Abstract
Objectives:
Elevated alanine aminotransferase can heighten concern for the presence of nonalcoholic fatty liver disease in obese children. Guidelines recommend alanine aminotransferase screening of obese children start at the age of 10 years. We examined alanine aminotransferase values routinely obtained for tertiary obesity care among preschool (2–5 years) and school-age children.
Methods:
Medical records of children attending a tertiary obesity clinic and with alanine aminotransferase measured within 6 months of the initial visit were reviewed. Children with known genetic abnormalities were excluded. Children were grouped by age to focus attention on groups not covered by screening guidelines. Associations with elevated alanine aminotransferase (>30 IU/L) were examined.
Results:
A total of 284 records were analyzed (73 preschool, 143 young school-age (6–9 years), 68 older school-age (10–11 years)). Children were primarily Hispanic and had body mass index ≥ 99th percentile (preschool children 92%, young school-age 73%, older school-age 59%). In all, 26% of preschool children had elevated alanine aminotransferase (young school-age 30%, older school-age 44%). Preschool children with elevated alanine aminotransferase had higher body mass index compared to preschool children with alanine aminotransferase ≤ 30 IU/L (median body mass index 27.8 kg/m2 vs 24.0 kg/m2; Mann–Whitney U test, p = 0.003), but there was no disparity for elevated alanine aminotransferase related to Hispanic ethnicity. For older children, Hispanic ethnicity, not body mass index, predicted elevated alanine aminotransferase.
Conclusion:
Alanine aminotransferase elevation was common in these preschool children. Screening severely obese children for elevated alanine aminotransferase should begin at the age of 2 years.
Keywords: Obesity, children, liver, alanine aminotransferase, nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of fat accumulation within the liver (fatty liver/hepatic steatosis) without a secondary cause for such fat accumulation.1–4 A recent analysis of data from The Third National Health and Nutrition Examination Survey (NHANES) (1988–1994) assessed prevalence of NAFLD in US adults.5 For these analyses, individuals were considered to have NAFLD if moderate to severe hepatic steatosis was present by ultrasonography in the absence of elevated alcohol consumption or use of medications associated with hepatic steatosis. Based on this analysis, NAFLD affects approximately 21% of US adults;5 prevalence increases with obesity; approximately 50% of US adults with body mass index (BMI) ≥ 35 kg/m2 have NAFLD.5 An autopsy study of children aged 2–19 years estimated the prevalence of fatty liver in children at 9.6%; however, fatty liver was present in 38% of children who were obese.6 The current prevalence of obesity in US children is almost 17%;7 therefore, a relatively large percentage of children may be at risk for NAFLD. Early identification of children with NAFLD is important as NAFLD may be an indicator of increased metabolic risk8 and because children with nonalcoholic steatohepatitis may experience progression of liver inflammation and fibrosis, which can lead to cirrhosis.2,9,10
Clinical guidelines recommend various strategies to screen obese children for NAFLD.11,12 The European Society of Paediatric Gastroenterology, Hepatology and Nutrition guideline on NAFLD notes that NAFLD usually does not occur in children younger than 3 years and is rare in children aged 3–10 years.13 The 2007 Expert Committee recommended that children with a BMI ≥ 95th percentile be screened for NAFLD biannually beginning at the age of 10 years.12 The Expert Committee also recommended using both alanine aminotransferase (ALT) and aspartate aminotransferase laboratory tests for NAFLD screening.12 However, among these two tests, ALT is more commonly used as a surrogate marker for fatty liver concerns in obese children.14,15
There is wide variability regarding the ALT value considered to be the upper limit of normal. A study of children’s hospitals identified an ALT upper limit ranging from 30 to 90 U/L (median 53 U/L).16 An examination of NHANES data identified much lower 95th percentile values for ALT measurements in children aged 12–17 years at low risk for liver disease (25.8 U/L for boys and 22.1 U/L for girls).16 Applying the NHANES 95th percentile ALT values compared to children’s hospitals upper limit median values increased sensitivity of ALT as a screen for NAFLD from approximately 35% to 85%.16 Approximately 51% of severely obese US adolescents (BMI ≥ 99th percentile) had an ALT value above these cut points.17 An evaluation of 371 healthy youths aged 7–18 years reported 95th percentile values for ALT boys and girls of 30 and 21 U/L, respectively.18 Upper limit of normal for ALT may be even lower for younger children.19
Studies investigating ALT elevations in obese children often focus on older children and adolescents. There is evidence that young obese children may also have elevated ALT. Two studies of children aged <10 years report elevated ALT at rates of 12.5% (for ALT ≥ 35 IU/L)20 and 24% (for ≥45 IU/L).14 Identifying children with elevated ALT values provides the opportunity to strengthen clinical management and enhance the call for health behavior change. Lifestyle interventions have been shown to improve ALT levels, liver histology, and steatosis (identified by ultrasonography) and are the first line intervention recommended for NAFLD.21,22 Since parental perception that their child’s weight is a health problem increases readiness to make changes,23 early detection of ALT elevations and focused counseling about this concern may enhance a family’s resolve to implement lifestyle changes. The aim of this study is to describe results of screening ALT values in children presenting for tertiary obesity care with a special emphasis on preschool children (age 2–5 years) as compared to young school-age children (age 6–9 years) and older school-age children (age 10–11 years).
Methods
Study population
This study is a retrospective review of a consecutive sample of children who presented for obesity care at the Wellness & Weight Management (W&WM) Clinic who (1) had completed an initial W&WM visit between April 2007 and December 2010, (2) were aged 2–11 years at the initial W&WM visit, (3) had BMI ≥ 95th percentile at their initial visit, (4) were not receiving enteral feeding at initial W&WM visit, and (5) did not have an identified genetic abnormality. Children are referred to this tertiary care clinic by primary care or subspecialty providers. There were 454 eligible children whose records were reviewed. Data elements, including demographics, anthropometric measurements, and laboratory measurements, were downloaded from the electronic medical record (EMR). The Ann & Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board (#2011-14528) approved this study. Waiver of Authorization was granted, which allowed evaluation of retrospective medical record data without signed authorization from each research subject.
Measurements
Anthropometric measurements were obtained at the initial W&WM clinic visit. Weight and height measurements were obtained by clinic staff members trained to apply hospital measurement technique protocols. Weight was measured to the nearest 0.1 kg using digital scales. Children were measured to the nearest 0.1 cm using a stadiometer.
Laboratory measurements, including fasting lipids, insulin, and glucose, and ALT are ordered as part of routine obesity care at the W&WM clinic. Laboratory values obtained by providers not providing care through the W&WM clinic (e.g. a child’s primary care pediatrician) were included if they had been scanned into the EMR. Laboratory tests ordered by primary care providers rarely include fasting insulin, which accounts for low responses for that test. If multiple test results were available, the laboratory value closest to the initial W&WM visit and within 6 months of that visit defined the initial ALT value (average time between initial W&WM visit and initial ALT value = 49 days, standard deviation (SD) = 47 days). Only those records of children with at least one ALT measurement (284/454, 63%) were analyzed.
An ALT value > 30 IU/L was considered elevated. This value corresponds to the 97th–98th percentile in a NHANES adolescent population,24 defines the upper limit at our hospital and at this clinic, and was used in another study which investigated younger obese children.25 An ALT value > 60 IU/L is the value that should prompt consultation with a pediatric hepatologist according to Expert Committee guidelines and was considered “very elevated” for this study.12 Other laboratory measurements that were downloaded and analyzed included fasting lipids, fasting glucose, and fasting insulin. Fasting lipid cutoffs were the same as those outlined in National Heart, Lung, and Blood Institute guidelines.26 Insulin resistance was measured using the homeostasis model assessment for insulin resistance (HOMA-IR).27 We used cutoffs developed by Kurtoglu et al. for pre-pubertal boys (≤11 years) 2.67, for pubertal boys (>11 years) 5.22, for pre-pubertal girls (≤10 years) 2.22, and for pubertal girls (>10 years) 3.82.28
Data analysis
Anthropometric interpretations, including BMI, BMI z-score, and BMI percentile, were calculated by applying the Centers for Disease Control and Prevention (CDC) 2000 growth references using Epi Info 3.5.3 (NationalCenter for Health Statistics, CDC, Atlanta, GA, 2011). Percent ideal body weight for height (%IBW) was calculated using Health Indicators Analyzer software (©2003, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL), which uses the McLaren method.29 Children were categorized by age at ALT testing (2–5 years, 6–9 years, and 10–11 years) in order to focus attention on findings for youngest children.
Data were analyzed using IBM SPSS Statistics, version 20.0.0. Significance was set at p < 0.05. Chi-square, Fisher’s Exact, and Mann–Whitney U tests were used, as appropriate, to examine differences between groups. Linear-by-linear association (LLA) was used to examine ALT and BMI ≥ 99th percentile groups by age groups.
Results
The 284 children analyzed included 73 preschool children (age 2–5 years), 143 young school-age children (6–9 years), and 68 older school-age children (≥10 years). Children were primarily Hispanic and Medicaid insurance recipients (Table 1). An elevated ALT (>30 IU/L) was identified in 26% of preschool children, 30% of young school-age children, and 44% of older school-age children (LLA, p = 0.023). Initial ALT values > 60 IU/L were found for 4%, 4%, and 13% of children in these age groups, respectively (Figure 1). Hispanic race/ethnicity was not significantly associated with ALT group status for preschool children, but was significantly associated with elevated ALT in school-age groups (Table 1). Sex was not significantly associated with ALT group status for any age group.
Table 1.
Demographic and anthropometric characteristics of children by alanine aminotransferase (ALT) and age groups.
| Total | ALT ≤ 30 IU/L | ALT > 30 IU/L | p value | |
|---|---|---|---|---|
| Ages 2–5 years, n (%) | 73 (100%) | 54 (74%) | 19 (26%) | |
| Demographics | ||||
| Sex | ||||
| Male, n (%) | 33 (45%) | 22 (67%) | 11 (33%) | 0.196a |
| Female, n (%) | 40 (55%) | 32 (80%) | 8 (20%) | |
| Ethnicity | ||||
| Hispanic, n (%) | 51 (70%) | 39 (75%) | 13 (25%) | 0.873a |
| Non-Hispanic, n (%) | 22 (30%) | 16 (73%) | 6 (27%) | |
| Insurance | ||||
| Medicaid, n (%) | 59 (81%) | 42 (71%) | 17 (29%) | 0.265b |
| Private, n (%) | 14 (19%) | 12 (86%) | 2 (11%) | |
| Anthropometrics | ||||
| BMI, kg/m2, median (range) | 24.4 (18.6–36.5) | 24.0 (18.6–36.5) | 27.8 (21.8–32.8) | 0.003d |
| BMI percentile, median (range)c | 100 (95.3–100) | 99.9 (95.3–100) | 100 (100–100) | 0.040d |
| BMI z-score, median (range)c | 3.4 (1.7–6.2) | 3.3 (1.7–6.2) | 3.6 (2.8–5.9) | 0.016d |
| %IBW, median (range) | 157.0 (119.3–234.9) | 154.1 (119.3–234.9) | 179.2 (140.0–211.1) | 0.004d |
| Ages 6–9 years, n (%) | 143 (100%) | 100 (70%) | 43 (30%) | |
| Demographics | ||||
| Sex | ||||
| Male, n (%) | 79 (55%) | 55 (70%) | 24 (30%) | 0.928a |
| Female, n (%) | 64 (45%) | 45 (70%) | 19 (39%) | |
| Ethnicity | ||||
| Hispanic, n (%) | 102 (71%) | 64 (63%) | 38 (37%) | 0.003a |
| Non-Hispanic, n (%) | 41 (29%) | 36 (88%) | 5 (12%) | |
| Insurance | ||||
| Medicaid, n (%) | 109 (76%) | 72 (66%) | 37 (34%) | 0.070a |
| Private, n (%) | 34 (24%) | 28 (82%) | 6 (18%) | |
| Anthropometrics | ||||
| BMI, kg/m2, median (range) | 27.4 (20.5–42.9) | 26.9 (20.5–38.2) | 27.6 (20.8–42.9) | 0.631d |
| BMI percentile, median (range)c | 99.4 (95.3–99.9) | 99.4 (95.3–100) | 99.3 (96.3–99.9) | 0.893d |
| BMI z-score, median (range)c | 2.5 (1.7–3.1) | 2.5 (1.7–3.1) | 2.5 (1.8–3.1) | 0.912d |
| %IBW, median (range) | 164.7 (122.1–262.7) | 163.9 (122.1–226.8) | 166.3 (127.6–262.7) | 0.649d |
| Ages 10–11 years, n (%) | 68 (100%) | 38 (56%) | 30 (44%) | |
| Demographics | ||||
| Sex | ||||
| Male, n (%) | 37 (54%) | 18 (49%) | 19 (51%) | 0.189a |
| Female, n (%) | 32 (46%) | 20 (64%) | 11 (35%) | |
| Ethnicity | ||||
| Hispanic, n (%) | 54 (79%) | 26 (48%) | 28 (52%) | 0.012a |
| Non-Hispanic, n (%) | 14 (21%) | 12 (86%) | 2 (14%) | |
| Insurance | ||||
| Medicaid, n (%) | 58 (85%) | 31 (53%) | 27 (47%) | 0.330c |
| Private, n (%) | 10 (15%) | 7 (70%) | 3 (30%) | |
| Anthropometrics | ||||
| BMI, kg/m2, median (range) | 31.0 (23.6–63.3) | 30.2 (24.3–44.1) | 32.2 (23.6–63.3) | 0.786d |
| BMI percentile, median (range)c | 99.2 (95.3–99.9) | 99.1 (95.3–99.8) | 99.2 (96.4–99.9) | 0.693d |
| BMI z-score, median (range)c | 2.4 (1.7–3.2) | 2.4 (1.7–2.9) | 2.4 (1.8–3.2) | 0.693d |
| %IBW, median (range) | 170.2 (132.2–347.9) | 167.9 (132.2–246.4) | 177.0 (132.6–347.9) | 0.630d |
BMI: body mass index; %IBW: percent ideal body weight for height.
Chi square test.
Fisher’s exact test.
Interpreted using Centers for Disease Control and Prevention (CDC) 2000 Growth References.
Mann–Whitney U test.
Figure 1.
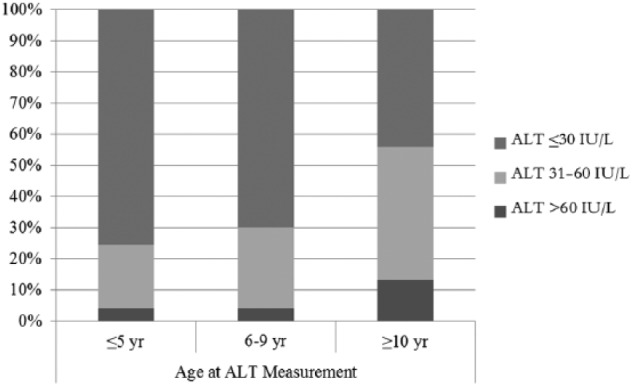
Alanine aminotransferase (ALT) values by age group.
BMI ≥ 99th percentile was present in 92% of preschool children, 73% of young school-age children, and 59% older school-age children (LLA, p < 0.001). Preschool children with elevated ALT more often had higher BMI value, BMI percentile, BMI z-score, and %IBW (Table 1) than those without elevated ALT. For school-age groups, anthropometric status did not significantly predict elevated ALT.
Overall, 48% of children with initial elevated ALT > 30 U/L had repeat ALT in the data acquisition interval (mean time between ALT measure-ments = 0.55 year; SD = 0.50 years). Elevated ALT was a persistent finding for 32/44 (73%) children, including 6/10 (60%) of preschool children, 14/19 (74%) of young school-age children, and 12/15 (80%) older school-age children. There were not any differences noted between age groups (data not shown).
For preschool children, measures of total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol, triglycerides (TG), and HOMA-IR did not significantly differ by ALT group status (data not shown). For children in the young school-age group, those with elevated ALT more frequently had low HDL (31/48 (78%) vs 54/94 (57%); p = 0.027). Other measures for young school-age children (total cholesterol, low-density lipoprotein cholesterol, TG, and HOMA-IR) and all of these measures (including HDL) for older school-age children did not differ by ALT group status (data not presented). Therefore, we next evaluated differences in frequency of abnormal lipid and HOMA-IR measures by age groups (Table 2). Preschool children less often had elevated HOMA-IR as compared to older children (Table 2); however, approximately half of the children in each age group did not have fasting glucose and insulin levels measured the same day as the ALT measurement. Frequency of abnormal lipid levels did not significantly differ by age group (Table 2). Notably, low levels of HDL and high levels of TG were common for all age groups.
Table 2.
Laboratory characteristics of obese children aged 2–11 years by age group.
| Total |
Aged 2–5 years |
Aged 6–9 years |
Aged 10–11 years |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 284 |
n = 73 |
n = 143 |
n = 68 |
||||||
| n | %a | n | %a | n | %a | n | %a | ||
| Total cholesterol | 0.259 | ||||||||
| ≥170 mg/dL | 109 | 26 | 22 | 34 | 56 | 43 | 31 | 48 | |
| <170 mg/dL | 151 | 74 | 43 | 66 | 74 | 57 | 34 | 52 | |
| Not evaluatedb | 24 | 8 | 13 | 3 | |||||
| Triglycerides | 0.108 | ||||||||
| ≥75 mg/dL (≤9 years) or ≥90 mg/dL (≥10 years) | 169 | 65 | 35 | 55 | 93 | 70 | 41 | 63 | |
| <75 mg/dL (≤9 years) or <90 mg/dL (≥10 years) | 93 | 35 | 29 | 45 | 40 | 30 | 24 | 37 | |
| Not evaluatedb | 22 | 9 | 10 | 3 | |||||
| LDL | 0.055 | ||||||||
| ≥110 mg/dL | 93 | 36 | 16 | 25 | 48 | 37 | 29 | 45 | |
| <110 mg/dL | 165 | 64 | 48 | 75 | 82 | 63 | 35 | 55 | |
| Not evaluatedb | 26 | 9 | 13 | 4 | |||||
| HDL | 0.160 | ||||||||
| ≤45 mg/dL | 173 | 66 | 40 | 62 | 85 | 63 | 48 | 76 | |
| >45 mg/dL | 88 | 34 | 24 | 38 | 49 | 37 | 15 | 24 | |
| Not evaluatedb | 23 | 9 | 9 | 5 | |||||
| HOMA-IR | 0.007 | ||||||||
| Elevated | 80 | 62 | 12 | 39 | 49 | 69 | 19 | 73 | |
| Normal | 48 | 37 | 19 | 61 | 22 | 31 | 7 | 27 | |
| Not evaluatedb | 156 | 42 | 72 | 42 | |||||
LDL: low-density lipoprotein cholesterol; HDL: high-density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment for insulin resistance.
Column percentage.
Not included in percentages.
Discussion
This study highlights the high frequency of elevated ALT in preschool children receiving obesity care. Ours is the first study to focus on preschool children. While the older school-age children we tested had the highest frequency of elevated ALT (44%), 26% of preschool children and 30% of young school-age children had elevated ALT. Importantly, we found that in preschool children, risk for elevated ALT was similar between Hispanic and non-Hispanic children. We did find that preschool children with ALT values > 30 IU/L had significantly higher BMI, BMI percentile, BMI z-score, and %IBW than preschool children with normal ALT. These results suggest that young children who are most obese may be at greater risk for liver abnormalities. However, since anthropometric values of preschool children with normal ALTs were also extremely high (median BMI percentile 99.9), it would be difficult to establish clinical distinctive features based on anthropometry.
The Expert Committee Guidelines recommend that screening for NAFLD begin at the age of 10 years; therefore, it is likely that many young obese children are not presently screened. Our results suggest that severely obese children should have their ALT values evaluated at least once as early as 2 years of age. While risk factors for elevated ALT have been identified,12,14 our data show that these are not sensitive indicators across age groups. Recommendations to limit ALT screening based on age, ethnicity, or BMI will miss the opportunity for identification of elevated ALT and subsequent counseling that may lead to important health behavior change. Since NAFLD-related fibrosis is related to longer disease duration and more severe degree of obesity,13 identifying children with elevated ALT provides an opportunity for clinicians to offer families treatment through counseling, behavioral change, and weight optimization. In the primary care setting, ALT screening of young obese children may also identify children who would benefit from weight management care; there is evidence that a multidisciplinary program can stabilize BMI and decrease elevated ALT values.30
Other studies have noted increasing frequency of elevated ALT by age31 and reported frequencies in general agreement with our findings for school-age children, although they used higher cutoff values to categorize abnormal ALT values.14,20,31 Similar to our findings, 29% of overweight or obese children aged 1–12 years presenting for obesity care had an ALT value above the upper limit of normal (ALT upper limit adjusted for age and test method), with higher ALT values among obese children.19 In our school-age sample, Hispanic children more frequently had an initial ALT value > 30 IU/L compared to children of other ethnic backgrounds. However, we did not find a sex disparity, as others have reported.14,31
Information from lipids, ALT, and measures of insulin sensitivity testing strengthens understanding of the metabolic risk of a patient. We find that such test results aid in health behavior counseling. In preschool children, elevated ALT was not significantly associated with any other metabolic parameter we evaluated. However, among young school-age children, we identified a significant association between low HDL and elevated ALT. This association has previously been reported in a sample of 143 obese children aged 3–18 years.25 Unlike others,25 in our sample, elevated ALT was not significantly associated with elevated TG. Several studies report an association of elevated ALT with the presence of metabolic syndrome and reduced insulin sensitivity.8,20,25 Data from our clinical sample were insufficient to identify those with metabolic syndrome and many lacked data to determine insulin sensitivity.
In the W&WM clinic, we use elevated ALT levels to reinforce the need for behavior changes. We first inform families that additional testing will be necessary to clarify the diagnosis. We discuss with parents the likely meaning and potential consequences of an elevated ALT in their obese young child and that the treatment for suspected NAFLD is attaining a healthy weight. In order to promote family-based healthy lifestyle changes, we discuss the high heritability of NAFLD.32 We also counsel about lowering sugar (and particularly fructose) intake as a potential strategy to impact liver health.33,34 Our further management of elevated ALT is guided by additional testing done 3–6 months after the first elevated ALT. We refer children to hepatology for ALT levels persistently elevated for 6–12 months if the child is not making progress in lowering their BMI.3 In our study, most children completing a repeat ALT measurement continued to have an elevated ALT at follow-up.
While degree of ALT elevation and ALT trajectory continue to be used to quantify obese patients needing further evaluations for NAFLD,15 hepatic steatosis has been noted across the spectrum of ALT values. Importantly, in a sample of 219 preschool children who had become overweight or obese within the prior 12 months, 31% had ultrasound diagnosis of NAFLD, with mean ALT of 28 U/L among the NAFLD group.35 Among children aged 5–18 years with suspected NAFLD enrolled in the Nonalcoholic Steatohepatitis Clinical Research Network, 4% (17/409) had ALT in the normal range (<26 U/L for boys; <23 U/L for girls) and 18% (74/409) had ALT in the high normal range (26–50 U/L boys, 23–44 U/L girls).36 Additional work is needed to understand the sensitivity and specificity of strategies to identify and monitor NAFLD in young obese children and to assess the impact of such information on family ability to implement healthy lifestyle changes.
A limitation of this study is that many of the children only had ALT measured once. While repeatedly abnormal ALT values increase suspicion for chronic liver injury, some children with fatty liver will have normal ALT or ALT that fluctuates between normal and elevated.36–38 ALT elevation may accompany viral infections or dehydration; however, these children were having laboratory testing done for an obesity evaluation not because of infectious conditions. This study population was drawn from a tertiary obesity clinic and may not be generalizable to the general obese pediatric population. Further evaluations in children at the 95th–98th BMI percentile are needed. Children with already-established laboratory abnormalities may be more likely to be seen in this clinic; therefore, there may be a referral bias. ALT values were drawn as part of an obesity comorbidity screening strategy, and investigation into the exact etiology of ALT elevation was beyond the scope of such a screening strategy; in particular, data evaluated did not include exposures to hepatotoxic drugs. Additionally, data were gathered retrospectively, so bias may exist related to which patients completed testing. However, even given these limitations, the age distribution and frequency of ALT elevations among very young very obese children is notable. Further work is needed to identify cost-effective additional testing needed to identify NAFLD in this high risk population and to assess whether the obesity comorbidity identification leads to family-based adoption of healthier behaviors and subsequent improvement of the child’s obesity status.
Conclusion
ALT elevations were relatively common in this population of obese young children, including one-fourth of preschool children. Given this relatively large percentage of young children with elevated ALT values, initiation of ALT screening in severely obese children should begin at 2 years of age. For school-age children, Hispanic ethnicity was associated with elevated ALT, but many children with elevated ALT would have been missed if screening was limited to those of Hispanic ethnicity.
Acknowledgments
The authors would like to thank George C. Lales, MS, of the Stanley Manne Children’s Research Institute for assistance with data extraction from the electronic medical record, and Joshua S. Rosen for his help in chart review and construction of the original database. These data are protected by institutional review board (IRB) regulations. Individuals would need to contact the author for further information.
Dr Helen J Binns had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Daniel R Beacher, Adolfo J Ariza, Helen J Binns
Acquisition of data: Daniel R Beacher, Helen J Binns
Statistical analysis: Daniel R Beacher, Helen J Binns
Analysis and interpretation of data: Daniel R Beacher, Adolfo J Ariza, Helen J Binns, Mark H Fishbein
Drafting of the manuscript: Daniel R Beacher
Critical revision of the manuscript for important intellectual content: Daniel R Beacher, Adolfo J Ariza, Helen J Binns, Mark H Fishbein
Study supervision: Helen J Binns
Footnotes
Declaration of conflicting interests: None of the authors have any conflicts to disclose.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005; 42: 641–649. [DOI] [PubMed] [Google Scholar]
- 2. Patton HM, Sirlin C, Behling C, et al. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr 2006; 43: 413–427. [DOI] [PubMed] [Google Scholar]
- 3. Lerret SM, Skelton JA. Pediatric nonalcoholic fatty liver disease. Gastroenterol Nurs 2008; 31: 115–119. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- 5. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013; 178: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012; 307: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wicklow BA, Wittmeier KD, MacIntosh AC, et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care 2012; 35: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manton ND, Lipsett J, Moore DJ, et al. Non-alcoholic steatohepatitis in children and adolescents. Med J Aust 2000; 173: 476–479. [DOI] [PubMed] [Google Scholar]
- 10. Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr 2000; 30: 48–53. [DOI] [PubMed] [Google Scholar]
- 11. August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008; 93: 4576–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barlow SE; Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007; 120: S164–S192. [DOI] [PubMed] [Google Scholar]
- 13. Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012; 54: 700–713. [DOI] [PubMed] [Google Scholar]
- 14. Leung DH, Williams K, Fraley JK, et al. Age- and ethnic-specific elevation of ALT among obese children at risk for nonalcoholic steatohepatitis (NASH): implications for screening. Clin Pediatr 2009; 48: 50–57. [DOI] [PubMed] [Google Scholar]
- 15. Estrada E, Eneli I, Hampl S, et al. Children’s Hospital Association consensus statements for comorbidities of childhood obesity. Child Obes 2014; 10: 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010; 138: 1357–1364.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr 2013; 162: 496–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poustchi H, George J, Esmaili S, et al. Gender differences in healthy ranges for serum alanine aminotransferase levels in adolescence. PLoS One 2011; 6: e21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engelmann G, Hoffmann GF, Grulich-Henn J, et al. Alanine aminotransferase elevation in obese infants and children: a marker of early onset non alcoholic fatty liver disease. Hepat Mon 2014; 14: e14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calcaterra V, Muratori T, Klersy C, et al. Early-onset metabolic syndrome in prepubertal obese children and the possible role of alanine aminotransferase as marker of metabolic syndrome. Ann Nutr Metab 2011; 58: 307–314. [DOI] [PubMed] [Google Scholar]
- 21. Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008; 48: 119–128. [DOI] [PubMed] [Google Scholar]
- 22. Koot BGP, van der Baan-Slootweg OH, Tamminga-Smeulders CLJ, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child 2011; 96: 669–674. [DOI] [PubMed] [Google Scholar]
- 23. Rhee KE, De Lago CW, Arscott-Mills T, et al. Factors associated with parental readiness to make changes for overweight children. Pediatrics 2005; 116: e94–e101. [DOI] [PubMed] [Google Scholar]
- 24. Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr 2000; 136: 727–733. [PubMed] [Google Scholar]
- 25. Vliet M, Rosenstiel I, Schindhelm R, et al. The association of elevated alanine aminotransferase and the metabolic syndrome in an overweight and obese pediatric population of multi-ethnic origin. Eur J Pediatr 2009; 168: 585–591. [DOI] [PubMed] [Google Scholar]
- 26. Expert Panel on Integrated Guidelines for Cardiovascular Health Risk Reduction in Children and Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128: S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 28. Kurtoglu S, Hatipoglu N, Mazicioglu M, et al. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol 2010; 2: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLaren D, Read W. Classification of nutritional status in early childhood. Lancet 1972; 2: 146–148. [DOI] [PubMed] [Google Scholar]
- 30. DeVore S, Kohli R, Lake K, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. J Pediatr Gastroenterol Nutr 2013; 57: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiegand S, Keller KM, Robl M, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes 2010; 34: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 32. Schwimmer JB, Celedon MA, Lavine JE, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 2009; 136: 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012; 96: 727–734. [DOI] [PubMed] [Google Scholar]
- 34. Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010; 51: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shashaj B, Bedogni G, Graziani MP, et al. Origin of cardiovascular risk in overweight preschool children: a cohort study of cardiometabolic risk factors at the onset of obesity. JAMA Pediatr. Epub ahead of print 11 August 2014. DOI: 10.1001/jamapediatrics.2014.900. [DOI] [PubMed] [Google Scholar]
- 36. Molleston JP, Schwimmer JB, Yates KP, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr 2014; 164: 707–713.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fishbein MH, Miner M, Mogren C, et al. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr 2003; 36: 54–61. [DOI] [PubMed] [Google Scholar]
- 38. Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: a pediatric report. Hepatology 2008; 48: 2087–2088. [DOI] [PubMed] [Google Scholar]


