Abstract
Objectives:
Self-management is widely promoted but evidence of effectiveness is limited. Policy encourages health care professionals to support people with long-term conditions to learn self-management skills, yet little is known about the extent to which both parties share a common understanding of self-management. Thus, we compared health care professional and lay understandings of self-management of long-term conditions.
Methods:
Systematic review and narrative synthesis of qualitative studies identified from relevant electronic databases, hand-searching of references lists, citation tracking and recommendations by experts.
Results:
In total, 55 studies were included and quality was assessed using a brief quality assessment tool. Three conceptual themes, each with two subthemes were generated: traditional and shifting models of the professional–patient relationship (self-management as a tool to promote compliance; different expectations of responsibility); quality of relationship between health care professional and lay person (self-management as a collaborative partnership; self-management as tailored support) and putting self-management into everyday practice (the lived experience of self-management; self-management as a social practice).
Conclusion:
Self-management was conceptualised by health care professionals as incorporating both a biomedical model of compliance and individual responsibility. Lay people understood self-management in wider terms, reflecting biomedical, psychological and social domains and different expectations of responsibility. In different ways, both deviated from the dominant model of self-management underpinned by the concept of self-efficacy. Different understandings help to explain how self-management is practised and may help to account for limited evidence of effectiveness of self-management interventions.
Keywords: Self-management, long-term conditions, health care professionals, lay people, narrative synthesis
Introduction
Self-management programmes are increasingly promoted as a policy response to the growing number of people living with long-term conditions (LTCs), and the associated economic implications of this.1 The meaning of self-management, however, is contested, with no agreed upon definition of the term. A commonly used definition of self-management proposed by Barlow et al.2 is ‘an individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent with living with a chronic condition’ (p. 366). Other definitions encompass social support from family, friends and wider community ties.3–5 Furthermore, self-management has been characterised as a key feature of contemporary health care systems, at the heart of which are collaborative partnerships between patients and health care professionals (HCPs), aimed at promoting a culture of care fostering self-management.6–9
Self-management has also been proposed as a sub-category of the broader term ‘self-care’.2 Whereas the former relates to the ways in which individuals and their families manage the impact and effects of living with a chronic condition, the latter refers more generally to health promoting strategies used by lay people to maintain or optimise a state of health and well-being.3 However, the two terms have often been used synonymously in the policy10,11 and health services research literature.3,12 This slippage in terminology makes it difficult to understand what is actually meant by self-management of LTCs.
Self-management interventions may include lay or professionally led and group-or individual-based approaches,13,14 but commonly they target people with a range of chronic conditions as part of a group programme modelled on the Chronic Disease Self-Management Programme (CDSMP) developed by Lorig and colleagues15,16 in the United States. This largely biomedical model of self-management aims to empower people with LTCs to learn new skills and knowledge (e.g. problem solving, shared decision making, utilisation of resources) in partnership with HCPs. Such an approach has been adapted in the United Kingdom as the Expert Patient Programme (EPP)17 and in Australia as the ‘Sharing Heath Care’ programme.18 These interventions are based on Bandura’s19 social cognitive theory of behaviour, in which individuals learn self-efficacy enhancing skills to improve their ability to manage the everyday challenges of living with a chronic condition. Drawing on self-regulation theory20 (also rooted in social cognitive theory), self-regulation models of chronic disease self-management proposed by Clark and colleagues21–23 have further informed the self-efficacy literature. Such scholars propose that individuals’ perception of ability to change or modify behaviours occurs through self-regulatory mechanisms, for example, in terms of their judgement of the benefits and importance of a particular goal, and beliefs about health and illness, which is partly influenced by a person’s social environment. Thus, self-regulation in turn influences self-efficacy, decision making processes and ongoing engagement in self-management behaviours.
Programmes based on psychological models of self-management have been criticised for their individualistic, biomedical and prescriptive focus on disease management, that fall short of addressing lay understandings of self-management and the social context shaping self-management practices.18,24–26 Such approaches have not incorporated phenomenological perspectives that draw our attention to the lived experience of self-management.27 Nor do they integrate the extensive body of sociological research on ‘illness narratives’, depicting the varied ways in which lay people make sense, cope and manage a chronic condition in their everyday lives.24 Illness narratives are embedded in social practices and social support from family and other social networks.5,24 In line with social support theory, however, this process may involve both positive and negative influences,28,29 which in turn may impact on self-management practices.
Current evidence for the benefits of self-management programmes remains equivocal30–32 and patient engagement is limited.30 For example, a Cochrane Review of 17 randomised controlled trials of lay-led self-management programmes for individuals with a range of LTCs (including, diabetes, arthritis and chronic pain) found only short-term improvements in some patient outcomes, including self-rated health and self-efficacy, but little or no effect on other outcomes, such as psychological health, quality of life and use of health services.30 Possible explanations for these mixed results relate to methodological issues, such as the heterogeneous nature and delivery of trials and outcome measures used,2 as well as the characteristics of participants taking part, mostly well-educated women, with limited engagement among men and those from minority ethnic groups.30,33 Other explanations proposed by Osborne et al.34 point to the lack of attention given to addressing social and structural factors shaping self-management practices, including inequitable access to services, social disadvantage and social isolation, and the lack of integration of self-management programmes in routine health care.
State-driven interventions such as the EPP10 designed to support lay people to learn self-management skills are based on an implicit shift in responsibility from professional to lay person in terms of managing the burden of disease and psychosocial impact of living with a chronic condition.7,35 It is often assumed that individuals living with one or more LTCs have a prior interest in managing their health through such an approach, arguably attracting those who might ‘buy into’ the notion of self-management and who are already good ‘self-managers’.36 However, the lack of patient engagement in such initiatives suggests that many lay people may not share the policy’s concern to manage their health in this way.
Increasingly, HCPs are encouraged to support individuals with LTCs to learn self-management skills.11 This implies a shared understanding of the concepts of self-management; something about which little is known. Thus, the aim of the current review is to conduct a systematic review and narrative synthesis of qualitative studies to compare lay and HCP understandings of self-management of LTCs.
Methods
We used a number of methods to search for relevant studies, which is recommended when looking for qualitative evidence from varied sources.37 This included searching electronic databases, hand-searching reference lists, citation tracking and recommendations by experts in the field. We included qualitative studies examining lay and/or HCP understandings of self-management published in peer-reviewed English language journals. We excluded studies that used only questionnaire designs and quantitative analyses and intervention studies as our focus was not to look at why self-management interventions worked or not. We also excluded conference papers and solely theoretical articles.
First, we searched SCOPUS, Web of Science, CINAHL and PsycINFO electronic databases from inception until May 2013. The search strategy used MeSH and free text terminology combining the terms: (‘Self-Management’ OR ‘Self-Care’ OR ‘Self-Help’) AND ‘Qualitative’ AND (‘Long Term Condition*’ OR ‘Chronic Disease*’ OR ‘Chronic Illness*’ OR ‘Diabetes’ OR ‘Arthritis’ OR ‘Asthma’ OR ‘Cancer’ OR ‘Chronic Obstructive Pulmonary Disease’ OR ‘Stroke’ OR ‘Multiple Sclerosis’ OR ‘Traumatic Brain Injury’ OR ‘Acquired Brain Injury’) AND (‘Health Care Professional*’ OR ‘General Practitioner*’ OR ‘Nurse*’ OR ‘Allied Health Professional*’ OR ‘Patient*’ OR ‘Carer*’ OR ‘Caregiver*’). Titles of all articles were read, non-relevant studies excluded (for example, intervention studies) and duplicates removed. Remaining abstracts were then reviewed and potentially relevant articles retrieved and read in full. Studies which did not meet the inclusion criteria after full reading were discarded (for example, those not directly focusing on self-management or more narrowly focusing on self-management of specific disease symptoms only, such as control of blood glucose levels or breathlessness).
Second, we hand-searched reference lists of included articles for further studies that met the inclusion criteria. Again, potentially relevant articles were retrieved and read in full to assess whether they met the inclusion criteria. Third, we used citation tracking in Google Scholar of all relevant studies retrieved from the above electronic and hand searches to look for further articles up until March 2014. Finally, recommendations were made of relevant articles by experts working in the fields of health services research and primary care.
Data extraction and method of synthesis
We used a narrative synthesis approach to provide a narrative, textual account of similarities and differences in HCP and lay understandings of self-management of chronic conditions. We drew on the Economic Social Research Council’s (ESRC) research programme methods guidance38 on using different methods during synthesis which aims to improve the quality and transparency of the review process. In line with their guidance, we adopted a flexible, iterative approach to conducting the stages of the synthesis, and we used tabulation and thematic analysis to compare similarities and differences among HCPs and lay people. E.S. constructed tables using relevant sub-headings (i.e. author, country, sample, method, theoretical approaches used and main themes) (see Table 1). Once tabulation was complete, E.S. and C.M. undertook a thematic analysis of key findings from included studies using the ‘one sheet of paper method’.39 This involved first individually coding the findings of included studies from tables into initial themes and then using the principles of the constant comparative method40 to look for similarities and differences between themes, grouping these into broader conceptual themes visually displayed on one sheet of paper. E.S. and C.M. then discussed relationships between themes and also considered the theoretical basis for the approaches used, which led to a joint consensus on the main emerging conceptual themes and subthemes of the synthesis.
Table 1.
Included studies in the synthesis.
| Author | Country | Sample | Method | Theoretical approach | Theme |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compliance | Responsibility | Collaborative partnership | Tailored support | Lived experience | Social practice | |||||
| Hunt and Arar44 | USA | 51 Mexican–American patients with type 2 diabetes; 35 HCPs recruited from public clinics | Interviews | Medical anthropological (explanatory models) | X | X | X | |||
| Carbone et al.45 | USA | 37 Latino patients with type 2 diabetes; 15 HCPs recruited from a community health centre | Focus groups | – | X | X | ||||
| Crowe et al.46 | New Zealand | 64 patients with chronic low back pain; 22 HCPs recruited through advertisement, newsletter and physiotherapy clinics | Interviews | – | X | X | X | |||
| Blakeman et al.47 | UK | 12 patients with range of LTCs (including diabetes, IHD, stroke and asthma); 17 HCPs recruited from GP practices | Interviews and observations of clinical consultations | – | X | X | ||||
| Kirby et al.48 | Australia | 33 patients with range of LTCs (e.g. chronic respiratory problems or heart failure); 18 clinicians recruited from hospital and primary care | Interviews | Psychological (social cognitive theory) | X | X | ||||
| Pooley et al.49 | UK | 47 patients with type 2 diabetes; 38 HCPs recruited from GP practices | Interviews | – | X | X | ||||
| Yen et al.50 | Australia | 88 primary care HCPs working with patients with a range of LTCs (COPD, diabetes and CHF) recruited from primary care settings | Focus groups | – | X | X | ||||
| Lake and Staiger51 | Australia | 31 HCPs (e.g. GPs, nurses, psychologists) working with patients with range of LTCs recruited from acute, primary and community health care settings | Group and individual interviews | – | X | X | X | X | ||
| Blakeman et al.52 | UK | 16 GPs working with patients with a range of LTCs recruited from two primary care trusts | Interviews | Social science (Howie’s theoretical model applied to GP consultations) | X | |||||
| Macdonald et al.53 | UK | 25 nurses working with individuals with range of LTCs recruited from chronic disease clinics | Interviews | Psychosocial (‘trajectory model’ and ‘personal construct’ theories) | X | X | X | |||
| Phillips et al.54 | Australia | 14 clinicians working with patients with range of LTCs recruited from hospital and community urban settings | Telephone interviews and survey | – | X | X | ||||
| Guidetti et al.55 | Sweden | 12 occupational therapists working with stroke and SCI patients recruited from a rehabilitation setting | Interviews | Phenomenological | X | X | ||||
| Oftedal et al.56 | Norway | 19 people with type 2 diabetes recruited from a hospital, educational programme, local diabetes association and GP practices | Focus groups | – | X | X | ||||
| Thorne and Paterson57 | Canada | 22 adults with type 1 diabetes identified as experts in self-care nominated by clinicians | Multiple interviews. Grounded theory approach | – | X | X | ||||
| Guidetti et al.58 | Sweden | 5 stroke patients and 6 patients with SCI recruited from a rehabilitation setting | Interviews | Phenomenological | X | |||||
| Guidetti et al.59 | Sweden | 5 stroke patients and 6 patients with SCI recruited from a rehabilitation setting | Interviews | Phenomenological | X | X | ||||
| Opal Cox et al.60 | USA | 39 older stroke survivors (aged 62 years and over) recruited from a rehabilitation hospital | Interviews | – | X | X | X | X | ||
| Pound et al.61 | UK | 40 stroke survivors living in a working-class area of London identified from a stroke register of hospital admissions | Interviews | Sociological (illness narratives) | X | X | ||||
| Audulv et al.62 | Sweden | 26 people with range of LTCs (diabetes, MS, rheumatic disease and IBD) recruited from an outpatient clinic | Narrative interviews | Psychological (‘responsibility attribution’) | X | X | X | X | ||
| Kralik et al.63 | Australia | 9 middle-aged older-aged adults with chronic arthritis recruited from an advertisement in a community newsletter | Focus group, written autobiographical accounts and telephone interviews | Psychosocial (experience of transitions) | X | X | ||||
| Atkin et al.64 | UK | 23 individuals with encephalitis recruited from a national voluntary organisation | Interviews | Sociological (illness narratives) | X | X | ||||
| Kielman et al.65 | UK | 31 patients with chronic respiratory conditions recruited through primary care organisations | Focus groups, diaries and telephone interviews | Social science (‘self-care’, ‘lay knowledge’ and ‘patient-provider interactions’) | X | X | ||||
| Koch et al.66 | Australia | 24 older people (aged 60 years and over) with asthma recruited using range of methods (including newspapers and contacting community health workers) | Interviews, questionnaires and participatory action research groups | – | X | X | X | X | ||
| Collins et al.67 | Ireland | 17 patients with type 1 and 2 diabetes recruited from GP practices and diabetic clinics | Interviews | – | X | X | ||||
| Townsend et al.68 | UK | 23 people with multiple LTCs taking part in a community health survey | Repeat narrative interviews | Sociological (illness narratives) | X | |||||
| Schulman-Green et al.69 | USA | 15 women with advanced breast cancer recruited from a hospital cancer centre | Interviews | – | X | X | X | |||
| Cooper et al.70 | UK | 24 patients with IBD aged between 30 and 40 years recruited from outpatient clinics | Interviews | – | X | X | ||||
| Clark et al.71 | USA | 23 older individuals (aged 65 years and over) from white and black ethnic groups with range of LTCs from low-income groups and 12 with private health insurance recruited from primary care settings | Interviews | Psychological (identity theory) | X | X | X | |||
| Chen et al.72 | Taiwan | 18 patients with COPD recruited from a hospital ward, outpatient and rehabilitation settings | Interviews | – | X | X | ||||
| Hjelm et al.73 | Sweden | 15 Swedish-born and 13 Yugoslavian-born women with diabetes recruited from primary health care centres | Focus groups | Medical anthropological (lay models of illness causation and health-care seeking behaviours) | X | X | ||||
| Ploughman et al.74 | Canada | 18 people aged 55 years and over with MS recruited from MS clinics and outpatient rehabilitation setting | Narrative interviews | Life-course perspective | X | X | X | X | X | |
| Dixon et al.75 | USA | 27 people with at least one LTC recruited from non-faculty university staff | Interviews | – | X | X | X | X | ||
| Furler et al.76 | Australia | Ethnically diverse sample of 52 people with type 2 diabetes recruited from community health service and support groups | Focus groups | – | X | |||||
| Gunn et al.77 | United Kingdom | 45 people with type 1 or type 2 diabetes living in urban and rural areas admitted to hospital and identified by specialist practitioners | Interviews | – | X | |||||
| Gallant et al.78 | USA | 84 people aged 65 years and over from white and African American ethnic backgrounds with range of LTCs (arthritis, diabetes and heart disease) recruited from a range of community organisations | Focus groups | Psychosocial (social cognitive theory and social support theories) | X | |||||
| McLaughlin and Zeeberg79 | Denmarkand USA | 51 community-dwelling Danish and 35 American adults with MS | Ethnographic approach: participant observations, interviews and self-reported self-care behaviour questionnaire | – | X | |||||
| Stamm et al.80 | Austria | 10 people with RA recruited from rheumatology outpatients clinic | Repeat narrative interviews | Sociological (biographical approach) | X | |||||
| Lindsay81 | UK | 53 people with multiple LTCs recruited from CHD registries, as part of a larger RCT study | Focus groups | Sociological (‘chronic illness trajectories’) | X | |||||
| Cicutto et al.82 | Canada | 42 individuals with COPD recruited through range of methods (posters in physician and rehabilitation clinics, newsletter and newspaper advertisements) | Focus groups | – | X | X | X | |||
| Loignon et al.83 | Canada | 24 people with asthma from low- and middle-income backgrounds recruited from a general hospital and snowball sampling techniques | Interviews | Sociological (illness narratives) | X | X | ||||
| Audulv et al.84 | Sweden | 26 people with range of LTCs (RA, diabetes, IBS, MS, IHD, kidney failure) | Narrative interviews, grounded theory approach | – | X | X | ||||
| Goodacre85 | UK | 12 women with chronic arthritis recruited from local arthritis self-help groups | Repeat interviews, diaries and focus groups | Sociological (illness narratives) | X | |||||
| Kidd et al.86 | UK | 11 patients with colorectal cancer recruited from a cancer centre | Repeat interviews | Psychological (self-regulation theory) | X | X | ||||
| Chiu-Chu et al.87 | Taiwan | 41 patients with type 2 diabetes recruited from hospital clinics | Focus groups | Psychological (self-regulation theory) | X | |||||
| Corbin and Strauss88 | USA | 60 people with a range of LTCs (mainly cardiac disease, cancer, stroke, SCI) and their carers | Interviews | Sociological (‘illness trajectories’) | X | |||||
| Kenning et al.89 | UK | 20 patients with multiple LTCs (CHD, diabetes, osteoarthritis, COPD and depression) identified from disease registries and HCPs recruited from GP practices, as part of a larger cohort study | Interviews | – | X | X | ||||
| Hinder and Greenhalgh90 | UK | Ethnically diverse sample of 30 people with type 1 and type 2 diabetes recruited from patient groups, primary and secondary health care clinics, community contacts and ethnic minority groups | Ethnographic approach, including home and community observations and interviews | Sociological (‘structuration theory’) | X | X | X | |||
| Morris et al.91 | UK | 21 individuals with multiple LTCs recruited from two GP practices (part of a qualitative study within an RCT) | Interviews | – | X | X | X | |||
| Greenhalgh et al.92 | UK | 82 individuals with type 1 and type 2 diabetes from minority ethnic groups taking part in an RCT study on storytelling | Ethnographic approach: observations and field notes of group discussions | Biomedical and sociological (illness narratives) | X | X | ||||
| Ong et al.93 | UK | 22 individuals aged 50 years and over with chronic knee pain, drawn from a cohort study | Repeat interviews and diaries | Sociological (illness narratives) | X | |||||
| Clarke and Bennett94 | Canada | 35 older people (aged 73–91 years) with multiple LTCs recruited through newspaper advertisements and database of participants taking part in a previous study | Multiple interviews | Sociological (illness narratives) | X | |||||
| Pickard and Rogers95 | UK | Patients with multiple LTCs (including diabetes, kidney disease and hypertension) and their carers recruited from patient records in GP practices | Multiple interviews, focuses on one case study | Phenomenological and sociological (illness narratives) | X | X | X | X | X | |
| Audulv et al.96 | Sweden | 21 people with range of LTCs recruited from an outpatient hospital clinic | Multiple interviews. Grounded theory approach | – | X | X | ||||
| Samuel-Hodge et al.97 | USA | 35 African American adults with type 2 diabetes and 32 of their family members recruited through flyers by ‘community recruiters’ | Focus groups | – | X | |||||
| Essue et al.98 | Australia | Ethnically diverse sample of 14 carers of patients with a range of LTCs (CHF, COPD, diabetes), who took part in wider study | Secondary qualitative analysis of interviews | – | X | |||||
HCP: health care professional; GP: general practitioner; LTC: long-term condition; IHD: ischaemic heart disease; COPD: coronary obstructive pulmonary disease; CHD; congestive heart disease; CHF: congestive heart failure; SCI: spinal cord injury; RA: rheumatoid arthritis; MS: multiple sclerosis; IBD: inflammatory bowel disease; IBS: irritable bowel syndrome; RCT: randomised controlled trial.
Robustness of the synthesis
Two authors (E.S. and C.M.) first independently considered the quality of included articles using the five-point checklist by Dixon-Woods et al.41 (see Box 1). This checklist is a brief way of evaluating the quality of studies in relation to their aims, methods, results and conclusions and involved giving each study a score out of 5 based on whether they were judged to meet five specific criteria (Box 1). For example, a score of 5 meant that all five criteria were met in one study. E.S. and C.M. then discussed together the individual quality scores given. Overall, there were no disagreements between the two authors in terms of quality scores ascribed to individual studies.
Box 1. Criteria used to assess quality in included studies.41.
Are the aims and objectives of the research clearly stated?
Is the research design clearly specified and appropriate for the aims and objectives of the research?
Do the researchers provide a clear account of the process by which their findings were reproduced?
Do the researchers display enough data to support their interpretations and conclusions?
Is the method of analysis appropriate and adequately explicated?
As quality appraisal methods for assessing the quality of qualitative studies is a contested area of debate,42 we did not exclude articles based on lower ascribed quality scores, but we decided to use this information to appraise the robustness of the findings in the results and discussion. To reduce potential bias affecting the robustness of the synthesis and findings, we further undertook a sensitivity analysis reanalysing the results after removing studies with lower quality scores (i.e. those with a score of 3 or less out of 5). As the checklist focuses on appraisal of the technical quality of the research design, rather than theoretical approaches used,43 we aimed to consider in the discussion how the theoretical approaches used could have impacted on the quality of results.
Results
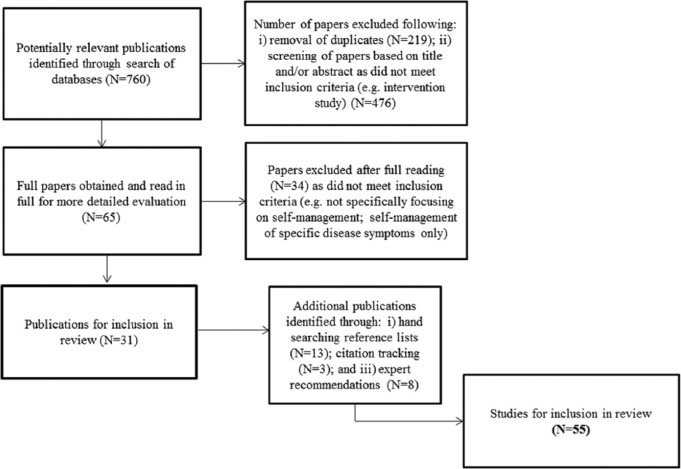
In all, 760 articles were identified through electronic searching; following the removal of 219 duplicates, 541 articles were left. Titles and abstracts of these articles were read, and 476 articles were excluded as they did not meet the inclusion criteria (e.g. intervention study). Full-text articles were obtained for 65 studies that appeared to meet the inclusion criteria. Following full reading of these articles, a further 34 studies were discarded for not meeting the inclusion criteria, which left 31 studies. Through hand-searching reference lists of these studies, 13 additional articles were then identified. Finally, a further three articles were obtained through citation tracking and eight through recommendations by experts in the field. In total, 55 studies were included in the review (Figure 1).
Figure 1.
Flow of studies through the stages of the review.
Overview of included studies
Table 1 summarises the main characteristics of the articles included in the review. Studies were from 11 countries, most from the United Kingdom (19); then the United States (9); Australia (8); Sweden (7); Canada (5); Taiwan (2) and one each from New Zealand; Norway; Ireland; Austria; as well as one cross-cultural study (United States/Denmark). Overall, authors tended to use the terms self-management or self-care synonymously. The majority of studies (N = 41) investigated understandings of self-management or self-care among individuals with one (N = 34) or multiple conditions (N = 7) (three studies also included carers); one focused on carers’ perspectives only; six on HCPs (including general practitioners (GPs), nurses, psychologists and allied health professionals) and seven both lay people and HCPs.
Over one-third of studies (N = 22) investigated self-management or self-care among people with a range of LTCs (such as diabetes, heart disease, arthritis, asthma, chronic obstructive pulmonary disease (COPD), stroke); 13 focused on individuals with diabetes; 7 stroke/other neurological conditions; 2 asthma; 3 COPD/other chronic respiratory conditions; 3 arthritis (osteoarthritis or rheumatoid arthritis); 2 chronic pain; 2 cancer and 1 inflammatory bowel disease (IBD).
Robustness of findings
Overall, the quality of included articles was high, with a mean score of 4.7 out of 5. Only three of the 55 articles were rated by both authors as lower quality (scoring 3 out of 5). A sensitivity analysis on the main themes emerging from the synthesis suggested that the findings were robust after removing these three lower quality score studies. In terms of theoretical approaches used, under one-half of included studies (N = 26) did not explicitly use a theoretical framework. The other 29 studies drew on theoretical approaches to investigate lay and, to a lesser extent, HCP understandings of self-management or self-care: 11 used sociological theory (e.g. illness narratives), 5 psychological approaches (e.g. social cognitive theory, self-regulation theory), 3 phenomenological, 2 medical anthropological, 2 other social science approaches, 3 psychosocial and 1 each of the following: a combination of biomedical and sociological; phenomenological and sociological and life-course approaches.
The synthesis
We generated three main conceptual themes, each with two subthemes, which we now discuss.
Traditional and shifting models of the professional–patient relationship
The studies suggest that HCP and lay understandings of self-management encompass traditional or paternalistic models of the professional–patient relationship based on compliance. They also entailed shifting models of this relationship based on different expectations of responsibility.
Self-management as a tool to promote compliance
Self-management was commonly interpreted among HCPs as a tool to promote compliance with expert advice and medical regimes, to encourage people with LTCs to monitor and control symptoms of disease, engage in healthy lifestyle behaviours44–46,48,50,54,89 and learn psychological skills, such as problem solving and goal setting.53,55 It was assumed by some medical practitioners that lay people lack the ability to self-manage, and require information to educate, motivate and build confidence in their ability to self-manage and thus comply with recommended strategies. For example, a hospital doctor in one study working with patients with a range of chronic conditions noted,
If you educate patients about their condition, about their medications, they have a great familiarity and that familiarity breeds confidence, that confidence means that they’re comfortable with their condition. It means they’ll self- manage.50 (p. 14)
Poor compliance was attributed by HCPs to negative psychological characteristics of patients. This was often assumed to be related to poor motivation and a lack of willingness to engage in self-management practices in the longer term:44,45,50,54,89
… unless [patients] are motivated and engaged they will not get anywhere. This is critical to the success of any intervention.54 (p. D)
Compliance is a big issue and they have to get their head around that idea that it’ll be OK if they do what they’re supposed to do, but they’re not used to doing that.50 (p. 13)
Two studies also found that medical practitioners considered compliance with professionally ascribed self-management practices required the ‘right’ cultural context.44,45 For example, a hospital doctor in one study of Mexican–Americans with type 2 diabetes commented,
I’d say more than 90 percent of them are noncompliant. I understand because of the culture, the diet is very difficult for them to adjust to.44 (p. 355)
Lay people similarly sometimes understood self-management as compliance with professionally recommended behaviours in terms of following medication, exercise or diet regimes.60,66,71–75,83 Expectations for a professionally led compliance approach to self-management among lay people was influenced by individual factors, such as lower perceived levels of activation75 (i.e. skills, knowledge and confidence) and emotional difficulties,76 but also social factors, such as low education71 and cultural beliefs that placed trust in professional expertise and knowledge.66,72,73,83 For example, one participant with COPD in a study conducted in Taiwan said,
Now, when I am told to exercise, I listen. They are doctors and nurses who have experience. We know nothing. You should just follow the orders.72 (p. 599)
In several studies people with a range of LTCs, such as osteoarthritis, cancer, asthma and COPD, spoke about their attempts to comply with professional advice regarding self-management practices, but experienced difficulties doing so due to a number of factors. Individual factors included emotional difficulties,69,76,89,90 poor health83,89 and cognitive impairment.90 Organisational and structural factors limiting compliance included a lack of information,69,95 poorly coordinated or fragmented community services,48 limited consultation time with HCPs49 and poor socio-economic circumstances.44,45,83,90
Different expectations of responsibility
Lay and HCP understandings of self-management also encompassed shifting models of the professional–patient relationship based on different expectations of responsibility. HCPs commonly expected patients to take increased personal responsibility to manage their own health.44,48,50,51 There was some evidence that they made moral assessments of people’s willingness and ability to take responsibility, with a lack of willingness regarded as problematic for effective self-management.44,48,89 In one study, such a viewpoint was particularly evident among hospital clinicians and GPs:
They don’t want to take responsibility. A lot of them think someone else will look after their health. [they] expect someone else to fix it.48 (p. 225)
Some GPs regarded self-management as conflicting with traditional health care practices and professional values of responsibility to patients.52 Self-management was also found in one study to be a marginalised topic of discussion in routine clinical encounters between GPs/nurses and patients, shaped by mutual expectations to maintain traditional professional–patient boundaries in such relationships.47
Lay people’s understandings of self-management suggested variations in expectations of responsibility. Some people, but not all, welcomed greater personal responsibility to manage their conditions.46,60,62,63,65–67,71,73,75,83 Some interpreted self-management as compliance with professional advice,60,65 while others preferred to combine professional advice with personal experience46,62,74 or made their own decisions regarding treatments, such as the use of alternative medicines.73,83
Lay people who interpreted self-management in terms of expectations of increased personal responsibility reported wanting to take an active role in decision making and problem solving,62,67,71 adopt health promoting strategies,71 use a higher number of coping strategies,75 integrate self-management practices into daily routines62 and learn through trial and error.63,74 For example, a participant with arthritis in one study said,
If you want to do something then work out how to do it and what you need to do it … you can usually find a way to get things done.63 (p. 263)
Lay understandings of self-management sometimes entailed expectations that responsibility in learning self-management skills would be shared with HCPs.60,66,74,95 For example, one stroke survivor in another study noted,
It’s their responsibility to use their expertise with respect for us individuals, and our responsibility to meet them halfway.60 (p. 84)
In contrast, some participants in a number of studies refused the idea of self-management, preferring instead to follow orders from doctors and other HCPs.62,65–67,71,74,75,89 A dependence on HCPs to provide support in learning self-management skills was influenced by beliefs that one had little control over the course of illness or that illness was attributed to external causes (e.g. supernatural causes),73 reduced knowledge, skills and confidence,75 low education71 and lack of social support.74 This sometimes led to feeling abandoned by HCPs when services had come to an end,65 as one participant with asthma in a further study said,
I said ‘Well why are you only seeing me every month or two?’ ‘Oh well, you always seem to be able to manage’. And so well, I can, but should I be, should I be just doing it all on my own, do you know what I mean? I don’t know, the more independent and able to manage you are the less keen they are to see you sometimes.65 (p. 58)
Quality of relationship between HCP and lay person
Lay people interpreted self-management in terms of the quality of relationship with a HCP, which was much less often reported among professionals. The quality of relationship included two aspects: self-management as a collaborative partnership and tailored support to meet the needs and situation of the individual.
Self-management as a collaborative partnership
In 14 studies, individuals with a range of LTCs, and those living with multiple chronic conditions, regarded self-management as a practice that was achieved through a collaborative partnership with a HCP.49,56,57,59,60,65,66,69,70,74,76,82,91,95 They identified the quality of this partnership as an important contextual factor fostering self-management practice. This included an expectation of being jointly involved in decision making,56,66 building a relationship based on mutual respect60,74 and trust,60,65 and that HCPs should endeavour to understand the ‘whole’ needs of the person to better integrate self-management practices into their everyday lives.82,95 Lay people further placed importance on their views, preferences, knowledge and expertise being part of the process of learning self-management skills.49,65,66,74
Other aspects of a collaborative partnership identified by lay people as part of self-management pointed to HCP relationships that were encouraging,57,60,70 empathetic,56 flexible and accessible,57,65 based on good communication57,59,69,91 and involving the provision of emotional support, reassurance76,82 and choice74. For example, in one study, a participant with multiple sclerosis (MS) commented,
We understood each other. He listened to what I had to say and paid attention to what I said. He told me about everything that was going on, but he left it to me to decide if I wanted to take part or not.74 (p. 13)
HCPs less commonly alluded to a collaborative partnership as part of self-management.51,53,55 In one study, this was interpreted among HCPs across acute, primary and community care settings as working together as part of an equal alliance.51 In two other studies, nurses53 and rehabilitation therapists55 regarded nurturing such a relationship as integral to supporting lay self-management practices. This entailed developing a trusting, enabling and guiding relationship;55 listening to individual needs and concerns;53,55 involving carers in providing appropriate support53 and recognising patients’ own expertise.55 For example, one occupational therapist working in a rehabilitation setting with stroke and spinal cord injury (SCI) patients said,
Maybe I am the person who has supported them in this process – but it is absolutely not me who is the expert any more.55 (p. 265)
Self-management as tailored support
A proportion of participants in several studies understood self-management as the provision of tailored support to meet individual needs and situations, which was seen as a further marker of the quality of the HCP–patient relationship.45,49,56,57,91,95 This reflected expectations for professional guidance and support in terms of diet,49 exercise,45 practical56 or emotional support45 and information.56,91,95 Furthermore, lay people expected HCPs to provide information that would meet changing learning needs over time57 and support self-management regimes for those with multiple conditions.91 Provision of consistent information provided by a range of HCPs was identified as a valued component of self-management, but this was perceived as not always forthcoming, as a participant with diabetes in one study said,
It is obvious that the level of knowledge varies, and they tell me different things. It is a big problem.56 (p. 1505)
HCP understandings of self-management similarly entailed tailored responses to meet individual needs of people with different chronic conditions.49,51,54,55 However, professionals spoke about organisational challenges limiting the provision of tailored support for those with LTCs, including fragmentation of community services50 and lack of professional training.51 Occasionally, this reflected the recognition among professionals that learning self-management skills should be adapted to individual ability, preferences, prior roles and activities.55 In one study, HCP understandings of self-management encompassed a flexible and eclectic approach and access to a diversity of self-management support initiatives to meet the varied and changing needs of people with a range of LTCs.51 One clinician defined self-management as
… an individual approach, looking at the stages of change and allowing an individual program. Having different strategies and different interventions is important.51 (p. 64)
Putting self-management into everyday practice
Lay understandings of self-management entailed variations in how individuals with chronic conditions attempted to put self-management into everyday practice. This process involved learnt biomedical and psychological strategies, but also aspects largely unacknowledged by professionals, including different preferences for self-management, the construction of meaning-making frameworks, social strategies and practices. Two interrelated emerging subthemes were the lived experience of self-management and self-management as a social practice.
The lived experience of self-management
In a number of studies, lay people understood self-management in terms of biomedical and psychological strategies to modify behaviours. Biomedical strategies comprised monitoring and managing bodily symptoms44,46,79,80,85,92 and levels of activity61,63,72, and engagement in diet,44,45,60,62,73,86,87,92 exercise45,46,60,61,62,72,92 and medication regimes.44,46,71,83,86,91,92 Psychological strategies involved problem solving,46,70,74 action planning,96 evaluation and reflection44,79,87,93 in relation to self-management of daily tasks. Self-management was also understood to involve managing the emotional impact of living with a chronic condition, for example, in dealing with fear and uncertainty of the future.72,76,86,90
Lay understandings of self-management involved different preferences for engagement in self-management practices.68,69,70,72,81,84,86,89,90 Variations were shaped by a number of factors, including individual ability,90 changes in the course of illness,69 cultural beliefs in health and illness,73,84 perceived level of disruption to one’s life and biography89,91 and competing social roles.63,70,84 In terms of the latter, this sometimes led to a tension in perceived priorities, in relation to balancing self-management activities with other valued social roles, which had implications for health, as a woman with rheumatoid arthritis with other health problems in one study said,
I tend to overdo it sometimes and I suffer badly for it as well … like, say, my daughter’s moving house there, giving her a hand recently, we were doing cleaning of the place and I suffered for about three days after it … I was in absolute agony, agony … the doctor gave me painkillers … But at the end of the day, it’s your daughter and you do it and that’s it, it’s your family … The illness comes secondary to your family …68 (p. 191)
The lived experience of self-management further involved the construction of different meaning frameworks as part of illness narratives,24 which lay people drew upon to make sense of, and cope with, the challenges of living with a chronic condition.63,64,66,70,74,75,79,80,82,86,92 These included the following: maintaining a positive attitude,64,74,80 finding a sense of purpose,82,92 restoring order in the face of uncertainty,58,63,79 managing identity63,64,86 and biographical disruption,47,63,81,92 maintaining a sense of ‘normality’86 and exercising personal control.66,70,75,79 Such meaning frameworks helped individuals to cope with sometimes challenging and uncertain self-management practices in the context of their everyday lives, as a woman with encephalitis in one study said,
I know the answers but it’s putting them into practice, I still have to. I’ll get there eventually. The only advice I can say is try to be as positive, try to get your interests and think outside of it and not dwell on, ‘oh, this happened to me and why?’ and so on and so forth. Consider it as part of the past. Just try to get on with life.64 (p. 390)
In several other studies, self-management was further understood as a demanding process which required hard work: physical, emotional, psychological or biographical.88,90,93,95 Those with multiple conditions considered self-management to entail a dynamic process of prioritising one chronic condition over another91 involving unpredictability80 and significant challenges to integrating self-management practices in everyday lives.96 Self-management of multiple conditions for some also had moral implications.68,94 Participants in one study interpreted self-management as a moral responsibility to maintain one’s health, with self-management practices involving self-discipline and personal effort in response to the limitations of professional intervention.94 For example, one woman living with a number of chronic conditions, including arthritis, chronic pain and heart disease said,
You’ve only got yourself and you have to self-discipline yourself […] You’re your own policeman.94 (p. 222)
Self-management as a social practice
Lay understandings of self-management commonly entailed social practices and strategies which required social support from family and friends;59–61,63,69,74,77,78,82,84,86,90,92,95,97,98 a theme that was much less often reported among HCPs.51,53 In line with social support theory,28,29 we found that perceived support from family and friends comprised both positive59,61,62,64,69,74,77,78,82,84,86,90,92,95 and negative64,78,97,98 influences, which in turn had an impact on self-management practices. Positive influences from family and friends involved the provision of support in activities of daily living and personal care,77,84,96 practical support in medication regimes78 and transport for GP visits,77,78 offering health-related advice and information69,74,77,78,86 and financial support.84
Other social strategies included peer support and information exchange with other patients86 or among those with similar conditions attending social and community groups.90 People living with conditions such as arthritis, heart disease and diabetes identified family members and friends as an important source of emotional support for self-management practices.78,84,96 Thus, family and friends provided ongoing encouragement, motivation and reassurance,96 and opportunities to reinforce self-identity,64 share concerns about long-term physical symptoms and management.75,78 For example, participants with multiple LTCs in two studies commented,
After she and I talk on the phone, we both feel better when we get finished talking. We talked about taking our medication, we talk about the pains that we have but we can’t do nothing about it so we deal with it.78 (p. 394)
I try to get someone to confirm that I have done the right thing somehow, to hear that from someone else, as well.96 (p. 341)
In several other studies, lay people interpreted self-management as the ability to mobilise emotional and practical support, information and advice from family and friends as part of a proactive approach to their self-management practices.57,69,74,82,84,90,92,95,96 Mobilising social support was identified as part of a process of long-term adjustment among people with conditions such as stroke,61 encephalitis64 and MS.74
Individuals with chronic conditions also reported negative social support influences from family members on self-management behaviours. This included overprotective family members who discouraged self-management practices,78,96 those providing unhelpful advice78,96 or who were undermining and lacked understanding and empathy.64,78 A proportion of participants in three studies reported that lack of family support shaped a greater dependence on HCP support in learning self-management skills.82,83,92 The availability of social support from family members to assist in self-management practices, however, was at times perceived as a taken-for-granted assumption among HCPs77, as one socially isolated male participant with diabetes and no family ties said,
I think the way the medical people see it … is that you are attached to someone, and that there’s somebody there to watch over you … No, no, there’s no family, so it’s not that they wouldn’t, it’s just that there’s no-one there …77 (p. 596)
Finally, a minority of studies highlighted how ongoing support in self-management activities had long-term negative effects on carers themselves. This included a negative impact on personal well-being, related to juggling a number of different roles,98 family conflicts due to blurring of boundaries with normative kinship practices97 and difficulties establishing the perceived correct level of support in self-management practices.98
Discussion
Summary of main findings
The aim of this article was to compare lay and HCP understandings of self-management of LTCs, through a systematic review and narrative synthesis of qualitative studies. We found some similarities, but mainly differences in the ways in which these two stakeholder groups interpreted self-management. HCPs and lay people understood self-management in terms of traditional or paternalistic professional–patient relationships premised on compliance with professional advice, but also shifting models of this relationship based on different expectations of responsibility.
Lay people interpreted self-management in terms of the quality of relationship with health professionals. Variations in how individuals put self-management into everyday practice were shaped by differences in preferences for engagement in self-management practices, and how individuals constructed different meaning frameworks and drew on social strategies to cope with, and manage, the challenges of living with chronic conditions. These aspects of self-management were largely not reported by professionals.
Robustness, strengths and limitations of the synthesis
Overall, the quality of studies included met most of the methodological criteria proposed by Dixon-Woods et al.41 However, one limitation of this brief quality assessment tool is that it did not encourage a critique of the research question posed in the original studies. The quality checklist also focuses on an appraisal of the technical quality of the research design of a study, and it did not allow for a consideration of theoretical approaches used. We found that over half of studies used one or more theoretical approaches, mostly sociological. This arguably enhanced quality, in that such studies went beyond taking self-management as a given and attempted to understand and contextualise self-management as a social construct. However, just under one-half of studies were largely atheoretical in their scope, reporting only emerging themes. Another aspect likely to have had an impact on the quality of included studies is that the terms self-management and self-care were often used synonymously, despite attempts in the literature to delineate similarities and differences between the two concepts.2,3
A further potential limitation is the inclusion of studies with different methodologies (interviews, focus groups or mixed methods), which could have implications for synthesising findings from these studies. The robustness of the synthesis might also have been affected by only one author (E.S.) conducting the search strategy selection and synthesis of studies. However, the validity of the synthesis was strengthened by using two authors during quality appraisal and data analysis. We recognise that people with LTCs represent a diverse group. Although we focused on the perspectives of individuals with different chronic conditions, including those with multi-morbidity, and the views of a range of HCPs, the synthesis led to a number of recurrent themes.
How our findings relate to the existing literature
Our findings show that, in different ways, lay and HCP understandings of self-management practice deviated from the dominant model of self-management underpinned by the concept of self-efficacy, as promoted in the policy10,11 and health care15–17 fields. HCP understandings of self-management based on a model of compliance is contrary to an ethos of self-management premised on empowering lay people with LTCs to make their own decisions in terms of managing their health in partnership with HCPs.16,18 However, lay people reported variations in expectations of the patient–professional relationship and interpreted self-management in different ways.
In line with studies that have shown how professional attitudes serve to construct tacit notions of what makes a ‘good patient’,99,100 our synthesis suggests that HCPs seemed to hold normative values concerning what makes a ‘good self-manager’,7,36 based on ideal values related to compliance, motivation and personal responsibility. As Lawn et al.7 and Kendall and Rogers18 have argued, the lack of such characteristics implicitly constructs individuals with LTCs as deficient, such that teaching self-management arguably becomes a moral intervention.
Despite self-efficacy being a dominant trope in the theory of a widespread model of self-management,15 we found that lay people only partly understood self-management in terms of self-efficacy enhancing strategies. In an Australian study, Kendall et al.101 similarly found a lack of support for the impact of self-efficacy on engagement in self-management behaviours following long-term adjustment after stroke. As proposed by self-regulation models of self-management,21–23 we also found some evidence that lay beliefs about health and illness shaped preferences for engagement in self-management practices. However, our findings support that people with chronic conditions understood self-management to include a number of domains not typically addressed by existing CDSMPs, including differences in expectations of responsibility and the importance of developing collaborative partnerships with health professionals. Lay people further understood self-management as encompassing different meaning frameworks and embedded in social practices. In line with the large body of sociological literature documenting the experiences of individuals living with chronic illness, self-management appeared to be part of the lay construction of illness narratives, enabling people to make sense of, cope with, and adapt to chronic conditions in their everyday lives.24 Self-management further reflected an appraisal of the quality of social support provided from one’s immediate social group, which supports other qualitative studies that have found the quality of social support from informal networks is important in the process of coping and longer term adaptation to chronic illness.102–104
Conclusion and implications for policy and future research
Self-management programmes are increasingly promoted as a panacea for under-resourced health care systems, although evidence of effectiveness of such programmes is lacking. This review found some similarities, but important differences between lay and HCP understandings of self-management of LTCs. This may help to account for the apparently limited effectiveness of self-management interventions, suggesting a mismatch between what is intended and practised.
The review raises a number of implications for policy and future research. First, self-management approaches should not be interpreted only as a way of improving compliance to professional advice and regimes, as this is likely to reinforce traditional practices based on paternalistic health professional–patient relationships, rather than empowering lay people to cope with, and manage the challenges of living with one or multiple chronic conditions. Second, our findings support calls made by others18,24–26,34 for the need to develop and evaluate approaches based on social models of self-management, rather than only psychological approaches which largely underpin existing state CDSMPs in health care15,17,18. This means greater attention needs to be paid to the different ways in which lay people understand and interpret self-management and the social context shaping self-management practices.
Third, individual or group self-management interventions need to address differences in lay expectations and abilities to take responsibility in terms of learning self-management skills and to tailor professional support to this end. Fourth, although collaborative professional–patient relationships are considered a mainstay of person-centred care,105 this was less often recognised among HCPs as an important component of self-management. Given that a significant proportion of people with chronic conditions regarded collaborative partnerships with HCPs as an integral part of their self-management practices, interventions which target improving the quality of professional–patient relationships to foster self-management are warranted.
Acknowledgments
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was funded by the Stroke Association as part of a Senior Research Training Fellowship (reference number: TSA SRTF 2011/01).
References
- 1. Imison C, Naylor C, Goodwin N, et al. Transforming our health care system: ten priorities for commissioners. Report, The King’s Fund, London, 2011. [Google Scholar]
- 2. Barlow J, Wright C, Sheasby J, et al. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2002; 48: 177–187. [DOI] [PubMed] [Google Scholar]
- 3. Richard A, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh 2011; 43: 255–264. [DOI] [PubMed] [Google Scholar]
- 4. Ryan P, Sawin K. The individual and family self-management theory: background and perspectives on context, process, and outcomes. Nurs Outlook 2009; 57: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grey M, Knafl K, McCorkle R. A framework for the study of self- and family management of chronic conditions. Nurs Outlook 2006; 54: 278–286. [DOI] [PubMed] [Google Scholar]
- 6. Trappenburg J, Jaarsma T, Van Os-Medendorp H, et al. Self-management: one size does not fit all. Patient Educ Couns 2013; 92: 134–137. [DOI] [PubMed] [Google Scholar]
- 7. Lawn S, McMillan J, Pulvirenti M. Chronic condition self-management: expectations of responsibility. Patient Educ Couns 2011; 84: e5–e8. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Innovative care for chronic conditions. Building blocks for action (Global report). Geneva: WHO, 2002. [Google Scholar]
- 9. Wagner E, Austin B, Von Korff M. Organising care for patients with chronic illness. Milbank Q 1996; 74: 511–542. [PubMed] [Google Scholar]
- 10. Department of Health. The expert patient: a new approach to chronic disease management in the 21st century. Report, Crown, London, 2001. [Google Scholar]
- 11. Skills for Care and Skills for Health. Common core principles to support self-care. A guide to support implementation, www.dh.gov.uk (2008, accessed 15 May 2013).
- 12. Wilkinson A, Whitehead L. Evolution of the concept of self-care and implications for nurses: a literature review. Int J Nurs Stud 2009; 46: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 13. Lawn S, Schoo A. Supporting self-management of chronic health conditions: common approaches. Patient Educ Couns 2011; 80: 205–211. [DOI] [PubMed] [Google Scholar]
- 14. Norris M, Kilbride C. From dictatorship to reluctant democracy: stroke therapists talking about self-management. Disabil Rehabil 2014; 36: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorig K, Ritter P, Stewart A, et al. Chronic disease self-management program: 2 year health status and health care utilization following disability. Med Care 2001; 39: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 16. Holman H, Lorig K. Patients as partners in managing chronic disease. BMJ 2000; 320: 526–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogers A, Kennedy A, Bower P, et al. The United Kingdom Expert Patients Programme: results and implications from a national evaluation. Med J Aust 2008; 189: S21–S24. [DOI] [PubMed] [Google Scholar]
- 18. Kendall E, Rogers A. Extinguishing the social? State sponsored self-care policy and the Chronic Disease Self-Management Programme. Disabil Soc 2007; 22: 129–143. [Google Scholar]
- 19. Bandura A. Human agency in social cognition theory. Am Psychol 1989; 44: 1175–1184. [DOI] [PubMed] [Google Scholar]
- 20. Leventhal H, Nerenz DR, Steel DJ. Illness representations and coping with health threats. In: Baum A, Taylor SE, Singer JE. (eds) Handbook of psychology and health, vol. 4 Hillsdale, NJ: Erlbaum, 1984, pp. 219–252. [Google Scholar]
- 21. Clark NM, Becker MH, Janz NK, et al. Self-management of chronic disease by older adults: a review and questions for research. J Aging Health 1991; 3: 3–27. [Google Scholar]
- 22. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav 2001; 28: 769–782. [DOI] [PubMed] [Google Scholar]
- 23. Clark NM. Management of chronic disease by patients. Annu Rev Publ Health 2003; 24: 289–313. [DOI] [PubMed] [Google Scholar]
- 24. Newbould J, Taylor D, Bury M. Lay-led self-management in chronic illness: a review of the evidence. Chronic Illn 2007; 2: 249–261. [DOI] [PubMed] [Google Scholar]
- 25. Vassilev I, Rogers A, Sanders C, et al. Social networks, social capital and chronic illness self-management: a realist review. Chronic Illn 2011; 7: 60–86. [DOI] [PubMed] [Google Scholar]
- 26. Ong B, Rogers A, Kennedy A, et al. Behaviour change and social blinkers? The role of sociology in trials of self-management behaviour in chronic conditions. Sociol Health Illn 2014; 36: 226–238. [DOI] [PubMed] [Google Scholar]
- 27. Husserl E. Phenomenological psychology. The Hague: MartinusNijhoff Publishers, 1970. [Google Scholar]
- 28. Wortman CB, Conway TL. The role of social support in adaptation and recovery from physical illness. In: Cohen S, Syme SL. (eds) Social support and health. Orlando, FL: Academic Press, 1985, pp. 281–302. [Google Scholar]
- 29. Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav 2003; 30: 170–195. [DOI] [PubMed] [Google Scholar]
- 30. Foster G, Taylor SJC, Eldridge S, et al. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2009; 4: CD005108. [DOI] [PubMed] [Google Scholar]
- 31. Nolte S, Osborne RH. A systematic review of outcomes of chronic disease self-management interventions. Qual Life Res 2012; 22: 1805–1816. [DOI] [PubMed] [Google Scholar]
- 32. Griffiths C, Foster G, Ramsay J, et al. How effective are expert patient (lay led) education programmes for chronic disease. BMJ 2007; 334: 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reavley N, Livingston J, Buchbinder R, et al. A systematic grounded approach to the development of complex interventions: the Australian Work Health Program – arthritis as a case study. Soc Sci Med 2010; 70: 342–350. [DOI] [PubMed] [Google Scholar]
- 34. Osborne RH, Jordan JE, Rogers A. A critical look at the role of self-management for people with arthritis and other chronic diseases. Nat Clin Pract Rheumatol 2008; 4: 224–225. [DOI] [PubMed] [Google Scholar]
- 35. Newman S, Mulligan K, Steed L. What is meant by self-management and how can its efficacy be established? Rheumatology 2001; 40: 1–6. [DOI] [PubMed] [Google Scholar]
- 36. Kennedy A, Rogers A, Gateley C. Assessing the introduction of the expert patients programme into the NHS: a realistic evaluation of recruitment to a national lay-led self-care initiative. Prim Health Care Res Dev 2005; 6: 137–148. [Google Scholar]
- 37. Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005; 331: 1064–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme, version 1, April, http://www.lancs.ac.uk/shm/research/nssr/research/dissemination/publications/NS_Synthesis_Guidance_v1.pdf (2006, accessed 20 August 2012).
- 39. Ziebland S, McPherson A. Making sense of qualitative data analysis: an introduction with illustrations from DIPEx (personal experiences of health and illness). Med Educ 2006; 40: 405–414. [DOI] [PubMed] [Google Scholar]
- 40. Strauss AL, Corbin J. Basics of qualitative research. Techniques and procedures for developing grounded theory. 2nd ed. Newbury Park, CA: SAGE, 1998. [Google Scholar]
- 41. Dixon-Woods M, Cavers D, Agarwal S, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs 2006; 53: 304–310. [DOI] [PubMed] [Google Scholar]
- 43. Hannes K. Chapter 4. Critical appraisal of qualitative research. In: Noyes J, Booth A, Hannes K, et al. (eds) Supplementary guidance for inclusion of qualitative research in Cochrane systematic reviews of interventions, version 1 (updated August 2011). Cochrane Collaboration Qualitative Methods Group, http://cqrmg.cochrane.org/supplemental-handbook-guidance (2011, accessed 14 April 2014).
- 44. Hunt LM, Arar NH. An analytical framework for contrasting patient and provider views of the process of chronic disease management. Med Anthropol Q 2001; 15: 347–367. [DOI] [PubMed] [Google Scholar]
- 45. Carbone EL, Rosal MC, Idali M, et al. Diabetes self-management: perspectives of Latino patients and their health care providers. Patient Educ Couns 2007; 66: 202–210. [DOI] [PubMed] [Google Scholar]
- 46. Crowe M, Whitehead L, Gagan MJ, et al. Self-management and chronic low back pain: a qualitative study. J Adv Nurs 2010; 66: 1478–1486. [DOI] [PubMed] [Google Scholar]
- 47. Blakeman T, Bower P, Reeves D, et al. Bringing self-management into clinical view: a qualitative study of long-term condition management in primary care consultations. Chronic Illn 2010; 6: 136–150. [DOI] [PubMed] [Google Scholar]
- 48. Kirby SE, Dennis SM, Bazeley P, et al. What distinguishes clinicians who better support patients for chronic disease self-management? Aust J Prim Health 2012; 18: 220–227. [DOI] [PubMed] [Google Scholar]
- 49. Pooley CG, Gerrard C, Hollis S, et al. ‘Oh it’s a wonderful practice… you can talk to them’: a qualitative study of patients’ and health professionals’ views on the management of type 2 diabetes. Health Soc Care Community 2001; 9: 318–326. [DOI] [PubMed] [Google Scholar]
- 50. Yen L, Gillespie J, Jeon Y-H, et al. Health professionals, patients and chronic illness policy: a qualitative study. Health Expect 2010; 14: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lake AJ, Staiger PK. Seeking the views of health professionals on translating chronic disease self-management models into practice. Patient Educ Couns 2010; 79: 62–68. [DOI] [PubMed] [Google Scholar]
- 52. Blakeman T, Macdonald W, Bower P, et al. A qualitative study of GPs’ attitudes to self-management of chronic disease. Br J Gen Pract 2006; 56: 407–414. [PMC free article] [PubMed] [Google Scholar]
- 53. MacDonald W, Rogers A, Blakeman T, et al. Practice nurses and the facilitation of self-management in primary care. J Adv Nurs 2008; 62: 191–199. [DOI] [PubMed] [Google Scholar]
- 54. Phillips RL, Short A, Dugdale P, et al. Supporting patients to self-manage chronic disease: clinicians’ perspectives and current practices. Aust J Prim Health. Epub ahead of print 23 May 2013. DOI: 10.1071/PY13002. [DOI] [PubMed] [Google Scholar]
- 55. Guidetti S, Tham K. Therapeutic strategies used by occupational therapists in self-care training: a qualitative study. Occup Ther Int 2002; 9: 257–276. [DOI] [PubMed] [Google Scholar]
- 56. Oftedal B, Karlsen B, Bru E. Perceived support from healthcare practitioners among older adults with type 2 diabetes. J Adv Nurs 2010; 66: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 57. Thorne SE, Paterson BL. Health care professional support for self-care management in chronic illness: insights from diabetes research. Patient Educ Couns 2001; 42: 81–90. [DOI] [PubMed] [Google Scholar]
- 58. Guidetti G, Asaba E, Tham K. The lived experience of recapturing self-care. Am J Occup Ther 2007; 61: 303–310. [DOI] [PubMed] [Google Scholar]
- 59. Guidetti S, Asaba E, Tham K. Meaning of context in recapturing self-care after stroke or spinal cord injury. Am J Occup Ther 2009; 63: 323–332. [DOI] [PubMed] [Google Scholar]
- 60. Opal Cox E, Dooley A, Liston M. Coping with stroke: perceptions of elderly who have experienced stroke and rehabilitation interventions. Top Stroke Rehabil 1998; 4: 76–88. [Google Scholar]
- 61. Pound P, Gompertz P, Ebrahim S. Social and practical strategies described by people living at home with stroke. Health Soc Care Community 1999; 7: 120–128. [DOI] [PubMed] [Google Scholar]
- 62. Audulv A, Asplund K, Norbergh K-G. Who’s in charge? The role of responsibility attribution in self-management among people with chronic illness. Patient Educ Couns 2010; 81: 94–100. [DOI] [PubMed] [Google Scholar]
- 63. Kralik D, Koch T, Price K. Chronic illness self-management: taking action to restore order. J Clin Nurs 2004; 13: 259–267. [DOI] [PubMed] [Google Scholar]
- 64. Atkin K, Stapley S, Easton A. No one listens to me, nobody believes me: self management and the experience of living with encephalitis. Soc Sci Med 2010; 71: 386–393. [DOI] [PubMed] [Google Scholar]
- 65. Kielmann T, Huby G, Powell A, et al. From support to boundary: a qualitative study of the border between self-care and professional care. Patient Educ Couns 2010; 79: 55–61. [DOI] [PubMed] [Google Scholar]
- 66. Koch T, Jenkin P, Kralik D. Chronic illness self-management: locating the ‘self’. J Adv Nurs 2004; 48: 484–492. [DOI] [PubMed] [Google Scholar]
- 67. Collins MM, Bradley CP, O’Sullivan T, et al. Self-care coping strategies in people with diabetes: a qualitative study. BMC Endocr Disord 2009; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Townsend A, Wyke S, Hunt K. Self-managing and managing self: practical and moral dilemmas in accounts of living with chronic illness. Chronic Illn 2006; 2: 185–194. [DOI] [PubMed] [Google Scholar]
- 69. Schulman-Green D, Bradley EH, Knobf T, et al. Self-management and transitions in women with advanced breast cancer. J Pain Symptom Manage 2011; 42: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cooper JM, Collier J, Jaes V, et al. Beliefs about personal control and self-management in 30-40 year olds living with Inflammatory Bowel Disease: a qualitative study. Int J Nurs Stud 2010; 47: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 71. Clark DO, Frankel RM, Morgan DL, et al. The meaning and significance of self-management among socioeconomically vulnerable older adults. J Gerontol B Psychol Sci Soc Sci 2008; 63: S312–S319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen K-L, Chen M-L, Lee S, et al. Self-management behaviours for patients with chronic obstructive pulmonary disease: a qualitative study. J Adv Nurs 2008; 64, 6: 595–604. [DOI] [PubMed] [Google Scholar]
- 73. Hjelm K, Nyberg P, Isacsson A, et al. Beliefs about health and illness essential for self-care practice: a comparison of migrant Yugoslavian and Swedish diabetic females. J Adv Nurs 1999; 30: 1147–1159. [DOI] [PubMed] [Google Scholar]
- 74. Ploughman M, Austin MW, Murdoch M, et al. The path to self-management: a qualitative study involving older people with multiple sclerosis. Physiother Can 2012; 64: 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dixon A, Hibbard J, Tusler M. How do people with different levels of activation self-manage their chronic conditions? Patient 2009; 2: 257–268. [DOI] [PubMed] [Google Scholar]
- 76. Furler J, Walker C, Blackberry I, et al. The emotional context of self-management in chronic illness: a qualitative study of the role of health professional support in the self-management of type 2 diabetes. BMC Health Serv Res 2008; 8: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gunn KL, Seers K, Posner N, et al. ‘Somebody there to watch over you’: the role of the family in everyday and emergency diabetes care. Health Soc Care Community 2012; 20: 591–598. [DOI] [PubMed] [Google Scholar]
- 78. Gallant MP, Spitze GD, Prohaska TR. Help or hindrance? How family and friends influence chronic illness self-management among older adults. Res Aging 2007; 29: 375–409. [Google Scholar]
- 79. McLaughlin J, Zeeberg I. Self-care and multiple sclerosis: a view from two cultures. Soc Sci Med 1993; 37: 315–329. [DOI] [PubMed] [Google Scholar]
- 80. Stamm T. I have mastered the challenge of living with a chronic disease: life stories of people with Rheumatoid Arthritis. Qual Health Res 2008; 18: 658–669. [DOI] [PubMed] [Google Scholar]
- 81. Lindsay S. Prioritizing illness: lessons in self-managing multiple chronic diseases. Canadian J Soc 2009; 34: 983–1002. [Google Scholar]
- 82. Cicutto L, Brooks D, Henderson K. Self-care issues from the perspective of individuals with chronic obstructive pulmonary disease. Patient Educ Couns 2004; 55: 168–176. [DOI] [PubMed] [Google Scholar]
- 83. Loignon C, Bedos C, Sévigny R, et al. Understanding the self-care strategies of patients with asthma. Patient Educ Couns 2009; 75: 256–262. [DOI] [PubMed] [Google Scholar]
- 84. Audulv A, Norbergh K-G, Asplund K, et al. An ongoing process of inner negotiation – a grounded theory study of self-management among people living with chronic illness. J Nurs Health Chron Illn 2009; 1: 283–293. [Google Scholar]
- 85. Goodacre L. Women’s perceptions on managing chronic arthritis. Br J Occup Ther 2006; 69; 7–14. [Google Scholar]
- 86. Kidd L, Kearney N, O’Carroll, et al. Experiences of self-care in patients with colorectal cancer: a longitudinal study. J Adv Nurs 2008; 64: 469–477. [DOI] [PubMed] [Google Scholar]
- 87. Chiu-Chu L, Anderson R, Hagerty B, et al. Diabetes self-management experience: a focus group study of Taiwanese patients with type 2 diabetes. J Nurs Health Chron Illn 2006; 17: 34–42. [DOI] [PubMed] [Google Scholar]
- 88. Corbin J, Strauss A. Managing chronic illness at home: three lines of work. Qual Sociol 8: 224–247. [Google Scholar]
- 89. Kenning C, Fisher L, Bee P, et al. Primary care practitioner and patient understanding of the concepts of multimorbidity and self-management: a qualitative study. SAGE Open Med. Epub ahead of print 29 October 2013. DOI: 10.1177/2050312113510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hinder S, Greenhalgh T. ‘This does my head in’. Ethnographic study of self-management by people with diabetes. BMC Health Serv Res 2012; 12, http://biomedcentral.com/1472-6963/12/83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Morris R, Sanders C, Kennedy A, et al. Shifting priorities in multimorbidity: a longitudinal qualitative study of patient’s prioritization of multiple conditions. Chronic Illn 2011; 7: 147–161. [DOI] [PubMed] [Google Scholar]
- 92. Greenhalgh T, Collard A, Campbell-Richards D, et al. Storylines of self-management: narratives of people with diabetes from multiethnic inner city population. J Health Serv Res Policy 2011; 16: 37–43. [DOI] [PubMed] [Google Scholar]
- 93. Ong B, Jinks C, Morden A. The hard work of self-management: living with chronic knee pain. Int J Qual Stud Health Well-Being 2011; 6 DOI: 10.3402/qhw.v6i3.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Clarke L, Bennett E. Constructing the moral body: self-care among older adults with multiple chronic conditions. Health 2012; 17: 211–228. [DOI] [PubMed] [Google Scholar]
- 95. Pickard S, Rogers A. Knowing as practice: self-care in the case of chronic multi-morbidities. Soc Theory Health 2012; 10: 101–120. [Google Scholar]
- 96. Audulv A, Asplund K, Norbergh K-G. The integration of chronic illness self-management. Qual Health Res 2012; 22: 332–345. [DOI] [PubMed] [Google Scholar]
- 97. Samuel-Hodge CD, Cene CW, Corsino L, et al. Family diabetes matters: a view from the other side. J Gen Intern Med 2012; 28: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Essue BM, Jowsey T, Jeon Y-H, et al. Informal care and the self-management partnerships: implications for Australian health policy and practice. Aust Health Rev 2010; 34: 414–422. [DOI] [PubMed] [Google Scholar]
- 99. Jeffrey R. Normal rubbish: deviant patients in casualty departments. Sociol Health Illn 1979; 1: 90–107. [DOI] [PubMed] [Google Scholar]
- 100. Maclean N, Pound P, Wolfe C, et al. The concept of patient motivation. A qualitative analysis of stroke professionals’ attitudes. Stroke 2002; 33: 444–448. [DOI] [PubMed] [Google Scholar]
- 101. Kendall E, Catalano T, Kuipers P, et al. Recovery following stroke: the role of self-management education. Soc Sci Med 2007; 64: 735–746. [DOI] [PubMed] [Google Scholar]
- 102. Bloom J, Spiegel D. The effect of two dimensions of social support on the psychological well-being and social functioning of women with advanced breast cancer. Soc Sci Med 1984; 19, 831–837. [DOI] [PubMed] [Google Scholar]
- 103. McCabe MP, Marita P, Elodie J. Why are some people with neurological illness more resilient than others? Psychol Health Med 2012; 17: 17–34. [DOI] [PubMed] [Google Scholar]
- 104. Dunkel-Schetter C. Social Support in cancer: findings based on patient interviews and their implications. J Soc Issues 1984; 40: 77–98. [Google Scholar]
- 105. Kennedy A, Gask L, Rogers A. Training professionals to engage with and promote self-management. Health Educ Res 2005; 20: 567–578. [DOI] [PubMed] [Google Scholar]