgery for older patients has been shown to be safe and effective for EWL and in improvement of obesity comorbidities, especially type II diabetes and blood pressure with trends in greater improvement for older patients than younger patients after RYGB.10 Individuals who underwent bariatric surgery had a significantly reduced number of total and fatal cardiovascular events compared with matched obese controls who did not undergo surgery.24 Furthermore, cancer rates have been reduced to nearly 50% in post-surgical bariatric patients.24
Quality of life measures and physical mobility
Regardless of age, improved mobility, reduced comorbidities, pain reduction, and enhanced psychological functioning such as improvements in mood, self-esteem, social functioning, and sexuality led to improved quality of life enrichment in bariatric patients.59,60 In all, 10 years after weight loss surgery, patients had significantly better health perceptions, social interactions, psychosocial functioning, and reduced depression.61 Improved mobility and less medications alone led many participants who underwent bariatric surgery to state they had experienced improved mood, regardless of whether all weight-loss goals were met, and would opt to have the surgery again.10,59–61
Wheelchair-bound older patients were often fully ambulatory within months post-surgery.15 Even modest weight-loss improved overall physical functioning of older adults.15 For example, patients with lower extremity arthritis experienced reduced knee and hip pain.60,62 Many obese patients who have type II diabetes experience normalization of blood sugars within days post-surgery.10 Patients can frequently stop taking diabetes medications before leaving the hospital after surgery.10 Being able to reduce or eliminate daily diabetes glucose testing and insulin injections leads to improved quality of life. Currently, research on glucose metabolism is underway to better understand this outcome phenomenon.10,63 However, nutrient deficiencies negatively affect quality of life by requiring extra doctor visits, vitamin supplements, iron infusions, B-12 injections, and physical symptoms of lower energy.26,30
Food intolerances and physical mobility challenges
Food intolerance and lack of vitamin and mineral absorption can be a problem for all patients after weight loss surgery, regardless of type, and can result in osteoporosis and anemia.6,48 This can be a more critical concern for the elderly. Since there is an increased risk of bone fractures for those who developed osteoporosis, recovery from hip fracture can prove to be problematic for aging patients.10 If left untreated, low intake of heme iron foods can increase the risk of anemia, which can lead to frailty and malnutrition in older patients.64 Patients report that they have a sense of control of food intake that they never had before but certain food intolerances such as meat and coarse vegetables can make choosing foods difficult.48,65
Since weight loss post-surgery is initially rapid, losing muscle and fat mass is a valid concern in older patients. As one ages, the loss of muscle mass, known as sarcopenia, usually happens as part of the aging process.34 But losing additional muscle mass through rapid weight loss has the potential to result in more mobility issues for older adults.34 Post-op diet and exercise regimes are recommended for all surgeries and all patients, but certain health challenges may make it difficult for older adults to adhere to strict programs.66 Obesity was once believed to be bone protective, but more recent research has introduced evidence of greater risk for metabolic bone disease due to lack of vitamin D and inadequate calcium intake, sedentary lifestyle, chronic dieting, and underlying diseases.34 After bariatric surgery, the risk of bone-related diseases increases due to restrictive intake, malabsorption, and poor compliance to vitamin and mineral supplements and dramatic weight loss.5,6,27,34
Costs of bariatric surgery
Healthcare utilization and healthcare costs for the morbidly obese are 81% above those of the nonobese population.65 Analysis of bariatric surgery cost of 3651 patients showed a strong return on investment up to 5 years post-operatively.67 Estimated costs in 2010 of laparoscopic and open gastric bypass are US$17,000 and US$26,000, respectively.57 Even with these high surgical expenditures, cost savings start accruing by the third postoperative month.68 The short-term return on investment associated with bariatric surgery is consistent with decrease in multiple comorbid conditions, including diabetes, coronary artery disease, hypertension, and sleep apnea.67,69 The cost reductions in these diseases take into account prescription drug usage, hospital stays, and physician visits.67 Type II diabetes is greatly improved by bariatric surgery69 and estimated annual costs of managing a diabetes patient (US$13,243) are five times more than a patient without diabetes (US$2,560).70 People age 65 years and older represent 10.9 million Americans. Approximately 27% of all people in this age group have diabetes but not all of these older dietetics will necessarily improve with bariatric surgery. It is estimated that one-third of all Medicare dollars are spent on the cost of care of people with diabetes.28
Conclusion
Bariatric surgery can offer patients an effective and long-lasting treatment for obesity and its related diseases. There does not appear to be any one bariatric surgical procedure that is recommended for older adults, so individual needs should be taken into consideration when exploring options. Literature is scarce on the long-term success of older adults and bariatric surgery. This may be due to the NIH removal of age limitations and Medicare inclusion of bariatric surgery since 2006. A number of studies have demonstrated that bariatric surgery is safe for the aging population; additionally, comorbidities improve. Many other research opportunities remain. Research questions should delve into motivating older adults and their younger counterparts to choose bariatric surgery and the effects of that surgery on their relationships. Questions remain concerning side effects of bariatric surgery on social relationships, the role of social support, quality-of-life issues post-surgery, and predictor differences for bariatric success in older versus younger adult patients. Other questions might include the effect of retirement as a help or hindrance on bariatric surgery recovery, older patients and diet adherence, the effect of nutritional deficiency complications, or weight regain concerns. Specific for older adults, research questions may include the effects of muscle and bone loss as a result of bariatric surgery and the long-term outcome for mobility for older adults.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research was not supported by any participating grant or foundation. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Salihu HM, Bonnema S, Alio AP. Obesity: what is an elderly population growing into? Maturitas 2009; 63(1): 7–12. DOI: 10.1016/j.maturitas.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2. Leveille SG, Wee CC, Iezzoni LI. Trends in obesity and arthritis among baby boomers and their predecessors, 1971-2002. Am J Public Health 2005; 95(9): 1607–1613. DOI: 10.2105/AJPH.2004.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zevin B, Aggarwal R, Grantcharov TP. Volume-outcome association in bariatric surgery: a systematic review. Ann Surg 2012; 256(1): 60–71. DOI: 10.1097/SLA.0b013e3182554c62. [DOI] [PubMed] [Google Scholar]
- 4. Han TS, Tajar A, Lean MEJ. Obesity and weight management in the elderly. Br Med Bull 2011; 97(1): 169–196. DOI: 10.1093/bmb/ldr002. [DOI] [PubMed] [Google Scholar]
- 5. Mathus-Vliegen EMH. Obesity and the elderly. J Clin Gastroenterol 2012; 46(7): 533–544. DOI: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 6. Mathus-Vliegen EMH, Basdevant A, Finer N, et al. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts 2012; 5(3): 460–483. DOI: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 7. Zamosky L. The obesity epidemic. Med Econ 2013; 90(4): 14–17. [PubMed] [Google Scholar]
- 8. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357(8): 753–761. DOI: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 9. Dorman RB, Abraham AA, Al-Refaie WB, et al. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg 2012; 16(1): 35–43. DOI: 10.1007/s11605-011-1749-6. [DOI] [PubMed] [Google Scholar]
- 10. Dunkle-Blatter SE, St Jean MR, Whitehead C, et al. Outcomes among elderly bariatric patients at a high-volume center. Surg Obes Relat Dis 2007; 3(2): 163–169. DOI: 10.1016/j.soard.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11. Hallowell P, Stellato T, Schuster M, et al. Avoidance of complications in older patients and Medicare recipients undergoing gastric bypass. Arch Surg 2007; 142(6): 506–510. [DOI] [PubMed] [Google Scholar]
- 12. O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients above age 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg 2010; 20(9): 1199–1205. DOI: 10.1007/s11695-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 13. Perry CD, Hutter MM, Smith DB, et al. Survival and changes in comorbidities after bariatric surgery. Ann Surg 2008; 247(1): 21–27. DOI: 10.1097/SLA.0b013e318142cb4b. [DOI] [PubMed] [Google Scholar]
- 14. Quebbemann B, Engstrom D, Siegfried T, et al. Bariatric surgery in patients older than 65 years is safe and effective. Surg Obes Relat Dis 2005; 1(4): 389–392. [DOI] [PubMed] [Google Scholar]
- 15. Sugerman HJ, DeMaria EJ, Kellum JM, et al. Effects of bariatric surgery in older patients. Ann Surg 2004; 240(2): 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Rutte PWJ, Smulders JF, de Zoete JP, et al. Sleeve gastrectomy in older obese patients. Surg Endosc 2013; 27(6): 2014–2019. DOI: 10.1007/s00464-012-2703-8. [DOI] [PubMed] [Google Scholar]
- 17. Varela JE, Wilson SE, Nguyen NT. Outcomes of bariatric surgery in the elderly. Am Surg 2006; 72(10): 865–869. [DOI] [PubMed] [Google Scholar]
- 18. Wool D, Bellatorre N, Wren S, et al. Male patients above age 60 and above have as good outcomes as male patients 50-59 years old at 1-year follow-up after bariatric surgery. Obes Surg 2009; 19(1): 18–21. [DOI] [PubMed] [Google Scholar]
- 19. Yuan X, Hawver LRM, Ojo P, et al. Bariatric surgery in Medicare patients: greater risks but substantial benefits. Surg Obes Relat Dis 2009; 5(3): 299–304. DOI: 10.1016/j.soard.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 20. Neff KJ, Olbers T, Le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med 2013; 11(1): 1–17. DOI: 10.1186/1741-7015-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013; 23(4):427–436. DOI: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 22. Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin North Am 2011; 95(5): 1009–1030. DOI: 10.1016/j.mcna.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 23. Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol 2011; 301(1): R15–R27. DOI: 10.1152/ajpregu.00038.2011. [DOI] [PubMed] [Google Scholar]
- 24. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012; 307(1): 56–65. DOI: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 25. Chakravarty PD, McLaughlin E, Whittaker D, et al. Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures; a systematic review of the randomised controlled trials. Surgeon 2012; 10(3): 172–182. DOI: 10.1016/j.surge.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 26. Jáuregui-Lobera I. Iron deficiency and bariatric surgery. Nutrients 2013; 5(5): 1595–1608. DOI: 10.3390/nu5051595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poirier P, Cornier MA, Mazzone T, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation 2011; 123(15): 1683–1701. [DOI] [PubMed] [Google Scholar]
- 28. Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA 2012; 308(11): 1132–1141. [DOI] [PubMed] [Google Scholar]
- 29. Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012; 308(11): 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saltzman E, Karl JP. Nutrient deficiencies after gastric bypass surgery. Annu Rev Nutr 2013; 33: 183–203. DOI: 10.1146/annurev-nutr-071812-161225. [DOI] [PubMed] [Google Scholar]
- 31. Hammer HF. Medical complications of bariatric surgery: focus on malabsorption and dumping syndrome. Dig Dis 2012; 30(2): 182–186. [DOI] [PubMed] [Google Scholar]
- 32. Myers VH, McVay MA, Adams CE, et al. Actual medical and pharmacy costs for bariatric surgery: 6-year follow-up. South Med J 2012; 105(10): 530–537. DOI: 10.1097/SMJ.0b013e318268c76d. [DOI] [PubMed] [Google Scholar]
- 33. Heinlein CR. Dumping syndrome in Roux-en-Y bariatric surgery patients: are they prepared? Bariat Nurs Surg Pat 2009; 4(1): 39–47. DOI: 10.1089/bar.2009.9992. [DOI] [Google Scholar]
- 34. Berarducci A. Bone loss—an emerging problem following obesity surgery. Orthop Nurs 2007; 26(5): 281–286. DOI: 10.1097/01.NOR.0000295953.74258.7b. [DOI] [PubMed] [Google Scholar]
- 35. Benotti P, Wood GC, Winegar DA, et al. Risk factors associated with mortality after Roux-en-Y gastric bypass surgery. Ann Surg 2014; 259: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis 2007; 3(2): 127–132. [DOI] [PubMed] [Google Scholar]
- 37. Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med 2008; 121(10):885–893. DOI: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 38. Thornton CM, Rozen WM, So D, et al. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009; 19(12):1702–1706. DOI: 10.1007/s11695-008-9672-y. [DOI] [PubMed] [Google Scholar]
- 39. Spivak H, Abdelmelek M, Beltran O, et al. Long-term outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass in the United States. Surg Endosc 2012; 26(7): 1909–1919. DOI: 10.1007/s00464-011-2125-z. [DOI] [PubMed] [Google Scholar]
- 40. Allen JW. Laparoscopic gastric band complications. Med Clin North Am 2007; 91(3): 485–497. [DOI] [PubMed] [Google Scholar]
- 41. Benaiges D, Goday A, Ramon JM, et al. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis 2011; 7(5): 575–580. DOI: 10.1016/j.soard.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 42. Aurora A, Khaitan L, Saber A. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc 2012; 26(6): 1509–1515. DOI: 10.1007/s00464-011-2085-3. [DOI] [PubMed] [Google Scholar]
- 43. Caiazzo R, Pattou F. Adjustable gastric banding, sleeve gastrectomy or gastric bypass. Can evidence-based medicine help us to choose? J Visc Surg 2013; 150(2): 85–95. DOI: 10.1016/j.jviscsurg.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 44. Hedberg J, Sundbom M. Superior weight loss and lower HbA1c 3 years after duodenal switch compared with Roux-en-Y gastric bypass—a randomized controlled trial. Surg Obes Relat Dis 2012; 8(3): 338–343. DOI: 10.1016/j.soard.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 45. Mitchell MT, Carabetta JM, Shah RN, et al. Duodenal switch gastric bypass surgery for morbid obesity: imaging of postsurgical anatomy and postoperative gastrointestinal complications. AJR Am J Roentgenol 2009; 193(6): 1576–1580. DOI: 10.2214/AJR.08.1941. [DOI] [PubMed] [Google Scholar]
- 46. Nilsson G, Hedberg P, Ohrvik J. Survival of the fattest: unexpected findings about hyperglycaemia and obesity in a population based study of 75-year-olds. BMJ Open 2011; 1(1): e000012 DOI: 10.1136/bmjopen-2010-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dorman RB, Rasmus NF, al-Haddad BJS, et al. Benefits and complications of the duodenal switch/biliopancreatic diversion compared to the Roux-en-Y gastric bypass. Surgery 2012; 152(4): 758–767. DOI: 10.1016/j.surg.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 48. Ziegler O, Sirveaux MA, Brunaud L, et al. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 2009; 35(6): 544–557. [DOI] [PubMed] [Google Scholar]
- 49. Prachand V, Ward M, Alverdy J. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. J Gastrointest Surg 2010; 14(2): 211–220. [DOI] [PubMed] [Google Scholar]
- 50. Iaconelli A, Panunzi S, De Gaetano A, et al. Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care 2011; 34(3): 561–567. DOI: 10.2337/dc10-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg 2012; 147(9): 847–854. DOI: 10.1001/archsurg.2012.1654. [DOI] [PubMed] [Google Scholar]
- 52. Yermilov I, McGory ML, Shekelle PW, et al. Appropriateness criteria for bariatric surgery: beyond the NIH guidelines. Obesity 2009; 17(8): 1521–1527. DOI: 10.1038/oby.2009.78. [DOI] [PubMed] [Google Scholar]
- 53. Van Hout GCM, Van Oudheusden I, Krasuska AT, et al. Psychological profile of candidates for vertical banded gastroplasty. Obes Surg 2006; 16(1): 67–74. DOI: 10.1381/096089206775222023. [DOI] [PubMed] [Google Scholar]
- 54. Pratt GM, McLees B, Pories WJ. The ASBS Bariatric Surgery Centers of Excellence program: a blueprint for quality improvement. Surg Obes Relat Dis 2006; 2(5): 497–503. [DOI] [PubMed] [Google Scholar]
- 55. Henrickson HC, Ashton KR, Windover AK, et al. Psychological considerations for bariatric surgery among older adults. Obes Surg 2009; 19(2): 211–216. DOI: 10.1007/s11695-008-9768-4. [DOI] [PubMed] [Google Scholar]
- 56. Buchwald H. Bariatric surgery: a systematic review and meta-analysis (Erratum in JAMA 2005; 293: 1728). JAMA 2004; 292(14): 1724–1737. [DOI] [PubMed] [Google Scholar]
- 57. Felix HC, West DS. Effectiveness of weight loss interventions for obese older adults. Am J Health Promot 2013; 27(3): 191–199. DOI: 10.4278/ajhp.110617-LIT-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dimick JB, Nicholas LH, Ryan AM, et al. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA 2013; 309(8): 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Folope V, Hellot MF, Kuhn JM, et al. Weight loss and quality of life after bariatric surgery: a study of 200 patients after vertical gastroplasty or adjustable gastric banding. Eur J Clin Nutr 2008; 62(8): 1022–1030. DOI: 10.1038/sj.ejcn.1602808. [DOI] [PubMed] [Google Scholar]
- 60. Van Hout G, Van Heck G. Bariatric psychology, psychological aspects of weight loss surgery. Obes Facts 2009; 2(1): 10–15. DOI: 10.1159/000193564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karlsson J, Taft C, Rydén A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes 2007; 31(8): 1248–1261. DOI: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 62. McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity 2006; 14(9): 1485–1497. DOI: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 63. Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013; 341(6144): 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heuberger RA. The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr 2011; 30(4): 315–368. DOI: 10.1080/21551197.2011.623931. [DOI] [PubMed] [Google Scholar]
- 65. Hensrud DD, Klein S. Extreme obesity: a new medical crisis in the United States. Mayo Clin Proc 2006; 81(Suppl. 10): S5–S10. [DOI] [PubMed] [Google Scholar]
- 66. Gremeaux V, Gayda M, Lepers R, et al. Exercise and longevity. Maturitas 2012; 73(4): 312–317. DOI: 10.1016/j.maturitas.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 67. Cremieux PY, Buchwald H, Shikora SA, et al. A study on the economic impact of bariatric surgery. Am J Manag Care 2008; 14(9): 589–596. [PubMed] [Google Scholar]
- 68. Bradley DW, Sharma BK. Centers of Excellence in Bariatric Surgery: design, implementation, and one-year outcomes. Surg Obes Relat Dis 2006; 2(5): 513–517. [DOI] [PubMed] [Google Scholar]
- 69. Klein S, Ghosh A, Cremieux PY, et al. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥ 35 kg/m2. Obesity (Silver Spring) 2011; 19(3): 581–587. DOI: 10.1038/oby.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campbell J, McGarry LJ, Shikora SA, et al. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care 2010; 16(7): e174–e187. [PubMed] [Google Scholar]