Abstract
Objective:
According to the guidelines for metastatic breast cancer, hormone therapy for hormone receptor–positive metastatic breast cancer without life-threatening metastasis should be received prior to chemotherapy. Previous trials have investigated the sensitivity of chemotherapy for preoperative breast cancer based on the efficacy of neoadjuvant hormone therapy. In this retrospective study, we investigated the efficacy of chemotherapy for metastatic breast cancer in hormone therapy–effective and hormone therapy–ineffective cases.
Methods:
Patients who received chemotherapy after hormone therapy for metastatic breast cancer between 2006 and 2013 at our institution were investigated.
Results:
A total of 32 patients received chemotherapy after hormone therapy for metastatic breast cancer. The median patient age was 59 years, and most of the primary tumors exhibited a T2 status. A total of 26 patients had an N(+) status, while 7 patients had human epidermal growth factor receptor 2–positive tumors. A total of 13 patients received clinical benefits from hormone therapy, with a rate of clinical benefit of subsequent chemotherapy of 30.8%, which was not significantly different from that observed in the hormone therapy–ineffective patients (52.6%). A total of 13 patients were able to continue the hormone therapy for more than 1 year, with a rate of clinical benefit of chemotherapy of 38.5%, which was not significantly different from that observed in the short-term hormone therapy patients (47.4%). The luminal A patients were able to continue hormone therapy for a significantly longer period than the non-luminal A patients (median survival time: 17.8 months vs 6.35 months, p = 0.0085). However, there were no significant differences in the response to or duration of chemotherapy.
Conclusion:
The efficacy of chemotherapy for metastatic breast cancer cannot be predicted based on the efficacy of prior hormone therapy or tumor subtype, and clinicians should administer chemotherapy in all cases of hormone receptor–positive metastatic breast cancer, if needed.
Keywords: Secondary breast neoplasms, drug therapy, hormone
Introduction
According to the results of trials investigating the efficacy of chemotherapy for breast cancer, hormone receptor (HR)-positive breast cancer is less sensitive to chemotherapy than HR-negative lesions.1,2 However, some patients with HR-positive breast cancer are thought to benefit from adjuvant chemotherapy as well as hormone therapy (HT).3 Moreover, patients with HR-positive metastatic breast cancer (MBC) often require chemotherapy due to disease progression after HT. Therefore, research regarding methods of predicting the sensitivity of chemotherapy for HR-positive breast cancer is important. Previous studies have investigated the efficacy of chemotherapy for preoperative breast cancer based on the efficacy of precedent neoadjuvant HT.4,5 These trials were conducted based on the hypothesis that chemotherapy can be effective if precedent HT is ineffective or may not be necessary if precedent HT is very effective.
According to the guidelines for MBC, such as the National Comprehensive Cancer Network (NCCN) guidelines or Hortobagyi’s algorithm, HT should be introduced in cases of HR-positive MBC prior to chemotherapy if the metastatic tumor is not life-threatening,6,7 with subsequent chemotherapy if the HT regimen is ineffective. If the sensitivity to chemotherapy could be predicted based on the efficacy of prior HT, and there are no differences in tumor biology between MBC and preoperative breast cancer, the sensitivity to chemotherapy among patients with MBC may be predicted based on the efficacy of the prior HT regimen.
In order to assess this hypothesis, we retrospectively investigated cases of HR-positive MBC in patients who received chemotherapy after HT and explored the efficacy of chemotherapy according to the efficacy of the prior HT regimen.
Patients and methods
The records of breast cancer patients who received chemotherapy after HT for MBC at the GifuPrefectural GeneralMedicalCenter between 2006 and 2013 were reviewed. The therapy in each case was investigated, and the efficacy of HT and chemotherapy was evaluated from the viewpoint of the objective response and time to treatment failure (TTF). If the patients received multi-line HT, the therapy was considered to be “effective” when one or more HTs resulted in tumor shrinkage or stable disease (SD) or when the total duration of HT was longer than 1 year.
The objective response to treatment was categorized into four groups: a complete response (CR), partial response (PR), SD, and progressive disease (PD). A CR indicates that the target lesion clinically disappeared, a PR indicates that the target lesion clinically reduced in size after treatment, SD indicates that the size of the target lesion appeared not to change, and SD lasting for more than 6 months was defined as “long SD.” Meanwhile, PD indicates that the target lesion increased in size, while a clinical benefit (CB) was defined as CR + PR + long SD. This categorization was determined based on the Response Evaluation Criteria In Solid Tumors (RECIST) criteria, although these criteria were not rigorously applied in this study.
In this study, the patients were divided into three groups according to the breast cancer subtype: luminal A (defined as HR-positive, human epithelial growth factor receptor 2 (HER2)-negative, and low or intermediate nuclear grade), luminal B (defined as HR-positive, HER2-negative, and high nuclear grade), and luminal HER2 (defined as HR-positive and/or HER2-positive with any nuclear grade). Because the levels of Ki67 were not investigated in most cases, the luminal A and luminal B patients were divided according to the “nuclear grade.” The nuclear grade refers to the tumor grading system used in Japan, which consists of a nuclear atypia score and mitotic count score. Nuclear atypia is given a score of 1 to 3 (score 1: regular uniform cells, score 2: moderate nuclear size and variation, and score 3: marked nuclear variation). The mitotic count is also given a score of 1 to 3 (score 1: 0–5 mitoses/10 high-power field (hpf), score 2: 5–10 mitoses/10 hpf, score 3: ≥10 mitoses/10 hpf). The nuclear grade is then given one of three grades based on the sum of these two items (low grade: 2–3, intermediate grade: 4, high grade: 5–6).
The CB rates were compared using the chi-square test. The TTF was analyzed using the Kaplan–Meier method, and the results were compared by the log-rank test. This study was approved by the ethical committee of Gifu Prefectural General MedicalCenter.
Results
Patient characteristics
A total of 32 patients received chemotherapy after HT for MBC. The median age of these patients was 59 years. Most of the primary tumors exhibited a size of 2–5 cm (T2). A total of 26 patients had an N(+) status, while 7 patients had HER2-positive tumors. The metastatic sites included the bones (13 patients), lungs (11 patients), liver (7 patients), lymph nodes (12 patients), and other organs (5 patients). The distribution of tumor subtype was 37.5% (12/32), 37.5% (12/32), and 21.9% (7/32) for luminal A, luminal B, and luminal HER2, respectively. The subtype was unknown in one case because the HER2 status was unknown (luminal B or luminal HER2). The details are shown in Table 1.
Table 1.
The patients’ characteristics.
| No | |
|---|---|
| Age (years) | |
| <49 | 10 |
| 50–59 | 5 |
| ≥60 | 17 |
| Menopausal status | |
| Premenopausal | 9 |
| Postmenopausal | 23 |
| T factor | |
| T1 | 1 |
| T2 | 18 |
| T3 | 5 |
| T4 | 6 |
| Unknown | 2 |
| Nodal status | |
| N(–) | 3 |
| N(+) | 26 |
| Unknown | 3 |
| Nuclear grade | |
| Low | 3 |
| Intermediate | 11 |
| High | 18 |
| HER2 status | |
| (–) | 24 |
| (+) | 7 |
| Unknown | 1 |
| Hormone receptor status | |
| ER(+)/PgR(+) | 25 |
| ER(+)/PgR(–) | 7 |
| ER(–)/PgR(+) | 0 |
| Metastatic sites | |
| Bone | 13 |
| Lung | 11 |
| Liver | 7 |
| Lymph node | 12 |
| Other | 5 |
| Subtypes | |
| Luminal A | 12 |
| Luminal B | 12 |
| Luminal HER2 | 7 |
| Unknown | 1 |
HER2: human epithelial growth factor receptor 2; ER: estrogen receptor; PgR: progesterone receptor.
Selection and efficacy of HT and selection of subsequent chemotherapy
The patients received 1.97 lines of HT after recurrence on average. A total of 24 patients received non-steroidal aromatase inhibitors (AIs), 17 patients received selective estrogen receptor modulators (SERMs), and 13 patients received steroidal AIs. The details are shown in Table 2.
Table 2.
The selection of hormone therapy and subsequent chemotherapy.
| No | |
|---|---|
| Prior hormonal therapy | |
| Exemestane | 13 |
| Anastrozole | 10 |
| Letrozole | 14 |
| High-dose toremifene | 14 |
| Tamoxifen | 3 |
| Fulvestrant | 9 |
| LHRH analogue | 12 |
| Subsequent chemotherapy | |
| Anthracycline | 2 |
| Taxane | 5 |
| Oral fluoropyrimidine | 20 |
| Vinorelbine | 2 |
| Trastuzumab | 3 |
LHRH: luteinizing-hormone-releasing hormone.
After HT, as the first-line chemotherapy, 20 patients received oral 5FU, 5 patients received taxanes, and 2 patients received anthracyclines. Patients with a HER2-positive status also received trastuzumab. The details are shown in Table 2.
The HTs for MBC produced 1 case of CR, 7 cases of PR, 5 cases of long SD, 4 cases of SD, and 15 cases of PD for all types of HT (Figure 1(a)), with a CB rate of 40.6% (13/32) (Figure 1(b)). The median survival time (MST) according to the Kaplan–Meier curve of the duration for all HT regimens was 9.1 months (Figure 1(c)).
Figure 1.
Selection and efficacy of HT and CT: (a) tumor response for all HT regimens, (b) clinical benefit rate of HT, (c) time to HT failure, (d) tumor response to the first-line CT after HT, (e) tumor response for all CT regimens, (f) clinical benefit rates for the first-line CT and all CT regimens, and (g) Kaplan–Meier curve of the time to CT failure.
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; MST: median survival time; CT: chemotherapy; HT: hormone therapy.
The first-line chemotherapy regimens administered after HT produced 1 case of CR, 17 cases of PR, 1 case of long SD, 5 cases of SD, and 11 cases of PD (Figure 1(d)), with a CB of 43.8% (14/32) (Figure 1(f)). We also investigated the best response to chemotherapy for all-line therapies after HT. The best responses included 1 case of CR, 17 cases of PR, 4 cases of long SD, 3 cases of SD, and 5 cases of PD (Figure 1(e)). The CB in this setting was therefore 68.8% (22/32) (Figure 1(f)). Furthermore, the MST of the duration for all chemotherapy regimens was 13.6 months (Figure 1(g)).
Efficacy of chemotherapy according to the response to prior HT
We analyzed the efficacy of the subsequent chemotherapy when HT was considered to be effective, if the HT regimen produced a CB. The number of HT-effective and HT-ineffective cases was 13 and 19, respectively. The CB was 30.8% (4 cases of PR, 3 cases of SD, and 5 cases of PD) among the HT-effective cases (Figure 2(a) and (c)), while the CB was 52.6% (1 case of CR, 8 cases of PR, 1 case of long SD, 2 cases of SD, and 6 cases of PD) among the HT-ineffective cases (Figure 2(b) and (c)); this difference was not significant (p = 0.22). For all chemotherapy regimens, the CB was 69.2% (8 cases of PR, 1 case of long SD, 1 case of SD, and 2 cases of PD) among the HT-effective cases (Figure 2(d) and (f)) and 68.4% (1 case of CR, 9 cases of PR, 3 cases of long SD, 2 cases of SD, and 3 cases of PD) among the HT-ineffective cases (Figure 2(e) and (f)); these differences were also not significant (p = 0.96). The MST of the duration for all chemotherapy regimens among the HT-effective and HT-ineffective cases was 18.3 and 5.0 months, respectively, which was not significantly different (Figure 2(g)).
Figure 2.
Efficacy of CT after HT according to the tumor response to prior HT. The tumor response to the first-line CT regimen in the patients (a) exhibiting a clinical benefit from HT (HT-effective) and (b) patients exhibiting no clinical benefits from HT (HT-ineffective). (c) Clinical benefit rate of the first-line CT. Tumor response for all CT regimens in the (d) HT-effective cases and (e) HT-ineffective cases. (f) Clinical benefit rate for all CT regimens. (g) Kaplan–Meier curve of the time to CT failure in the HT-effective and HT-ineffective cases.
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; MST: median survival time; CT: chemotherapy; HT: hormonetherapy.
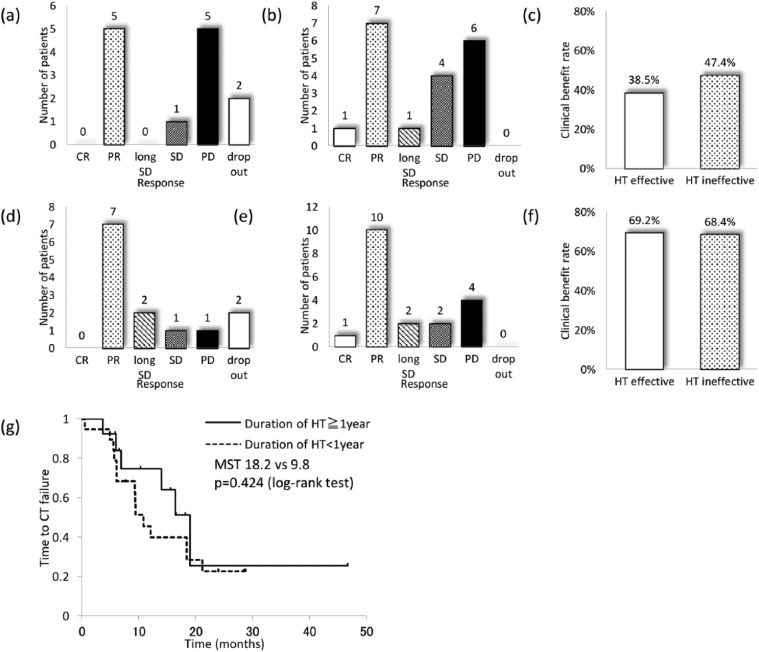
Efficacy of chemotherapy according to the duration of prior HT
Second, we analyzed the efficacy of the subsequent chemotherapy when HT was considered to be effective, if the duration of all HT regimens was longer than 1 year. The number of patients who received HT for more and less than 1 year was 13 and 19, respectively. The CB was 38.5% (5 cases of PR, 1 case of SD, and 5 cases of PD) in the HT-effective cases (Figure 3(a) and (c)) and 47.4% (1 case of CR, 7 cases of PR, 1 case of long SD, 4 cases of SD, and 6 cases of PD) in the HT-ineffective cases (Figure 3(b) and (c)); these differences were not significant (p = 0.62). For all chemotherapy regimens, the CB was 69.2% (7 cases of PR, 2 cases of long SD, 1 case of SD, and 1 case of PD) among the HT-effective cases (Figure 3(d) and (f)) and 68.4% (1 case of CR, 10 cases of PR, 2 cases of long SD, 2 cases of SD, and 4 cases of PD) among the HT-ineffective cases (Figure 3(e) and (f)); these differences were also not significant (p = 0.96). The MST of the duration for all chemotherapy regimens in the HT-effective and HT-ineffective cases was 18.2 and 9.8 months, respectively, which was not significantly different (Figure 3(g)).
Figure 3.
Efficacy of CT after HT according to the duration of prior HT. The tumor response to the first-line CT regimens in the patients whose (a) duration of HT was more than 1 year (HT-effective) and (b) less than 1 year (HT-ineffective). (c) Clinical benefit rate of the first-line CT. The tumor response for all CT regimens among the (d) HT-effective cases and (e) HT-ineffective cases. (f) Clinical benefit rate for all CT regimens. (g) Kaplan–Meier curve of the time to CT failure.
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; MST: median survival time; CT: chemotherapy; HT: hormone therapy.
Efficacy of chemotherapy according to the subtype of primary tumor
In this study, we divided the patients according to the tumor subtype based on the HER2 status and nuclear grade of the primary tumor. We defined luminal A as HER2(–) with a low or intermediate nuclear grade and evaluated the efficacy of HT and subsequent chemotherapy in the luminal A and non-luminal A cases.
The CB of HT was higher in the luminal A cases than in the non-luminal A cases (luminal A: 54.5%, non-luminal A: 35.5%). However, this difference was not significant (p = 0.29) (Figure 4(a)). Meanwhile, the duration of all HT regimens among the luminal A cases was significantly longer than that observed in the non-luminal A cases (MST 17.8 months vs 6.35 months, Figure 4(b)), suggesting that the tumor subtype adopted in this study could be used to predict the duration of HT.
Figure 4.
Efficacy of chemotherapy after HT according to the tumor subtype. (a) Clinical benefit rate of prior HT. (b) Kaplan–Meier curve of the time to HT failure. The tumor response to the first-line chemotherapy regimen in the (c) luminal A cases and (d) non-luminal A cases. (e) Clinical benefit rate of the first-line chemotherapy regimen. The tumor response for all chemotherapy regimens in the (f) luminal A cases and (g) non-luminal A cases. (h) Clinical benefit rate for all chemotherapy regimens. (i) Kaplan–Meier curve of the time to chemotherapy failure.
MST: median survival time; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; HT: hormone therapy.
The CB of the first chemotherapy after HT in the luminal A cases and non-luminal A cases was 33.3% (4/12) and 50.0% (10/20), respectively, which was not significantly different (p = 0.36) (Figure 4(c)–(e)).
The CB for all chemotherapy regimens after HT among the luminal A cases and non-luminal A cases was 58.3% (7/12) and 75% (15/20), respectively (p = 0.32) (Figure 4(f)–(h)), while the MST of the duration for all chemotherapy regimens among the luminal A cases and non-luminal A cases was 16.1 and 11.0 months, respectively (Figure 4(i)); these differences were also not significant.
Discussion
We evaluated the efficacy of chemotherapy after HT for MBC and found no differences between the HT-effective and HT-ineffective cases. The tumor subtype of luminal A was found to be related to the success of a longer duration of HT; however, the efficacy of subsequent chemotherapy did not differ between the luminal A and non-luminal A cases.
In the adjuvant setting, many previous trials have documented the superiority of novel chemotherapy regimens over conventional chemotherapy regimens; however, several trials have failed to prove such superiority in HR-positive cases, such as the CALGB 9344 and PACS01 trials.8,9 Moreover, later analyses of these adjuvant chemotherapy trials have revealed that HR-positive patients, particularly those with a luminal A status, are less responsive to therapy than patients with other subtypes.1,2 Many breast cancer specialists explain the ineffectiveness of chemotherapy for HR-positive breast cancer based on these results. However, some HR-positive breast cancer patients are considered to require adjuvant chemotherapy as well as adjuvant HT, and various tools to predict the need for adjuvant chemotherapy have been introduced. For example, the Oncotype DX is a tool that can help to predict the benefits of adjuvant chemotherapy based on the expression levels of 21 genes in breast cancer tissue whose usefulness has been accepted by many physicians.10 The St Gallen Consensus Conference recommends evaluating the tumor subtype categorized according to immunohistochemistry for estrogen receptor (ER), progesterone receptor (PgR), HER2, and Ki67 in order to determine the need for adjuvant therapy,11 with adjuvant chemotherapy being recommended in HR-positive and HER2-negative cases if the patient has a high Ki67 index. However, these methods are applicable to early breast cancer, and their use in cases of MBC has not been fully established, although clinicians often apply these methods in patients with MBC in daily clinical practice.
The Z1031B trial was conducted in order to identify AI-resistant cases after neoadjuvant HT based on the Ki67 index. In that trial, 51 patients were judged to have exhibited an ineffective response to HT according to the Ki67 index at rebiopsy after 2–4 weeks of HT, 36 of whom received chemotherapy after HT. The pathological complete response (pCR) rate among these cases was 5.5%, which is similar to that reported in other trials targeting HR-positive breast cancer.4
In Japan, the CSPOR N-SAS BC06 trial is currently ongoing to investigate the efficacy of adjuvant chemotherapy after surgery in cases of effective preoperative HT judged based on the therapeutic response; the results of this trial are anticipated. However, considering the findings of the Z1031B trial, clinicians are unlikely to select chemotherapy-effective cases based on the sensitivity to prior HT, even if patients who do not require chemotherapy can be identified.5
Even if HR-positive MBC is not as sensitive to chemotherapy as HR-negative lesions, the administration of chemotherapy for MBC is inevitable. Based on the present results, the use of chemotherapy after HT is effective to a certain extent, regardless of whether HR-positive MBC is sensitive to prior HT. Moreover, the tumor subtype is available to predict the efficacy of HT only, not chemotherapy.
Unfortunately, the sample size of our study was small, and we could not draw any definitive conclusions due to the small number of subjects evaluated. However, there have been no previous reports describing the relationship between the efficacy of prior hormonal therapy and the efficacy of subsequent chemotherapy for MBC, which therefore motivated us to investigate this issue. Further investigations are required to confirm the present findings.
In conclusion, the efficacy of chemotherapy cannot be predicted based on the efficacy of prior HT or the tumor subtype. Therefore, clinicians should administer chemotherapy in cases of HR-positive MBC, if needed.
Footnotes
Declaration of conflicting interests: The authors have no conflicts of interest to disclose.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 2009; 27: 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 2006; 295: 1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012; 379: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellis MJ, Suman V, McCall L, et al. Z1031B Neoadjuvant Aromatase Inhibitor Trial: a Phase 2 study of Triage to Chemotherapy based on 2 to 4 week Ki67 level >10%. Cancer Res 2012; 72: Abstract nr PD07-01. [Google Scholar]
- 5. Iwata H. Neoadjuvant endocrine therapy for postmenopausal patients with hormone receptor-positive early breast cancer: a new concept. Breast Cancer 2011; 18: 92–97. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network. Clinical practice guidelines in oncology, breast cancer. Version 3, 2013, http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PubMed]
- 7. Hortobagyi GN. Treatment of breast cancer. N Engl J Med 1998; 339: 974–984. [DOI] [PubMed] [Google Scholar]
- 8. Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003; 21: 976–983. [DOI] [PubMed] [Google Scholar]
- 9. Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol 2006; 24: 5664–5671. [DOI] [PubMed] [Google Scholar]
- 10. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24: 3726–3734. [DOI] [PubMed] [Google Scholar]
- 11. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]