Abstract
Co-transplantation of hematopoietic stem cells with those engineered to express leukemia-reactive T cell receptors (TCRs) and differentiated ex vivo into precursor T cells (preTs) may reduce the risk of leukemia relapse. Since expression of potentially self-(leukemia-) reactive TCRs will lead to negative selection or provoke autoimmunity upon thymic maturation, we investigated a novel concept whereby TCR expression set under the control of an inducible promoter would allow timely controlled TCR expression. After in vivo maturation and gene induction, preTs developed potent anti-leukemia effects. Engineered preTs provided protection even after repeated leukemia challenges by giving rise to effector and central memory cells. Importantly, adoptive transfer of TCR-transduced allogeneic preTs mediated anti-leukemia effect without evoking graft-versus-host disease (GVHD). Earlier transgene induction forced CD8+ T cell development, was required to obtain a mature T cell subset of targeted specificity, allowed engineered T cells to efficiently pass positive selection and abrogated the endogenous T cell repertoire. Later induction favored CD4 differentiation and failed to produce a leukemia-reactive population emphasizing the dominant role of positive selection. Taken together, we provide new functional insights for the employment of TCR-engineered precursor cells as a controllable immunotherapeutic modality with significant anti-leukemia activity.
INTRODUCTION
Despite advances, several obstacles are remaining when considering the application of mature T cell transfer for the treatment of acute leukemias:1 (I) the challenge of obtaining sufficient numbers of mature T cells in patients receiving intensive chemotherapy; (II) poor in vivo persistence of transferred T cells, and (III) the time and cost to manufacture the required cell product on an individualized basis.
More recently Notch-based in vitro culture systems have been developed allowing the generation of progenitor T cells (preTs).2, 3 Upon co-transfer, preTs undergo final maturation in the recipient’s thymus and give rise to a naïve and fully functional T cell population. Preclinical data have shown that preTs of MHC-mismatched third party donors can be used.4 Since preTs are still subject to thymic maturation, they develop into fully functional T cells in vivo being tolerant to both donor and recipient.5 The anti-tumor effects of preTs can be improved by genetically enforced expression of chimeric antigen receptors (CARs).6 However, their antigen recognition pattern contains a target cell surface antibody-binding domain while many attractive leukemia-specific antigens7 represent intracellularly-processed antigens that are generally difficult to target by CARs.8, 9 Although very recent developments may allow the design of CARs recognizing selected peptides in their MHC pocket10, the introduction of T cell receptors (TCRs) has been classically used to target both intracellular antigens and cell surface bound antigens.11–14 Nevertheless, in vivo maturation of co-transplanted preTs still undergoing selection processes in the thymus represents a major obstacle for using TCR-engineered preTs. Not only for adoptive transfer of receptor-engineered preTs but envisioned clinical trials aiming to co-transplant stem cells as a T cell source, this problem has reached high clinical relevance.
Here we evaluated the novel concept of engineering preTs with a leukemia-reactive TCR whose expression can be controlled by an antibiotic-inducible promoter. We show that the co-transfer of engineered preTs gives rise to mature T cells that display specific antigen recognition upon induction in leukemia-bearing mice. After antigen exposure, effector memory and central memory populations are generated. We further show that early induction of the TCR is a prerequisite for the development of a mature T cell population with defined TCR-specificity by favoring the differentiation into CD8+ T cells and allowing a leukemia-reactive T cell subset to escape negative selection. Here, putting an introduced therapeutic gene under the control of an inducible promoter allows important functional and kinetic insights for further translational development of cellular products for clinical use.
MATERIALS AND METHODS
Mice
Animals in the experiments were used under protocols approved by the State Government of Lower Saxony, Germany. BALB/c (H-2d) and C57BL/6NCrl (B6, H-2b) mice were purchased from Charles River. Transgenic DsRed (H-2b)15, B6.PL-Thy1a/CyJ (Thy1.1, H-2b) and OT-I (H-2b)16 mice were obtained from the Jackson laboratory. B10.A (H-2a) mice were purchased from Taconic laboratories. R26-M2rtTA (B6-Rosa, express a reverse tetracycline-controlled transactivator protein, H-2b) and Rip-OVAhi [express a secreted form of ovalbumin, (OVA)] mice were kindly provided by Andreas Krüger and Reinhold Förster (Hannover, Germany). R26-M2rtTA mice were backcrossed onto B10.A mice to create an allogeneic B10.A-R26-M2rtTA (B10.A-Rosa) background. For TCR induction, doxycycline (1mg/ml) was added to the drinking water
Hematopoietic cell transplantation (HCT)
B6 recipients received total body irradiation of 10.5 Gy from a linear accelerator. After 24 hours, bone marrow (BM) was reconstituted with 3 × 106 syngeneic T cell-depleted bone marrow cells (TCDMB).17
Lentiviral constructs, cell lines and murine cell transduction
Encoding sequences of the OVA-reactive, CD8 OT-I TCR were derived from a construct described earlier.18 This gene was codon-optimized and set under the control of a Tetracycline-inducible (Tet-On) promoter.19 For achieving higher viral titers, the hPGK promoter and the M2 cassette were deleted from the original construct.
C1498-OVA is a myeloid leukemia cell line (H-2b, B6) expressing the experimental surrogate antigen OVA as described previously20 and was injected at a dose of 1.2 × 106 cells via the lateral tail vein to induce 100% lethality. 293T-cells, the viral plasmids encoding for, pMD.G, pRSV.Rev, pcDNA3.GP.4×CTE (PlasmidFactory), and the Calcium Phosphate Transfection Kit (Sigma-Aldrich) were used for the generation of lentiviral supernatant. A TCR-negative hybridoma cell line, 58α−β−, was used for viral titer determination after transduction with the Tet-responsive transactivator variant M2.
To isolate murine hematopoietic stem and progenitor cells, the lineage negative, Sca-1 and c-kit positive (LSK) population was sorted using a FACSAria™ cell sorter (BD Biosciences). LSK cells were then cultured and transduced in the presence of mSCF (10–50 ng/mL), TPO (20–50 ng/mL), IGFII (20 ng/mL), and FGFI (10 ng/mL) (Pepro Tech). To generate preTs, TCR-transduced LSK cells were transferred onto T cell development-supporting murine stromal cells OP-9, expressing the notch ligand, delta-like ligand 1 (OP9-DL1) monolayer cells3, and supplemented with FLT3-L (5 ng/mL), IL-7 (5 ng/mL) (Pepro Tech), L-glutamine, and antibiotics. After 24 to 28 days of co-culture, preTs were harvested and injected i.v. together with the TCDBM graft. Unless otherwise specified, 8 × 106 preTs/mouse were used.
Flow cytometry
The following Fluorochrome-conjugated antibodies were purchased from eBiosciences or Biolegend: CD3ε-(PerCP/Cy5.5), CD4-(PE/APC/Brilliant Violet 421™/Brilliant Violet 570™), CD8α-(PE/APC/APCH7/Brilliant Violet 421 /Brilliant Violet 570 ), CD25-(PE/APC), CD44-(PE/APC), B220-(PE/APCCy7), CD62L-PE, CD11c-APC, IFNγ-PE, Vα2-(APC/PerCP/Cy5.5), Vβ2-PE, Vβ3-PE, Vβ4-PE, Vβ5-PE, Vβ8.1-PE, PD1-PE, c-kit-APC, Sca-1-PE, and lineage markers (all in FITC). Flow cytometry was performed using a FACSCanto or LSR-II (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Assessment of GVHD
GVHD was clinically assessed in a blinded fashion. The scoring system was based on skin integrity, fur texture, activity, posture, and weight loss.21 For pathologic assessment, GVHD target organs were fixed in 3.9% formalin and paraffin-embedded sections were stained with hematoxylin and eosin for microscopic evaluation.
Intracellular cytokine staining and ELISPOT
Intracellular interferon-γ (IFNγ) staining of ex vivo-cultured splenocytes was performed as described previously.17 For ELISPOT analysis, splenocytes were cultured in 96-well plates in the presence of the CD8 OT-1 TCR reactive peptide, SIINFEKL, and doxycycline, transferred to IFNγ-antibody-coated MultiScreen-IP 96-well Plates (Millipore), and stained with a biotinilated anti-IFNγ antibody. Plates were analyzed by an EliScan ELISPOT reader (AELVIS).
In vitro cytotoxicity and mixed leukocyte reaction test (MLR)
In vitro cytotoxicity and the MLR assays were performed as described previously.22
Statistical analysis
The Kaplan-Meier product-limit method was used to calculate survival rates. Differences between the groups were determined using Log-rank statistics. In other cases, statistical analyses were performed by Mann-Whitney test or by Student’s t-test (two-tailed). P values < 0.05 were considered to be significant and were designated by *. P values < 0.01 were designated by **, p values less than 0.001 were designated by ***. P values less than 0.0001 were designated by ****.
RESULTS
Large numbers of preTs containing a specific TCR transgene under the regulation of an inducible promoter are generated in vitro
To generate a construct wherein transgene expression is controllable, we used a tetracycline-inducible (Tet-On) system within a self-inactivating (SIN) lentiviral backbone. A major histocompatibility complex class I (MHC-I)-restricted TCR specific for the OVA peptide SIINFEKL (OT-I TCR) was cloned into this vector and enhanced green fluorescent protein (eGFP) used as a reporter. The reverse tet-responsive transactivator variant M2 (rtTA-M2) and the human PGK promoter were removed for construct size reduction (figure 1A) and rtTA-M2 knock-in mouse-derived LSK cells used for further experiments.
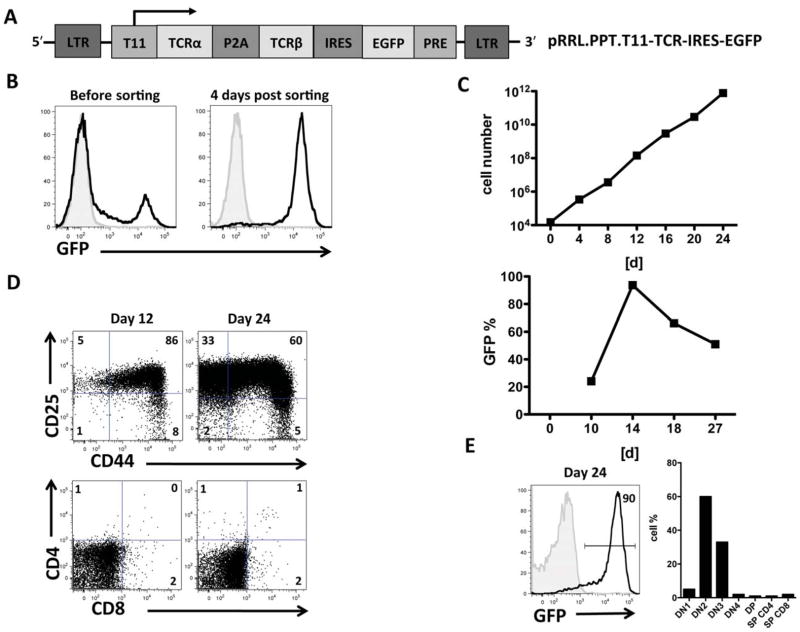
Figure 1. Large numbers of preTs containing the transgene of a specific TCR under the regulation of an inducible promoter were generated in vitro.
(A) Schematic figure of the pRRL.PPT.T11-TCR-IRES-EGFP construct. (B) Prestimulated murine LSK cells were transduced with concentrated lentiviral supernatant and co-cultured with OP9-DL1 cells. Transduction efficacy was determined based on eGFP expression on day 10. This was followed by cell sorting for eGFP to increase the purity of transgene-positive cells. (C) Expansion kinetics of preTs on OP9-DL1 stromal cells is graphed in the upper panel. Transduction efficiency was determined by day 10. Immediately thereafter cell sorting was performed. The purity of the product was reassessed after another four days of culture (day 14). The lower panel depicts the decreasing fraction of transgene-positive preTs during the differentiation and expansion process. Results of one representative experiment are shown. (D) The phenotype of in vitro-generated preT cells was serially determined between days 12 and 24 using flow cytometry. Results of a representative experiment are shown. (E) The phenotype of the final cell product is shown that was used for adoptive cell transfer studies. Cells were sorted for eGFP a second time on day 20 of culture and used for animal experiments on day 24. LTR, long terminal repeat; P2A, self-cleaving 2A peptide; IRES, internal ribosome entry site; eGFP, enhanced green fluorescent protein; PRE, Woodchuck hepatitis virus posttranscriptional regulatory element.
To generate TCR gene-engineered preTs, LSK cells were transduced and transferred to OP9-DL1 stromal cells to support preT development. EGFP-positive cells were enriched by a cell sorter (figure 1B). Over a period of 24 days, large numbers of preTs were generated in vitro (figure 1C; upper graph). During in vitro differentiation, a proliferative advantage of transgene negative cells was observed such that eGFP negative cells outproliferated eGFP positive cells, requiring a second sorting step (figure 1C; lower graph). The majority of preTs at day 12 had a phenotype resembling double negative (DN)-2 (CD4neg/CD8neg/CD44pos/CD25pos) thymocytes, which further developed into a DN3 (CD4neg/CD8neg/CD44neg/CD25pos) stage. Only a small number (less than 4%) of preTs expressed more mature T cell markers (CD4 and/or CD8) (figure 1D). A preT product containing approximately 90% transgene positive cells and displaying a DN2/DN3-like phenotype was used for further in vivo experiments (figure 1E).
Co-transplanted preTs enhance T cell reconstitution and are subject to negative selection in the thymus
To investigate the in vivo kinetics of preTs after adoptive transfer, lethally irradiated B6 recipients received B6 TCDBM either with or without DsRed-derived preTs. After two, four, and eight weeks, cellular immune recovery within the thymus and the spleen was assessed. As shown in figure 2A, large numbers of thymocytes were derived from co-transplanted preT cell progenies 14 days after HCT. Numbers decreased over the following weeks. Progenies of preTs accumulated in the spleen reaching a peak four weeks post-HCT. The majority of the preT-derived progeny in the periphery represented CD4+ and CD8+ T cells; however, a low number of γδT cells, NKT cells, and B220+ B-cells were identified as well (data not shown). 8 weeks post-transfer higher numbers of thymic DN1, DN2, DN3, double positive, single-positive CD8+, γδT-cells, and CD11c+ dendritic cells were found in preT cell recipients as compared to controls not receiving preTs (figure 2B, C). To assess the inducibility of the Tet-On system in vivo, TCR-transduced preTs were transferred to irradiated recipients and doxycycline was added on day 0. Three weeks later the progenies of TCR gene-engineered preTs constituted ~60% of peripheral blood CD8+ cells continuously decreasing to 20% by 4 weeks post-HCT as the hematopoietic stem cells engrafted and differentiated in the thymus (figure 2D).
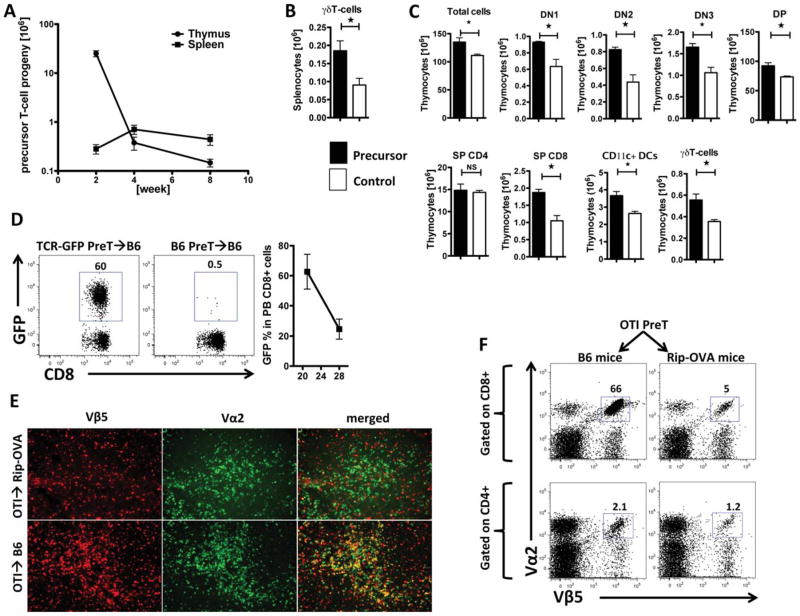
Figure 2. Co-transplanted preTs can enhance T cell reconstitution and are subject to negative selection in the thymus.
(A) Lethally-irradiated B6 mice received 3 × 106 syngeneic TCDBM cells together with 8 × 106 DsRed preTs. Thymi and spleens were harvested two, four and eight weeks later and the number of preT-derived progenies was determined by flow cytometric analysis (n = 4 for each time point). (B) Lethally-irradiated B6 mice received 3 × 106 syngeneic TCDBM either with (black bars) or without (white bars) 8 × 106 DsRed-derived preTs. Spleens were harvested two weeks after transplantation. T cell reconstitution was determined based on cell count and flow cytometric analysis (n = 4 for each group at each time point). (C) Thymi were harvested and analyzed eight weeks after transplantation. Values are shown as mean ± SEM. (D) Lethally-irradiated B6 mice received 3 × 106 syngeneic TCDBM cells and either 8 × 106 non-transduced (B6) or TCR gene-transduced (TCR-GFP) preTs. Doxycycline was added to the drinking water on day 0. Peripheral blood was monitored for transgene positive CD8+ T cells by flow cytometry 21 and 28 days later (n = 3). (E) Lethally irradiated wild-type B6 and Rip-OVAhi mice received 3 × 106 syngeneic TCDBM cells together with 8 × 106 OT-I-derived preTs. Two weeks after transplantation, thymi were harvested and stained using 4′,6-diamidino-2-phenylindole (DAPI) and fluorochrome-labeled antibodies against Vα2 (alophycocianin, APC) and Vβ5 (phycoerythrin, PE). Photomicrographs were generated by a Zeiss Axio Imager-2 microscope and analyzed by AxioVision 4.8 software. (F) Spleens of remaining mice were harvested one month later and analyzed by flow cytometry for the extent of thymic negative selection if the leukemia-associated antigen represents a self-antigen. Events shown in the upper row are gated on splenic CD8+ T cells whereas gating was done on CD4+ cells in the lower row. The right column depicts results that were derived from mice expressing the leukemia-associated antigen as a self-antigen (Rip-OVA mice), recipients represented by the left column do not express the antigen of interest on their tissues (wild-type B6 mice).
Since most tumor-associated antigens represent self-antigens, forced expression of a TCR in preTs was expected to render them susceptible to thymic negative depletion. To assess the extent of preT depletion in a potentially auto-reactive setting, we transferred preTs derived from OT-I-transgenic mice to either irradiated Rip-OVAhi or wild type recipients. Immunofluorescence studies of the thymus showed almost complete depletion of OT-I preTs in Rip-OVAhi recipients two weeks after transplantation. In mice not expressing the respective “self-antigen”, OT-I preTs matured into T cells exhibiting the TCR of interest (figure 2E). Corresponding results were observed in secondary lymphoid organs 28 days after transplantation (figure 2F).
The progenies of TCR gene-manipulated preTs containing an inducible TCR of defined specificity are functional ex vivo
Since preTs are not yet expected to express a TCR during in vitro differentiation, the inducibility of the TCR gene was assessed first using Jurkat cells that express CD3 signaling machinery but not intact TCRs. As shown in figure 3A, both TCR and eGFP expression could be repeatedly switched on and off. Maximal expression level of the TCR and the reporter gene was achieved after four days and reached baseline again four days after the inducing agent had been removed. To examine this kinetic profile in LSK-derived preTs, engineered hematopoietic stem cells were differentiated into preTs in vitro. During maturation the eGFP signal could be switched on and off displaying an expression pattern similar to that observed in Jurkat cells. Importantly, 93% of these T cells expressed the introduced TCR after in vivo maturation (figure 3B).
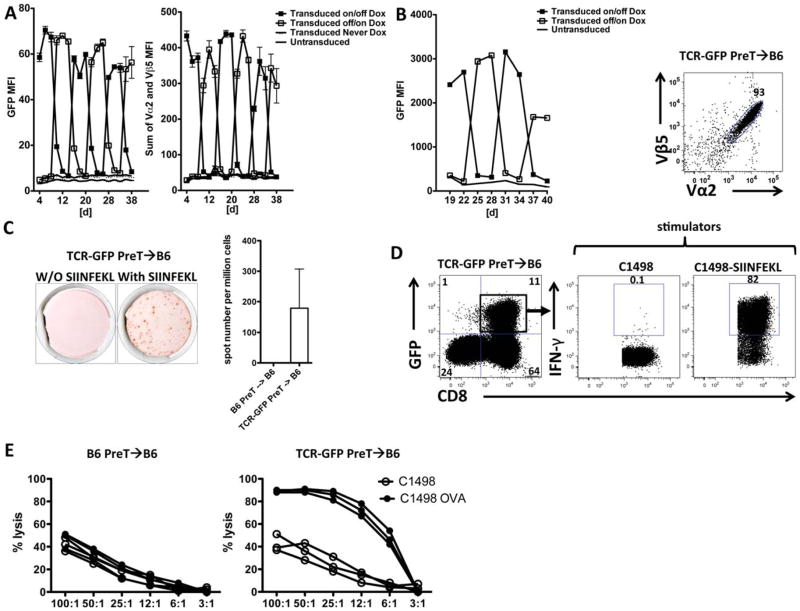
Figure 3. The progenies of TCR gene-manipulated preTs containing an inducible TCR are functional ex vivo.
(A) Jurkat cells were transduced with a vector containing the inducible promoter system, model TCR, and eGFP cassette. Doxycycline (1μg/mL) was added to the medium and cells were analyzed for transgene expression. Two groups (Transduced on/off and off/on Dox) of transduced Jurkat cells were alternatively exposed to (filled square) or deprived from (open square) doxycycline for 6-day periods and analyzed by flow cytometry. Transduced Jurkat cells that never received doxycycline (Transduced never Dox) and non-transduced Jurkat cells were used as controls. (B) TCR gene-transduced preTs, (B6-Rosa-derived, transgenic for M2rtTA), were generated. Two groups (Transduced on/off and off/on Dox) were alternatively exposed to or deprived from the inducing agent and analyzed for eGFP expression (preTs cannot yet express a TCR at this stage of differentiation) every three days (left panel). The majority (93%) of eGFP+ preT-derived T cells expressed the TCR of interest 25 days after co-transplantation into mice (right panel). Non-transduced preTs were used as controls. (C, D and E) Lethally irradiated B6 mice received 3 × 106 syngeneic TCDBM cells together with either 8 × 106 non-transduced (B6 recipients only) or TCR gene-transduced preTs. One month after transplantation, splenocytes were harvested and cultured ex vivo. (C) Antigen-specific functionality was assessed by ELISPOT assays in the presence or absence of the corresponding antigen SIINFEKL. (D) In addition, flow cytometry-based intracellular IFNγ detection assays were performed using either naïve or antigen-coated (SIINFEKL) leukemia cells (C1498) as stimulators (middle and right panel). For determining the IFN-γ response CD8+/GFP+ double positive cells were gated and analyzed (right upper quadrant of the left panel). (E) Specificity of targeted cytotoxic capacity of non-transduced (left panel) or TCR gene-engineered (right panel) preTs was assessed against either C1498 or C1498-OVA. Results represent an overlay of three mice per group. MFI, mean fluorescent intensity.
To assess the functionality of the progenies of TCR-transduced preTs, irradiated B6 recipients received syngeneic TCDBM with either non-transduced or TCR-transduced preTs. One month after transplantation, splenocytes were harvested and stimulated with or without the cognate peptide SIINFEKL. Their ability to produce IFNγ was assessed in ELISPOT and intracellular IFNγ-production assays. Figure 3C and 3D show that the progenies of TCR-transduced preTs (GFP/CD8 positive) produce IFNγ upon antigen-specific stimulation. Most importantly, splenocytes harvested from recipients of gene-engineered preTs lysed C1498-OVA significantly better ex vivo than C1498 leukemia cells (figure 3E). Although the majority of TCR-transduced preTs was deleted in Rip-OVAhi recipients, a small fraction escaped negative deletion. Of note, in vitro stimulation followed by flow cytometric IFN-γ analysis showed that these cells indeed remain functional and produce IFN-γ upon SIINFEKL stimulation (supplementary figure S1).
Co-transplantation of engineered preTs provide potent anti-leukemia effects upon TCR-induction in vivo
One of the safety concerns of adoptive cell transfer studies for cancer is off-target or on-target off-tumor activity. Therefore, we investigated to what extent the removal of the inducing agent could switch off transgene expression in vivo. Irradiated recipients of TCDBM were co-transplanted with TCR-transduced preTs and induced immediately after transplantation. On day 17, doxycycline was discontinued and peripheral blood monitored by flow cytometry for two consecutive days. 24 hours after doxycycline had been stopped, the percentage of exogenous TCR-bearing T cells declined and reached the background level 50 hours later (figure 4A, B). Next, we explored whether the progenies of co-transplanted preTs could protect transplanted recipients from a lethal leukemia challenge. TCDBM-transplanted B6 mice received OT-I-derived transgenic preTs. 28 days later, recipients were challenged with C1498-OVA leukemia cells and followed for survival. Co-transplantation of as few as 25 × 104 OT-I-transgenic preTs significantly improved survival (supplementary figure S2). We then investigated whether the transfer of TCR gene-engineered syngeneic preTs would also enhance survival. Transplant recipients of syngeneic TCDBM received either 8 × 106 non-transduced or TCR-transduced preTs and were challenged with C1498-OVA 28 days later. As shown in figure 4C, co-transfer of TCR gene-transduced preTs significantly improved survival over non-transduced preTs. Even when leukemia cells were given subcutaneously one day prior to transplantation significant anti-leukemia effects were achieved as determined by weight of the subcutaneous tumor inocula (figure 4E). Having observed the anti-leukemia effect of syngeneic preTs, we set to apply completely MHC-mismatched preTs as a potential readily available “off the shelf product”. Co-transfer of allogeneic TCR-engineered preTs (B10.A (H2a)) with B6 TCDBM and doxycycline improved survival significantly, however, results were inferior to those after syngeneic preT transfer achieving only ~40% long-term survival at day 90 as contrasted to 0% in recipients given the same cells but without doxycycline (figure 4D). Importantly, preTs of completely MHC-mismatched donors did not evoke GVHD.
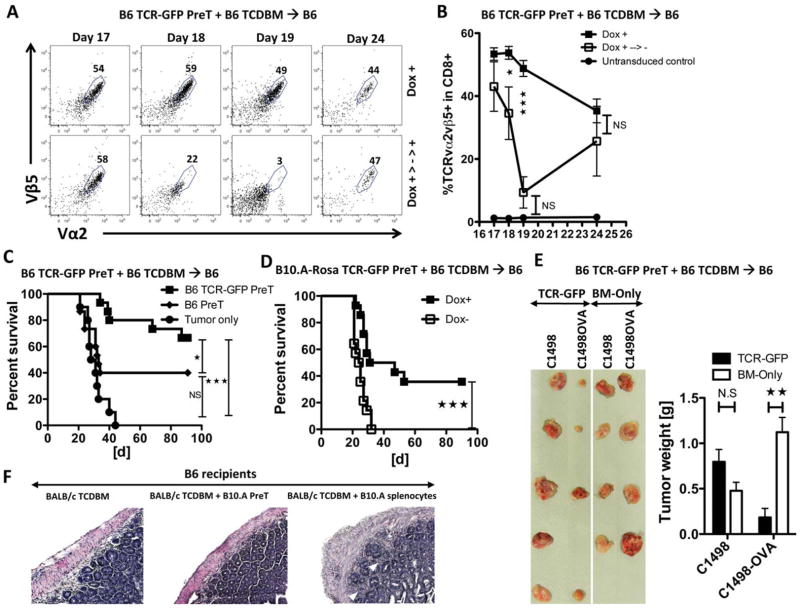
Figure 4. Co-transplantation of engineered preTs provides potent anti-leukemia effects upon TCR-induction in vivo.
(A and B) B6 recipients received 3 × 106 TCDBM together with 8 × 106 engineered preTs and early Doxycycline. Peripheral blood was analyzed by flow cytometry 17 days later and doxycycline discontinued for one group. Peripheral blood was assessed on day 18 and 19 for GFP and the co-expression of Vα2/Vβ5. Doxycycline was re-administered on day 20 and blood was assessed on day 24. n = 3 or 4 per group. (C) B6 mice received 3 × 106 syngeneic TCDBM cells and either 8 × 106 non-transduced or engineered preTs. Doxycycline was given starting the day of transplantation. One month later, 1.2 × 106 C1498-OVA leukemia cells were intravenously injected. Controls received non-transduced preTs. n = 10 to 15 per group. NS, not significant. (D) B6 recipients received 3 × 106 syngeneic TCDBM cells and 8 × 106 TCR gene-transduced B10.A-Rosa-derived preTs. Doxycycline was given to one group only. 1.2 × 106 C1498-OVA leukemia cells were injected intravenously 28 days later. (E) Here, 1 × 106 C1498-OVA were given subcutaneously to one flank and 1 × 106 C1498 to the contralateral flank of mice one day after lethal irradiation. One day later, 3 × 106 syngeneic TCDBM cells were given either without adding preTs (BM-Only) or after enrichment with 8 × 106 engineered preTs (TCR-GFP). Developing leukemia nodules were harvested 17 days later and consecutively weighted. (F) B6 mice received 15 × 106 BALB/c-derived allogeneic BM together with either 8 × 106 B10.A-derived preTs or 8 × 106 B10.A splenocytes serving as positive controls. Representative histology sections of colon slides are shown. White arrowheads point at lymphocyte infiltrations and the typical colon crypt destruction of acute GVHD. Photomicrographs were generated by a Zeiss Axio Imager-2 microscope, and were analyzed by AxioVision 4.8 software.
In other studies, B10.A-derived preTs were co-transplanted into B6 recipients of BALB/c-derived TCDBM, which were then followed for the development of clinical signs of GVHD and/or signs of autoimmunity (Figure 4F). Whereas positive controls developed severe GVHD, no clinical GVHD (by weight loss and clinical score) was observed in preT recipients (supplementary figure S3). Colon sections in recipients of B10.A preTs but not B10.A splenocytes lacked typical morphologic features associated with GVHD such as extended lymphocyte infiltration and destruction of the colon crypt structure supported these findings (figure 4F, right slide).
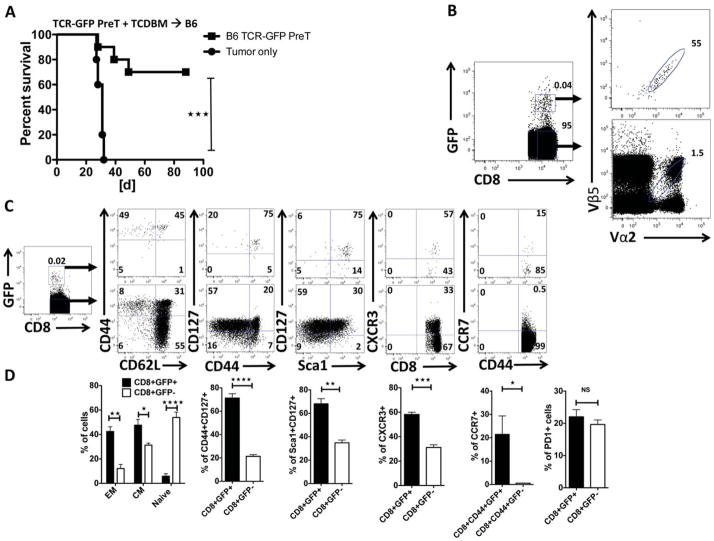
TCR gene-engineered preTs confer long-term anti-leukemia protection in vivo by memory cell formation
To assess preT-mediated long-term immunity against leukemic cells, we challenged leukemia-surviving mice with a second dose of leukemia cells. Almost two-third of the animals survived a second leukemia challenge (supplementary figure S4). 100 days after the second challenge we asked whether the survivors could stand against this third round of leukemia. Strikingly, about 40% of these mice survived this third challenge (supplementary figure S4).
In a different cohort of mice surviving a primary and secondary challenge of C1498-OVA cells, studies were performed to assess preT cell progeny in secondary lymphoid organs. As shown in figure 5A, 70% of primary C1498-OVA survivors were alive after a second challenge. The secondary lymphoid organs of surviving mice sacrificed after 100 days of rechallenge were analyzed for preT progeny that had been co-transplanted 7 months previously. As depicted in figure 5B, a tiny fraction of splenocytes did stem from the transferred preTs and approximately half expressed the introduced TCR. Analysis of T cell memory marker expression three months after the second leukemia challenge revealed that almost all of these splenic preT progenies were either of effector memory (CD8+GFP+CD44+CD62L−) or central memory (CD8+GFP+CD44+CD62L+) phenotype (figure 5C). The expression of memory markers such as CD127, CXCR3, Sca1 and CCR7 was significantly more prominent on preT progeny than on the remaining CD8+ splenocytes (figure 5C–D). Importantly, the exhaustion marker PD1 was not upregulated on the progenies of TCR-engineered preTs (figure 5D).
Figure 5. TCR gene-engineered preTs confer long-term anti-leukemia protection in vivo by memory cell formation.
(A) Surviving mice of the co-transplantation experiments were re-challenged with 1.2 × 106 C1498-OVA leukemia three months after the first challenge. Non-transplanted mice were used as controls. Pooled data of two independent transplantations (n = 10) are shown. (B–D) 95 days after the second challenge, spleens of surviving animals were harvested (n = 4) and analyzed for the progenies of co-transplanted preTs. The expression of eGFP, Vα2, Vβ5 (B), and memory T-cell markers (C and D) was determined by flow cytometry. EM, effector memory; CM, central memory
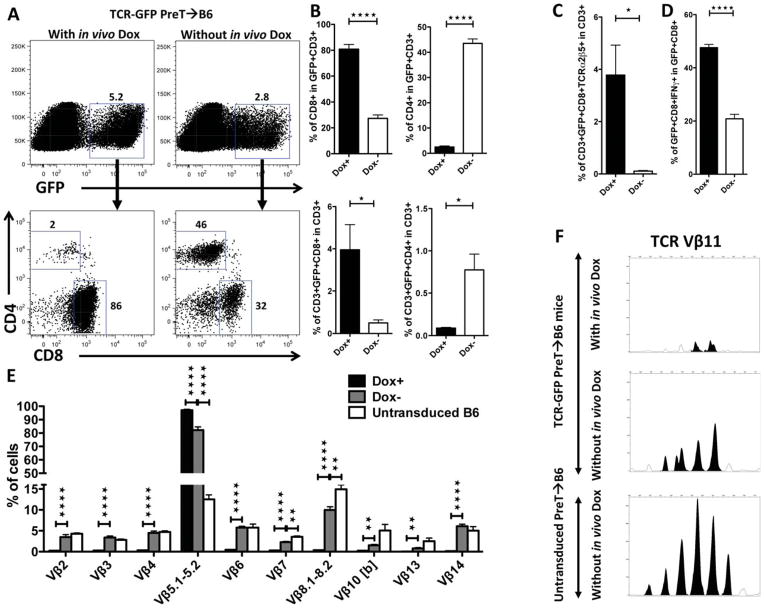
Early transgene expression in engineered preTs is required for their in vivo survival and directs final differentiation towards CD8 T cells
To further assess the impact of genetic engineering on in vivo fate of preTs, we exploited the Tet-On system for timely-controllable transgene expression. TCDBM-transplanted B6 mice received TCR gene-manipulated preTs. In one group, doxycycline was added from the day of transplantation while the controls did not receive doxycycline. Spleens were harvested one month after transplantation, exposed to doxycycline ex vivo, phenotypically analyzed by flow cytometry, and functionally tested. More than 80% of the progenies of preTs had differentiated into CD8+ T cells in the presence of doxycycline, whereas 2% only became CD4+ T cells. In contrast, when doxycycline was not administered in vivo, preTs had a higher propensity towards CD4 T cell lineage commitment (figure 6A–B). Further analysis of the thymus 14 days later revealed that high expression of the introduced TCR forced CD8 lineage commitment while low transgene expression favored CD4 development by failing to express the TCR of interest (supplementary figure S5). These observations are in line with the instructive model of T cell lineage commitment wherein MHC-I-restricted TCR expression at the double-positive stage instructs downregulation of CD4 and commitment towards CD8 development. Vice versa MHC-II-restriction is reported to drive CD4 commitment and to suppress CD8 development 23.
Figure 6. Early transgene expression in engineered preTs is required for their in vivo survival and directs final differentiation towards CD8 T cells.
(A–E) Lethally irradiated B6 mice received 3 × 106 syngeneic TCDBM cells together with either 8 × 106 non-transduced or model TCR gene-transduced preTs. For one group doxycycline was added early starting the day of transplantation while the second group did not receive any doxycycline. One month after transplantation, splenocytes were harvested and cultured ex vivo for four more days in the presence of doxycycline. (B and C) Expression of CD4 and CD8 T cell markers were assessed on T cell progenies of precursor preTs. (D) The ability for intracellular IFNγ generation was assessed upon stimulation with SIINFEKL. (E) Expression levels of a broad range of TCR Vβ families (other than Vβ5) on eGFP+ progenies of preTs were quantified by flow cytometry. (F) One month after transplantation, splenocytes were harvested and cultured ex vivo in the presence of doxycycline. After four days of culture 105 GFP+CD3+ cells were sorted and analyzed by complete TCR Vβ spectratyping as described previously17. Data were further analyzed by GeneMapper software (Life Technologies) comparing the area under the curve (AUC) representing various CDR3-size lengths. Peak Scanner software (Life Technologies) was used for calculations. Statistical analysis was done applying the Student’s t-test. A representative example is shown.
Timely-controlled transgene expression showed that early in vivo induction of the introduced TCR led to a 30-fold increase of preT-derived CD8+ T cells that expressed the introduced TCR (figure 6C). When comparing the IFNγ producing capacity of GFP+/CD8+ T cells in both groups (for with versus without in vivo doxycycline) upon SIINFEKL stimulation, we observed, that the fraction of IFNγ-releasing cells was up to 2.5-fold higher when TCR expression was induced during thymic maturation (figure 6D). Collectively, these data support the necessity of TCR expression for successful positive selection.
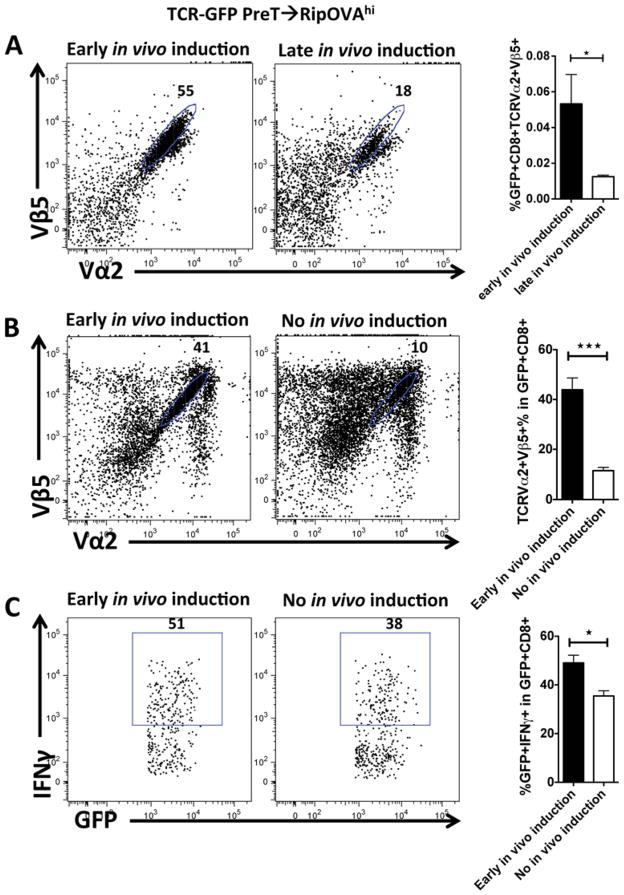
To assess the role of negative selection for an inducible TCR against a potential self-antigen in an autoreactive thymic environment we sought to keep the TCR in an off state thereby avoiding negative selection and then inducing its expression after thymus emigration. Irradiated RipOVAhi mice received B6 TCDBM cells together with TCR gene-transduced preTs. In one group mice received doxycycline early after transplantation while in the other group recipients received doxycycline starting day 22. Splenocytes were harvested at day 26 and analyzed by flow cytometry. Unexpectedly, delayed TCR induction led to a 4-fold reduction of antigen-specific CD8+ cells (figure 7A). Hypothesizing that an overwhelming presence of antigen might lead to early T cell exhaustion in vivo, mice received the inducing agent from the day of transplantation whereas controls did not receive doxycycline. Splenocytes were harvested from both groups at day 28, cultured for four days in the presence of doxycycline ex vivo, and consecutively analyzed. Again, a 4-fold reduction of antigen-specific CD8+ T cells was observed in the non-induced group (figure 7B). These data were supported by functional assays showing that the percentage of IFNγ-producing progenies of engineered GFP+ preTs upon stimulation with the cognate antigen was lower in non-induced as compared to induced recipients (figure 7C).
Figure 7. Early induction of an autoreactive TCR in engineered preTs yields the highest number of functional progenies escaping thymic selection.
(A–C) Lethally irradiated RipOVAhi mice received 3 × 106 B6 TCDBM cells together with 8 × 106 model TCR gene-transduced preTs. (A) In one group recipients received doxycycline from day 10 of transplantation (early in vivo induction) while in a second group doxycycline was given 22 days later (late in vivo induction). Spleens were harvested and assessed for functional preT-derived mature T cells on day 26 using flow cytometry. Panels are gated on CD8+GFP+ cells. (B–C) In one transplant group mice were again induced early while the other group received no doxycycline at all (no in vivo induction). After 28 days, splenocytes were isolated, cultured for four days in the presence of doxycycline, and analyzed for TCR expression by flow cytometry (B). Additionally, IFNγ-responsiveness upon stimulation with SIINFEKL was assessed (C). Panels are gated on CD8+GFP+ cells.
To further understand the functional consequences of induced TCR expression during thymic maturation, we evaluated the impact of the exogenously introduced TCR on the formation of the endogenous TCR repertoire. GFP+ splenic progenies of engineered preTs were assessed for the presence of a broad spectrum of TCR Vβ families one month after transplantation both by FACS analysis on the protein level and by spectratyping analysis on the mRNA level (figure 6E, F, and supplementary figure S6). On the protein level we observed a profound reduction of all tested endogenous TCR Vβ chains in comparison to those in the doxycycline negative group. The latter was almost comparable to progenies of non-transduced preTs. Interestingly, this difference was far less pronounced on the mRNA level.
Collectively these data underline the therapeutic relevance of the exogenous TCR expression on developing preTs even in a potentially autoreactive setting as its absence dramatically compromises their in vivo maturation.
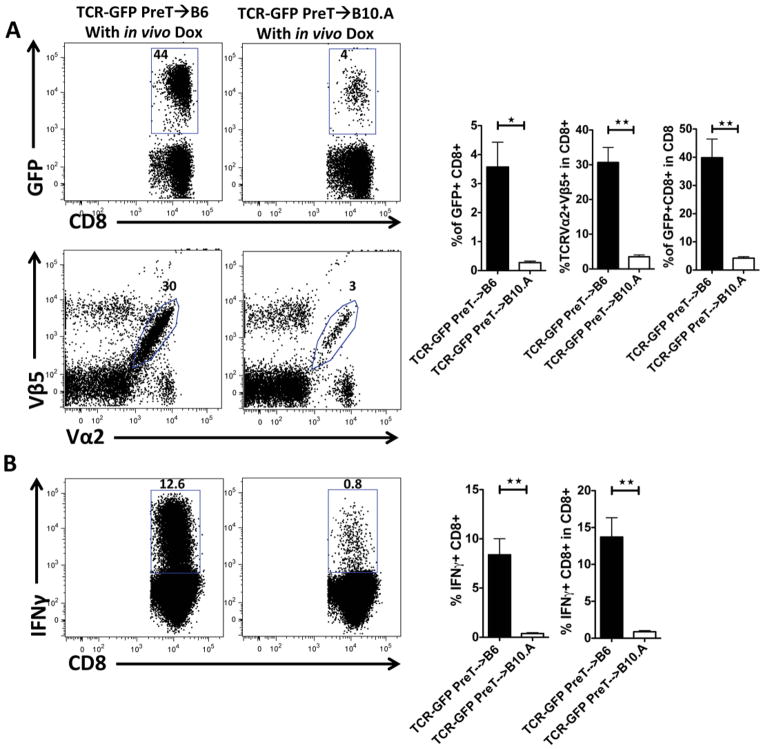
Recipient-type MHC restriction of the introduced TCR is a prerequisite for the generation of mature leukemia-reactive T cells
In order to estimate the relative contribution of negative versus positive selection in an adoptive transfer model with TCR-transduced preTs, we set to determine the role of MHC restriction of the introduced TCR to guide preTs through the positive thymic selection processes. Therefore, irradiated B6 or B10.A mice received B6 TCDBM cells together with B6-derived preTs engineered to express the model leukemia-reactive TCR and in vivo doxycycline beginning on the day of HCT. Flow cytometric analysis one month after transplantation showed that functional progenies of transferred preTs were reduced 21-fold in non-selecting B10.A as compared to positive-selecting B6 recipients (figure 8). These data help to acknowledge the dominant role of positive selection and its impact on preT therapies during thymic maturation.
Figure 8. Recipient-type MHC restriction of the introduced TCR is a prerequisite for the generation of mature leukemia-reactive T cells.
(A–B) Lethally irradiated B6 or B10.A mice received 3 × 106 or 15 × 106 B6 TCDBM cells together with 8 × 106 model TCR gene-transduced preTs. All recipients received doxycycline from the day of transplantation. After one month, splenocytes were isolated either immediately analyzed by flow cytometry (A) or cultured for four more days in the presence of doxycycline ex vivo (B) before assessing the IFNγ-responsiveness upon stimulation with SIINFEKL.
DISCUSSION
The treatment of leukemia being refractory to conventional treatment remains challenging and the risk of relapse is substantial especially for patients not transplanted in complete remission. Technologies providing potent anti-leukemic effects very early after transplantation without triggering GVHD could be highly advantageous for overcoming the propensity for relapse post-HCT.
The central role of Notch signaling for T cell development has been identified24 over the last years and has initiated significant efforts to generate a therapeutically applicable cell product in vivo.2, 3, 25 The OP9-DL1 system has shown its value for adoptive transfer of T cell-committed preTs. Among the broad array of thymocyte-like cells produced in this system the so-called DN2 cells are of particular use, since they seed the recipient’s thymus early after co-transplantation in irradiated animals and consecutively give rise to a naïve but mature T cell population of donor type in vivo.4, 5 While there are reports of co-transferring TCR gene-engineered murine hematopoietic stem cells or induced pluripotent stem cells26–29, based on our knowledge, this is the first study describing the adoptive transfer of TCR gene-modified preTs derived from murine HSCs and functionally assessing the role of a genetically engineered TCR during thymic maturation. We evaluated the concept of placing a TCR directed against a leukemia-associated antigen under the control of a drug-inducible promoter.19 It proved to be a valuable tool by shedding light on thymic maturation processes mediated by an introduced TCR in both, self-reactive and non-self-reactive environments. The immigration of preTs into the thymus is a gated process of individual thymic niches.30, 31 In our hands, large numbers of thymocytes did stem from co-transplanted preTs 14 days after co-transplantation. Thymic content of co-transplanted preTs decreased thereafter and mature, predominately CD8+/GFP+ T cells appeared in the peripheral blood, lymph nodes, and spleen. This CD8 predominance of developing TCR engineered preTs is in line with the current understanding of the development of αβ T cell lineage. It is at the double positive stage (DP) that the association among the CD4 or CD8 co-receptor, αβ TCR specificity, and function appears to be established. According to this understanding, cells expressing an αβ TCR that recognizes peptide in the context of the antigen-presentation molecule class I MHC are positively selected, down regulate the expression of CD4 and activate the gene program specific to a CD8 cytotoxic T cell. Conversely, class II MHC recognition will lead to suppression of CD8 development and activate the developmental CD4 program 23.
Challenging recipients of engineered preTs 28 days after co-transfer with a lethal dose of C1498-OVA rescued up to 70% of mice. Of note, the relative fast recovery of a leukemia-reactive T cell response after transplantation addresses an important clinical concern regarding the direct application of TCR-gene modified hematopoietic stem cells whereby gene-specific T cells reached their peak expression after 4–6 months.32 When the cell dose of TCR-transgenic preTs required for significant leukemia protection was retrospectively compared to that of TCR-transduced mature T cells in a similar leukemia model as published earlier, a more-than 2-log smaller cell dose was needed.33 This would have significant translational implications in regard of GMP-compatible scale-up.
Several leukemia-associated antigens are self-antigens. Therefore, targeting them via adoptive transfer of TCR-transduced preTs would risk cell deletion through thymic negative selection. Hence, we aimed to control transgene expression during thymic maturation of TCR-transduced preTs to protect them from deletion in an autoreactive setting. For transgene expression control, we employed a tetracycline-inducible system whose architecture was significantly optimized by one of us.19 The resulting reduction of “leakiness” minimized high background activity of earlier versions34 and allowed the expression of a tightly regulated therapeutic gene.
We then co-transplanted TCR-modified preTs in either doxycycline-exposed or doxycycline-negative RipOVAhi recipients, thereby modeling an autoreactive scenario. When the splenic progenies of preTs were quantified one month later, they were unexpectedly 4-times more frequent in the doxycycline-positive group as compared to the doxycycline-negative recipients. These results highlight the importance of early TCR expression during thymic maturation even in an autoreactive setting. This is suggestive of positive selection playing a dominant role for developing preTs even overriding negative selection. To exclude possible interference with negative selection, similar experiments were performed using wild type recipients wherein OVA is not expressed as a self-antigen. Strikingly, a 30-fold increase in antigen-specific progenies of preTs occurred upon early in vivo induction of the introduced TCR.
We now hypothesized that upon forced TCR expression preTs would effectively pass positive selection while withholding TCR induction would channel developing preTs into rearrangement processes of the endogenous TCR repertoire. The latter is believed to be random allowing a final passage of about 5% only.35 Positive selection requires MHC recognition in the thymus. We therefore tested our hypothesis by transferring TCR-transduced preTs (whereby the model leukemia-reactive TCR was H-2b restricted) to either TCDBM-transplanted B6 (H-2b) or B10.A (H-2a) recipients. A 21-fold reduction of functional preT progenies in B10.A as compared to B6 recipients was found confirming the hypothesis. This recapitulates the importance of MHC restriction for efficient positive selection. The clinical relevance in the context of genetically TCR-engineered hematopoietic stem cells has recently been underlined in a humanized mouse model demonstrating that preTs expressing a TCR against an HLA-A*0201-resticted melanoma peptide produced higher numbers of mature genetically engineered CD8+ and CD4+ T cells if the thymic epithelium of the recipient was positive for human HLA-A*0201.26
Decreasing thymic function either caused by age-dependent involution or direct damage by irradiation or chemotherapy has raised concerns regarding the effectiveness of adoptive preT transfer. Although it became evident, that the generation of new T cells in the human thymus continues throughout life 36, 37, the quantitative capacity of new T cell maturation is dependent upon a re-establishment of thymic structure. 38 Recent murine studies however have shown that in aged and even athymic HCT-recipients, extrathymic T cell maturation can support T cell development 39. Additionally, new strategies are being employed to enhance thymic function by intrathymic injection of cellular and pharmaceutical products. 40
Despite of important advantages, the therapeutic use of receptor-engineered hematopoietic precursor cells remains a challenging field requiring a receptor to I) be expressed during thymic maturation for positive selection, II) show restriction to the recipient’s MHC molecules, and III) aim to recognize preferentially leukemia-specific antigens. Targeting tumor mutations such as fusion proteins, neo-non-self mutations, and oncoviral antigens make this an attractive strategy, especially for leukemias and lymphomas 7, 13, 41–44.
Supplementary Material
Acknowledgments
The authors would like to thank R. Schwinzer and W. Baars for providing their radioisotope laboratory and the Cell Sorting Core Facility of the Hannover Medical School for their assistance. This work was supported by Deutsche Forschungsgemeinschaft, the IFB-TX (grants SFB-738 and CBT_6, M.G.S.), the Ph.D. program Molecular Medicine of the Hannover Medical School (HBRS) and the National Institutes of Health (R01 CA72669, B.R.B.).
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
AUTHORSHIP CONTRIBUTIONS
Contribution: S.S.H. designed research, performed experiments, analyzed and interpreted data, and drafted and edited the manuscript; M.H., J.H. and N.H. performed experiments; A.S. and D.V. contributed vital new reagents; M.R.M.v.d.B., D.V., R.B., B.S., and B.R.B analyzed and interpreted data and edited the manuscript; and M.G.S. designed research, analyzed, and interpreted data, drafted and edited the manuscript.
Supplementary information is available at Leukemia’s journal website.
References
- 1.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004 Aug 16;200(4):469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004 Apr;5(4):410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002 Dec;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 4.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006 Sep;12(9):1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 5.Zakrzewski JL, Goldberg GL, Smith OM, van den Brink MR. Enhancing T cell reconstitution after hematopoietic stem cell transplantation: a brief update of the latest trends. Blood Cells Mol Dis. 2008 Jan-Feb;40(1):44–47. doi: 10.1016/j.bcmd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008 Apr;26(4):453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012 Oct;26(10):2186–2196. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 8.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins RE, Gilham DE, Debets R, Eshhar Z, Taylor N, Abken H, et al. Development of adoptive cell therapy for cancer: a clinical perspective. Hum Gene Ther. 2010 Jun;21(6):665–672. doi: 10.1089/hum.2010.086. [DOI] [PubMed] [Google Scholar]
- 10.Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med. 2013 Mar 13;5(176):176ra133. doi: 10.1126/scitranslmed.3005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002 Jul;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 12.Finn OJ. Cancer immunology. The New England journal of medicine. 2008 Jun 19;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 13.Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev. 2014 Jan;257(1):145–164. doi: 10.1111/imr.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MC, Riddell SR. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev. 2014 Jan;257(1):127–144. doi: 10.1111/imr.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004 Dec;40(4):241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 16.Kelly JM, Sterry SJ, Cose S, Turner SJ, Fecondo J, Rodda S, et al. Identification of conserved T cell receptor CDR3 residues contacting known exposed peptide side chains from a major histocompatibility complex class I-bound determinant. Eur J Immunol. 1993 Dec;23(12):3318–3326. doi: 10.1002/eji.1830231239. [DOI] [PubMed] [Google Scholar]
- 17.Koestner W, Hapke M, Herbst J, Klein C, Welte K, Fruehauf J, et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2011 Jan 20;117(3):1030–1041. doi: 10.1182/blood-2010-04-283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006 Mar;3(3):191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 19.Heinz N, Schambach A, Galla M, Maetzig T, Baum C, Loew R, et al. Retroviral and transposon-based tet-regulated all-in-one vectors with reduced background expression and improved dynamic range. Human gene therapy. 2011 Feb;22(2):166–176. doi: 10.1089/hum.2010.099. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Wolenski M, Klein C, Welte K, Blazar BR, Sauer MG. Cytotoxic T cells reactive to an immunodominant leukemia-associated antigen can be specifically primed and expanded by combining a specific priming step with nonspecific large-scale expansion. J Immunother. 2008 Feb-Mar;31(2):121–131. doi: 10.1097/CJI.0b013e31815aaf24. [DOI] [PubMed] [Google Scholar]
- 21.Cooke KR, Kobzik L, Martin T, Brewer J, Delmonte JJ, Crawford J, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 22.Ghosh A, Koestner W, Hapke M, Schlaphoff V, Langer F, Baumann R, et al. Donor T cells primed on leukemia lysate-pulsed recipient APCs mediate strong graft-versus-leukemia effects across MHC barriers in full chimeras. Blood. 2009 Apr 30;113(18):4440–4448. doi: 10.1182/blood-2008-09-181677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Current opinion in immunology. 2002 Apr;14(2):207–215. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 24.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999 May;10(5):547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 25.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zuniga-Pflucker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010 Jul 15;185(2):867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 26.Giannoni F, Hardee CL, Wherley J, Gschweng E, Senadheera S, Kaufman ML, et al. Allelic exclusion and peripheral reconstitution by TCR transgenic T cells arising from transduced human hematopoietic stem/progenitor cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2013 May;21(5):1044–1054. doi: 10.1038/mt.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha SP, Klemen ND, Kinnebrew GH, Brandmaier AG, Marsh J, Hangoc G, et al. Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity. The Journal of clinical investigation. 2010 Dec;120(12):4273–4288. doi: 10.1172/JCI43274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005 Mar 22;102(12):4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, et al. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer research. 2011 Jul 15;71(14):4742–4747. doi: 10.1158/0008-5472.CAN-11-0359. [DOI] [PubMed] [Google Scholar]
- 30.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001 Feb 5;193(3):365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004 Aug 1;173(3):1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 32.Ha SP, Klemen ND, Kinnebrew GH, Brandmaier AG, Marsh J, Hangoc G, et al. Transplantation of mouse HSCs genetically modified to express a CD4-restricted TCR results in long-term immunity that destroys tumors and initiates spontaneous autoimmunity. J Clin Invest. 2010 Dec;120(12):4273–4288. doi: 10.1172/JCI43274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koestner W, Hapke M, Herbst J, Klein C, Welte K, Fruehauf J, et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host-disease after delayed adoptive transfer of T cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2010 Nov 9; doi: 10.1182/blood-2010-04-283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proceedings of the National Academy of Sciences of the United States of America. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aliahmad P, Kaye J. Commitment issues: linking positive selection signals and lineage diversification in the thymus. Immunol Rev. 2006 Feb;209:253–273. doi: 10.1111/j.0105-2896.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 36.Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000 Feb 25;18(16):1638–1641. doi: 10.1016/s0264-410x(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 37.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, et al. Generation of functional thymocytes in the human adult. Immunity. 1999 May;10( 5):569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 38.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005 Apr;115(4):930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland AM, Zakrzewski JL, Tsai JJ, Hanash AM, Dudakov JA, Smith OM, et al. Extrathymic development of murine T cells after bone marrow transplantation. J Clin Invest. 2012 Dec 3;122(12):4716–4726. doi: 10.1172/JCI60630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuckett AZ, Thornton RH, Shono Y, Smith OM, Levy ER, Kreines FM, et al. Image-guided intrathymic injection of multipotent stem cells supports lifelong T-cell immunity and facilitates targeted immunotherapy. Blood. 2014 May 1;123(18):2797–2805. doi: 10.1182/blood-2013-10-535401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer Immunotherapy Based on Mutation-Specific CD4+T Cells in a Patient with Epithelial Cancer. Science. 2014 May 9;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler JH, Melief CJM. Identification of T-cell epitopes for cancer immunotherapy. Leukemia. 2007 Sep;21(9):1859–1874. doi: 10.1038/sj.leu.2404787. [DOI] [PubMed] [Google Scholar]
- 43.Van Driessche A, Gao L, Stauss HJ, Ponsaerts P, Van Bockstaele DR, Berneman ZN, et al. Antigen-specific cellular immunotherapy of leukemia. Leukemia. 2005 Nov;19(11):1863–1871. doi: 10.1038/sj.leu.2403930. [DOI] [PubMed] [Google Scholar]
- 44.Sensi M, Anichini A. Unique tumor antigens: Evidence for immune control of genome integrity and immunogenic targets for T cell-mediated patient-specific immunotherapy. Clinical Cancer Research. 2006 Sep 1;12(17):5023–5032. doi: 10.1158/1078-0432.CCR-05-2682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.