Abstract
Background
The clinical significance of subcentimeter nodules identified on staging chest Computed Tomography (CT) for sarcoma remains unknown. Our goal was to evaluate the effect of initial pulmonary nodule size and number on survival rates in young, newly diagnosed sarcoma patients.
Methods
Medical records were reviewed for all patients ≤50 years of age with primary, high-grade bone or soft tissue sarcoma at our institution over a 10-year period. This population was divided into patients with no nodules (Group 1); 1 nodule <5 mm (Group 2); >1 nodule <5 mm (Group 3); and ≥1 nodule ≥5 mm (Group 4). Kaplan-Meier analyses with log rank tests were performed to compare overall and disease free survival between these 4 groups, as well as between patients with unilateral and bilateral nodules.
Results
There were 74 patients in Group 1 (59.2%), 26 in Group 2 (21%), 11 in Group 3 (9%), and 13 in Group 4 (10%). Mean follow up was 74 (range 6-191) months. Survival was only slightly worse with larger nodules but significantly worse with multiple nodules. In addition, patients with bilateral nodules had a significantly worse prognosis than those with multiple unilateral nodules.
Conclusions
This data suggests that in young patients with high grade sarcoma, the number and distribution of subcentimeter pulmonary nodules are an important prognostic factor, while nodule size may be less relevant.
Keywords: Sarcoma, Osteosarcoma, Neoplasm Metastasis, Solitary Pulmonary Nodule, Multiple Pulmonary Nodules, Prognosis, Survival, Disease-Free Survival
Introduction
In patients newly diagnosed with sarcoma, classification of the disease as localized or metastatic has several important implications with respect to surgical intervention, inclusion in clinical trials, and counseling regarding prognosis. Because the lungs are the most common site for metastases of bone and soft tissue sarcomas, routine staging includes Computed Tomography (CT) exams of the chest to screen for pulmonary involvement. However, since 1996 the COG has had an evolving but inconsistent and unvalidated definition of ‘pulmonary metastasis’ based on initial CT imaging for sarcoma. While it is generally agreed that multiple or large nodules represent metastases, the significance and optimal management of subcentimeter lesions remains unclear.1
The availability of thin slice CT technology introduced further uncertainty, increasing the frequency of positive tests by detecting nodules <5 mm in diameter.2 The ability of even experienced radiologists using this enhanced imaging modality to identify metastatic pulmonary nodules has been shown to be unreliable.3 Subcentimer pulmonary lesions may regress or resolve during chemotherapy; however, this does not resolve the question of their etiology. These lesions may represent responsive metastasis, transient benign findings (such as recent infection or artifact of sedation such as atelectasis), or simply different degrees of sensitivity between scanners and radiologists. In spite of this, lung biopsy prior to treatment is often not recommended or accepted and, in our study, only 1of 50 patients underwent initial biopsy of a subcentimeter nodule. The resulting uncertainty surrounding the clinical significance of these pulmonary nodules continues to pose a challenge in the management of sarcoma patients.
At the COG Annual Meeting in 2008, the Children's Oncology Group (COG) identified “small pulmonary nodules” as an area requiring additional research (COG Annual Meeting, October, 2008); to date, however, the literature remains scant. The few studies that address this issue do not include all patients under the age of 50 years, a group that is the target of most of current COG sarcoma protocols.4 Our institution has maintained a combined adult and pediatric oncology sarcoma program for 30 years, treating all patients under 50 according to standard COG protocols. Over this period of time, the definition of pulmonary metastasis in sarcoma has not been uniform in either COG or the medical community. For example, certain COG protocols have considered any number of nodules > 0.5 mm or multiple nodules > 3 mm as “evidence” of metastasis, with smaller and fewer nodules considered “questionable.” Definitions of metastatic disease have been especially inconsistent as they relate to more than one nodule ranging from 1-4 mm in diameter.(5-23)
Given the impossibility of biopsying every lesion, and in the absence of a large comprehensive study, we sought to better understand the clinical significance of indeterminate pulmonary nodules by examining the cohort of young sarcoma patients treated at our institution. Our purpose was to determine the long term outcome of subcentimeter pulmonary nodules identified on initial thin cut CT scan in patients age 0-50 years who were newly diagnosed with sarcoma and treated uniformly according to standard protocols from COG or NCCN guidelines.
Methods
Under an IRB approved protocol, a review was performed of young patients who were diagnosed with high-grade bone or soft tissue sarcoma and treated at a single referral center. Patients were identified through a clinical database maintained by the institution's sarcoma practitioners, as well as a search of the pathology database for all patients with sarcoma diagnoses. The 10 year study period began in 1998 with the introduction of thin slice CT scans to our institution and ended in 2008 to allow for appropriate length of follow up. In concordance with COG, the study populations included all patients aged ≤50 years with diagnosis of high grade sarcoma, treated with chemotherapy, and without obvious metastatic disease (nodules >1 cm or non-pulmonary lesions) at the time of diagnosis. Subjects were subsequently excluded for history of a second malignancy or treatment prior to presentation.
Electronic medical records and patient charts were reviewed for patient demographics (age and gender) and oncologic data (sarcoma type, primary tumor size, and location). Initial CT scan reports were reviewed for diameter, location, and number of pulmonary nodules/masses. Patients were then categorized according to presence and size of nodules as follows: no nodules (Group 1); 1 nodule <5 mm (Group 2); more than one nodule <5 mm (Group 3); and any nodule ≥5 mm (Group 4). All patients underwent surgical excision of the primary tumor with adjuvant and neoadjuvant chemotherapy according to COG or National Comprehensive Cancer Network (NCCN) standard of care. Ninety-six patients were either enrolled in or following COG therapeutic trials, while 30 were not. All osteosarcoma patients were treated with methotrexate, adriamycin, and either cis or carboplatinuum; of these, several received additional ifosfamide or ifosfamide and etoposide according to the standard treatments of the time. Ewing sarcoma patients were all treated with vincristine, doxorubicin, cyclphosphomide, ifosfamide and etoposide, while soft tissue sarcoma patients recieved ifosfamide and doxorubicin. Only one patient underwent nodule biopsy at time of diagnosis; in addition, subcentimeter nodule(s) that arose or progressed during or following initial treatment were managed with excisional biopsy according to COG/NCCN standards.
Kaplan-Meier survival curves for months of overall survival and DFS (no evidence of disease on physical exam and pulmonary imaging) were constructed for Groups 1-4 and compared using a log rank test. Osteosarcoma was the only histology subgroup large enough for separate analysis. Among patients with multiple nodules (groups 3 and 4), Kaplan-Meier curves and log rank test were used to compare overall and DFS in those with unilateral versus bilateral nodules. Patient age, gender, diagnosis, primary tumor size (≥5 vs <5 cm), and primary tumor location (axial vs appendicular and bone vs soft tissue) and were recorded and compared between groups using chi squared testing. Data was analyzed using the SPSS version 10.0.5 (SPSS Inc, Chicago, Illinois), and an alpha value of 0.05 was considered significant.
Results
Of the 134 young sarcoma patients that met the inclusion criteria, 8 were excluded (4 for history of previous malignancy, 3 for treatment prior to presentation, and 1 for two simultaneous primary malignancies), leaving 126 available for study. Mean age was 23.5 years (range 1.8-49.8 years), and mean overall follow-up was 74 months (range 6-191 months). The study population was comprised of 50 females and 76 males; 99 primary bone and 27 soft tissue sarcomas; 101 appendicular and 25 axial tumors. Diagnoses included osteosarcoma (66), Ewing's sarcoma (24), fibrosarcoma (8), synovial sarcoma (7), undifferentiated pleiomorphic sarcoma (6), leiomyosarcoma (3), chondrosarcoma (2), liposarcoma (2), and other (8). Seventy-four patients (59.2%) presented with no nodules on CT (Group 1); 26 (21%) had a single nodule <5 mm (Group 2); 11 (9%) had >1 nodules <5 mm (Group 3); and 13 (10%) had 1 or more nodules ≥5 mm (Group 4). There was no statistically significant difference between Groups 1-4 with respect to patient age, gender, diagnosis, primary tumor size, and primary tumor location.
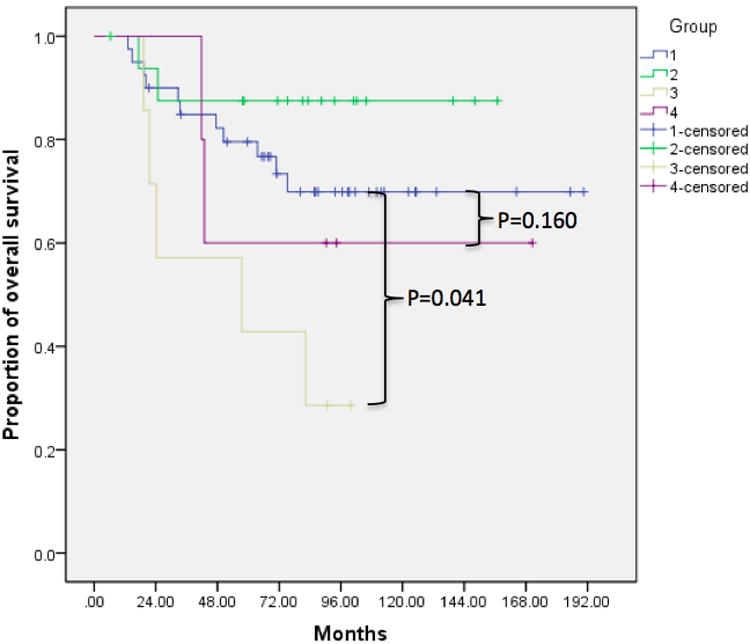
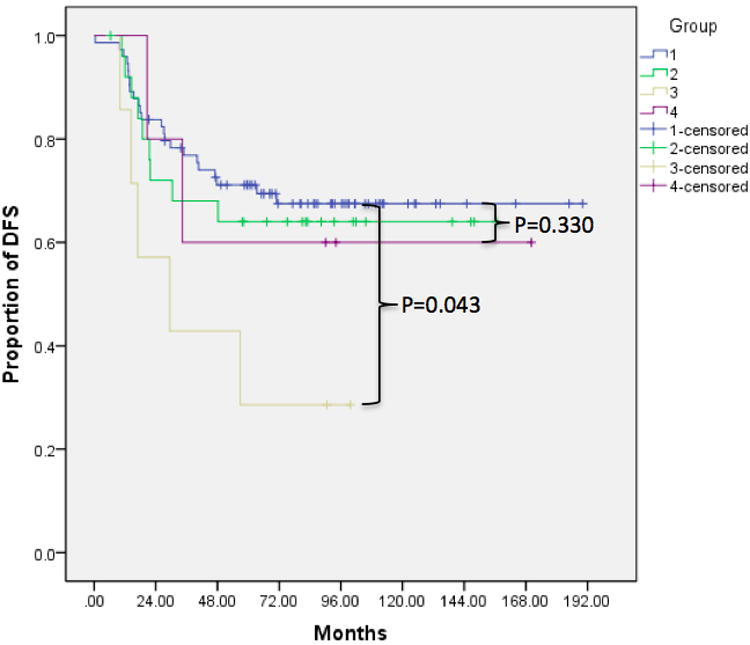
Kaplan-Meier analysis with log rank test revealed statistically decreased survival in patients with multiple small nodules (Group 3 compared to Group 1) for all sarcomas (mortality p=0.022, DFS p=0.015) as well as only osteosarcomas (mortality p=0.041, DFS p=0.043). In addition, we observed a trend toward worse prognosis with larger nodules (Group 4 compared to Group 1) for all sarcomas (mortality p=0.116, DFS p=0.231) and osteosarcomas (mortality p=0.160, DFS p=0.330). Compared to patients with no pulmonary nodules, those with a single <5 mm nodule (Group 2 compared to Group 1) did not show significant improvement in prognosis (mortality p=0.682 and DFS p=0.662 for all sarcomas; mortality p=0.685, DFS p=0.804 for osteosarcomas) (Figure 1, Table 2). The 2- and 5-year survival rates for patients in each group are shown in Table 3. Finally, we observed significantly worse prognosis in patients with multiple bilateral compared to multiple unilateral nodules (p=0.023 for overall survival, p=0.014 for DFS) (Figure 2).
Figure 1.
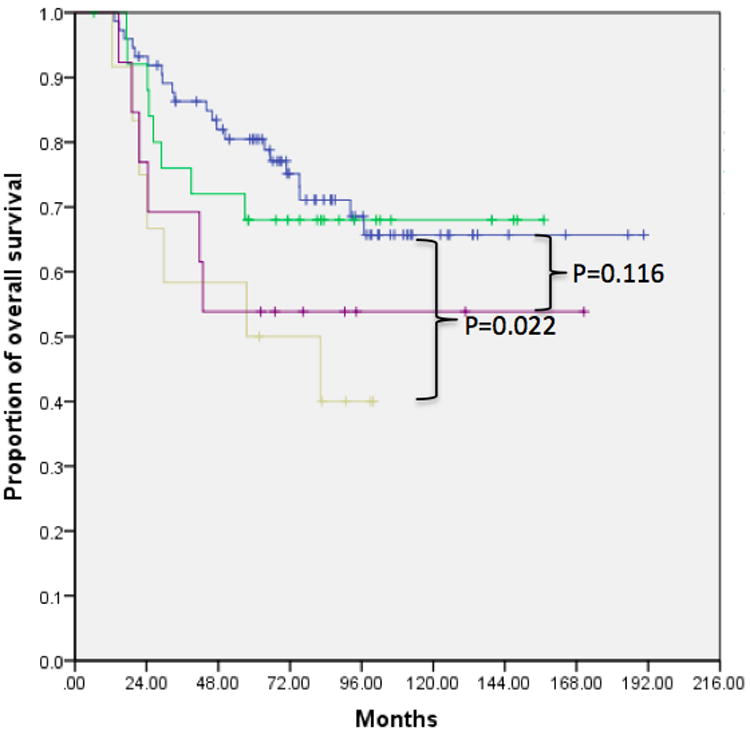
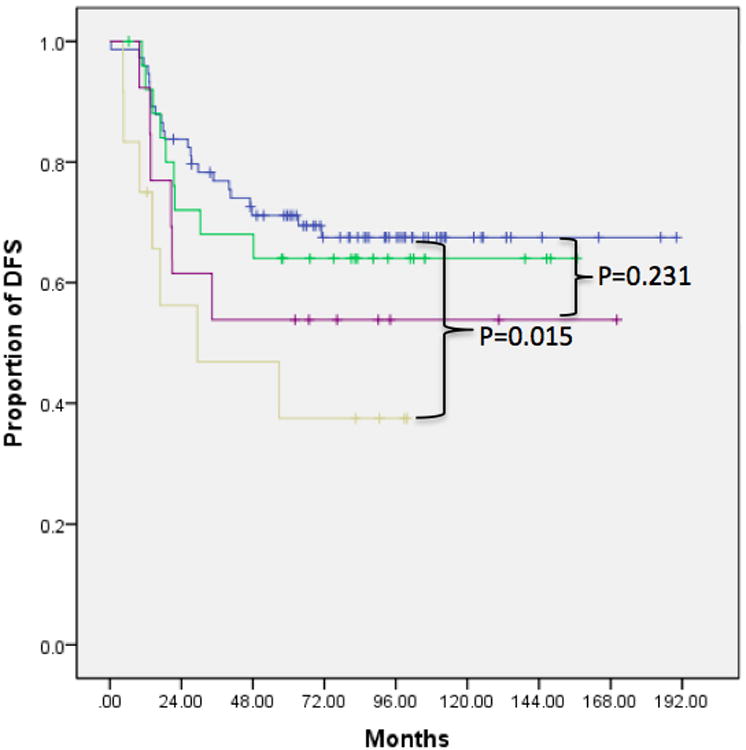
Kaplan Meier survival curves demonstrating decreased rates of overall survival and DFS in patients with multiple ≤5 mm nodules (Group 3) compared to those with no nodules (Group 1), a single ≤5 mm nodules (Group 2), or any number of nodules ≥5 mm (Group 4). This finding was consistent across all sarcoma patients as well as the subset of osteosarcoma patients. A) Overall survival in all sarcoma patients, B) DFS in all sarcoma patients, C) overall survival in osteosarcoma patients, D) DFS in osteosarcoma patients.
Table 2.
P values for log rank test comparing Kaplan Meier survival curves in Figure 1. These confirm that survival and DFS were significantly decreased in Group 3, but not Groups 2 or 4, for all sarcomas as well as osteosarcomas.
| All Sarcomas | Osteosarcomas | |||
|---|---|---|---|---|
| Survival | DFS | Survival | DFS | |
| Group 1 vs 2 | 0.682 | 0.662 | 0.685 | 0.804 |
| Group 1 vs 3 | 0.022 | 0.015 | 0.041 | 0.043 |
| Group 1 vs 4 | 0.116 | 0.231 | 0.160 | 0.330 |
Table 3.
Percent 2 and 5 year survival rates for Groups 1-4 in patients with all sarcoma types and the subset of osteosarcomas.
| 2 year survival(%) | 5 year survival (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| All sarcomas | Overall survival | 92 | 83 | 67 | 69 | 80 | 68 | 50 | 54 |
| Disease free survival | 83 | 71 | 56 | 61 | 70 | 64 | 38 | 54 | |
| Osteosarcomas | Overall survival | 90 | 88 | 57 | 100 | 80 | 88 | 43 | 60 |
| Disease free survival | 84 | 71 | 57 | 80 | 70 | 64 | 29 | 60 | |
Figure 2.
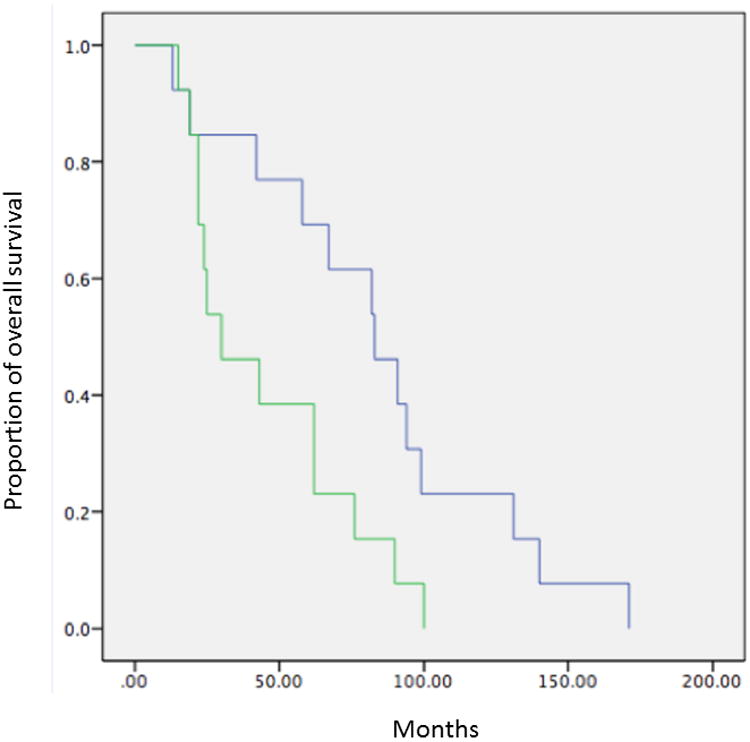
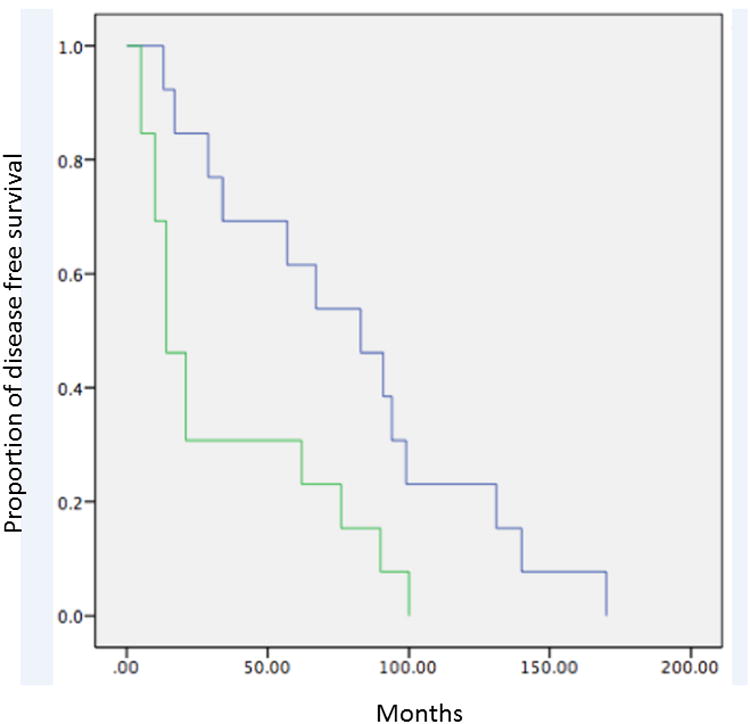
Kaplan Meier survival curves demonstrating decreased rates of A) overall survival and B) DFS in patients with multiple bilateral compared to multiple unilateral nodules.
One nodule in a patient from Group 2 was biopsied at the time of diagnosis and proven benign. In addition, 24 pulmonary nodules that developed or progressed during or following chemotherapy underwent excisional biopsy; of these, 23 (96%) were confirmed to be malignant. Late biopsy was performed in 11 (15%) of Group 1, 8 (31%) of Group 2, 1 (9%) of Group 3, and 4 (31%) of Group 4 patients (Table 4). Patients who underwent excision/biopsy tended to have a poorer prognosis in terms of both mortality and DFS than those who did not, likely because progressive disease was the indication for which they underwent the procedure.
Table 4.
Biopsied lung nodules persisting or arising during or following chemotherapy treatment.
| Patient group | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Patients with nodules (n, % of total population) | 75 | 59.5 | 26 | 20.6 | 12 | 9.5 | 13 | 10.3 |
| Biopsied nodules (n, % of patients in group) | 11 | 14.7 | 9 | 34.6 | 1 | 8.3 | 4 | 30.8 |
| Malignant nodules (n, % of biopsied nodules) | 11 | 100 | 7 | 77.8 | 1 | 100 | 4 | 100 |
| Benign nodules (n, % of biopsied nodules) | 0 | 0 | 2 | 22.2 | 0 | 0 | 0 | 0 |
Discussion
The results of our study suggest that, in young sarcoma patients treating according to standard COG protocols, the number of pulmonary nodules detected on thin-slice CT scan at the time of diagnosis may have greater prognostic significance than their size. We observed no significant difference in outcome between patients without nodules and those with a single nodule <5 mm on initial CT, suggesting that for some patients chemotherapy may be treating the “microscopic disease” that we have long suspected was present in the lungs of sarcoma patients but had been unable to visualize with the imaging tools of the day. However, multiple pulmonary nodules <5 mm were associated with significantly decreased mortality and DFS in all patients suggesting that disease burden, now evident with modern imaging, is also important.
The COG definition of clinically metastatic disease differs between sarcoma types and continues to evolve. For osteosarcoma, a single nodule >10 mm or more then 3 nodules >5 mm is considered evidence of metastasis. Metastatic Ewing's sarcoma is currently defined as a solitary nodule >5 mm or multiple nodules >3 mm, unless biopsied and proven benign; previously, however, the criteria were a single nodule >10 mm or multiple nodules > 5 mm. Finally, soft tissue sarcoma does not have imaging definitions based on size or number but relies on biopsy to establish proof of metastasis. In all protocols, for lesions below the ‘absolute’ thresholds, the diagnosis can only be established through biopsy, which is left to the discretion of the treating physician.5-23
Historically, the presence of sarcoma metastases at the time of diagnosis limited chemotherapeutic treatment options and was associated with extremely poor survival.24 Chemotherapy is responsible for improved survival of “non-metastaic” sarcoma since the 1980s, and the importance of adjuvant treatment to control residual microscopic lung disease in sarcoma for children and young adults has been well established.25-28 The observation that lung metastasis commonly occurred despite negative imaging and resection of the primary, suggests there may be a threshold effect for the size of “microscopic disease” that would allow chemotherapy to control or even eradicate disease that could not be seen by imaging tools of the day.
Just as adjuvant treatments have improved, the sensitivity of cross sectional imaging has increased dramatically in recent years, allowing for the detection of pulmonary micronodules. Three prior studies of patients undergoing screening spiral CT exams upon sarcoma diagnosis reported the prevalence of pulmonary nodules <1cm as 17-34%.(4,29,30) We observed pulmonary nodule(s) in 41% of all sarcoma patients and 45% of osteosarcoma patients at the time of diagnosis. This rate is substantially greater than those reported in the prior studies, which we hypothesize may be related to further advances in CT sensitivity in recent years. While advancing imaging technology will likely continue to increase sensitivity, pursuing a diagnosis and treatment of possibly in significant nodules may cause undue morbidity and anxiety for patients. Therefore, understanding the oncologic relevance of subcentimeter nodules is critically important.
Few comprehensive studies have examined the relationship between pulmonary nodules and mortality. In their retrospective chart review of 210 subjects under the age of 25 years, Absalon et al reported that patients who presented with any nodules at diagnosis had a lower estimated survival compared to those with none (57.8 vs. 82.8 months). They also found an association between the presence of more than 3 nodules or bilateral distribution and the likelihood of pulmonary recurrence/progression in the first 3 years after diagnosis, but there was no significant relationship to the size of largest pulmonary nodule.4 Conversely, in another retrospective CT scan review of 331 primarily older adult sarcoma patients, Rissing et al found no association between number of nodules and survivorship, but they did observe increased 3-year mortality in patients with nodule(s) ≥5 mm. This study, however, contained primarily (90%) adult subjects, and young patients were not analyzed as a separate subpopulation.30 Additional studies addressing this topic from varied perspectives have been published in the Radiology and Orthopedic Surgery literature. Some correlate radiological and surgical or pathological findings 1,3,31 while others evaluate the use of CT scan compared to other modalities,29, 32 however, variations in age range, nodule definition, and outcome measures make comparison between these data challenging. Thus, while the previous literature provides valuable information, our study offers the most comprehensive analysis of the relationship between subcentimeter pulmonary nodules and mortality in a young sarcoma patients treated with consistent chemotherapy protocols and management paradigms.
Certain limitations of this study must be acknowledged. The distribution of patients within our study population was uneven, with more in Groups 1 and 2 than in Groups 3 and 4, which prevented us from analyzing the difference between single and multiple nodules >5 mm. Larger subject numbers would be required for evaluation of nodule characteristics as risk factors for disease progression and mortality, as well as subgroup analysis by type of sarcoma. In addition, this study was designed to report outcomes for patients treated according to a common protocol, including aggressive resection of progressive pulmonary nodules whenever feasible. This could introduce an element of treatment bias, as some patients with multiple nodules may not have been surgical candidates; however, this accurately reflects the relationship between number of nodules, disease resectability, and prognosis when managed in a uniform way at an experience sarcoma treatment center.
Conclusions
Our results indicate that the number and distribution of subcentimeter pulmonary nodules on initial screening CT may be a more important prognostic factor than nodule size in young sarcoma patients treated according to standard chemotherapy protocols. Compared to patients without pulmonary lesions at presentation, we observed no difference in outcome for patients with a single nodule <5 mm. Patients with multiple nodules, especially when bilateral, had significantly reduced overall and DFS regardless of nodule size. These results suggest that metastatic disease and its prognosis may be more of a spectrum than is recognized by current treatment protocols. Additional research will be needed to further define these intermediate levels of disease and determine their optimal form of management.
Table 1.
Demographic and disease characteristics of the study population.
| Age in years at diagnosis (mean, range) | 23.5 | 1.8-49.8 | |
|---|---|---|---|
|
| |||
| Gender (n, %) | Male | 77 | 61.1 |
| Female | 49 | 38.9 | |
|
| |||
| Ethnicity(n, %) | Caucasian | 88 | 69.8 |
| African American | 14 | 11.1 | |
| Hispanic | 20 | 15.9 | |
| Other | 4 | 3.2 | |
|
| |||
| Diagnosis (n, %) | Osteosarcoma | 66 | 52.4 |
| Ewing sarcoma | 24 | 19.0 | |
| Soft tissue sarcomas | 27 | 21.4 | |
| Other | 9 | 7.1 | |
|
| |||
| Tumor Location (n, %) | Axial | 25 | 19.8 |
| Appendicular | 101 | 8.0 | |
|
| |||
| Tumor size (n, %) | Small (<5 cm) | 21 | 16.7 |
| Large (≥5 cm) | 105 | 83.3 | |
|
| |||
| Number of nodules at diagnosis (n, %) | None | 75 | 59.5 |
| Solitary | 26 | 20.6 | |
| Multiple unilateral | 16 | 12.7 | |
| Multiple bilateral | 10 | 7.9 | |
|
| |||
| Size of nodules at diagnosis (n, %) | <3 mm | 22 | 43.1 |
| 3-5 mm | 16 | 31.4 | |
| 5-10 mm | 13 | 25.5 | |
|
|
|||
| Chemotherapy regimen (n, %) | MAP | 52 | 49.2 |
| MAPI | 4 | 3.2 | |
| MAPIE | 10 | 7.9 | |
| VACIE | 24 | 19 | |
| AI | 26 | 20.6 | |
| None | 10 | 7.9 | |
|
| |||
| Total | 126 | ||
M = Methotrexate, A = Adriamycin, P = Cis/carboplatinuum, I = Ifosfamide, E = Etoposide, C = Cyclophosphamide, V = Vincristine
Acknowledgments
The authors would like to thank Patti Piasecki, BSN, MS, RN for her assistance with data collection, and Anthony Griffin, BSc for his assistance with statistical analysis.
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
No external sources of funding were used in this research.
All work was performed at Rush University Medical Center, Chicago, IL, United States.
References
- 1.Picci P, Vanel D, Briccoli A, et al. Computed tomography of pulmonary metastases from osteosarcoma: the less poor technique. A study of 51 patients with histological correlation. Ann Oncol. 2001 Nov;12(11):1601–1604. doi: 10.1023/a:1013103511633. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MS, Panicek DM. Subcentimeter pulmonary nodules detected in patients with sarcoma. Sarcoma. 2000;4(3):135. doi: 10.1080/13577140020008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006 May;239(2):514–520. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 4.Absalon MJ, McCarville MB, Liu T, Santana VM, Daw NC, Navid F. Pulmonary nodules discovered during the initial evaluation of pediatric patients with bone and soft-tissue sarcoma. Pediatr Blood Cancer. 2008 Jun;50(6):1147–1153. doi: 10.1002/pbc.21454. [DOI] [PubMed] [Google Scholar]
- 5.1996 COG 7921, Paul Meyers PI: Trial of ADR, CDDP and MTX With and Without Ifos, With and Without MTP-PE for Treatment of Osteogenic Sarcoma(POG-9351).
- 6.1999 POG 9754: Cindy Schwartz, MD MPH PI “Protocol for Patients with Newly-Diagnosed, Non-metastatic Osteosarcoma: A Pilot Study.”
- 7.2001 COG AOST0121: David Ebb, MD PI ‘A Groupwide Phase II Study of Trastuzumab (Herceptin) in Metastatic Osteosarcoma Patients with Tumors that Overexpress HER2.’
- 8.2005: COG AOST0331: Neyssa Marina, MD PI “A Randomized Trial of the European and American Osteosarcoma Study Group to Optimize Treatment Strategies for Resectable Osteosarcoma Based on Histological Response to Pre-Operative Chemotherapy.”
- 9.2008: COG AOST06P1: Robert Goldsby, MD PI, “Feasibility and Dose Discovery Analysis of Zoledronic Acid with Concurrent Chemotherapy in the Treatment of Newly Diagnosed Metastatic Osteosarcoma.”
- 10.1995 CCG 7942: Linda Granowetter, MD PI, “Evaluation of Intensified VCR, DOXO, CPM, IFOS, and VP16 in the Treatment of Newly-Diagnosed Ewing's Sarcoma or PNET of Bone or Soft Tissue (POG-9354).”
- 11.2001 COG AEWS0031: Richard Womer, MD PI, “Trial of Chemotherapy Intensification through Interval Compression in Ewing's Sarcoma and Related Tumors.”
- 12.2008 COG AEWS07P1: V, PI “A Pilot Study of Chemotherapy Intensification by adding Vincristine, Topotecan and Cyclophosphamide to Standard Chemotherapy Agents with an Interval Compression Schedule in Newly Diagnosed Patients with Localized Ewing Sarcoma Family of Tumors.”
- 13.2010 COG AEWS1031: Patrick Leavey, MD, PI, “A Phase III Randomized Trial of Adding Vincristine-Topotecan-Cyclophosphamide to Standard Chemotherapy in Initial Treatment of Non-metastatic Ewing Sarcoma.”
- 14.2014 (CURRENT): AEWS121: Steven DuBois, MD “Randomized Phase II Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008, IND# 120449) to Multiagent Chemotherapy for Patients with Newly Diagnosed Metastatic Ewing Sarcoma.
- 15.1996 IRSG 9602: David Walterhouse, MD PI, “Actinomycin D and VCR with or without CPM and RT, for Newly Diagnosed Patients with Low-Risk Embryonal/Botryoid Rhabdomyosarcoma: IRS-V Protocol.”
- 16.1999 IRSG 9802: Alberto Pappo, MD, “D9802, A Phase II “Up-Front Window Study” of Irinotecan (CPT-11) Combined with Vincristine followed by Multimodal, Multiagent, Therapy for Selected Children and Adolescents with Newly Diagnosed Stage 4/Clinical Group IV Rhabodomyosarcoma: An IRS-V/STS Study.”
- 17.1999 IRSG 9803: Carola Arndt, MD PI “Randomized Study of VCR, Actinomycin-D, and CPM (VAC) vs. VAC alternating with VCR, Topotecan, and CPM (VTC) for Patients with Intermediate Risk RMS.”
- 18.2004: COG ARST0331: David Walterhouse, MD PI, “Vincristine, Dactinomycin, and Lower Doses of Cyclophosphamide With or Without Radiation Therapy for Patients with Newly Diagnosed Low-Risk Embryonal/Botryoid/Spindle Cell Rhabdomyosarcoma.”
- 19.2006: COG ARST0431: Brenda Weigel, MD, PI, “Intensive Multi-Agent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide (IE) and Vincristine/Doxorubicin/Cyclophosphamide (VDC) for Patients with High-Risk Rhabdomyosarcoma.”
- 20.2006: COG ARST0531: Douglas Hawkins, MD, PI, “Randomized Study of Vincristine, Dactinomycin and Cyclophosphamide (VAC) versus VAC Alternating with Vincristine and Irinotecan (VI) for Patients with Intermediate-Risk Rhabdomyosarcoma (RMS).”
- 21.2007 COG ARST0332: Sheri Spuint, MD, PI, “Risk-Based Treatment for Non-Rhabdomyosarcoma Soft Tissue Sarcomas in Patients Under 30 Years of Age.”
- 22.2010 COG ARST08P1: Suman Malempati, MD, PI “A Pilot Study to Evaluate Novel Agents (Temozolomide and Cixutumumab [IMC-A12, Anti-IGF-IR Monoclonal Antibody, IND #100947, NSC #742460]) in Combination with Intensive Multi-Agent Interval Compressed Therapy for Patients with High-Risk Rhabdomyosarcoma.”
- 23.2014 COG ARST 1321: Aaron Weiss, DO. PI, “Pazopanib Neoadjuvant Trial In Non-Rhabdomyosarcoma Soft Tissue Sarcomas (PAZNTIS): A Phase II/III Randomized Trial of Preoperative Chemoradiation or Preoperative Radiation Plus or Minus Pazopanib (NSC# 737754, IND# 118613).”
- 24.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19(3):292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 25.Arndt C, Crist W. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999 Jul;341(5):342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 26.Link, Gorin AM, Miser AW, Green AA, et al. The effect of Adjuvant chemotherapy on relapse free survival in osteosarcoma of the extremities. N Engl J Med. 1986 Jun;314(25):1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 27.Rafique A, Kent P. Standard chemotherapy for malignant bone tumors in children and young adults. Curr Probl Cancer. 2013;(37):207–214. doi: 10.1016/j.currproblcancer.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Arndt CA, Rose PS, Folpe AL, Laack NN, et al. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–87. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jürgens H, Schober O, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol. 2001 Apr;12(4):479–486. doi: 10.1023/a:1011111322376. [DOI] [PubMed] [Google Scholar]
- 30.Rissing S, Rougraff BT, Davis K. Indeterminate pulmonary nodules in patients with sarcoma affect survival. Clin Orthop Relat Res. 2007 Jun;459:118–121. doi: 10.1097/BLO.0b013e31805d8606. [DOI] [PubMed] [Google Scholar]
- 31.Kayton ML, Huvos AG, Casher J, Abramson SJ, Rosen NS, Wexler LH, Meyers P, LaQuaglia MP, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg. 2006 Jan;41(1):200–206. doi: 10.1016/j.jpedsurg.2005.10.024. discussion 200-206. [DOI] [PubMed] [Google Scholar]
- 32.Fleming JB, Cantor SB, Varma DG, Holst D, Feig BW, Hunt KK, Patel SR, Benjamin RS, Pollock RE, Pisters PW, et al. Utility of chest computed tomography for staging in patients with T1 extremity soft tissue sarcomas. Cancer. 2001 Aug 15;92(4):863–868. doi: 10.1002/1097-0142(20010815)92:4<863::aid-cncr1394>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]


