Abstract
The neuromuscular system of helminths controls a variety of essential biological processes and therefore represents a good source of novel drug targets. The neuroactive substance, acetylcholine controls movement of Schistosoma mansoni but the mode of action is poorly understood. Here, we present first evidence of a functional G protein-coupled acetylcholine receptor in S. mansoni, which we have named SmGAR. A bioinformatics analysis indicated that SmGAR belongs to a clade of invertebrate GAR-like receptors and is related to vertebrate muscarinic acetylcholine receptors. Functional expression studies in yeast showed that SmGAR is constitutively active but can be further activated by acetylcholine and, to a lesser extent, the cholinergic agonist, carbachol. Anti-cholinergic drugs, atropine and promethazine, were found to have inverse agonist activity towards SmGAR, causing a significant decrease in the receptor’s basal activity. An RNAi phenotypic assay revealed that suppression of SmGAR activity in early-stage larval schistosomulae leads to a drastic reduction in larval motility. In sum, our results provide the first molecular evidence that cholinergic GAR -like receptors are present in schistosomes and are required for proper motor control in the larvae. The results further identify SmGAR as a possible candidate for antiparasitic drug targeting.
Keywords: Schistosome, G protein-coupled receptor (GPCR), constitutive activity, acetylcholine (ACh), G protein-coupled acetylcholine receptor (GAR), RNA interference (RNAi)
Graphical Abstract
1. Introduction
Schistosomiasis is a debilitating, chronic infection that affects over 200 million people in 74 endemic countries. Trematodes of the genus Schistosoma are the causative agents of the disease [1], with S. mansoni responsible for nearly half the infections. Currently, there is a single therapeutic option, praziquantel, and no vaccine is available. Reports of emerging resistance to praziquantel [reviewed in 2], as well as its lack of efficacy against the migratory larval stages of the parasite [3] underpin the need to develop new therapeutic targets. One area that has been especially productive in the search for new drug targets is the parasite nervous system, exemplified by the success of ivermectin, pyrantel and the more recently discovered octadepsipeptides [4].
The schistosome nervous system is involved in a variety of processes that are essential to parasite survival including migration, attachment, feeding and reproduction [5]. It is hypothesized to play a role in signal transduction via synaptic and paracrine mechanisms, as schistosomes lack a circulatory system and thus the capability for classical endocrine signaling. The key interaction controlling neuronal signaling in schistosomes involves neuroactive compounds binding to their cognate receptors and eliciting effects directly or via second messenger cascades [reviewed in 6–8]. These receptors fall into two broad classes: the Cys-loop ligand-gated ion channels and the metabotropic, heptahelical G protein-coupled receptors (GPCR). Sequencing of the S. mansoni genome [9, 10] has provided a large complement of putative neuroreceptors from both classes. Several have been cloned and characterized, including receptors for dopamine, histamine, glutamate and serotonin [11–17]. Relatively less, however, is known about the cholinergic system of schistosomes.
Acetylcholine (ACh) is a quaternary amine neurotransmitter that elicits a variety of biological effects. In vertebrates, ACh acts primarily as an excitatory neurotransmitter and controls processes such as muscular contraction, glandular secretion and memory formation [18]. ACh plays a similar excitatory role among invertebrates and its role in nematode motor function is well characterized. A notable exception to the excitatory role of ACh occurs in schistosomes, where there is evidence of ACh acting as a major inhibitory neurotransmitter or modulator. Activation of ACh receptors in S. mansoni manifests as muscular relaxation resulting in flaccid paralysis [19, 20]. Schistosomes have several putative ACh receptors that may be responsible for this phenomenon [reviewed in 7]. The majority of these receptors are nicotinic ion channels, some of which have been cloned and characterized in vitro [21, 22]. However, two muscarinic cholinergic receptors are also predicted. One of these appears to be truncated (Smp_152540) [10] but the other has all the structural features of a full-length GPCR and is worthy of further investigation.
Muscarinic acetylcholine receptors (mAChRs) are members of the heptahelical GPCR superfamily and are structurally related to rhodopsin (Family A GPCRs). They mediate their effects by interaction with heterotrimeric G proteins, causing changes in intracellular Ca2+ or cyclic adenosine monophosphate (cAMP). The term “muscarinic” is derived from these receptors’ preferential binding and activation by the fungal toxin muscarine [23]. There are 5 subtypes of mAChRs in vertebrate organisms [reviewed in 24]. Vertebrate mAChRs are located in both the central and peripheral nervous systems and are involved in a vast array of physiological processes such as memory, smooth muscle contraction and regulation of neurotransmitter release. Invertebrate mAChRs, also known as G protein-coupled acetylcholine receptors (GARs), share this functional diversity with their vertebrate homologs. Three GAR subtypes have been identified in parasitic and free-living nematodes [25–28]. Similar to vertebrate receptors, they may act in either an excitatory or inhibitory manner and are located on neurons contributing to several important nematode activities, such as muscular contraction, sensory perception and reproduction. Although structural similarity and broad expression patterns define the invertebrate GARs and vertebrate mAChRs as homologs, there are significant differences in their pharmacological profiles [28, 29]. This unique pharmacology, combined with their functional importance, marks helminth GARs as promising targets for antiparasitics.
In the present work, we describe the first functional analysis of a schistosome GAR (SmGAR), possibly the only full-length G protein-coupled acetylcholine receptor in S. mansoni. SmGAR is distantly related to nematode GARs and its expression is predicted to be highly up regulated during the early larval stages of the parasite [10]. Functional analysis in a heterologous system determined that SmGAR has high basal activity, consistent with a constitutively active receptor, but it is further activated by cholinergic agonists. Furthermore, RNAi phenotypic assays revealed that silencing of SmGAR causes significant disruption of larval motility, suggesting a potentially important role in early parasite migration.
2. Materials and Methods
2.1 Parasites
Biomphalaria glabrata snails infected with a Puerto Rican strain of S. mansoni were generously provided by the Biomedical Research Institute and BEI Resources, MD, USA. Cercariae were obtained by exposing 6–8 week-old snails to bright light [30] for 2 hours. Cercariae were then transformed into larval schistosomulae in vitro by mechanical shearing [30]. Schistosomulae were washed with Opti-MEM containing antibiotics (100 μg/ml streptomycin, 100 units/ml penicillin and Fungizone 0.25 μg/ml) and cultured for 1–3 days in Opti-MEM (no antibiotics) supplemented with 6% fetal bovine serum at 37°C/5% CO2. Adult worms were recovered by portal perfusion [30] from adult female CD1 mice 7 weeks post-infection with 250 freshly shed cercariae/mouse.
2.2 Cloning of SmGAR
Total RNA was extracted from either pooled adult worms or 24-hour-old schistosomulae, using Trizol (Invitrogen) or the RNeasy Micro Kit (Qiagen), according to manufacturers’ instructions. RNA was reverse-transcribed (RT) using MML-V and Oligo-dT primer (Invitrogen). A negative control reaction lacking MML-V reverse transcriptase (-RT), was used to rule out the possibility of contamination of cDNA with genomic DNA. Primers to amplify the full length, predicted coding sequence of Smp_145540 (SmGAR) were designed using Oligo 6.2 [31]. Primer sequences were as follows: Forward 5′-ATGAATCTATTATTTTGTTTTC-3′ and Reverse 5′-TTATAATCTTCTAAAATCACC-3′. A proofreading Phusion High Fidelity Polymerase (New England Biolabs) was used for PCR amplification according to standard protocols. Cycling conditions were as follows: 98°C/30s, 30 cycles of 98°C/10s, 54°C/60s, 72°C/60s and a final extension of 72°C/5min. All PCR products were ligated to the pJet1.2 Blunt cloning vector (Thermo Scientific) and verified by DNA sequencing of at least two independent clones.
2.3 Bioinformatics
The predicted protein sequence of SmGAR (Smp_145540) was used as a query for a BLASTp search of the NCBI non-redundant protein dataset. Homologs were aligned with SmGAR using PROMALS3D [32] and the resulting multiple sequence alignment was then inspected manually to ensure the correct alignment of highly conserved Family A GPCR transmembrane (TM) motifs. Residues of interest are described both according to their numerical position in the primary SmGAR sequence and the Ballesteros and Weinstein numbering system for Family A GPCRs [33], which is shown as a superscript. The Ballesteros and Weinstein designator describes the TM helix where the residue is located (TM 1–7) and its position within the helix relative to a reference residue. The reference is an invariant amino acid of each TM helix, which is arbitrarily given the number 50. Thus, for example, the invariant reference for TM 3 in the schistosome receptor is Arg2483.50 (position 3.50) and Asp2303.32 is a TM 3 aspartate located eighteen residues upstream from the conserved reference (position 3.32). This system is used throughout the study to compare equivalent TM residues from different receptors. Identification of TM regions was performed by TMHMMv2.0 [34] and comparison of SmGAR with crystal structures of vertebrate GPCRs available in the general Protein Database (PDB), including the human β2-adrenergic receptor (PDB Accession# 2rh1) and the rat M3 muscarinic receptor (4daj). A neighbor-joining phylogenetic tree with 1000 bootstrap replicates was built from the multiple sequence alignment and visualized with FigTree 3.0 (http://tree.bio.ed.ac.uk/software/figtree/). Accession numbers of the sequences used in the alignment can be found in Table S1.
2.4 Yeast Expression
Full-length SmGAR was ligated into a previously described yeast expression vector, Cp4258 [28, 35]. The resulting construct (Cp4258-SmGAR) was confirmed by DNA sequencing and used to transform Saccharomyces cerevesiae strain Cy13393 (MATαPFUS1-HIS3 GPA1-Gαi2(5) can1 far1Δ1442 his3 leu2 lys2 sst2Δ2 ste14::trp1::LYS2 ste18γ6-3841 ste3Δ1156 tbt1-1 trp1 ura3); kindly provided by J. Broach, Penn State University). This strain expresses the HIS3 gene under the control of the FUS1 promoter [35] and also includes an integrated copy of a chimeric Gα gene in which the first 31 and last 5 codons of the native yeast Gα (GPA1) were replaced with those of human Gαi2 subunit. Strains containing Gαq and Gαs were also tested but found to yield no receptor activity when compared to Cy13393. Yeast was cultured according to a previously established protocol [28] until mid-log phase. Yeast (200 μl) were then transformed by the lithium acetate method using 200 μg of carrier DNA and 1 μg of Cp4258-SmGAR or empty plasmid as a negative control and positive transformants were selected on synthetic complete (SC) media containing 2% glucose and lacking leucine (SC/leu−)
2.5 Yeast Receptor Activity Assays
The principle of the receptor activity assay is based upon the protocols of Wang et al. [35] as previously described [13, 14, 28]. Briefly, single colonies carrying the Cp4258-SmGAR construct or empty plasmid (mock control) were grown in SC/leu− overnight at 30°C, 250 rpm. Cells were then washed 3 times in SC medium lacking leucine and histidine (SC/leu−/his−) and finally resuspended in SC/leu−/his− supplemented with 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS), pH 6.8 and 1.5 mM 3-Amino-1, 2, 4-Triazole (3-AT). The addition of 3-AT reduces background signaling-induced basal yeast growth by inhibiting the gene product of HIS3 [36]. Yeast cells were plated at a density of 3000 cells/well to a flat-bottom 96-well plate with either test agonist at the specified concentration, vehicle alone, or SC/leu−/his+ media at a final volume of 200 μl and incubated at 30°C for a period of 24–30 hours, after which 20 μl of Alamar Blue dye (Invitrogen) was added to each well. Plates were returned to 30°C incubator until Alamar Blue began to turn pink (2–4 hours) and fluorescence (560nm excitation/590 emission) was measured every 30 minutes for a total of 4 hours using a Synergy H4 microplate fluorometer (BioTek, USA). Baseline fluorescence values from cell-free wells were subtracted from test wells and fluorescence for each test group was normalized to water-treated control cells. All results are derived from 2–3 experiments each with 6 replicates. Statistical analysis and curve fitting was done using Prism v5.0 (GraphPad Software).
2.6 Synthesis of pooled SmGAR siRNAs
A unique 219 bp fragment of SmGAR sequence was identified using BLAST analysis and amplified using Phusion High Fidelity Polymerase (New England Biolabs). Amplification primers were designed using Oligo 6.2 [31] and are as follows: Forward 5-CGAAAACAACCAAACTTGGGG-3′ and Reverse 5′-CGGTTTCTGGAACTTCATTTAAACG-3′. Products were ligated to pJET 1.2Blunt vector (Fermentas, USA) and verified by DNA sequencing. For synthesis of long double stranded RNA (dsRNA), a T7 promoter site (5′-TAATACGACTCACTATAGGGAGA-3′) was added to each end of the target fragment by PCR. The T7-flanked target sequence was used as a template for in vivo transcription of both DNA strands by the MegaScript T7 Transcription Kit (Ambion), according to the manufacturer’s instructions. The resulting dsRNA was digested by RNAseIII (Invitrogen) and purified using a Centricon YM-30 filter unit (Millipore) in order to generate a heterogeneous pool of specific siRNA. The purity and concentration of pooled siRNA were measured using a Nanodrop ND1000 spectrophotometer.
2.7 RNAi and Motility Assay
Cercariae were shed from snails and transformed in vitro by the standard protocol [30] with a slight modification. Following the final wash step, parasites were resuspended in Opti-MEM containing no antibiotics or FBS and plated at a density of ≈100 animals/well in a 24-well culture plate. Transfection of schistosomulae with SmGAR or non-relevant negative control siRNA (Silencer scrambled siRNA negative control, Ambion) was performed as previously described [37] in the presence of siPORT Neo FX Transfection Agent (Ambion, USA) at a final concentration of 50 nM. Animals were cultured for 24 hours and then assayed for motor phenotypes or harvested for quantitative PCR (qPCR). The principle of the motility assay is based upon a previously established protocol [38]. Schistosomulae were filmed for a period of 60 seconds using a Nikon SMZ1500 microscope equipped with a digital video camera (QICAM Fast 1394, mono 12 bit, QImaging) and SimplePCI version 5.2 (Compix Inc.) software. Parasite motility was then calculated using the Fit Ellipse function in the ImageJ software package (version 1.41, NIH, USA), as previously described [38] except that the definition of a body movement was revised to include any change in body length (shortening or elongation) of 5% or more. Three distinct fields were recorded for each well and a minimum of 12 animals per treatment group were measured in each experiment. The data shown are the result of three independent experiments.
2.8 Quantitative PCR (qPCR)
Total RNA was extracted from siRNA-treated schistosomulae using the RNeasy Micro RNA Extraction Kit (Qiagen) as per manufacturer’s instructions with the following modification. Animals were washed in 1X phosphate-buffered saline (PBS) and resuspended in the provided lysis buffer prior to sonication for 1 minute (6 pulses of 10s/each), as described in [38]. Total RNA was then extracted from the resulting lysate and assessed for quantity and purity using a Nanodrop ND 1000 spectrophotometer. RT reactions were performed as above, using 100 ng of RNA template per reaction. Primers to amplify a unique 250 bp fragment separate from the region used to generate the siRNA of SmGAR were designed using Oligo [31] and are as follows: Forward 5′-CAGCCTGTTTAACCTCCC-3′ and Reverse 5′-TTGAAGATAGGGTCCGTT-3′. Quantitative real-time PCR (qPCR) was performed using Platinum SYBR Green UDG SuperMix (Invitrogen) in a 25 μl reaction volume on a RotorGene RG3000 (Corbett Life Sciences, Australia). Cycling conditions were 50°C/2 min, 95°C/2 min, followed by 50 cycles of 94°C/15 s, 57°C/30 s, 72°C/15 s. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Accession # M92359) was used as an internal control and qPCR primers were: Forward 5′-GTTGATCTGACATGTAGGTTAG-3′ and Reverse 5′-ACTAATTTCACGAAGTTGTTG-3′. Relative gene expression was calculated using the Pfaffl’s method [39]. Results shown are derived from three separate experiments, each done in triplicate.
2.9 Homology Modeling
Homology modeling of SmGAR was carried out using the UCSF Chimera Package (Computer Graphics Laboratory, University of California, San Francisco) [40] and Modeller v9.12 [41]. SmGAR was aligned with several GPCR crystal structures available in the general Protein Database (PDB accession numbers 2rh1, 4daj, 1u19, 3eml) and the rat (R. norvegicus) M3 muscarinic receptor (4daj) [42] was selected as the best template, according to similarity scores. The alignment between SmGAR and the M3 receptor was edited to remove areas of low structural resolution, including portions of the N-terminal and the divergent third intracellular (il3) loop. Deletion of the il3 loop also removed the portion of the M3 structure containing the T4 lysozyme structure [42]. The default automodel feature of Modeller v9.12 was used for subsequent modeling steps and final evaluation of model accuracy. The rat M3 structure (4daj) and resulting model of SmGAR were superimposed using the Matchmaker tool.
3. Results
3.1 SmGAR is related to nematode and predicted flatworm GARs
SmGAR (Smp_145540) was amplified from oligo-dT reverse-transcribed cDNA using PCR primers that targeted the beginning and end of the predicted Smp_145540 coding sequence. Based on the most recent annotation of the S. mansoni genome [10], Smp_145540 spans 3 exons and 1938 bp, which would result in a 74 kDa protein. However, the RT-PCR consistently amplified a longer product with a single continuous reading frame of 2805 bp and a predicted protein size of 106 kDa. This was confirmed by sequencing of multiple clones. The cloned sequence is identical to the Smp_145540 transcript except for the highly variable third intracellular loop region (il3), which is longer in the cloned cDNA. Further analysis revealed that SmGAR matches exactly the Smp_145540 genomic sequence and the extra bases correspond to the two predicted introns, which are retained in the SmGAR cDNA. The absence of amplification in the negative –RT control lacking reverse transcriptase rules out possible genomic DNA contamination of the sample. Thus we conclude that the SmGAR (Smp_145540) is expressed without introns, similar to mammalian Family A GPCRs [43]. We cannot, however, rule out the possibility of a shorter SmGAR species, possibly the product of differential splicing in the il3 loop, which could not be detected in this study.
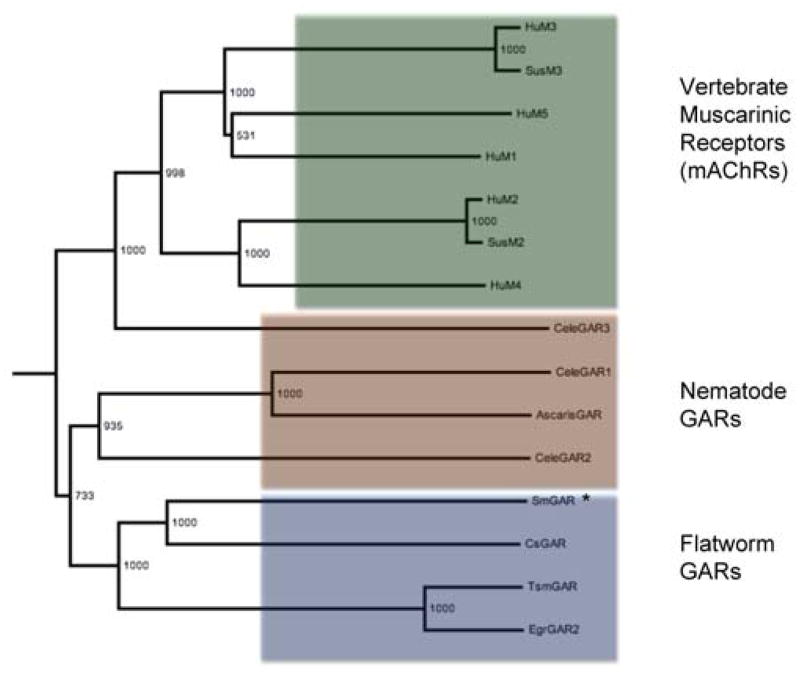
In order to identify homologs, the sequence of SmGAR was used as a query for a BLASTp search of the NCBI non-redundant protein dataset and aligned with the resulting hits. SmGAR shared the highest homology with a putative GAR-2 receptor from the trematode Clonorchis sinensis (42%), Caenorhabditis elegans GAR-2 (44%) and Ascaris suum GAR-like receptor (35%,). Although the C. elegans GAR shares a higher percent similarity with SmGAR, the Clonorchis receptor has higher coverage (96%), even across the highly divergent il3 loop. Putative homologs of SmGAR also appear in the genomes of the cestodes Taenia solium and Echinococcus granulosus [44, 45]. Phylogenetic analysis (Fig. 1) shows helminth (nematode and flatworm) GARs clearly diverging from vertebrate muscarinic receptors and the flatworm GARs form their own distinct clade within the helminth branch. All members of this clade, including SmGAR, have longer amino acid sequences than their human and nematode counterparts.
Figure 1. SmGAR is structurally related to acetylcholine receptors from other species.
A bootstrapped, neighbor-joined phylogenetic tree was generated from a structural alignment of SmGAR and putative homologs from vertebrates, nematodes and Platyhelminthes. The tree is outgroup-rooted to the human serotonergic 5-HT2 receptor (Accession# P28223) and was visualized using FigTree v3.0. Two larger groupings of receptors can be seen. The vertebrate muscarinic acetylcholine receptors (mAChRs) (green box) separate into their canonical subtypes, M1/M3/M5 (top) and M2/M4 (bottom) and also include the closely related C. elegans GAR-3 receptor. The remaining invertebrate G-protein-linked acetylcholine receptors (GARs) show a further division into the nematode GARs and predicted flatworm GARs, including SmGAR (starred). Species abbreviations are as follows: Hu, Homo sapiens; Sus, Sus scofra; Cele, Caenorhabditis elegans; Ascaris, Ascaris suum, Cs, Clonorchis sinensis; Tsm, Taenia solium; Egr, Echinococcus granulosus; Sm, Schistosoma mansoni (see Table S1 for accession numbers of aligned sequences).
3.2 SmGAR is a constitutively active acetylcholine receptor
The activity of SmGAR was assessed using a previously described yeast functional assay [28, 35]. Briefly, a plasmid containing the 2805 bp coding sequence for SmGAR was transformed into S. cerevesiae yeast that are auxotrophic for histidine. Activation of the heterologously expressed GPCR with the appropriate ligand allows for expression of the HIS3 reporter gene, which is coupled to the yeast endogenous pheromone pathway and allows the yeast to grow in histidine-deficient media. Receptor activity is then measured by yeast growth in selective media using the fluorometric redox indicator Alamar Blue (Invitrogen). Coupling to the correct guanosine nucleotide-binding protein (G protein) alpha subunit is important for receptor function. Therefore, yeast strains expressing different Gα subunits were tested and a strain expressing inhibitory Gαi (CY13393) produced the strongest response when compared to mock-transfected controls (not shown). This suggests that SmGAR couples to Gαi and agrees with our prediction of SmGAR as a GAR-2 homolog. GAR-2 was previously described as a Gi/o-coupled receptor [26].
Further analysis of SmGAR revealed that the receptor has high basal activity when expressed in yeast, suggesting a propensity towards spontaneous activation (Fig. 2A). SmGAR-expressing cells treated with vehicle (water) exhibited high levels of receptor activity, whereas no measurable activity was seen in cells transformed with empty plasmid (mock control). Once thought of as an artifact of heterologous expression, there is mounting evidence for the biological relevance of constitutively active GPCRs [reviewed in 46]. Moreover, constitutively active GPCRs often retain their ability to respond to agonists and signal above their elevated baseline [47]. In order to test this, SmGAR-expressing cells were treated with several neuroactive substances, each at a concentration of 100 μM. The results show that SmGAR is significantly activated by ACh (P < 0.0001) but not biogenic amines (tyramine and histamine) or glutamate (Fig. 2A), suggesting the response is specific. The effect of ACh was seen above the elevated baseline of SmGAR and was dose-dependent (Fig. 2B). SmGAR could also be activated by the classical cholinergic agonist, carbachol (Fig. 2C) but the response was weaker than that observed with ACh.
Figure 2. SmGAR forms a constitutively active receptor that responds selectively to acetylcholine.
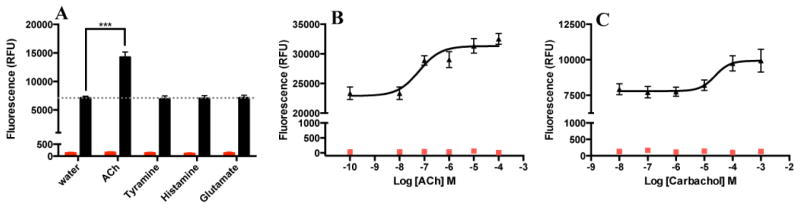
(A) Yeast cells were transformed with SmGAR expression plasmid (black bars) or empty plasmid (mock, red bars). Cells were then assayed for receptor activity in the presence of test substances each at 10−4 M or vehicle (water). The results are from a single clone of SmGAR but are representative of 3 separate clones. *** Significantly different from water-treated control at P< 0.0001. ACh (B) and carbachol (C) activate SmGAR-expressing cells (black triangles) in a concentration-dependent manner. No effect was observed in the mock control at any of the concentrations tested (red squares).
One way to characterize the pharmacology of constitutively active receptors is through the use of inverse agonists. Inverse agonists are compounds that inhibit the ligand-independent signaling of constitutively active GPCRs [48]. Often inverse agonists act as neutral antagonists on non-spontaneously activated receptors. We therefore decided to test several known cholinergic drugs for inverse agonism on SmGAR activity. Yeast cells expressing SmGAR were treated with varying concentrations of atropine, promethazine or pirenzepine in the absence of ACh. Atropine and pirenzepine are classical muscarinic receptor antagonists. Promethazine has mixed antagonist activity towards muscarinic and H1 histamine receptors [49]. The results of the functional assay show that both atropine (Fig. 3A) and promethazine (Fig. 3B) are able to decrease the high basal activity of SmGAR in a dose-dependent manner, atropine being the most potent of the two. In contrast, pirenzepine had no significant effect up to a concentration of 100 μM. There is some inhibition by pirenzepine at 1mM but the effect is quite small (< 50% inhibition) (Fig. 3C). To verify that the drug effects were receptor-mediated and not the result of generalized cytotoxicity, cells were plated in non-selective media (SC supplemented with histidine) containing the highest concentration of antagonist tested (1 mM). There was no inhibition of growth in these control cells or in the mock-transfected, antagonist-treated control cells.
Figure 3. Atropine and promethazine inhibit SmGAR constitutive activity.
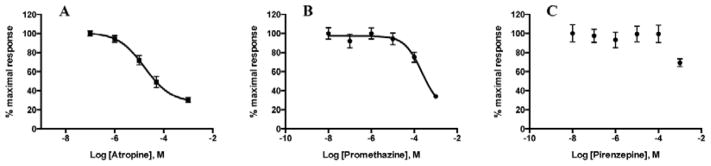
SmGAR-expressing yeast were treated with increasing concentrations of anticholinergic drugs in the absence of ACh in order to assay for inverse agonism. Atropine (A) and promethazine (B) both inhibited the high basal activity of SmGAR in a dose-dependent manner whereas pirenzepine (C) had no significant effect up to 100 μM.
3.3. SmGAR sequence analysis and homology modeling
In order to examine whether SmGAR contained unique structural features that might explain its high basal activity, we compared the schistosome receptor to cholinergic receptors from other species. A structural alignment of mAChRs and GAR-like receptors from humans, nematodes and other Platyhelminthes was generated. A homology model of SmGAR was also generated, using the crystal structure of the rat M3 muscarinic receptor (4daj) as a template. These results show that SmGAR has the typical heptahelical architecture of Family A GPCRs, and the TM regions align closely with the M3 template (Fig. 4A). The structural alignment identified several of the amino acids previously implicated in ligand binding, including the three lid-forming tyrosines of the receptor’s binding pocket (SmGAR Tyr2313.33, Tyr8696.51, Tyr8987.39) and the highly conserved aspartate of TM3 (SmGAR Asp2303.32), which is essential for binding the positively charged headgroup of ACh and related ligands [42]. However the analysis also revealed potentially important amino acid substitutions that could affect binding affinity and/or signaling activity. For example, the signature TM6 asparagine of mammalian muscarinic receptors (Asn6.52) [50, 51], which is directly involved in hydrogen bonding within the ligand binding pocket [42] is replaced with a histidine in SmGAR (His8706.52). Other important substitutions were detected at the TM3/il2 interface and the cytoplasmic end of TM6 (Fig. 4B), two regions known to play a key role in the conformational activation of GPCRs and subsequent G protein-coupling [reviewed in 50]. Of note are substitutions involving residues of the so-called “ionic lock” between TM3 and TM6, which stabilizes the inactive conformation of some GPCRs. The lock is formed in part by a salt bridge between an invariant arginine of TM3 (Arg3.50) and an acidic residue at the cytosolic interface of TM6 (Glu6.30) [50]. The TM3 arginine is conserved in SmGAR (Arg2483.50) but there is a non-conservative substitution near the interacting site (Phe →Cys2503.52) and the acidic residue of TM6 is replaced with an alanine (Glu →Ala8486.30) (Fig. 4B). As discussed later, these differences are expected to impact on receptor signaling activity and could contribute to the high basal activity of SmGAR.
Figure 4. Non-conservative amino acid substitutions may contribute to constitutive activity in SmGAR.
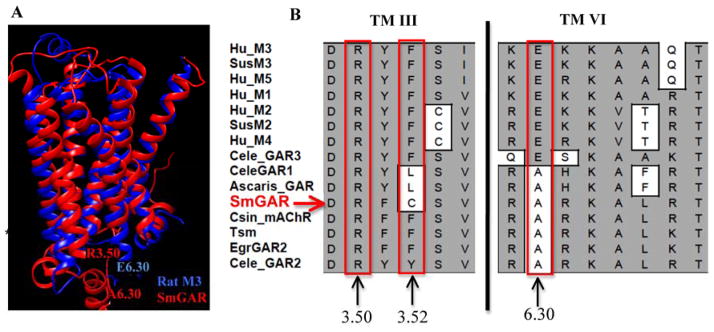
(A) A homology model of SmGAR (red) was superimposed onto the crystal structure of the rat M3 muscarinic receptor (4daj) (blue). The SmGAR model shows a typical topology of Family A GPCRs, including the canonical 7 transmembrane (TM) domains. Residues of the predicted “ionic lock” of Family A GPCRs are shown at the cytosolic ends of TM 3 and TM 6. Residue R3.50 corresponds to Arg2483.50 in SmGAR and Arg1653.50 in the rat M3 receptor. E6.30 refers to Glu4856.30 of the rat M3 receptor and A6.30 is the corresponding alanine in SmGAR (Ala8486.30). (B) Structural alignment of vertebrate and invertebrate muscarinic receptors showing a portion of TM 3 and TM6. The numbers describe the relative positions of amino acids of interest in each TM helix, according to the Ballasteros and Weinstein system [33], as described in the text. Mammalian muscarinic receptors are identified according to subtype, M1–M5; Invertebrate receptors are listed as GAR (G protein-coupled acetylcholine receptor) or mAChR (muscarinic acetylcholine receptor). Species abbreviations are as described in Fig. 1 and accession numbers are provided in Table S1.
3.4 SmGAR is plays a role in larval parasite motility
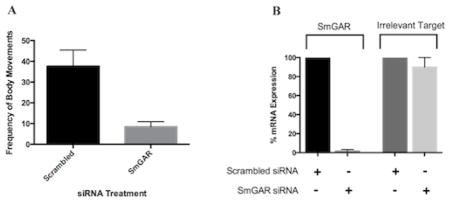
C. elegans GAR receptors are known to play an important role in worm locomotion [52, 53]. Here, we used an RNAi phenotypic assay to determine whether SmGAR has a similar function in schistosomes. Freshly transformed S. mansoni schistosomulae were treated with a pool of heterogeneous SmGAR-specific siRNA and the effect on parasite motility was measured with a quantitative imaging assay. The expression of SmGAR is predicted to be highly up-regulated in early-stage schistosomulae [10] and therefore we measured motility at 24 hours post-transfection with siRNAs. Animals treated with nonsense scrambled siRNA were also included as a negative control. Treatment with SmGAR siRNAs significantly (P < 0.01) decreased parasite motility by approximately 70% when compared to the negative control (Fig. 5A) and the silencing was confirmed by qRT-PCR (Fig. 5B). Treatment with gene-specific siRNA completely silenced the expression of SmGAR relative to the scrambled siRNA control. However, there was no change in the expression of an unrelated gene, SmACC-1 (Accession# KF694748), indicating that suppression of SmGAR was specific, at least at the RNA level.
Figure 5. Silencing of SmGAR affects the motor behavior of S. mansoni schistosomulae.
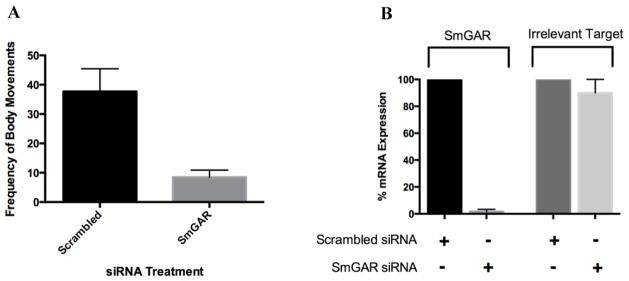
Freshly transformed schistosomulae were treated with 50 nM of either SmGAR-specific siRNA or nonsense (irrelevant) scrambled siRNA. 24 hours post-transfection schistosomulae were assayed for motor phenotype or collected for confirmation of silencing at the transcript level (A) Suppression of SmGAR in early larval schistosomulae produces a hypomotile phenotype. Animals treated with SmGAR-specific siRNA show a 70% reduction in the frequency of body movements when compared to the scrambled siRNA negative control. (B) RNA from treated parasites was oligo-dT reverse-transcribed and the resulting cDNA was used as a template for quantitative real-time PCR (qPCR). Primers targeting SmGAR or an off-target (irrelevant) schistosome gene (SmACC-1 Accession# KF694748) were used for qPCR amplification and the data were normalized to a housekeeping gene (GAPDH, Accession # M92359). Expression of SmGAR and the irrelevant off-target control were calculated as % remaining expression in the siRNA-treated samples relative to the scrambled control, using the Pfaffl’s method.
4. Discussion
Cholinergic neurotransmission is a key pathway in both nematode and flatworm motor control [5–7]. The effects of ACh are mediated through two types of receptors- ionotropic nicotinic receptors and the metabotropic muscarinic receptors or GARs. Fast cholinergic neurotransmission is mediated by the nicotinic receptors, which are members of the Cys-loop superfamily of ligand-gated ion channels. These channels are typically cation-selective, though they can also be anion-selective in invertebrates, and are expressed both neuronally and directly on muscle. Due to their importance as antiparasitic drug targets, several nematode nicotinic receptors have been cloned and pharmacologically characterized [reviewed in 54]. Among the flatworms, putative cholinergic channel subunits have been cloned from S. haematobium [21] and we have recently described a first nicotinic chloride channel in S. mansoni [22].
Invertebrate GARs belong to the GPCR superfamily and are homologs of the muscarinic cholinergic receptors in mammals. Studies primarily of C. elegans have shown that GARs control a variety of processes in nematodes, including the modulation of sensory perception, locomotion and reproductive behaviors. Three GAR receptor subtypes have been identified in free-living and parasitic nematodes [25–28] and, similar to vertebrate mAChRs, GARs may behave in either an excitatory or inhibitory manner, depending on where they are expressed and signaling mechanism. It is important to note that while nematode GARs are activated by ACh, they display a divergent pharmacological profile compared to human mAChRs. This unique pharmacology and the control exerted over motor function by cholinergic signaling make GAR homologs found in parasitic worms potential therapeutic targets [28].
In comparison to nematodes, relatively little is known about the structure or function of GARs in flatworms. Pharmacological studies carried out on the free-living flatworm Dugesia do suggest the involvement of muscarinic receptors in the control of motor function [55] and SmGAR is the first of these receptors to be characterized in a flatworm at the molecular level. Our bioinformatics analysis indicate that SmGAR shares significant homology with known GARs from nematodes as well as predicted GARs of fellow Platyhelminthes, including other trematodes and recently described tapeworm sequences [44, 45]. Interestingly, the flatworm GARs all appear to have exceptionally long il3 regions, resulting in longer protein sequences than those of nematodes or vertebrates. The il3 region of Family A GPCRs is known to play important roles in conformational activation of the receptor, binding to the G protein and regulation of signaling. It will be of interest to determine if the increased length of the il3 among flatworm GARs impacts on receptor function.
SmGAR was cloned and expressed in yeast, using a previously described GPCR functional assay [35]. Initial studies showed that cholinergic agonists selectively activated the schistosome receptor, ACh being more potent than carbachol. Interestingly, our investigation also showed that SmGAR was partially activated in the absence of agonist. Untreated SmGAR-expressing yeast cells consistently exhibited a high level of basal receptor activity when compared to cells transformed with empty vector, suggesting that SmGAR is capable of spontaneous activation. Constitutive (ligand-independent) activation of GPCRs has been well documented, not only in mammals but also invertebrates [56] and cholinergic receptors, in particular, are known to exhibit constitutive activity [46, 50]. Like SmGAR, these receptors can be further activated by a specific agonist (e.g. ACh) but their baseline is elevated. Spontaneous GPCR activity may be caused by overexpression of the receptor (or G protein partner) in recombinant systems. However, it is now generally accepted that some GPCRs have a natural propensity towards spontaneous activation and the resulting, continuous signaling can have important (patho)physiological consequences in vivo [46, 48, 57]. Extensive research into the structural basis of constitutive activity has identified regions of particular importance that affect inter- and intra-helical bonding interactions, which in turn affect the conformational shift between inactive and active states. One of these key regions is the well-described “ionic lock” of rhodopsin-like receptors. An electrostatic interaction formed between an invariant arginine of TM3 and a highly conserved acidic residue (glutamate) of TM6 holds the cytosolic ends of the two helices together, which, in turn, helps to stabilize the receptor in an inactive conformation. Disruption of this interaction is a known cause of constitutive GPCR activity [58]. Interestingly, SmGAR contains several amino acid substitutions that could interfere with formation of the ionic lock. Of note is the absence of the critical TM6 glutamate, which is replaced with an alanine in SmGAR, and a Phe→Cys substitution near the interacting site of TM3. These differences could help to explain the receptor’s propensity towards spontaneous activation. In vertebrate muscarinic receptors, Phe→Cys and Glu→Ala point mutations at these same positions both caused significant constitutive activity, most likely due to destabilization of bonding interactions that favor the off-state of the receptor [58]. Interestingly, the Ascaris GAR receptor carries nonconservative substitutions at these two sites and also displays a significant level of constitutive activity in the yeast expression system [28]. Moreover, our sequence analyses show that most invertebrate GAR-like sequences analyzed carry the Glu→Ala substitution in TM6. This conserved substitution may suggest a family of constitutively active GARs in invertebrates, or point to a fundamental difference in the mechanism of conformational activation between invertebrates and vertebrates.
To further investigate the high basal activity of SmGAR we repeated assays in the presence of predicted cholinergic inverse agonists. As explained earlier, inverse agonists are compounds that display a negative intrinsic activity - they preferentially bind to and stabilize the inactive conformation of a receptor, thus reducing basal activity [48]. Often compounds that act as competitive antagonists will display inverse agonism when tested on constitutively active receptors [48]. Here we examined the response of SmGAR to three drugs with known anti-cholinergic activity, atropine, pirenzepine and promethazine. The latter was tested because it is a potent inhibitor of the Ascaris GAR-like receptor [28] and we questioned whether the drug might also recognize SmGAR. The results showed that atropine and promethazine abrogated the high basal activity of SmGAR in a dose-dependent manner. These effects are consistent with inverse agonism (rather than antagonism) because the inhibition occurred in the absence of added ACh. Moreover the drug effects are selective. Whereas atropine and promethazine decreased basal activity, pirenzepine had no significant effect, possibly because it does not recognize the inactive form of the receptor. Combined with the unusual structural features of SmGAR discussed above, the response to these drugs strongly suggests that the schistosome receptor has intrinsic constitutive activity. However, we cannot rule out the possibility of other factors contributing to high basal activity, such as receptor overexpression or possibly non-specific activation by some unknown factor in the yeast system. Though the full significance of these findings remains unclear, the possibility that SmGAR has constitutive activity merits further investigation, especially if the receptor is responsive to inverse agonists as suggested by our data. Inverse agonists are increasingly recognized for their therapeutic benefits in a variety of human diseases [48, 59] and are potential tools for anthelmintic drug discovery. Future studies of SmGAR pharmacology will need to incorporate inverse agonist screens, together with conventional (competitive) antagonist assays, so as to identify specific receptor inhibitors.
Having characterized SmGAR in vitro, we began to investigate the potential role of the receptor in the worm using RNAi. Given that the receptor is upregulated in early larval stages [10] and ACh is well known to control worm movement, we questioned whether SmGAR might play a role in the control of larval motility. RNAi is well established in schistosomes [60] and has been successful in elucidating the role of neuronal proteins in motor function [17, 22, 38]. Here, we modified a previously developed RNAi behavioral assay [17, 38] to assess the role of SmGAR in larval schistosomulae. Transcriptional profiling of SmGAR indicates that it is most highly expressed in cercariae and first 24 hours of the schistosomulae stage [10]. Therefore, transfected schistosomulae were assayed for motility within the first 24 hours of transformation. Treatment of schistosomulae with SmGAR siRNA caused a significant decrease in motor activity when compared to control animals, and this correlated with near complete knockdown of the transcript, as determined by RT-qPCR. The results suggest that SmGAR does play a role in motor control in the young larvae and its activity stimulates larval movement, since the RNAi phenotype was clearly hypoactive.
The RNAi phenotype of SmGAR may seem surprising at first, given that ACh is known to inhibit schistosome movement. Removal of an inhibitory mechanism would be expected to increase motility, whereas the opposite was observed. To explain the results it is important to keep in mind that SmGAR is one of many cholinergic receptors in schistosomes [7] and the flaccid paralysis caused by ACh in vitro [19, 20] is likely due to stimulation of multiple receptors all at once. Some of these receptors do have inhibitory effects on movement [22] but there could be stimulatory pathways that were missed in the earlier studies. Based on the RNAi data, SmGAR is the first example of a cholinergic receptor that stimulates schistosome motor activity, at least in the young larvae. How the receptor is able to influence movement is unknown at present. In C. elegans, GARs control locomotion indirectly by modulating neuronal output to the muscles. The C. elegans GAR-2 receptor, in particular, is expressed on cholinergic motorneurons and acts to suppress the activity of ACh as a form of negative feedback mechanism [52]. If SmGAR functions in a similar manner, then the hypoactive RNAi phenotype could be explained by loss of the negative feedback provided by the receptor, which would result in increased ACh signaling and reduced movement in the RNAi-abrogated animals. However more studies are needed to elucidate the exact mode of action of SmGAR.
In conclusion, we have described the cloning and first functional characterization of a new cholinergic GPCR in S. mansoni. The results identify a receptor that has intrinsic constitutive activity but can be further and selectively activated by ACh. SmGAR shares high structural homology with GAR receptors in C. elegans and RNAi experiments suggest an important role in motor control, similar to nematode GARs. Given these unique qualities and the importance of motor function to schistosome survival, we believe that SmGAR merits further investigation as a novel antischistosomal drug target.
Supplementary Material
Highlights.
SmGAR is a new G protein-coupled acetylcholine receptor of Schistosoma mansoni
SmGAR has constitutive activity and can be further activated by acetylcholine
The cholinergic drug atropine has inverse agonist activity towards SmGAR
RNAi targeting SmGAR produced a hypoactive phenotype in cultured schistosomula
SmGAR is a potential candidate for anti-schistosomal drug targeting.
Acknowledgments
B. glabrata snails infected with S. mansoni were kindly provided by the Biomedical Research Institute (Bethesda, MD, USA) via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I. This research was funded by NIH Grant No. R01 AI093703-01A1 to MJK, TAD and PR and a Natural Sciences and Engineering Research Council of Canada (NSERC) Grant No. 169998-2012 to PR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human Schistosomiasis. Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111(5):1871–7. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- 3.Sabah AA, Fletcher C, Webbe G, Doenhoff J. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 4.Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. The effect of the anthelminthic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/s0031182002002639. [DOI] [PubMed] [Google Scholar]
- 5.Maule AG, Day TA, Chappell CH. Parasite neuromusculature and its utility as a drug target. Parasitology. 2005;131:S1–S2. [Google Scholar]
- 6.Ribeiro P, El-Shehabi F, Patocka N. Classical transmitters and their receptors in flatworms. Parasitology. 2005;131:S19–S40. doi: 10.1017/S0031182005008565. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro P, Geary T. Neuronal signaling in schistosomes: Current status and prospects for post-genomics. Can J Zool. 2010;88:1–22. [Google Scholar]
- 8.Ribeiro P, Gupta V, El-Sakkary N. Biogenic amines and the control of neuromuscular signaling in schistosomes. Invert Neurosc. 2012;12 (1):53–61. doi: 10.1007/s10158-012-0132-y. [DOI] [PubMed] [Google Scholar]
- 9.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis. 2012;6(1):e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamdan F, Abramovitz M, Mousa A, Xie J, Durocher Y, Ribeiro P. A novel Schistosoma mansoni G protein – coupled receptor is responsive to histamine. Mol Biochem Parasitol. 2002;119:75–86. doi: 10.1016/s0166-6851(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 12.El-Shehabi F, Vermeire J, Yoshino T, Ribeiro P. Developmental expression analysis and immunolocalization of a G protein coupled receptor in Schistosoma mansoni. Exp Parasitol. 2009;122:17–27. doi: 10.1016/j.exppara.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Shehabi F, Ribeiro P. Histamine signaling in Schistosoma mansoni: Immunolocalization and characterization of a new histamine-responsive receptor (SmGPR-2) Int J Parasitol. 2010;40:1395–1406. doi: 10.1016/j.ijpara.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 14.El-Shehabi F, Taman A, Moali LS, El-Sakkary N, Ribeiro P. A novel G protein-coupled receptor of Schistosoma mansoni (SmGPR-3) is activated by dopamine and is widely expressed in the nervous system. PLoS Negl Trop Dis. 2012;6(2):e1523. doi: 10.1371/journal.pntd.0001523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taman A, Ribeiro P. Investigation of a dopamine receptor in Schistosoma mansoni: Functional studies and immunolocalization. Mol Biochem Parasitol. 2009;168:24–33. doi: 10.1016/j.molbiopara.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Taman A, Ribeiro P. Glutamate-mediated signaling in Schistosoma mansoni: a novel glutamate receptor is expressed in neurons and the female reproductive tract. Mol Biochem Parasitol. 2011;176(1):42–50. doi: 10.1016/j.molbiopara.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Patocka N, Sharma N, Rashid M, Ribeiro P. Serotonin signaling in Schistosoma mansoni: A Serotonin–activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 2014;10(1):e1003878. doi: 10.1371/journal.ppat.1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 19.Barker LR, Bueding E, Timms AR. The possible role of acetylcholine in Schistosoma mansoni. Brit J Pharmacol. 1966;26:656–665. doi: 10.1111/j.1476-5381.1966.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day TA, Chen GZ, Miller C, Tian M, Bennett JL, Pax RA. Cholinergic inhibition of muscle fibers isolated from Schistosoma mansoni (Trematoda: Digenea) Parasitology. 1996;113(Pt. 1):55–61. doi: 10.1017/s0031182000066270. [DOI] [PubMed] [Google Scholar]
- 21.Bentley GN, Jones AK, Oliveros Parra WG, Agnew A. ShAR1alpha and ShAR1beta: novel putative nicotinic acetylcholine receptor subunits from the platyhelminth blood fluke Schistosoma. Gene. 2004;329:27–38. doi: 10.1016/j.gene.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald K, Buxter S, Kimber M, Day T, Robertson AP, et al. Functional characterization of a novel family of acetylcholine-gated chloride channels in Schistosoma mansoni. PLoS Pathog. 2014;10(6):e1004181. doi: 10.1371/journal.ppat.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dale HH. The action of certain esters and ethers of choline, and their relation to muscarine. J Pharmacol Exp Ther. 1914;6:147–190. [Google Scholar]
- 24.Eglen RM. Overview of muscarinic receptor subtypes. Handb Exp Pharmacol. 2012;208:3–28. doi: 10.1007/978-3-642-23274-9_1. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Park YS, Chang DJ, Hwang JM, Min CK, et al. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Park YS, Nam S, Suh SJ, Lee J, et al. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 2000;75:1800–1809. doi: 10.1046/j.1471-4159.2000.0751800.x. [DOI] [PubMed] [Google Scholar]
- 27.Hwang JM, Chang DJ, Kim US, Lee YS, Park YS, et al. Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptors Channels. 1999;6:415–424. [PubMed] [Google Scholar]
- 28.Kimber MJ, Sayegh L, El-Shehabi S, Song C, Zamanian M, et al. Identification of an Ascaris G protein-coupled acetylcholine receptor with atypical muscarinic pharmacology. Int J Parasitol. 2009;39(11):1215–22. doi: 10.1016/j.ijpara.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannan F, Hall LM. Muscarinic acetylcholine receptors in invertebrates: comparisons with homologous receptors from vertebrates. EXS. 1993;63:98–145. doi: 10.1007/978-3-0348-7265-2_6. [DOI] [PubMed] [Google Scholar]
- 30.Lewis F. Schistosomiasis. Current Protocols in Immunology. 2001;28:19.1.1–19.1.28. doi: 10.1002/0471142735.im1901s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rychlik W. OLIGO 7 Primer Analysis Software. In: Yuryev A, editor. Methods in Molecular Biology Vol. 402: PCR Primer Design. Totowa: Humana Press; 2007. pp. 35–59. [DOI] [PubMed] [Google Scholar]
- 32.Pei J, Kim BH, Grishin NV. PROMALS3D: a tool for multiple sequence and structure alignment. Nucleic Acids Res. 2008;36(7):2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballasteros J, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Meth Neurosc. 1995;25:366–428. [Google Scholar]
- 34.Krogh B, Larssen B, von Heijne G, Sonnehammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Molec Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZX, Broach JR, Peiper SC. Functional expression of CXCR4 in Saccharomyces cerevisiae in the development of powerful tools for the pharmacological characterization of CXCR4. In: Ali H, Haribabu B, editors. Methods in Molecular Biology, vol. 332: Transmembrane Signaling Protocols. 2. Humana Press Inc; Totowa, NJ: 2006. pp. 115–127. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson BJ, Rhodes N, Errede B, Sprague GF., Jr Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- 37.Nabhan JF, El-Shehabi F, Patocka N, Ribeiro P. The 26S proteasome in Schistosoma mansoni: bioinformatics analysis, developmental expression, and RNA interference (RNAi) studies. Exp Parasitol. 2007;117(3):337–47. doi: 10.1016/j.exppara.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Patocka N, Ribeiro P. The functional role of a serotonin transporter in Schistosoma mansoni elucidated through immunolocalization and RNA interference (RNAi) Mol Biochem Parasitol. 2013;187:32–42. doi: 10.1016/j.molbiopara.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. UCSF Chimera - A Visualization System for Exploratory Research and Analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 41.Eswar N, Marti-Renom MA, Webb B, Madhusudhan MS, Eramian D, et al. Comparative Protein Structure Modeling With MODELLER. Current Protocols in Bioinformatics. 2006;S15:5.6.1–5.6.30. doi: 10.1002/0471250953.bi0506s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fridmans D, Fredriksson R, Kappa I, Sciöth HB, Klovins J. Formation of new genes explains lower intron density in mammalian Rhodopsin G protein-coupled receptors. Mol Phylogenet Evol. 2007;43(3):864–80. doi: 10.1016/j.ympev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Chen W, Wang X, Liu H, Chen Y, et al. The carcinogenic liver fluke, Clonorchis sinensis: new assembly, reannotation and analysis of the genome and characterization of tissue transcriptomes. PLOS One. 2013;8(1):e54732. doi: 10.1371/journal.pone.0054732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496(7443):57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spalding TA, Burstein ES. Constitutive activity in muscarinic acetylcholine receptors. J Recept Signal Transduction. 2006;26:61–85. doi: 10.1080/10799890600567349. [DOI] [PubMed] [Google Scholar]
- 47.Burstein ES, Spalding TA, Brann MR. The second intracellular loop of the m5 muscarinic receptor is the switch which enables G-protein coupling. J Biol Chem. 1998;273:24322–24327. doi: 10.1074/jbc.273.38.24322. [DOI] [PubMed] [Google Scholar]
- 48.de Ligt RA, Kourounakis AP, Ijzerman AP. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br J Pharmacol. 2000;130(1):1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanai K. Anticholinergic activity of antihistamines. Clin Neurophysiol. 2012;123(4):633–4. doi: 10.1016/j.clinph.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Leach K, Simms J, Sexton PM, Christopoulos A. Structure-function studies of muscarinic acetylcholine receptors. Handb Exp Pharmacol. 2012;208:29–48. doi: 10.1007/978-3-642-23274-9_2. [DOI] [PubMed] [Google Scholar]
- 51.Blüml K, Mutschler E, Wess J. Functional role in ligand binding and receptor activation of an asparagine residue present in the sixth transmembrane domain of all muscarinic acetylcholine receptors. J Biol Chem. 1994;269(29):18870–6. [PubMed] [Google Scholar]
- 52.Dittman JS, Kaplan JM. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J Neurosci. 2008;28(28):7104–14. doi: 10.1523/JNEUROSCI.0378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keating CD, Kriek N, Daniels M, Ashcroft NR, Hopper NA, et al. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Current Biol. 2003;13(19):1715–20. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Holden-Dye L, Joyner M, O’Connor V, Walker RJ. Nicotinic acetylcholine receptors: A comparison of the nAChRs of Caenorhabditis elegans and parasitic nematodes. Parasitol Int. 2013;62(6):606–15. doi: 10.1016/j.parint.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Butarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125(2):225–31. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 56.Bouchard C, Ribeiro P, Dube F, Anctil M. A new G protein-coupled receptor from a primitive metazoan shows homology with vertebrate aminergic receptors and displays constitutive activity in mammalian cells. J Neurochem. 2003;86:1149–1161. doi: 10.1046/j.1471-4159.2003.01924.x. [DOI] [PubMed] [Google Scholar]
- 57.Meye FJ, Ramakers GMJ, Adan RAH. The vital role of constitutive GPCR activity in the mesolimbic dopamine system. Transl Psychiatry. 2014;4:e361. doi: 10.1038/tp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel R, Mahalingam M, Lüdeke S, Huber T, Siebert F, et al. Functional role of the “ionic lock”-an interhelical hydrogen-bond network in family A heptahelical receptors. J Mol Biol. 2008;4:648–55. doi: 10.1016/j.jmb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends in Pharmacol Sci. 2006;27(2):92–6. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Kreutz-Peterson G, Radwanska M, Ndegua D, Shoemaker CK, Skelly PJ. Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol. 2007;153(2):194–202. doi: 10.1016/j.molbiopara.2007.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.