Abstract
Objectives:
This study aimed to evaluate the benefits of an interactive and visual flowchart-type leaflet for head and neck cancer inpatients who received induction chemotherapy, docetaxel, cisplatin, and 5-fluorourasil (DCF), or docetaxel, cisplatin, and S-1 (DCS) from September 2009 to April 2012. The flowchart-type leaflet group used a flowchart-type leaflet during chemotherapy, while the non-flowchart-type leaflet group did not.
Methods:
A retrospective cohort study was performed using patient records. The endpoints of this study were to determine the following: the number of emergency hospital admissions/visits, incidence of Grade 2 or higher non-haematological adverse drug reactions, nonadherence to treatment, and the number of telephone calls from subjects.
Results:
A total of 109 subjects were identified as follows: 49 in the flowchart-type leaflet group (139 chemotherapy sessions) and 60 in the non-flowchart-type leaflet group (163 chemotherapy sessions). No significant differences were observed in age, performance status, or chemotherapy regimen. The incidence of emergency hospital admissions was significantly lower in the flowchart-type leaflet than in the non-flowchart-type leaflet group (1% vs 10%, p < 0.01). No difference was seen between groups (12% vs 19%, p = 0.1) in the nonadherence rate of supportive medication for adverse drug reactions. Telephone call rates were significantly higher in the flowchart-type leaflet (16%, 30 calls) than in the non-flowchart-type leaflet group (7%, 11 calls) in each chemotherapy regimen. Of the 30 calls from patients in the FCL group, 24 (80%) were made to the hospital, compared with only 5 (45%) of the 11 calls from patients in the non-flowchart-type leaflet group.
Conclusions:
Our results suggest that the flowchart-type leaflet can reduce nonadherence and improve patient judgment during chemotherapy, leading to a decrease in emergency hospital admissions.
Keywords: Flowchart, leaflet, adherence, severe adverse events
Introduction
Medication nonadherence, a deviation from the prescribed medication regimen sufficient to adversely influence the regimen’s intended effect,1 is an important problem for clinical pharmacists. Adherence, which involves the self-care skills learned by the patient, has been recognized as an important component of both chemotherapy and supportive therapies. Therefore, a high level of adherence supports the independence of cancer patients, whereas nonadherence can lead to serious consequences. In an adjuvant hormone medication setting, Hershman et al.2 reported that nonadherence to adjuvant hormonal therapy was associated with increased mortality in women with breast cancer. Moreover, a meta-analysis3 showed that patients who were adherent had better outcomes than those who were not; therefore, attention is currently focused on nonadherence in cancer chemotherapy. The factors influencing patient adherence are difficult to clarify due to the variety of backgrounds and demographic factors; however, it is important to continue improving patient adherence in order to promote more successful treatment.
When pharmacists explain patients’ medication, information sheets are important tools for improving adherence, especially in cancer chemotherapy. Most information sheets cover the schedule of chemotherapy sessions and the adverse reactions of the respective drugs; however, the leaflets do not provide sufficient information to instruct patients on how to take their supportive medications while at home when adverse drug reactions (ADRs) occur. Bhattacharya et al.4 reported that ADRs affected patients’ capecitabine adherence, even though adherence was high due to a strong conviction that the capecitabine was necessary. Cancer chemotherapy causes severe ADRs that may require emergency hospital admissions. Mayer et al.5 reported more than half of emergency hospital visits caused by ADRs resulted in admittance. McKenzie et al.6 also suggested follow-up of cancer chemotherapy patients because they tend to have frequent, unplanned presentations and emergency admissions due to ADRs.
Fever is an important ADR that may be febrile neutropaenia due to multiple cytotoxic agents. In a previous report, only 20% of subjects adhered to the instructions of medical professionals when they had a fever.7 In another study, we observed sorafenib (an oral anticancer agent) nonadherence, and found that most patients had errors adhering to both sorafenib and its supportive medications.8 In that study, we found that most nonadherence occurred when patients had ADRs. Patients tended to rely on self-judgement rather than the instructions they were given, especially in an outpatient setting. Currently, the number of chemotherapy outpatients is increasing, and thus, it is increasingly important to manage various ADRs, especially severe adverse events that require emergency hospital admissions. From our experience, we have found that conventional chemotherapy information sheets consisting of multiple descriptions are difficult for patients to understand in relation to management of chemotherapy ADRs, even if they are well written.7,8 One of the ways to provide practical and clear information is to use visual tools that instruct patients on how to manage their ADRs. Therefore, we developed a leaflet using a visual flowchart (FC) as a potential tool to improve patient adherence. This retrospective cohort study was designed to determine the benefits of a FC-type leaflet (FCL) in cancer chemotherapy.
Methods
Subjects and study design
This was a retrospective chart review of head and neck cancer patients consecutively treated with induction chemotherapy; subjects received standard dose regimens of either docetaxel, cisplatin, and 5-fluorourasil9,10 (DCF) or docetaxel, cisplatin, and S-111 (DCS). Subjects were identified via a computer-generated list printed from the pharmacy database at the National Cancer Center Hospital East from 1 September 2009 through 30 April 2012. The DCF regimen (docetaxel 70 mg/m2, cisplatin 70 mg/m2, and 5-fluorourasil 750 mg/m2) was administered intravenously every 3 weeks, and the DCS regimen (docetaxel 70 mg/m2 and cisplatin 70 mg/m2) was administered intravenously with S-1 of 60 mg/m2 oral chemotherapy for 2-week periods every 3 weeks. Subjects received three courses of either regimen before chemoradiation. Data were extracted from the electronic medical record and the pharmacy electronic database. If subjects had serious ADRs, their doctors could reduce or delay their chemotherapy. Both chemotherapy regimens included the same amount of hydration and standard supportive care.
The FCL was introduced in January 2012; therefore, the study is categorized as a time-series study. In the first cycle of the FCL group’s chemotherapy, subjects received the FCL accompanied by an explanation by a pharmacist. If the FCL was subsequently lost, pharmacists gave the FCL to the subject again. Pharmacist explanation of how to use the FCL lasted about 15 min. During this time, they evaluated whether subjects were able to read and understand the concept of the FCL. During chemotherapy, pharmacists asked subjects about their adherence and ADRs. If subjects had a problem, the pharmacist recorded the event on the subject’s electronic record. There were no specific tools to measure adherence; rather, nonadherence was defined as the incorrect use of medications. Subject’s phone calls to their doctors were then to be documented in the subject’s electronic medical records. All subject telephone calls in the electronic medical records were reviewed for this study.
The endpoints of this study were to determine the following: the number of emergency hospital admissions/visits, incidence of Grade 2 or higher non-haematological ADRs, nonadherence to treatment, and the number of telephone calls from subjects. The study was approved by the National Cancer Center Institutional Review Board (Approval number (2011-182)). Due to the fact that this was a retrospective study, informed consent was not required. Subject-specific information was collected from electronic databases. ADRs were evaluated by oncologists according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0).11
Development of the FCL
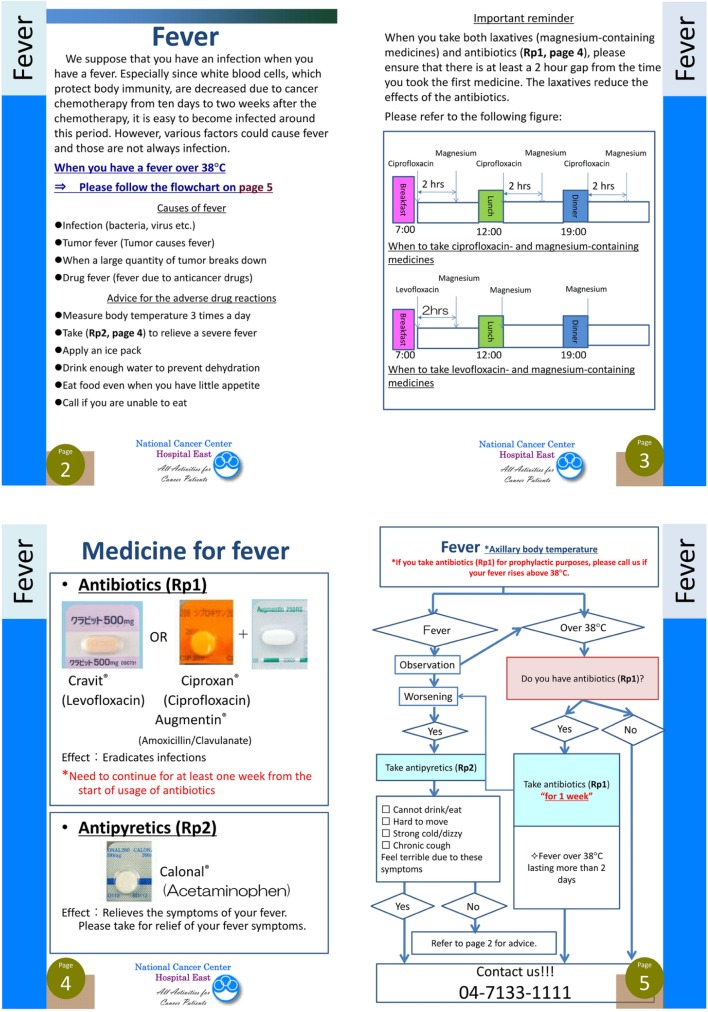
The development of the FCL was started in June 2011. Using a flowchart design, it was composed of ADR descriptions and their related management. The goal of the FCL was to make the management of important ADRs more easily understood by the patient. Therefore, the flowchart consisted of yes/no questions to guide patients on how to take supportive medicine for ADRs and when to call the hospital. An example of the FCL is shown in Figure 1. The FCL was designed for patients receiving cancer chemotherapy; therefore, the pharmacists reached a consensus regarding supportive medicine for cancer chemotherapy (e.g. antiemetic medications). We chose 12 ADRs which would frequently cause emergency hospital admissions, and made a flowchart for each that explained how to manage them. We had several meetings with oncologists, nurses, and pharmacists to share ideas on supportive medicines for cancer chemotherapy. We did not focus on specific chemotherapy regimens or anticancer agents; however, we tried to make the FCL based on patients’ symptoms in order to increase patient understanding and identification of ADRs. The final version of the FCL was 51 pages. It consisted of flowcharts and descriptions of 12 ADRs approved by the National Cancer Center Hospital East in January 2012. We have used the FCL with head and neck chemotherapy patients since its approval. It is necessary to investigate how new tools work in daily practice; therefore, we performed this retrospective study to evaluate the benefits of the FCL.
Figure 1.
An example of the flowchart-type leaflet used in this study.
Data analysis
Bivariate analyses were employed to examine differences in demographic characteristics between the FCL and non-FCL groups using t-tests for continuous variables (e.g. age), and chi-square tests for categorical variables (e.g. sex). Similarly, chi-square tests were also used to examine differences in patient-specific information, including type of induction chemotherapy and their ADRs. All data were analysed using SPSS version 16.0 (SPSS Inc., Chicago, IL). A p-value <0.05 was considered statistically significant.
Results
Demographics and ADRs
A total of 109 subjects were identified as follows: 49 in the FCL group (139 chemotherapy sessions) and 60 in the non-FCL group (163 chemotherapy sessions). There was no subject in this study evaluated by pharmacists as not being able to read and understand the FCL. No significant differences were apparent between the two groups in age, performance status, or chemotherapy regimen (Table 1). Overall, the incidence of Grade 2 or higher non-haematological ADRs occurring due to the induction of chemotherapy was higher in the FCL group than in the non-FCL group (40% vs 26%, p < 0.05). However, the incidence rate included ADRs that the FCL or other tools were unable to manage, such as hiccup and renal dysfunction. When ADRs that were covered in the FCL (i.e. fever, nausea, diarrhoea, etc.) were analysed, no difference was found between the two groups in the incidence of the non-haematological ADRs (Table 2).
Table 1.
Patients’ characteristics.
| FCL |
Non-FCL |
p-value* | |
|---|---|---|---|
| n = 49 | n = 60 | ||
| Sex | |||
| Male | 43 | 52 | NS |
| Female | 6 | 8 | |
| Age (years) | |||
| Median | 60 | 62 | NS |
| Range | 20–75 | 22–76 | |
| ECOG PS | |||
| 0 | 42 | 55 | |
| 1 | 6 | 5 | NS |
| 2 | 1 | 0 | |
| Chemotherapy cycles (total) | 139 | 163 | |
| Chemotherapy cycles, n (%) | |||
| DCF | 45 (32) | 74 (45) | NS |
| DCS | 94 (68) | 89 (55) | |
FCL: flowchart-type leaflet; ECOG PS: Eastern Cooperative Oncology Group Performance Status; DCF: docetaxel, cisplatin, and 5-fluorourasil; DCS: docetaxel, cisplatin, and S-1.
Mann-Whitney’s U test, χ2 test, and Fisher’s exact test.
Table 2.
Adverse drug reactions.
| FCL |
Non-FCL |
p-value* | |
|---|---|---|---|
| 139 cycles/49 pts | 163 cycles/60 pts | ||
| Grade 2, 3 non-haematologic toxicities: number of patients (%) | 29 (59) | 30 (50) | NS |
| Grade 2, 3 non-haematologic toxicities: number of events (%) | 67 (48) | 77 (47) | NS |
| Adverse events | |||
| Anorexia | 23 | 28 | |
| Nausea/vomiting | 8 | 13 | |
| Stomatitis | 7 | 6 | |
| Fatigue | 6 | 14 | |
| Diarrhoea | 6 | 3 | |
| Fever/FN | 5 | 2 | |
| Hand foot syndrome | 5 | 1 | |
| Skin rash | 4 | 0 | |
| Constipation | 3 | 10 | |
FCL: flowchart-type leaflet; cycles: number of chemotherapy cycles; pts: number of patients; FN: febrile neutropaenia.
χ2 test.
Emergency hospital admissions/visits rate
Emergency hospital admissions or visits were identified as hospital admissions or visits that were not planned as part of chemotherapy. All counted emergency hospital admissions or visits were due to ADRs. The incidence of emergency hospital visits was significantly lower in the FCL group (3%) than in the non-FCL group (10%, p < 0.01). The incidence of emergency hospital admission was also significantly lower in the FCL group (1%) than in the non-FCL group (10%, p < 0.001) (Table 3).
Table 3.
Emergency hospital admissions and telephone calls.
| FCL |
Non-FCL |
p-value* | |
|---|---|---|---|
| 139 cycles/49 pts | 163 cycles/60 pts | ||
| Emergency hospital admissions, n (%) | 1 (1) | 16 (10) | 0.0015 |
| Emergency hospital visits, n (%) | 4 (3) | 17 (10) | 0.01 |
| Nonadherence (inpatients), n (%) | 17 (12) | 31 (19) | 0.1 |
| Nonadherence (outpatients), n (%) | 5 (4) | 23 (14) | 0.002 |
| Telephone calls, n (%) | 22 (16) | 11 (7) | 0.01 |
| Total calls | 30 | 11 | |
| Calls according to pharmacists’ instruction | 24 (80) | 5 (45) | |
| Calls before emergency admissions | 1 (100) | 6 (37) | |
| Calls before emergency visits | 4 (100) | 6 (37) |
FCL: flowchart-type leaflet; cycles: number of chemotherapy cycles; pts: number of patients.
Fisher’s exact test.
Nonadherence rate
No difference was observed in the nonadherence rate in supportive medication for ADRs due to chemotherapy between the two groups in inpatients (FCL, 12% vs non-FCL, 19%; p = 0.1); however, the nonadherence rate was significantly lower in the FCL group (4%) than in the non-FCL group (14%, p < 0.01) in outpatients (Table 3).
Telephone calls
Telephone call rates were significantly higher in the FCL group (16%, 30 calls) than in the non-FCL group (7%, 11 calls) in each chemotherapy regimen (p < 0.01). Of the 30 calls from patients in the FCL group, 24 (80%) were made to the hospital, compared with only 5 (45%) of the 11 calls from patients in the non-FCL group. All of the patients in the FCL group called the hospital before emergency hospital admissions, compared with 37% in the non-FCL group (Table 3).
Discussion
The anticancer and molecular-targeted agents used in cancer chemotherapy regimens have severe and unique ADRs when compared to other medications; therefore, ADR management is very important. Of 4190,911 emergency hospital visits in 2008 in a population-based survey of emergency hospital visits in North Carolina, 37,760 were by 27,644 cancer patients,5 indicating that cancer patients receiving chemotherapy frequently experience severe ADRs. The data also showed that emergency situations occurred after business hours, during the night, and on holidays. Patients’ adherence to their drug regimens is an important factor for safe and effective treatment, and researchers have studied various tools and systems to improve it.12 Active intervention by healthcare professionals could decrease patient emergencies; however, the number of available healthcare professionals is limited. New systems to improve patient safety in regard to chemotherapy are needed.
Information sheets and instruction leaflets could be an effective tool to supplement explanations by health-care professionals on how to manage symptoms, especially in an outpatient setting. There are many cautions and instructions given during treatment, and it is difficult for patients to memorize them all. An instruction leaflet is effective when it is explained to patients by health-care professionals; however, these leaflets typically have a lot of text and few figures. Pictures or video tend to be preferred over reading text, especially during illness. Based on our clinical experience, even though they have been provided with instruction via a text-style leaflet from their pharmacists, patients tend to forget how to manage their symptoms when they have ADRs. In outpatient settings, symptom management is critical due to the lack of home medical care in Japan.
We developed an instruction leaflet for managing ADRs and instructing patients when to call the hospital in emergency situations that was visually easy to understand. In this study, the incidence of Grade 2 or higher non-haematological ADRs was 40% in the FCL group and 26% in the non-FCL group, indicating that the chemotherapy was toxic. In the non-FCL group, there was a 10% emergency admission rate; however, the incidence was significantly lower in the FCL group (Figure 1). The FCL improved subjects’ knowledge of how to manage certain symptoms by either taking medication or calling the hospital. In the study, telephone call rates were significantly higher in the FCL group than in the non-FCL group. Of the 30 calls from patients in the FCL group, 24 (80%) were made to the hospital, compared with only 5 (45%) of the 11 calls from patients in the non-FCL group, demonstrating that the FCL triaged emergency conditions due to chemotherapy. It is difficult to establish a new system that will identify patients who have emergency conditions over multiple chemotherapy sessions; however, the FCL has the potential both to enhance treatment adherence through adequate supportive medicine and to remind patients how to manage severe ADRs during cancer chemotherapy.
It is known that adherence to treatment is one of the important factors in determining successful treatment. There have been some reports that pharmacists contribute to improving patients’ adherence and clinical outcomes,13,14 especially in cancer treatment that includes cytotoxic agents and unique molecular-targeted medicines.15 In this study, we found the FCL improved not only emergency admission rates but also adherence in an outpatient setting. The rate of telephone calls when the subjects had severe ADRs was significantly improved by using the FCL. In the inpatient chemotherapy setting, we did not use a telephone follow-up method to support chemotherapy patients; however, our other study showed that telephone follow-up identified half of the nonadherence in sorafenib therapy.8 In addition, we also found that patients who called the hospital when they had a fever could maintain their oral antibiotic adherence in outpatient chemotherapy. Several studies that proved the benefits of telephone triage have been reported,16,17 and we believe that triage is important during chemotherapy because the duration of inpatient chemotherapy is getting shorter while outpatient chemotherapy is increasing. Obviously, cancer chemotherapy has numerous severe ADRs, and the incidence of emergency hospital admissions or visits is high;5,6 therefore, timely telephone intervention could be effective in managing ADRs.
DCF and DCS were toxic regimens, and we expected that early self-management by subjects might reduce the incidence of severe grade ADRs. In this study, the FCL did not reduce the incidence of Grade 2 or higher non-haematological ADRs; however, the incidence of adherence to supportive medicine (which reflects subjects’ self-management) was significantly superior in the FCL group than in the non-FCL group. In our hypothesis, the FCL enhanced subjects’ understanding towards their symptoms, and consequently, their emergency hospital admission rates were lower.
In this study, no subjects refused using the leaflet; however, we were unable to analyse subject satisfaction or quality of life due to the retrospective nature of the study. In our daily practice, we explain how to use the FCL to both patients and their family members. Therefore, the implication is that patients’ family members also might be able to use the tool; however, we did not collect any data from family members in this study. Moreover, the FCL helped pharmacists share information with oncologists on how to treat patients. Some pharmacists and oncologists used the FCL as an educational textbook because it described how to manage ADRs and emergency settings. The incidence of emergency hospital visits and admissions was significantly lower in the FCL group than in the non-FCL group. This is probably because pharmacists are likely to have provided more thorough explanations to subjects in the FCL group; however, a bias which affected the quality of the pharmacists’ intervention was evident.
This retrospective study was limited to a specific chemotherapy regimen, and therefore, a prospective pilot study to evaluate the FCL is needed. Another limit of our study was that we could not measure subject variables such as beliefs about medication, access to medication, and forgetfulness, and so on; therefore, subjects’ education levels might also have affected the results of the study.
Conclusion
In this study, no difference was observed in the incidence of ADRs between the FCL and the non-FCL group. Our data showed that the FCL did not reduce ADRs, but it did support subjects’ telephone calls during emergencies due to ADRs. This suggests that the FCL could contribute to reducing emergency hospital admissions by discouraging nonadherence and improving patients’ judgment during chemotherapy. This is the first report that a visually instructive FCL is feasible to use in cancer chemotherapy. Currently, text-style leaflets are commonly provided; however, an easier to understand FCL may provide greater patient benefit in the future.
Acknowledgments
The authors wish to thank the National Cancer Center Hospital East and members of its staff for reviewing the FCL, approving it as an official document, and offering support to publish it for the patients.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant 2009; 9: 35–41. [DOI] [PubMed] [Google Scholar]
- 2. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011; 126: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robin DM, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care 2002; 40: 794–811. [DOI] [PubMed] [Google Scholar]
- 4. Bhattacharya D, Easthall C, Willoughby KA, et al. Capecitabine non-adherence: exploration of magnitude, nature and contributing factors. J Oncol Pharm Pract 2012; 18: 333–342. [DOI] [PubMed] [Google Scholar]
- 5. Mayer DK, Travers D, Wyss A, et al. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 2011; 29: 2683–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKenzie H, Hayes L, White K, et al. Chemotherapy outpatients’ unplanned presentations to hospital: a retrospective study. Support Care Cancer 2011; 19: 963–969. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki S, Muranaga A, Matsui R, et al. Adherence in use of oral antibiotics for fever in outpatient docetaxel chemotherapy. Jpn J Pharm Health Care Sci 2011; 37: 389–394. [Google Scholar]
- 8. Suzuki S, Odanaka M, Funazaki H, et al. Evaluation of improvement of adherence following pharmacist intervention for hand/foot skin reactions induced by sorafenib. Jpn J Pharm Health Care Sci 2011; 37: 317–321. [Google Scholar]
- 9. Posner MR, Glisson B, Frenette G, et al. Multicenter phase I-II Trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol 2001; 19: 1096–1104. [DOI] [PubMed] [Google Scholar]
- 10. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007; 357: 1695–1704. [DOI] [PubMed] [Google Scholar]
- 11. Tahara M, Araki K, Okano S, et al. Phase I trial of combination chemotherapy with docetaxel, cisplatin and S-1 (TPS) in patients with locally advanced or recurrent/metastatic head and neck cancer. Ann Oncol 2011; 22: 175–180. [DOI] [PubMed] [Google Scholar]
- 12. Hall J, Peat M, Birks Y, et al. Effectiveness of interventions designed to promote patient involvement to enhance safety: a systematic review. Qual Saf Health Care 2010; 19: e10. [DOI] [PubMed] [Google Scholar]
- 13. Lee JK, Grace KA, Taylor AJ, et al. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006; 296: 2563–2571. [DOI] [PubMed] [Google Scholar]
- 14. Bouvy M. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail 2003; 9: 404–411. [DOI] [PubMed] [Google Scholar]
- 15. Simons S, Ringsdorf S, Braun M, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer 2011; 19: 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Towle E. Telephone triage in today’s oncology practice. J Oncol Pharm Pract 2009; 5: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Purc-Stephenson RJ, Thrasher C. Nurses’ experiences with telephone triage and advice: a meta-ethnography. J Adv Nurs 2010; 66: 482–494. [DOI] [PubMed] [Google Scholar]