Abstract
Objectives
Shiga toxin–producing Escherichia coli (STEC) are an important cause of foodborne disease, yet global estimates of disease burden do not exist. Our objective was to estimate the global annual number of illnesses due to pathogenic STEC, and resultant hemolytic uremic syndrome (HUS), end-stage renal disease (ESRD), and death.
Materials
We searched Medline, Scopus, SIGLE/OpenGrey, and CABI and World Health Organization (WHO) databases for studies of STEC incidence in the general population, published between January 1, 1990 and April 30, 2012, in all languages. We searched health institution websites for notifiable disease data and reports, cross-referenced citations, and consulted international knowledge experts. We employed an a priori hierarchical study selection process and synthesized results using a stochastic simulation model to account for uncertainty inherent in the data.
Results
We identified 16 articles and databases from 21 countries, from 10 of the 14 WHO Sub-Regions. We estimated that STEC causes 2,801,000 acute illnesses annually (95% Credible Interval [Cr.I.]: 1,710,000; 5,227,000), and leads to 3890 cases of HUS (95% Cr.I.: 2400; 6700), 270 cases of ESRD (95% Cr.I.: 20; 800), and 230 deaths (95% Cr.I.: 130; 420). Sensitivity analyses indicated these estimates are likely conservative.
Conclusions
These are the first estimates of the global incidence of STEC-related illnesses, which have not been explicitly included in previous global burden of disease estimations. Compared to other pathogens with a foodborne transmission component, STEC appears to cause more cases than alveolar echinococcosis each year, but less than typhoid fever, foodborne trematodes, and nontyphoidal salmonellosis.
Applications
Given the persistence of STEC globally, efforts aimed at reducing the burden of foodborne disease should consider the relative contribution of STEC in the target population.
Introduction
Foodborne infections are a global public health issue (Stein et al., 2007; Majowicz et al., 2010; Torgerson et al., 2010; Fürst et al., 2012). Shiga toxin–producing Escherichia coli (STEC), including O157 and many non-O157 serotypes, are an important cause of foodborne disease. Outcomes range from mild intestinal discomfort, to hemolytic uremic syndrome (HUS), end-stage renal disease (ESRD), and death (Heyman, 2008; Gould et al., 2009). Although the reported incidence is often low, large outbreaks with serious consequences occur (Buchholz et al., 2011). While some countries have estimated the population health impacts of STEC (De Wit et al., 2001; Thomas et al., 2006; Scallan et al., 2011; Tam et al., 2012), there are no global estimates (Murray et al., 2012; Lozano et al., 2012). Our objectives were to estimate the global annual number of STEC infections and resulting cases of HUS, ESRD, and deaths, and the age distribution of cases to inform future disability-adjusted life year (DALY) calculations.
Materials and Methods
Search strategy
We searched peer-reviewed literature, gray literature, and publicly available notifiable disease data (i.e., nationally reported, laboratory-confirmed STEC infections) for information pertaining to STEC incidence in the general population. Due to temporal and geographic variation in terminology, we searched for any of “STEC,” “VTEC,” “EHEC,” or “E. coli O157.” We searched Medline, Scopus, SIGLE/OpenGrey, CABI databases (CAB Abstracts, VetMed Resource, Global Health, Animal Health and Production Compendium, Leisure, Recreation and Tourism, and Rural Development), and World Health Organization (WHO) regional databases for studies published between January 1, 1990 and April 30, 2012, in all languages. Database-specific search strings consisted of key words and Medical Subject Headings (search strings available on request).
We searched regional and national health institution websites for notifiable disease data and reports containing STEC-specific information. Additional studies, particularly non-English and prepublication sources, were identified via cross-referencing citations and consulting with experts from the WHO’s Foodborne Diseases Epidemiology Reference Group (FERG), the WHO’s Global Foodborne Infections Network, and the International Collaboration on Enteric Disease “Burden of Illness” Studies (Flint et al., 2005). We followed the PRISMA guidelines for systematic reviews (http://www.prisma-statement.org; 27-item checklist).
Study selection
Search results were combined, and duplicates eliminated, in a RefWorks database (2010, ProQuest, LLC). Two reviewers conducted the first relevance screen. Given high reviewer agreement (n = 1000; κ = 0.905 [95% confidence interval 0.867, 0.941]), articles were screened by one reviewer per reference, by reviewing titles, and abstracts if available. Articles were excluded if they pertained solely to laboratory methods for STEC isolation or diagnosis; microbiological characterization of STEC; the management or treatment of STEC infection or sequelae; or nonhuman populations. Non-English articles were screened using Google Translate (http://translate.google.com/).
Remaining articles were screened against the exclusion criteria, and the inclusion criteria, specifically: the study included all ages; results pertained to the general population; and either the article provided the incidence or prevalence of acute STEC illness, or the number of cases and both the relevant time period and source population were given or derivable. We required that STEC be identified via laboratory confirmation or epidemiological link to a laboratory-confirmed case. Investigations were included regardless of laboratory method, including the following: isolation of nonsorbitol fermenting E. coli O157; isolation of non-O157 E. coli carrying stx genes or producing Shiga toxin; detection of stx genes in clinical stool by polymerase chain reaction or other molecular methods; and detection of Shiga toxin in clinical stool by enzyme-linked immunosorbent assay or cell cytotoxicity assay. Urinary and asymptomatic infections were excluded. Inclusion criteria for notifiable disease data were as follows: data arose from a population-level, laboratory-based, routine notifiable disease system; and case ascertainment was done via laboratory confirmation, or epidemiological link to a laboratory-confirmed case. This and subsequent review steps were conducted on full texts, independently by two reviewers per reference, with differences resolved by a third reviewer, and with non-English articles single-reviewed by trained, fluent reviewers.
In the final characterization stage, we classified all remaining studies by design, and selected the highest quality design per WHO Sub-Region (Table 1) for extraction and inclusion in the analysis. This method was determined a priori, given the following hierarchical preference for different study designs. Prospective cohort studies, which follow a defined population over time and measure incidence via laboratory confirmation, were considered the criterion standard. Because these studies are expensive, many countries rely on data obtained from national, laboratory-based surveillance systems. However, such data under-report the true population incidence, since many cases do not seek medical care or undergo laboratory testing. To address this, some countries have calculated corrected incidence estimates, which adjust notifiable disease data for under-reporting. These “multiplier studies” were considered the next highest quality study design, followed by notifiable disease data (which we corrected for under-reporting using available information).
Table 1.
Data on Population-Level Incidence of Shiga Toxin–Producing Escherichia coli (STEC) Infection, and the Assumed Proportion of Cases That Are O157 (Versus Non-O157), Circa 2012, by World Health Organization (WHO) Sub-region
| WHO Sub-regiona | 2005 WHO Sub-region Population | Existing sources of incidence data
|
Assumed proportion STEC O157 (versus non-O157) | |||
|---|---|---|---|---|---|---|
| Type of datab | Source countries | Parameter values used to estimate the incidence per 100,000 person-years
|
||||
| Most likely value | Min.; max. values | |||||
| AFR D | 353,412,879 | Extrapolation | AFR E | 0.6 | 0.06; 6.0 | 0.10 |
| AFR E | 402,012,097 | Notification | South Africa (National Institute for Communicable Diseases, 2010) | 0.6 | 0.06; 6.0 | 0.10 |
| AMR A | 343,376,860 | Multiplier | Canada (Thomas et al., 2006); United States (Scallan et al., 2011) | 89 | 85; 120 | 0.36 |
| AMR B | 464,046,150 | Notification | Chile (Institute of Public Health, 2012) | 12 | 1.2; 116 | 0.36 |
| AMR D | 76,985,668 | Extrapolation | AMR A | 89 | 85; 120 | 0.10 |
| EMR B | 148,336,863 | Prospective | Iran (Aslani et al., 1998; Aslani and Bouzari, 2003) | 136 | 122; 249 | 0 |
| EMR D | 381,484,794 | Extrapolation | EMR B | 136 | 122; 249 | 0 |
| EUR A | 423,910,793 | Prospective | The Netherlands (De Wit et al., 2001); the United Kingdom (Tam et al., 2012) | 42 | 30; 86 | 0.36 |
| EUR B | 224,521,283 | Notification | Poland, Romania, Slovakia (European Centre for Disease Control and Prevention, 2011); Serbia (Lazic et al., 2006) | 1.6 | 0; 168 | 0.36 |
| EUR C | 236,910,762 | Notification | Estonia, Hungary, Latvia, Lithuania (European Centre for Disease Control and Prevention, 2011) | 1.1 | 0; 11 | 0.36 |
| SEAR B | 308,186,718 | Extrapolation | SEAR D | 30 | 0.01; 278 | 0.10 |
| SEAR D | 1,388,360,385 | Notification | Bangladesh (Islam et al., 2007); India (Sehgal et al., 2008) | 30 | 0.01; 278 | 0.10 |
| WPR A | 157,005,359 | Multiplier | Australia (Hall et al., 2008); New Zealand (Cressey and Lake, 2011) | 36 | 23; 101 | 0.36 |
| WPR B | 1,593,805,917 | Notification | Hong Kong (Centre for Health Protection, 2011); Republic of Korea (Korea Centres for Disease Control and Prevention, 2011) | 3.9 | 1.5; 4.2 | 0.10 |
| Global total | 6,502,356,528 | |||||
http://www.who.int/quantifying_ehimpacts/global/ebdcountgroup/en/, accessed: July 23, 2013.
Prospective, prospective population-based incidence study; Multiplier, multiplier study (laboratory-based incidence adjusted for under-ascertainment); Notification, disease notification data, adjusted for under-ascertainment using published multipliers; Extrapolation, extrapolation from regions in close geographic proximity.
AFR D: Algeria, Angola, Benin, Burkina Faso, Cameroon, Cape Verde, Chad, Comoros, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Madagascar, Mali, Mauritania, Mauritius, Niger, Nigeria, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Togo.
AFR E: Botswana, Burundi, Central African Republic, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Eritrea, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Uganda, United Republic of Tanzania Zambia, Zimbabwe.
AMR A: Canada, Cuba, United States.
AMR B: Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Brazil, Chile, Colombia, Costa Rica, Dominica, Dominican Republic, El Salvador, Grenada, Guyana, Honduras, Jamaica, Mexico, Panama, Paraguay, Saint Kitts and Newis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Uruguay, Venezuela.
AMR D: Bolivia, Ecuador, Guatemala, Haiti, Nicaragua, Peru.
EMR B: Bahrain, Cyprus, Iran (Islamic Republic of), Jordan, Kuwait, Lebanon, Libyan Arab Jamahiriya, Oman, Qatar, Saudi Arabia, Syrian Arab Republic, Tunisia, United Arab Emirates.
EMR D: Afghanistan, Djibouti, Egypt, Iraq, Morocco, Pakistan, Somalia, Sudan, Yemen.
EUR A: Andorra, Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, United Kingdom
EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovia, Bulgaria, Georgia, Kyrgyzstan, Poland, Romania, Slovakia, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan, Yugoslavia.
EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Republic of Moldova, Russian Federation, Ukraine.
SEAR B: Indonesia, Sri Lanka, Thailand.
SEAR D: Bangladesh, Bhutan, Democratic People’s Republic of Korea, India, Maldives, Myanmar, Nepal, Timor Leste.
WPR A: Australia, Brunei Darussalam, Japan, New Zealand, Singapore.
WPR B: Cambodia, China, Cook Islands, Fiji, Kiribati, Lao People’s Democratic Republic, Malaysia, Marshall Islands, Micronesia (Federated States of), Mongolia, Nauru, Niue, Palau, Papua New Guinea, Philippines, Republic of Korea, Samoa, Solomon Islands, Tonga, Tuvalu, Vanuatu, Viet Nam.
Knowledge synthesis
We synthesized study results (Table 1) using a version of the simulation model published for Salmonella (Majowicz et al., 2010). We used the 2005 WHO Sub-Region populations as point estimates (World Health Organization, 2005). For each Sub-Region, incidence estimates from the systematic review were modeled as PERT distributions (Vose, 2000). For Sub-Regions with prospective cohort studies, the average incidence, weighted by the respective national populations, was used as the most likely value in the corresponding PERT distribution, and the lowest and highest values were used as the minimum and maximum values, respectively. For Sub-Regions without prospective studies, we used data from multiplier studies, in the same manner.
In Sub-Regions without prospective cohort or multiplier studies, we estimated incidence using the annual number of cases reported in notifiable disease data and 2010 United Nations country population estimates (United Nations, 2010). For one study with surveillance from a single hospital (Islam et al., 2007), the estimated annual number of patients seen and catchment area population were available (International Centre for Diarrhoeal Disease Research, 2011), and were used to calculate incidence. In Sub-Regions with notifiable disease data from one country, the annual incidence estimate was used as the most likely value in the corresponding PERT distribution. To determine appropriate minimum and maximum values, we used a 10-fold decrease/increase (i.e., the range we calculated across multiple countries’ surveillance data in the EUR B and EUR C Sub-Regions). For Sub-Regions with notifiable disease data from more than one country, the average incidence, weighted to the respective national populations, was used as the most likely value, and the lowest and highest country-specific incidence rates were used as the minimum and maximum values, respectively. To account for known under-ascertainment in notifiable disease data, we multiplied the annual incidence within these Sub-Regions by published STEC-specific under-reporting estimates (Thomas et al., 2006; Hall et al., 2008; Scallan et al., 2011; Tam et al., 2012, Haagsma et al., 2012), which averaged to 36 (range: 7.4–106.8). We used these values as the mean, minimum, and maximum values in a PERT distribution, respectively. For Sub-Regions without prospective cohort or multiplier studies, or notifiable disease data, we extrapolated from Sub-Regions of geographic proximity, as has been done for salmonellosis (Majowicz et al., 2010) and congenital toxoplasmosis (Torgerson and Mastroiacovo, 2013). To estimate the global annual number of cases, the Sub-Regional incidences per person-year were multiplied by the Sub-Region populations, and summed.
To calculate the number of cases of HUS and deaths due to STEC per Sub-Region, we accounted for two sources of variation. First, both the proportion of cases experiencing sequelae and the case fatality rate are higher in persons infected with STEC O157 versus non-O157 STEC (Gould et al., 2013). Second, STEC O157 appears more prevalent in developed regions such as North America and Europe (Mead and Griffin, 1998). We assigned Sub-Regions to one of three categories, making conservative assumptions based on laboratory results, with extrapolation between similar Sub-Regions: “negligible O157” (0% O157, 100% non-O157); “some O157” (10% O157, 90% non-O157); and “recognized O157” (36% O157, 64% non-O157; Table 1). We multiplied these estimated proportions by the O157- and non-O157 specific proportions developing HUS and dying, to estimate the number of HUS cases and deaths per Sub-Region, adjusted for regional and clinical differences between O157 and non-O157 STEC.
Population-level information on the proportion of STEC cases who develop HUS and who die was limited. Most published estimates of sequelae and fatality are derived from notified, hospitalized, or other subsets of severe cases. Applying such estimates to population-level incidences overestimates the numbers of sequelae and deaths, since mild cases often do not seek care. Thus, we used data from the United States (Gould et al., 2013), where both the numbers of cases of HUS and numbers of deaths among notified cases, and under-reporting multipliers, were available specific to O157 and non-O157 STEC. We calculated the proportion of STEC cases with HUS by dividing the number of HUS cases (corrected for severe outcome under-reporting by a factor of two; Mead et al., 1999), by the total number of notified cases (corrected using the under-reporting multiplier for total notified cases). We did this calculation for the proportion of STEC O157 cases with HUS ([83*2]/[773*26.1] = 0.823%), and who die ([33*2]/[5688*26.1] = 0.045%), as well as for the proportion of non-O157 STEC cases with HUS ([4*2]/[301*106.8] =0.025%), and who die ([2*2]/[2006*106.8] = 0.002%), and modeled these values as Beta distributions. We estimated the proportion of HUS cases who develop ESRD using a 2003 systematic review and meta-analysis (Garg et al., 2003), which estimated that permanent ESRD occurs in 3% of post-diarrheal HUS cases (range: 0–30%); we used these as the most likely, minimum, and maximum values in PERT distribution.
We included age categories as point estimates. Given the lack of detailed age information on STEC-related illnesses and deaths, we used surveillance information from Australia (Vally et al., 2012), New Zealand (Ministry for Primary Industries, 2010), and the United States (Gould et al., 2009). We estimated that 29% of STEC cases occur in those 0–4 years of age, 20% in 5–15 years of age, 35% in 16–59 years of age, and 17% in ≥ 60 years of age; 42% of HUS cases occur in those 0–4 years of age, 18% in 5–15 years of age, 26% in 16–59 years of age, and 14% in ≥ 60 years of age; and 29% of deaths occur in those 0–4 years of age, 10% in 5–15 years of age, 5% in 16–59 years of age, and 57% in ≥ 60 years of age. We assumed the same age distribution for ESRD as for HUS.
Analyses were done in @RISK version 5.7 (Palisade Corporation, Ithaca, NY) as an add-on to Microsoft Excel 2010 (Microsoft Corporation), using Monte Carlo simulation and Latin Hypercube sampling, with 100,000 iterations. We conducted a sensitivity analysis by ranking correlation coefficients between the input parameters and the global annual number of cases, and running scenarios to explore various model assumptions.
Results
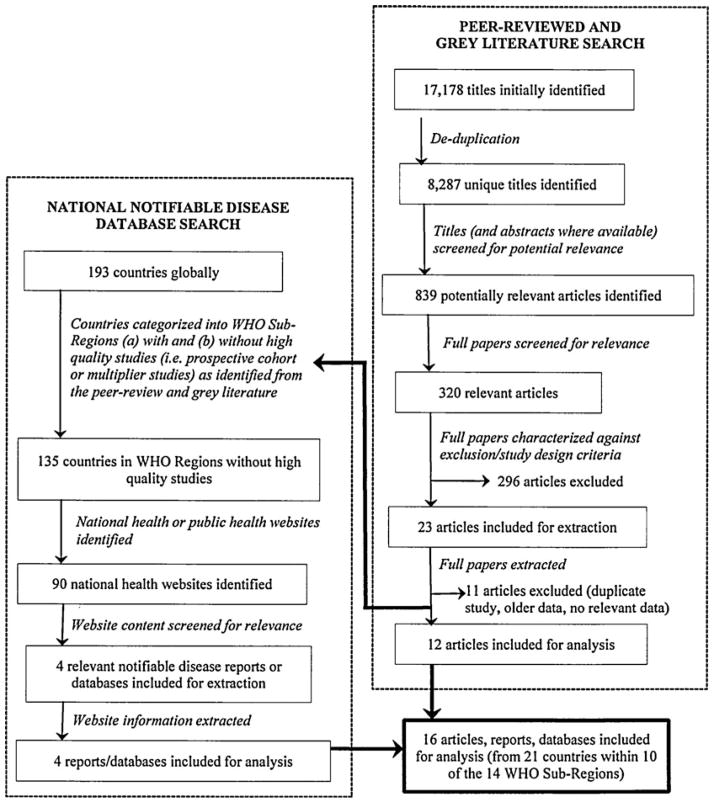
We identified 16 articles, reports, and databases containing information on 21 countries, from 10 of the 14 WHO Sub-Regions (Fig. 1 and Table 1), representing a cumulative population of 2.1 billion (~30% of the global population).
FIG. 1.
Results of the systematic review to identify studies and notifiable disease data, on the population-level incidence of Shiga toxin–producing Escherichia coli infection, published between January 1, 1990 and April 30, 2012, in all languages.
We estimated that STEC causes 2,801,000 acute illnesses annually, worldwide (95% credible interval [Cr.I.]: 1,710,000; 5,227,000), with 809,000 cases in those 0–4 years of age, 554,000 in those 5–15 years, 974,000 in those 16–59 years, and 464,000 in those ≥ 60 years. We estimated that STEC leads to 3890 cases of HUS (95% Cr.I.: 2400; 6700), with 1630 cases in those 0–4 years of age, 720 in those 5–15 years, 1010 in those 16–59 years, and 530 in those ≥ 60 years. We estimated that STEC leads to 270 cases of permanent ESRD (95% Cr.I.: 20; 800), with 110 cases in those 0–4 years of age, 50 in those 5–15 years, 70 in those 16–59 years, and 40 in those ≥ 60 years. We estimated that STEC leads to 230 deaths (95% Cr.I.: 130; 420), with 70 deaths in those 0–4 years of age, 20 in those 5–15 years, 10 in those 16–59 years, and 130 in those ≥ 60 years. Sub-Regional estimates are given in Table 2.
Table 2.
Estimated Global Burden of Shiga Toxin–Producing Escherichia coli, Circa 2012, by World Health Organization (WHO) Sub-regions
| WHO Sub-region | 2005 WHO Sub-region population | Estimated burden (mean value)a
|
||||
|---|---|---|---|---|---|---|
| No. cases | Incidence per 100,000 person-years | No. cases hemolytic uremic syndrome | No. cases end-stage renal disease | No. deaths | ||
| AFR D | 353,412,879 | 4800 | 1.4 | 5 | 0 | 0 |
| AFR E | 402,012,097 | 5400 | 1.4 | 5 | 0 | 0 |
| AMR A | 343,376,860 | 321,000 | 93.5 | 1000 | 70 | 60 |
| AMR B | 464,046,150 | 126,200 | 27.2 | 400 | 30 | 20 |
| AMR D | 76,985,668 | 71,900 | 93.5 | 80 | 5 | 5 |
| EMR B | 148,336,863 | 226,300 | 152.6 | 60 | 5 | 5 |
| EMR D | 381,484,794 | 582,000 | 152.6 | 160 | 10 | 15 |
| EUR A | 423,910,793 | 199,500 | 47.1 | 630 | 40 | 35 |
| EUR B | 224,521,283 | 6000 | 2.7 | 20 | < 5 | < 5 |
| EUR C | 236,910,762 | 6000 | 2.5 | 20 | < 5 | < 5 |
| SEAR B | 308,186,718 | 204,300 | 66.3 | 220 | 15 | 15 |
| SEAR D | 1,388,360,385 | 920,200 | 66.3 | 1000 | 70 | 60 |
| WPR A | 157,005,359 | 69,900 | 44.5 | 220 | 15 | 10 |
| WPR B | 1,593,805,917 | 56,100 | 3.5 | 60 | 5 | 5 |
| Global TOTALS b | 6,502,356,528 | 2,801,000 | 43.1 | 3890 | 270 | 230 |
Values were rounded to the nearest 5; values between 1 and 5 inclusive are represented as “ < 5.” Results reported here are mean values of the distributions generated from the simulation model, and as such will be different than the most likely values reported in Table 1.
Numbers may not add up due to rounding.
Sensitivity analysis
The incidence in the SEAR D Sub-Region had the greatest influence on the estimated annual number of cases (Spearman’s rank correlation coefficient [SRCC]: 0.94), followed by the incidence in the WPR B Sub-Region (SRCC: 0.63). The impacts of various modeling assumptions are shown in Table 3. We assumed that under-ascertainment multipliers, generated from developed Sub-Regions, were applicable to Sub-Regions with less well-established surveillance. To explore this, we increased the multiplier in the AFR D, AFR E, AMR B, EUR C, SEAR B, SEAR D, and WPR B Sub-Regions by factors of two and five (Scenarios 1 and 2, respectively). We also assumed that prospective cohort data were higher quality than data from surveillance systems, adjusted for under-ascertainment, from the same area. For the EUR A Sub-Region, both prospective cohort (De Wit et al., 2001; Tam et al., 2012) and multiplier study incidence estimates (Haagsma et al., 2012) were available; we investigated the effects of data source by replacing the prospective incidence with that from the multiplier study (50 cases per 100,000 population; Scenario 3).
Table 3.
Sensitivity Analysis Illustrating the Estimated Annual, Global Burden of Illness Due to Shiga Toxin–Producing Escherichia coli, Circa 2012, Under Different Modeling Assumptions
| No. cases | No. cases hemolytic uremic syndrome | No. cases end-stage renal disease | No. deaths | |
|---|---|---|---|---|
| Results from Table 2 | 2,801,000 | 3890 | 270 | 230 |
| Scenario | ||||
| (1) Under-ascertainment multiplier in AFR D, AFR E, AMR B, EUR C, SEAR B, SEAR D, and WPR B, increased by a factor of two | 4,028,000 | 5310 | 370 | 320 |
| (2) Under-ascertainment multiplier in AFR D, AFR E, AMR B, EUR C, SEAR B, SEAR D, and WPR B, increased by a factor of five | 7,715,000 | 9570 | 670 | 580 |
| (3) Estimated incidence in EUR A (prospective cohort study) replaced with estimate from multiplier study | 2,812,000 | 3930 | 280 | 230 |
| (4) Unweighted averages of incidence estimates within Sub-Regions | 4,192,000 | 5500 | 380 | 330 |
| (5) SEAR D incidence estimate applied to WPR B Sub-Region | 3,800,000 | 4970 | 350 | 300 |
| (6) South Australia surveillance data on percent HUS used in lieu of United States’ data. | 2,801,000 | 14,100 | 990 | 230 |
| (7) Additional uncertainty for Sub-Regions with only one study, or for whom data were extrapolated from a neighbouring Sub-Region | 2,834,000 | 3920 | 280 | 230 |
| (8) Extrapolating from AMR B to AMR D (instead of extrapolating from AMR A) | 2,749,000 | 3840 | 270 | 230 |
We assumed countries within a Sub-Region had different underlying rates of disease, therefore weighting incidence estimates to the corresponding national populations when estimating the Sub-Regional incidence. However, differences in incidences may reflect varying study methodologies, case ascertainment, and surveillance systems rather than varying disease rates. Thus, we removed the weighting on the average incidences (Scenario 4). We also assumed that countries within a Sub-Region best represented their entire Sub-Region. In the two most populous, developing Sub-Regions, we had data from Hong Kong and the Republic of Korea (WPR B), and India and Bangladesh (SEAR D). Since India and Bangladesh may more accurately represent the overall conditions of WPR B Sub-Region, we applied the SEAR D incidence estimate to the WPR B Sub-Region (Scenario 5).
We estimated the proportions of cases with HUS using United States’ data (i.e., published data that we could extrapolate to the population level, and stratify by O157 versus non-O157 STEC). To explore alternate estimates, we used surveillance data from the state of South Australia, where O157 and non-O157 surveillance is equally rigorous. From 2000 to mid-2013, there were 2 cases of HUS among 178 STEC O157 notifications, and 8 cases of HUS among 369 non-O157 STEC notifications (personal communication: E. Fearnley, South Australian Department for Health and Ageing, August 9, 2013). We adjusted for under-reporting of notified cases by a factor of eight (Hall et al., 2008), and HUS by a factor of two (Mead et al., 1999), and used the resulting estimates (0.28% HUS among STEC O157 cases; 0.54% HUS among non-O157 STEC cases) in Scenario 6.
To explore the impact of extrapolating from one study to an entire Sub-Region, and extrapolating between Sub-Regions of close geographic proximity, we expanded the range of uncertainty around the estimates (Scenario 7). For Sub-Regions that relied on one study’s results (AFR E, AMR B, EMR B), or that were extrapolated from a Sub-Region of close proximity (AMR D, SEAR B), we widened the range of uncertainty by 20%. For Sub-Regions meeting both conditions (AFR D, EMR D), we widened the range by 50%. We also explored the impact of using AMR B incidence data (versus AMR A), when extrapolating to AMR D (Scenario 8).
Discussion
We provide the first estimates of the global incidence of STEC infection, a foodborne pathogen of emerging importance over the past three decades (Mead and Griffin, 1998; Buchholz et al., 2011). We estimated STEC causes 2,801,000 acute illnesses, 3890 cases of HUS, 270 cases of permanent ESRD, and 230 deaths annually, worldwide. Compared to other pathogens with a foodborne transmission component, STEC appears to cause more cases than alveolar echinococcosis (18,200 cases; Torgerson et al., 2010) each year, but less than typhoid fever (21.7 million cases; 216,500 deaths; Crump et al., 2004), foodborne trematodes (7158 deaths; Fürst et al., 2012), and nontyphoidal salmonellosis (93.8 million cases; 155,000 deaths; Majowicz et al., 2010). Despite fewer cases and deaths, STEC leads to severe sequelae that must be accounted for when prioritizing foodborne pathogens for control efforts. Future DALY calculations that consider multiple pathogens simultaneously are needed to effectively prioritize foodborne agents for public health action.
The impacts of STEC are often greater in infants and children, compared to other ages. In developed countries, studies investigating all ages typically identify STEC more frequently and with greater severity in young children (see, for example: van Pelt et al., 2003; Eklund et al., 2005; Ruzante et al., 2011; Buvens et al., 2012). In less developed countries, where childhood diarrheal diseases are highly endemic, studies typically focus solely on children, but have also identified STEC as a pathogen of concern in this age group (see, for example: Okeke, 2009; Al Jarousha et al., 2011; Llanos et al., 2012; López et al., 2012; Bonkoungou et al., 2013; Lozer et al., 2013). Unfortunately, robust information on the age distribution of STEC cases in the overall population is scarce; thus, for this analysis we used information from three ongoing, national surveillance systems to estimate that 29% of acute cases, 42% of cases of HUS and ESRD, and 29% of deaths occurred in children between the ages of 0 and 4 years. These estimates, derived from developed countries’ surveillance data, are broad; the actual age distribution in developing countries may differ, and future studies should attempt to estimate the population age distribution of illness in a given country.
We relied on multiple data sources and assumptions to produce global estimates. For example, prospective cohort studies are considered the criterion standard for estimating incidence. However, relying solely on such studies would entail using data from the Netherlands (De Wit et al., 2001), United Kingdom (Tam et al., 2012), and Iran (Aslani et al., 1998, 2003). The unweighted and weighted averages of these studies’ results were 122 and 136 cases per 100,000 person-years, respectively, both of which are larger estimates than ours (43.1 cases per 100,000 person-years). Given the geographic variation in STEC in humans and reservoirs, the validity of such global generalization is unknown. We chose instead to use the best available data from each WHO Sub-Region, and make explicit the inherent uncertainty via stochastic modeling, as has been done with other pathogens with similar paucity of data (Majowicz et al., 2010; Torgerson et al., 2010). In the absence of evidence to determine the best alternative, we chose the conservative option, and illustrated other options in the sensitivity analyses.
We purposely included public health databases containing routinely collected notifiable disease data. Such information is a rich data source for systematic reviews aimed at estimating infectious disease incidence. Given that notifiable disease data in a given country may exist in lieu of population-level research, including such data in systematic reviews of disease incidence is necessary to minimize bias, particularly given the heterogeneity of Sub-Regions.
Our results are subject to several limitations. We did not account for Sub-Regional differences in health care access or standards. In Sub-Regions with poor health care, ESRD is likely synonymous with death, and thus our fatality results may be underestimates. We used a global case fatality rate, derived from United States’ data; realistically, case fatality rates in Sub-Regions with higher or lower standards of health care will be lower or higher, respectively. We assumed the proportion of cases developing HUS and ESRD is the same universally, although it is currently unknown whether the clinical course of illness varies by Sub-Region. Finally, as described above, we used data from three developed countries to generate broad age estimates; given that the risk factors and causal pathways likely vary between countries, our age estimates are rudimentary, and should be refined as new data emerge. Variation across Sub-Regions with respect to other factors (e.g., exposures, underlying health status) could also impact the results presented here. These limitations highlight existing knowledge gaps, in particular the lack of primary studies and notifiable disease data from several Sub-Regions, and the lack of population-level estimates on exposures, age distributions, and the clinical course of illness. These knowledge gaps persist for other foodborne pathogens (Majowicz et al., 2010; Torgerson et al., 2010), and future studies that address these gaps will contribute to more certain global burden estimates.
Conclusions
This study provides the first estimates of the global impact of STEC, a group of pathogens not explicitly included in previous global burden of disease estimations (Murray et al., 2012; Lozano et al., 2012). These results can underpin future DALY calculations, at the Sub-Regional, Regional, and global levels, which can be used to prioritize pathogens for public health action. Given the persistence of STEC globally, efforts aimed at reducing the burden of foodborne disease should consider the relative contribution of STEC in the target population.
Acknowledgments
The authors thank Janet Harris (Public Health Agency of Canada) for assistance creating the search strings and conducting the review and extraction. The School of Public Health and Health Systems and the Faculty of Applied Health Sciences (University of Waterloo) and the World Health Organization’s Foodborne Diseases Epidemiology Reference Group (FERG) supported this work. FERG was consulted during study design, assisted with obtaining identified references, provided feedback on preliminary results, and had the opportunity to comment on the final manuscript.
Footnotes
All authors had full access to all study data, and the analysis, interpretation, and the decision to publish were solely the responsibility of the authors. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure Statement
SEM, ES, AJB, JMS, JS, and DHY declare no conflicts of interest. FJA and MDK serve without compensation as members of FERG, and declare no other conflicts of interest.
References
- Al Jarousha AM, El Jarou MA, El Qouqa IA. Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J Pediatr. 2011;78:165–170. doi: 10.1007/s12098-010-0249-0. [DOI] [PubMed] [Google Scholar]
- Aslani MM, Badami N, Mahmoodi M, Bouzari S. Verotoxin-producing Escherichia coli (VTEC) infection in randomly selected population of Ilam province (Iran) Scand J Infect Dis. 1998;30:473–476. doi: 10.1080/00365549850161467. [DOI] [PubMed] [Google Scholar]
- Aslani MM, Bouzari S. An epidemiological study on verotoxin-producing Escherichia coli (VTEC) infection among population of northern region of Iran (Mazandaran and Golestan provinces) Eur J Epidemiol. 2003;18:345–349. doi: 10.1023/a:1023602416726. [DOI] [PubMed] [Google Scholar]
- Bonkoungou IJ, Haukka K, Österblad M, Hakanen AJ, Traoré AS, Barro N, Siitonen A. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13:36. doi: 10.1186/1471-2431-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Höhle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kühne M. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- Buvens G, De Gheldre Y, Dediste A, de Moreau AI, Mascart G, Simon A, Allemeersch D, Scheutz F, Lauwers S, Piérard D. Incidence and virulence determinants of verocytotoxin-producing Escherichia coli infections in the Brussels-Capital Region, Belgium, in 2008–2010. J Clin Microbiol. 2012;50:1336–1345. doi: 10.1128/JCM.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Health Protection. The Government of the Hong Kong Special Administrative Region. [accessed: July 12, 2013];Number of notifications for notifiable infectious diseases in 2011. 2011 Available at: http://www.chp.gov.hk/en/data/1/10/26/43/455.html.
- Cressey P, Lake R. MPI Technical Paper No: 2012/11. Christchurch, New Zealand: Institute of Environmental Science & Research Limited; 2011. Estimated incidence of foodborne illness in New Zealand: Application of overseas models and multipliers. [Google Scholar]
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- De Wit MAS, Koopmans MPG, Kortbeek LM, Wannet WJ, Vinjé J, van Leusden F, Bartelds AI, van Duynhoven YT. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: Incidence and etiology. Am J Epidemiol. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- Eklund M, Nuorti JP, Ruutu P, Siitonen A. Shigatoxigenic Escherichia coli (STEC) infections in Finland during 1998–2002: A population-based surveillance study. Epidemiol Infect. 2005;133:845–852. doi: 10.1017/S0950268805004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011: Reporting on 2009 Surveillance Data and 2010 Epidemic Intelligence Data. Stockholm, Sweden: ECDC; 2011. [Google Scholar]
- Flint JA, Van Duynhoven YT, Angulo FJ, DeLong SM, Braun P, Kirk M, Scallan E, Fitzgerald M, Adak GK, Sockett P, Ellis A, Hall G, Gargouri N, Walke H, Braam P. Estimating the burden of acute gastroenteritis, foodborne disease, and pathogens commonly transmitted by food: An international review. Clin Infect Dis. 2005;41:698–704. doi: 10.1086/432064. [DOI] [PubMed] [Google Scholar]
- Fürst T, Keiser J, Utzinger J. Global burden of human foodborne trematodiasis: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: A systematic review, meta-analysis, and meta-regression. JAMA. 2003;290:1360–1370. doi: 10.1001/jama.290.10.1360. [DOI] [PubMed] [Google Scholar]
- Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, Griffin P. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, Foodborne Diseases Active Surveillance Network sites, 2000–2006. Clin Infect Dis. 2009;49:1480–1485. doi: 10.1086/644621. [DOI] [PubMed] [Google Scholar]
- Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Lathrop S, Medus C, Spina NL, Webb TH, White PL, Wymore K, Gierke RE, Mahon BE, Griffin PM Emerging Infections Program Foodnet Working Group. Increased recognition of non-O157 Shiga toxin–producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis. 2013;10:453–460. doi: 10.1089/fpd.2012.1401. [DOI] [PubMed] [Google Scholar]
- Haagsma JA, Geenen PL, Ethelberg S, Fetsch A, Hansdotter F, Jansen A, Korsgaard H, O’Brien SJ, Scavia G, Spitznagel H, Stefanoff P, Tam CC, Havelaar AH Med-Vet-Net Working Group. Community incidence of pathogen-specific gastroenteritis: Reconstructing the surveillance pyramid for seven pathogens in seven European Union member states. Epidemiol Infect. 2012;141:1625–1639. doi: 10.1017/S0950268812002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G, Yohannes K, Raupach J, Becker N, Kirk M. Estimating community incidence of Salmonella, Campylobacter, and Shiga toxin–producing Escherichia coli infections, Australia. Emerg Infect Dis. 2008;14:1601–1609. doi: 10.3201/eid1410.071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman DL. Control of Communicable Diseases Manual. 19. Washington, DC: American Public Health Association (APHA) Press; 2008. [Google Scholar]
- Institute of Public Health. Report of laboratory monitoring of Shiga toxin–producing E. coli [in Spanish] [accessed July 12, 2013];Gobierno of Chile. 2012 Available at: http://www.ispch.cl/sites/default/files/stec_se_39_2011_2012.pdf.
- International Centre for Diarrhoeal Disease Research, Bangladesh (iccdr,b) Estimated incidence of cholera in the catchment area of two diarrhoeal diseases hospitals in Dhaka City. Health Science Bull. 2011;9:1–7. [Google Scholar]
- Islam MA, Heuvelink AE, de Boer E, Sturm PD, Beumer RR, Zwietering MH, Faruque AS, Haque R, Sack DA, Talukder KA. Shiga toxin-producing Escherichia coli isolated from patients with diarrhoea in Bangladesh. J Med Microbiol. 2007;56:380–385. doi: 10.1099/jmm.0.46916-0. [DOI] [PubMed] [Google Scholar]
- Korea Centres for Disease Control and Prevention. [accessed July 15, 2013];Infectious Disease Surveillance Yearbook. 2011 Available at: http://www.cdc.go.kr/CDC/notice/CdcKrInfo0301.jsp?menuIds=HOME001-MNU0004-MNU0036-MNU0037&cid=12796.
- Lazic S, Cobeljic M, Dimic B, Opacic D, Stojanovic V. Epidemiological importance of humans and domestic animals as reservoirs of verocytotoxin-producing Escherichia coli. Vojnosanit Pregl. 2006;63:13–9. doi: 10.2298/vsp0601013l. [DOI] [PubMed] [Google Scholar]
- Llanos A, Lee J, López F, Contreras C, Barletta F, Chea-Woo E, Ugarte C, Cleary TG, Ochoa TJ. Shiga toxin–producing Escherichia coli in Peruvian children with bloody diarrhea. Pediatr Infect Dis J. 2012;31:314–316. doi: 10.1097/INF.0b013e318244000c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López EL, Contrini MM, Glatstein E, Ayala SG, Santoro R, Ezcurra G, Teplitz E, Matsumoto Y, Sato H, Sakai K, Katsuura Y, Hoshide S, Morita T, Harning R, Brookman S. An epidemiologic surveillance of Shiga-like toxin–producing Escherichia coli infection in Argentinean children: Risk factors and serum Shiga-like toxin 2 values. Pediatr Infect Dis J. 2012;31:20–24. doi: 10.1097/INF.0b013e31822ea6cf. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozer DM, Souza TB, Monfardini MV, Vicentini F, Kitagawa SS, Scaletsky IC, Spano LC. Genotypic and phenotypic analysis of diarrheagenic Escherichia coli strains isolated from Brazilian children living in low socioeconomic level communities. BMC Infect Dis. 2013;13:418. doi: 10.1186/1471-2334-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM the International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. . The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998;352:1207–1212. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry for Primary Industries. [accessed August 7, 2013];Foodborne Disease Annual Reports. 2010 Available at: http://www.foodsafety.govt.nz/science-risk/human-health-surveillance/foodborne-disease-annual-reports.htm.
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- National Institute for Communicable Diseases. [accessed July 12, 2013];Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa: GERMS-SA Annual Report 2010. 2010 Available at: http://nicd.ac.za/assets/files/2010%20GERMS-SA%20Annual%20report%20Final.pdf.
- Okeke IN. Diarrheagenic Escherichia coli in sub-Saharan Africa: Status, uncertainties and necessities. J Infect Dev Ctries. 2009;3:817–842. doi: 10.3855/jidc.586. [DOI] [PubMed] [Google Scholar]
- Ruzante JM, Majowicz SE, Fazil A, Davidson VJ. Hospitalization and deaths for select enteric illnesses and associated sequelae in Canada, 2001–2004. Epidemiol Infect. 2011;139:937–945. doi: 10.1017/S0950268810001883. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal R, Kumar Y, Kumar S. Prevalence and geographical distribution of Escherichia coli O157 in India: A 10-year survey. Trans R Soc Trop Med Hyg. 2008;102:380–383. doi: 10.1016/j.trstmh.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Stein C, Kuchenmüller T, Hendrickx S, Prüss-Ustün A, Wolfson L, Engels D, Schlundt J. The Global Burden of Disease assessments—WHO is responsible? PLoS Negl Trop Dis. 2007;1:e161. doi: 10.1371/journal.pntd.0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS O’Brien SJ; IID2 Study Executive Committee. . Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MK, Majowicz SE, Sockett PN, Fazil A, Pollari F, Doré K, Flint JA, Edge VL. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxigenic Escherichia coli: Pathogen-specific community rates. Can J Infect Dis Med Microbiol. 2006;17:229–34. doi: 10.1155/2006/806874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: A systematic review. Bull World Health Organ. 2013;91:501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. [accessed July 23, 2013];World Population Prospects: The 2010 Revision, Volume I: Comprehensive Tables. 2010 ST/ESA/SER.A/313. Available at: http://esa.un.org/unpd/wpp/Documentation/pdf/WPP2010_Volume-I_Comprehensive-Tables.pdf.
- Vally H, Hall G, Dyda A, Raupach J, Knope K, Combs B, Desmarchelier P. Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000–2010. BMC Public Health. 2012;12:63. doi: 10.1186/1471-2458-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt W, de Wit MA, Wannet WJ, Ligtvoet EJ, Widdowson MA, van Duynhoven YT. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiol Infect. 2003;130:431–441. [PMC free article] [PubMed] [Google Scholar]
- Vose D. Risk Analysis: A Quantitative Guide. 2. West Sussex, England: John Wiley & Sons; 2000. [Google Scholar]
- World Health Organization. [accessed July 23, 2013];Population, Death Rates, and Reproductive Rates. 2005 Available at: http://www.who.int/choice/demography/pop_death_rates/en/index.html.