Abstract
Background
India accounts for one-fifth of the global TB incidence. While the exact burden of childhood TB is not known, TB remains one of the leading causes of childhood mortality in India. Bacteriological confirmation of TB in children is challenging due to difficulty in obtaining quality specimens, in the absence of which diagnosis is largely based on clinical judgement. While testing multiple specimens can potentially contribute to higher proportion of laboratory confirmed paediatric TB cases, lack of high sensitivity tests adds to the diagnostic challenge. We describe here our experiences in piloting upfront Xpert MTB/RIF testing, for diagnosis of TB in paediatric population in respiratory and extra pulmonary specimens, as recently recommended by WHO.
Method
Xpert MTB/RIF testing was offered to all paediatric (0–14 years) presumptive TB cases (both pulmonary and extra-pulmonary) seeking care at public and private health facilities in the project areas covering 4 cities of India.
Results
Under this pilot project, 8,370 paediatric presumptive TB & presumptive DR-TB cases were tested between April and–November 2014. Overall, 9,149 specimens were tested, of which 4,445 (48.6%) were non-sputum specimens. Xpert MTB/RIF gave 9,083 (99.2%, CI 99.0–99.4) valid results. Of the 8,143 presumptive TB cases enrolled, 517 (6.3%, CI 5.8–6.9) were bacteriologically confirmed. TB detection rates were two fold higher with Xpert MTB/RIF as compared to smear microscopy. Further, a total of 60 rifampicin resistant TB cases were detected, of which 38 were detected among 512 presumptive TB cases while 22 were detected amongst 227 presumptive DR-TB cases tested under the project.
Conclusion
Xpert MTB/RIF with advantages of quick turnaround testing-time, high proportion of interpretable results and feasibility of rapid rollout, substantially improved the diagnosis of bacteriologically confirmed TB in children, while simultaneously detecting rifampicin resistance.
Background
India has the world’s largest burden of tuberculosis (TB) and accounts for one-fifth of the global TB incidence [1]. While, globally the exact burden of childhood TB is not well documented, it is estimated that childhood TB constitutes about 10–20% of all TB cases, in high burden countries [3–4] and TB remains one of the leading cause of childhood mortality [2]. In 2013, 63,919 paediatric TB cases were notified accounting for 5% of notified TB cases [5] in India, under the Revised National Tuberculosis Control Programme (RNTCP).
Diagnosis of pulmonary TB in children is challenging, more so in resource-limited, tuberculosis- endemic countries and is largely based on clinical and radiological findings and medical history [6–7]. Bacteriological confirmation of pulmonary TB is challenging due to difficulty in obtaining good quality sputum specimens from children. In the absence of quality specimens, one has to rely on testing alternate types of specimens from children. Here again confirmation of TB becomes challenging due to difficulties in obtaining these specimens, inadequate clinical sample volumes and paucibacillary nature of biological samples [8–9]. Diagnostic efforts are also undermined by the lack of diagnostic tests with high sensitivity that are simple to use and can be applied at the point of clinical care [8]. Isolation of M tuberculosis by culture, while considered as gold standard for diagnosing TB, takes 4–8 weeks and often requires expensive and sophisticated laboratory facilities which cannot be afforded in most resource-limited settings [10].
Though there are various PCR based diagnostic tests available for TB diagnosis, the specificities and sensitivities of these tests are known to be quite variable [8, 11]. Further, these tests involve multiple manual steps and long turnaround time, making them unsuitable for decentralised deployment. A series of meta-analyses have shown Xpert MTB/RIF (here after called as Xpert) assay (Cepheid Inc., Sunnyvale, California), a cartridge based nucleic acid amplification test (NAAT) to have a high specificity with variable sensitivity in different type of specimens for TB diagnosis [8, 12–14]. In 2013, World Health Organization (WHO) endorsed the use of Xpert assay for TB diagnosis in paediatric presumptive pulmonary and extra-pulmonary tuberculosis (EPTB) cases [15–16]. Xpert, a tool with a quick turn-around time, which simultaneously detects TB and rifampicin resistance, offers a promising solution to achieve the global objective of improved TB care and control and early TB case detection [17]. In line with 2013 WHO recommendations a pilot project was undertaken under RNTCP in four major cities of India, offering upfront Xpert testing to all types of paediatric presumptive TB and DR-TB cases. The primary objectives of the project were:
To identify and document the feasibility of routine upfront implementation of Xpert assay for paediatric TB diagnosis.
To evaluate Xpert assay performance in different types of paediatric specimens such as gastric lavage, BAL, induced sputum, lymph node aspirates, etc., under routine programmatic conditions.
To assess the diagnostic yield of Xpert assay in terms of TB and DR-TB detection in different types of paediatric TB specimens (respiratory and extra-pulmonary) in comparison to smear microscopy under routine programme conditions.
Methods
Project Setting
The current pilot project provided upfront Xpert testing for presumptive paediatric TB and DR-TB cases by establishing high through put Xpert laboratories, within existing RNTCP reference laboratories. One such Xpert laboratories was established in each of the four cities, namely Chennai, Delhi, Hyderabad and Kolkata, covering a population of 14.3 million, for city wide project coverage. It may be noted here that while the exact paediatric population is not known, children < 15 years constitute about 25% of the population. Geographic coverage of the pilot was ensured by means of referral and free rapid specimen transport linkages between the Xpert lab and public and private institutions in the area. A number of sensitization workshops and advocacy meetings were organised with various health providers for participation under the current pilot. All the presumptive paediatric TB and DR-TB cases coming to collaborating facilities and hospitals (both public/private) were offered free of cost Xpert testing. Xpert test was performed on various types of specimen such as gastric lavage/aspirate, BAL, CSF, induced sputum, lymph node aspirates, etc.
Project Design
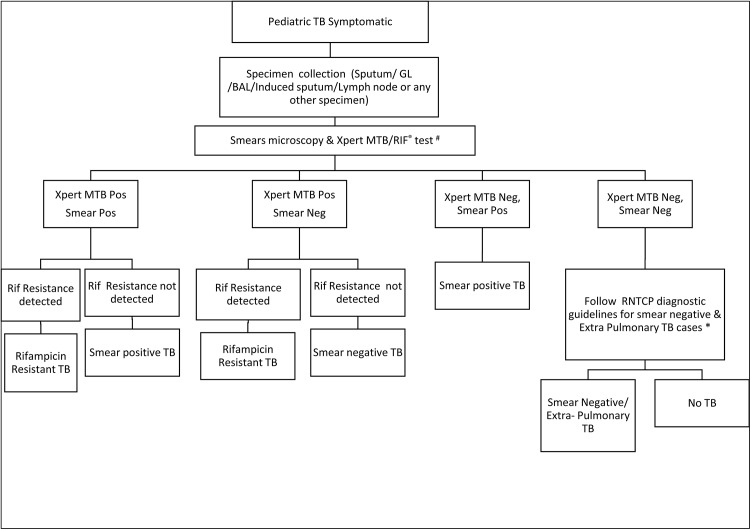
Children (age 0–14 years) presenting with signs and symptoms suggestive of TB to any of the health facilities in the project area between April’14 to November’14 were prospectively enrolled in the pilot. Respiratory and extra-pulmonary specimens were collected at the respective health facilities and transported to the Xpert lab established in the city. The specimen transportation mechanisms were micro planned across all 4 cities, taking into account feasible local transportation mechanism acceptable to various health facilities. The mechanisms deployed for rapid specimen transportation included, using commercial courier services and local volunteers whose incidental costs were reimbursed at a standard rate. The average cost of transportation covered under the project was one USD per specimen shipment received at the lab. The referring providers provided their contact details in the test request form. Ziehl Neelsen (ZN) smear microscopy and Xpert testing was done for all patient specimens. A rapid reporting mechanism was established to ensure that all test results were promptly communicated to providers by e-mail and short messaging service (SMS). Standard diagnostic algorithm approved by RNTCP was followed under the pilot for patient management (Fig 1)
Fig 1. Project diagnostic algorithm.
Definitions
Presumptive paediatric TB cases were defined as per the Indian RNTCP guidelines [16–17]. This includes children presenting with fever and/or cough for ≥2 weeks, with or without weight loss or no weight gain, or presenting with symptoms suggestive of pulmonary and/or extra-pulmonary TB.
Presumptive paediatric DR-TB cases were defined as previously-diagnosed paediatric TB cases based on smear-microscopy results and/or clinically; referred for drug susceptibility testing (DST) because of an elevated risk of drug-resistant TB. National guidelines used in the project define high-risk TB cases as those with previous history of anti-TB treatment, on treatment with positive sputum smear result at any follow up smear examination, diagnosed TB cases with HIV-co-infection, and contacts of a known MDR-TB case diagnosed with TB [18].
Specimen collection methods for different type of specimens
Standardised methodology for collection of various non-sputum specimens was followed. Specimens were collected at referral facilities which were linked with Xpert lab and equipped to perform different types of specimen collection procedures. The specimens were collected by trained medical personnel at these centres using standard methods.
Sample processing on Xpert MTB/RIF
Xpert testing was performed as per project diagnostic algorithm (Fig 1). In cases where specimens was less (<1ml), preference was given to Xpert testing ahead of smear microscopy in line with WHO recommendations [15–16]. For a given patient, whenever multiple types of specimen were available, all types of available specimen were tested. In case of ‘error’ and ‘no result’ test result on Xpert repeat test was performed on the remaining sample mix and in case of ‘invalid’ and ‘rifampicin resistance indeterminate’ test result, repeat testing was performed on a second specimen.
Sputum specimens were tested by adding of buffer in 1:2 proportions as recommended by the manufacturer Invalid source specified.. For non-sputum specimen, standard operating procedures (SOPs) developed by RNTCP [19] and WHO were adopted. Confirmatory DST for rifampicin resistant cases diagnosed on Xpert was performed on LPA and/or culture DST under the pilot. Confirmatory DST was performed either on the remnant specimen, or additional specimen, if available or on a freshly collected patient specimen. All the diagnosed TB and rifampicin resistant TB cases were initiated on appropriate anti TB regimens based on prior history of anti TB treatment as per RNTCP treatment guidelines [20].
Feasibility assessment
Feasibility of Xpert implementation was assessed both in terms of the ability of the assay to produce a valid result and the operational feasibility of providing upfront Xpert testing for paediatric presumptive TB cases though a single Xpert lab in each of the 4 cities.
The absence of a valid test result for any given assay initiated was defined as a ‘test failure’ regardless of the underlying reason. Information on frequency of various reasons for the occurrence of test failure and associated factors such as ambient temperature, power failure and or procedural error was collected and analysed.
The operational feasibility of offering upfront Xpert testing through referral and rapid specimen transport linkages between public and private institutions and the lab were assessed by analysing the turnaround time for specimen transportation, diagnosis and reporting of results to the provider.
Data management
Data for all presumptive TB and DR-TB cases was collected from RNTCP lab request form (Annexure I). The pilot was carried out under uncontrolled programmatic field conditions covering health facilities in the selected geographic area. No additional patient related clinical data, including details of X-Ray, histo-pathological findings, etc. was collected. However, for rifampicin resistant TB cases diagnosed under the pilot, additional information regarding history of contact past history of TB and BCG vaccination status was retrospectively collected through personal visit and one to one interview. Data was analysed using Microsoft Excel 2013 and EpiData Analysis (Version 2.1). All confidence intervals were calculated based on the binomial distribution with a 95% probability interval. For analytical purpose, paediatric patients were categorized into 3 groups: 0–4 years, 5–9 years, and 10–14 years of age. Odd’s ratio was calculated to determine the statistical significance and relation between two variables.
Ethical issues
The use of upfront Xpert testing for presumptive paediatric TB cases is an approved intervention under RNTCP. As such the results presented here are our experience sharing of the pilot of approved interventions in the programmatic settings within the existing accredited RNTCP TB diagnostic lab. Since the observations describe here are a part of implementation of approved interventions under RNTCP, a separate ethical clearance was not required.
Results
The project was implemented between April to November 2014, starting with one city in April 2014 and scaled up to all four cities by May’14. Within 3 months of project roll out, an average of 1,300 presumptive paediatric TB and DR-TB cases were being tested per month across the four cities (Figure A in S1 File).
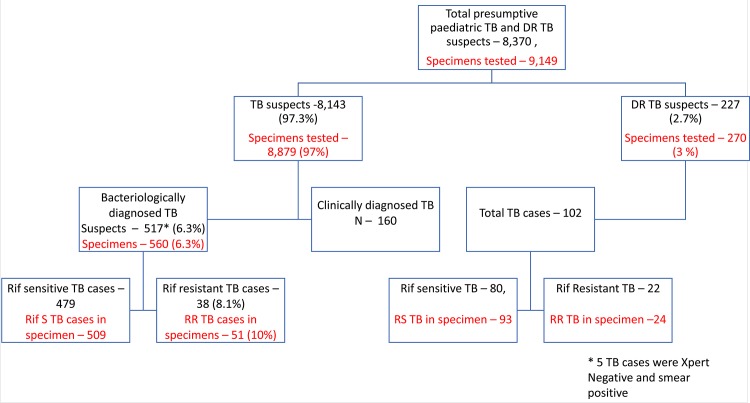
Overall 8,370 paediatric cases were tested in this period, of which, 8,143 (97.3%) were presumptive TB cases and 227 (2.7%) were presumptive DR-TB cases (Fig 2). Of the presumptive TB cases, 4,454 (54.7%) were male and 3,688 (45.3%) were female; 2,355 (28.9%) were in the age-group of 0–4 year, 2,794 (34.3%) in 5–9 years age group and 2,994 (36.8%) in the age group of 10–14 years. Of these, only 201 (2.5%) had past history of anti TB treatment (Table 1).
Fig 2. Flow chart of presumptive TB and DR-TB case enrolment and TB detection.
Table 1. Demographic profile of presumptive TB and DR-TB cases.
| Presumptive TB cases | % | Presumptive DR-TB cases | % | Total | |
|---|---|---|---|---|---|
| Total | 8143 | 97.3% | 227 | 2.7% | 8370 |
| Gender | |||||
| Female | 3688 | 45.3% | 116 | 51.1% | 3804 |
| Male | 4454 | 54.7% | 111 | 48.9% | 4565 |
| TG | 1 | 0.0% | 0 | 0.0% | 1 |
| Age group (yrs) | 0 | ||||
| Below 5 | 2355 | 28.9% | 30 | 13.2% | 2385 |
| 5 to 9 | 2794 | 34.3% | 64 | 28.2% | 2858 |
| 10 to 14 | 2994 | 36.8% | 133 | 58.6% | 3127 |
| Past History of anti TB treatment | 0 | ||||
| NA | 749 | 9.2% | 1 | 0.4% | 750 |
| NO | 7193 | 88.3% | 17 | 7.5% | 7210 |
| YES | 201 | 2.5% | 209 | 92.1% | 410 |
| Smear Microscopy | 0 | ||||
| NA | 424 | 5.2% | 17 | 7.5% | 441 |
| Negative | 7553 | 92.8% | 163 | 71.8% | 7716 |
| Positive | 166 | 2.0% | 47 | 20.7% | 213 |
| No TB | 7626 | 93.7% | 125 | 55.1% | 7751 |
| Bacteriologically confirmed TB | 517 | 6.3% | 102 | 44.9% | 619 |
| Xpert-positive; smear-negative | 329 | 63.6% | 48 | 47.1% | 377 |
| Xpert-positive; smear-NA | 22 | 4.3% | 7 | 6.9% | 29 |
| Xpert-positive; smear-positive | 161 | 31.1% | 47 | 46.1% | 208 |
| Xpert-negative; smear-positive | 5 | 1.0% | 0 | 5 | |
| Xpert-Indeterminate; smear-positive | 0 | 0 | 0 | ||
| Xpert-NA; smear-positive | 0 | 0 | 0 |
A total of 9,149 specimens from 8,370 children were tested and details of various specimen tested on Xpert are listed in Fig 3. Overall 4,445 (48.6%) of the 9,149 specimens tested were non-sputum specimens.
Fig 3. Various types of specimens tested on Xpert MTB/RIF.
Feasibility Assessment
Of 9,149 Xpert tests conducted, 8,774 (95.9%, CI 95.4–96.2) valid test result were obtained on a single test. Of the 375 (4.2%, CI 3.8–4.7) initial test failures, repeat testing was done on 332 (88.5%, CI 84.9–91.3) by retesting same or second available specimen. Of these, valid test results on retesting were obtained on 309 (93.0%, CI 89.8–95.3) specimens. Overall, 9,083 (99.2%, CI 99.0–99.4) yielded valid results with 66 (0.7%, CI 0.5–0.9) were test failures. Of the 66 test failures, 43 were not retested due to various programmatic and/or study limitations (Table B in S1 File)
Of the 375 test failures under the pilot, 135 (36%, CI 31.3–40.9) tests failed on account of PCR inhibition giving an invalid test result. Overall invalidity rate observed among different types of specimens tested under the pilot varied from 0% to 2% (Table A in S1 File). Of the total test failure on account of PCR inhibition, majority was observed in sputum (69/135; 51.1%) and gastric aspirate/lavage (53/135; 39.3%). Of these total 135 test failures, 102 (75.6%) were re-tested and 96 (94.1%) were resolved giving a valid result, by retesting a second specimen.
Specimen transportation, diagnostic and reporting turnaround time
Specimen of most of the cases, (8,050 (96.2%)), were received on the (same) day of collection while 213 (2.5%) were received a day later. Remaining 1.3% samples were received subsequently due to public holidays. Likewise, test results were made available for 6,718 (80.3%) on the (same) day of sample collection and another 6.6% a day later. Cumulatively, 99.3% (8,314) patients had received their results within a week. Overall, 7,002 (83.7%) of the results were sent to respective providers on the same day of testing and a cumulative total of 7,950 (95.0%) of the results were reported within one day of testing. (Figure B in S1 File)
Effect of upfront Xpert MTB/RIF on TB case detection
Of the 8,143 presumptive TB cases enrolled, 517 (6.3%, CI 5.8–6.9) were bacteriologically confirmed as TB cases. Of these 517 TB cases, 161 (31.1%) were positive by both Xpert and smear microscopy, while 329 (63.6%) were Xpert TB positive and smear negative; 22 (4.3%) were Xpert positive and smear not done. Additionally, 5 were smear positive and Xpert negative. (Table 1)
TB detection on Xpert was compared against smear microscopy among various types of specimens. Of the total 8,143 presumptive TB cases, smear microscopy was not done for 424 cases. For the remaining 7,719 (94.8%) presumptive TB cases for which both smear and Xpert tests was performed, total of 8,396 different specimens were tested. The additional gain in detection rate on different types of specimen tested under the project on Xpert as compared to microscopy is described in Table 2. Overall, more than 2 fold higher TB case detection was observed on Xpert as compared with smear microscopy irrespective of type of specimen. The increase in TB detection rate was highest while testing CSF, fine needle aspiration cytopathology samples, BAL and Pus specimen with Xpert as compared to smear microscopy (Table 2) Of the, 3,996 various non-sputum specimen tested under the project the overall positivity on Xpert was 299 (7.4%, CI 6.7–8.3), whereas positivity on microscopy was 64 (1.6%, CI 1.2–2.0). It was observed that none of the 52 ascetic fluid specimen tested had a positive test result either on Xpert or smear microscopy. Also, there was no significant gain in TB detection (OR 1.2 (95% CI 0.3–4.0) on Xpert over smear microscopy in case of pleural fluid.
Table 2. Additional gain on Xpert MTB/RIF over smear microscopy-specimen wise analysis.
| Type of Specimen | Total presumptive TB cases | Cases with simultaneous smear and Xpert MTB/RIF done | Specimen tested | Xpert MTB/RIF Positive | % | Smear Positive | % | Additional gain (Fold) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Sputum/IS | 4349 | 4156 | 4397 | 237 | 5.4% | 146 | 3.3% | 1.6 | 1.6 (1.3–2.0) |
| Gastric Aspirate/Lavage | 2648 | 2541 | 2806 | 167 | 6.0% | 41 | 1.5% | 4.1 | 4.2 (3.0–6.0) |
| CSF | 551 | 475 | 564 | 40 | 7.1% | 0 | 0.0% | ||
| Pleural Fluid | 195 | 183 | 186 | 6 | 3.2% | 5 | 2.7% | 1.2 | 1.2 (0.3–4.0) |
| BAL | 186 | 180 | 221 | 34 | 15.4% | 5 | 2.3% | 6.8 | 7.8 (3.0–20.4) |
| Pus | 73 | 58 | 67 | 24 | 35.8% | 5 | 7.5% | 4.8 | 6.9 (2.4–19.5) |
| FNAC | 54 | 49 | 59 | 21 | 35.6% | 4 | 6.8% | 5.3 | 7.5 (2.4–23.9) |
| Ascetic Fluid | 44 | 42 | 52 | 0 | 0.0% | 0 | 0.0% | 0.0 | |
| Tissue | 8 | 7 | 8 | 1 | 12.5% | 1 | 12.5% | 1.0 | |
| Urine | 8 | 5 | 6 | 0 | 0.0% | 0 | 0.0% | 0.0 | |
| Others * | 27 | 23 | 27 | 6 | 10.5% | 3 | 5.3% | 2.0 | 2.1 (0.5–8.9) |
| Total | 8143 | 7719 | 8396 | 536 | 6.4% | 210 | 2.5% | 2.6 | 2.6 2.2–3.1) |
*Others = Cervical Aspirate, Peritoneal Fluid, Tracheal aspirate, Abscess, Synovial Fluid, Bone, Chyle fluid, Nasal Aspirate, Pleural Biopsy, Thoracic swab, ET secretion, pericardial fluid.
Effect of upfront Xpert MTB/RIF on rifampicin-resistant TB case detection
Of the enrolled 8,143 presumptive paediatric TB cases, total 512 were found positive for TB on Xpert. Of these 38/512 (7.4%, CI 5.4–10.0) were resistant to rifampicin. Additionally, of the 227 presumptive DR-TB cases tested under the pilot, 102 were positive for TB on Xpert, of which 22/102 (21.5%, CI 14.7–30.0) were resistant to rifampicin. Thus, 60 rifampicin resistant TB cases were detected among all patients tested across four sites under this pilot.
Higher proportion of rifampicin resistance was observed in children less than 5 years as compared with children in age group 10–14 years [(12.9% vs 9.1%; OR 1.4 (CI 0.7–2.8)]; more cases detected in males as compared to females [(12.0% vs 8.5%; OR 1.4 (CI 0.8–2.5)] and also in children with past history of TB treatment [(15.6% vs 8.7%; OR 1.9 (CI 1.0–3.4)] (Table 3), though none of these differences were statistically significant.
Table 3. Effect of upfront Xpert MTB/RIF on rifampicin-resistant TB case detection.
| Presumptive TB cases | Xpert Positive | Rif Resistant TB | Presumptive DR-TB cases | Xpert Positive | Rif Resistant TB | Total Xpert Positive | Total Rif Resistant TB | OR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | 95% CI | ||||||
| Total | 8143 | 512 | 38 | 7.4% | 227 | 102 | 22 | 21.6% | 614 | 60 | 9.8% | |
| Age group (yrs) | ||||||||||||
| Below 5 | 2355 | 108 | 12 | 11.1% | 30 | 8 | 3 | 37.5% | 116 | 15 | 12.9% | 1.4 (0.7–2.8) |
| 05–09 | 2794 | 122 | 9 | 7.4% | 64 | 26 | 4 | 15.4% | 148 | 13 | 8.8% | 0.9 (0.4–1.8) |
| 10–14 | 2994 | 282 | 17 | 6.0% | 133 | 68 | 15 | 22.1% | 350 | 32 | 9.1% | 1 |
| Gender | ||||||||||||
| Female | 3688 | 327 | 20 | 6.1% | 116 | 62 | 13 | 21.0% | 389 | 33 | 8.5% | 1 |
| Male | 4454 | 185 | 18 | 9.7% | 111 | 40 | 9 | 22.5% | 225 | 27 | 12.0% | 1.4 (0.8–2.5) |
| TG | 1 | 0 | 0 | 0.0% | 0 | 0 | 0 | 0 | 0 | |||
| Past history of anti TB | ||||||||||||
| Unknown | 749 | 27 | 0 | 0.0% | 1 | 1 | 0 | 0.0% | 28 | 0 | 0.0% | |
| No | 7193 | 451 | 37 | 8.2% | 17 | 7 | 3 | 42.9% | 458 | 40 | 8.7% | 1 |
| Yes | 201 | 34 | 1 | 2.9% | 209 | 94 | 19 | 20.2% | 128 | 20 | 15.6% | 1.9 (1.0–3.4) |
| Smear Microscopy | ||||||||||||
| NA | 424 | 22 | 2 | 9.1% | 17 | 7 | 0 | 0.0% | 29 | 2 | 6.9% | |
| NEG | 7553 | 329 | 24 | 7.3% | 163 | 48 | 7 | 14.6% | 377 | 31 | 8.2% | 1 |
| POS | 166 | 161 | 12 | 7.5% | 47 | 47 | 15 | 31.9% | 208 | 27 | 13.0% | 1.6 (0.9–2.8) |
| History of contact with TB patient | ||||||||||||
| Yes | 22 | 17 | 39 | |||||||||
| No | 8 | 2 | 10 | |||||||||
| Unknown | 8 | 3 | 11 | |||||||||
Information on history of contact with a known TB/DR-TB patient was collected from all the rifampicin resistant cases. History of contact with a TB / DR-TB case could not be verified for 11/60 (18.3%) patients. In the remaining DR-TB cases, it was observed that, 39/49 (79.6%) had history of contact either with a known TB or DR-TB case. (Table 3) Of these, 39 rifampicin resistant TB cases with positive history of contact, 14 (35.9%) had no prior history of anti TB treatment.
Further analysis was done to assess rifampicin resistant detection rates among various specimens tested under the project. Of the total 9,149 specimens tested, total 677 were found to be positive for TB on Xpert, of which 75 (11.0%, CI 8.9–13.6) were rifampicin resistant. Positivity of rifampicin resistant detection observed in FNAC 7/37 (18.9%, CI 9.4–34.2), CSF 8/46 (17.3%, CI 9.0–30.7), gastric aspirate 25/194 (12.8%, CI 8.8–18.3), BAL 5/41(12.2%, CI 5.3–25.5), pleural fluid 1/9 (11.1%, CI 1.9–43.5), pus 4/39 (10.2%, CI 4.0–23.5) and sputum specimen 25/298 (8.3%, CI 5.7–12.0). (Table C in S1 File)
Of the 60 rifampicin resistant cases diagnosed under the pilot, confirmatory DST could be conducted for 57 cases. Additional or remnant samples from 3 cases were not available for testing (2 cases had died and 1 had defaulted). Of these 57 specimens, valid results were obtained on 40 specimens, of which 36 (90%, CI 76.9–96.4) were found to be rifampicin resistant on LPA or liquid culture DST while 4 (10%, CI 3.9–23) were found to be sensitive to rifampicin.
Treatment details on confirmed cases
Of the 517 bacteriologically confirmed TB case detected under the study, 38 were resistant to rifampicin. Additional 22 rifampicin resistant cases were detected among 227 presumptive DR-TB cases. Of the total 60 rifampicin resistant TB cases, 51 (85%) patients were initiated on second line treatment, of which 41 (80.4%) were initiated on treatment within 15 days of diagnosis after completing the pre-treatment evaluation as per RNTCP guidelines. Of the total 479 rifampicin sensitive TB patients, 392 (81.8%) patients were initiated on treatment while, 38 (7.9%) patients were initial loss to follow-ups and 11 (2.3%) died. Treatment information for remaining 38 (7.9%) cases was not available as most of them were referrals from private sector and could not be traced. Since patients enrolled under the pilot were from various sectors referred by different providers, diligent follow up on bacteriologically negative TB cases getting diagnosed later on clinical criteria was not feasible hence this aspect has been excluded from current manuscript.
Discussion
For the first time upfront Xpert testing was offered to all presumptive paediatric TB cases through centralised high through-put Xpert labs in defined geographic areas in India. Participating providers were linked through rapid specimen transportation linkages and rapid result reporting mechanisms. Another novel aspect of this pilot was that, for the first time Xpert testing was extended to non-sputum specimen under routine programmatic conditions in India, in line with the recent WHO recommendations [16]. This led to overall improvement in bacteriologically confirmed paediatric TB cases, as well as detection of significant numbers of rifampicin resistant TB cases in children. All the TB cases diagnosed under the project were notified under RNTCP irrespective of type of referring provider.
While upfront Xpert testing has been recommended by WHO for paediatric presumptive TB cases, implementation of such an intervention is challenging due to high cost of Xpert equipment limiting the test availability across a large number of health facilities; The current pilot project demonstrated the feasibility of rapidly rolling out upfront Xpert testing through a single laboratory exclusively for paediatric population in urban areas by enrolling more than 8,100 paediatric presumptive TB cases within eight months of implementation. Wide geographical coverage of the pilot achieved through rapid specimen transportation linkages with participating health facilities resulting with most specimens being transported on the same day of collection and provided results on the day of specimen collection. This implementation design piloted here provides a feasible methodology of providing upfront access to Xpert, considering that high cost of equipment, limits this test’s applicability for point of care usage covering large geographic areas. Considering that an unexpectedly high proportion of TB cases were found to be resistant to rifampicin, these findings are quite significant in view of much higher turnaround time on other available diagnostic tests for the diagnosis of drug resistance [10].
For wider geographic coverage a large number of sensitization workshops and advocacy meetings were organised with various health providers. These activities led to enhanced referrals from various providers and overall increase in presumptive TB case enrolment under the project. Though objective before and after comparative data could not be documented for the same, with the availability of free services, door to door pick up of specimens from linked heath providers and reporting of test results in a time bound manner, the pilot resulted in large number of providers utilizing these free Xpert testing services. All diagnosed cases were given an option of getting free treatment services and case management under RNTCP.
Under this pilot, Xpert testing for first time was extended to various types of non-sputum and non-respiratory specimens to assess the performance of this assay under uncontrolled field conditions. Xpert performance on both sputum and non-sputum was found to be highly satisfactory, with overall 99.3% cases getting valid results. These findings are similar to other studies conducted on Xpert assay on sputum [21–24] and non-sputum specimens [25]. However, proportion of interpretable results on Xpert obtained under the current study was observed to be higher than previous reports from India and Germany [10, 26]. The key factor contributing to the high proportion of interpretable results was rapid retesting thereby resolving test failures on the same specimen. This was similar to earlier reports from India [21].
Polymerase chain reaction inhibition leading to invalid test results is a major concern while testing specimens on various types of molecular assays, especially non-sputum specimen [27–29]. The invalidity rate observed in our study on account of PCR inhibition was lower than reported in another study on Xpert assay from India [10] and on other PCR based assays [30]. Invalid or false negative results in various PCR based tests are mostly due to the presence of inhibitors, sub-optimal assay conditions or omission of key steps [31]. However, this issue was seen to be of lesser concern on Xpert which can be attributed to the fact that this test automated and self-contained test that offers minimal hands-on manual manipulation of specimen which may be a key factor in leading to low PCR inhibition rates.
The current pilot assessed the diagnostic yield of Xpert, a test with already documented high specificity, in comparison to smear under routine programmatic conditions. The current study design limited the possibility of additional, parallel testing of the collected specimen on liquid culture, which might have provided useful performance information such as sensitivity and specificity of the test. However, the same was beyond the scope of the current pilot. Xpert detected more than twice TB cases over smear microscopy, these findings suggest better sensitivity of Xpert for TB diagnosis than smear microscopy in children. Our findings are in-line with the findings from recent meta-analysis by Anne Detjen et al [32]. Xpert performance in TB detection was excellent among various specimens tested as majority of specimen has yielded valid results. However, higher positivity was observed in specimens such as gastric aspirate/gastric lavage, CSF, FNAC, Pus and BAL specimens. These findings are similar to findings from other study conducted in India [10]. Our observation is similar to the findings from other similar studies from different countries [26, 33–38]. The positivity rate on ascetic fluid and pleural fluid observed in our pilot was very low, in-line with guidance issued by WHO [16] and observations in different studies [39–41] which suggests limited utility of testing these specimen on Xpert over and above smear microscopy.
By offering upfront Xpert to all presumptive TB and DR-TB cases, substantial numbers of rifampicin resistant TB patients were diagnosed under the study. Though limited data on levels of rifampicin resistance in paediatric population is available, our study findings are broadly similar to the findings from earlier studies conducted in India in the same age group [36, 42–43]. The levels of rifampicin resistance were high in all three age groups, i.e. 0–4, 5–9 & 10–14. Further, around half of the diagnosed rifampicin resistance cases were smear negative. Other WHO endorsed rapid tests for the diagnosis of rifampicin resistance have limited diagnostic utility on smear negative specimens [31, 20]. Though the current pilot was not designed to draw a representative estimate of rifampicin resistance levels in the diagnosed paediatric TB cases, the findings underscore the importance of considering upfront Xpert testing for presumptive TB cases in this highly vulnerable population.
The data from the current pilot shows that rifampicin resistance in paediatric TB cases correlated better with a positive history of contact, rather than a past history of TB treatment. We did not observe any correlation between the detection of rifampicin resistant and BCG vaccination status. While most of the rifampicin resistant TB cases had a positive history of contact with TB cases, a large proportion of them had no prior history of TB treatment. This finding suggests that history of contact with a TB patient can be considered as a more appropriate risk factor for rifampicin resistance in paediatric population, rather than just focusing on past history of TB treatment.
While cost implications are always important in implementation, the present study focuses solely on assessing the feasibility and impact of upfront Xpert testing on the pediatric TB & DR-TB detection rates. Results of this approach can contribute to the basis for assessment of cost-effectiveness. As a logical next step costing of the project interventions is being undertaken by the project team findings of which will be published separately.
Conclusion
Xpert with advantages of quick turnaround testing-time, high proportion of interpretable results and feasibility of rapid rollout, provides a promising solution to the TB diagnostic challenges in children, while simultaneously detecting rifampicin resistant TB. The pilot demonstrated the feasibility of extending Xpert testing to non-sputum specimens with a very high proportion of interpretable results (similar to sputum specimen) and the feasibility of linking a large number of providers with a single Xpert lab, efficiently. With more than a two fold increase in TB case detection over smear microscopy and detection of significant numbers of rifampicin resistant TB cases, the study demonstrates the utility of offering upfront Xpert testing to paediatric presumptive TB and DR-TB patients under programmatic conditions.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The project was funded by United States Agency for International Development (USAID) and Center for Disease Control (CDC). FIND was responsible for implementation, training, coordination, monitoring, data analysis and writing of the report in close coordination with Central TB Division. The funder had no participation in the design, implementation, analysis, or preparation of any reports or manuscript.
References
- 1. Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a New Pillar in Diagnosis of Extra-pulmonary Tuberculosis?, 2011; JOURNAL OF CLINICAL MICROBIOLOGY, July 2011, p. 2540–2545 Vol. 49, No. 7 0095-1137/11/$12.00 10.1128/JCM.02319-10 Copyright 2011, American Society for Microbiology. Available: http://jcm.asm.org/content/49/7/2540.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World health Organization (2013). Global Tuberculosis report 2013, Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1, Accessed 25 August 2014.
- 3. Marais B, Hesseling A, Gie R, Schaaf H, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. International Journal of Tuberculosis and Lung Disease, 2006; 10: 259–263. , Available: http://www.ncbi.nlm.nih.gov/pubmed/16562704. [PubMed] [Google Scholar]
- 4. Kumar A, Gupta D, Nagaraja S, Singh V, Sethi GR, Prasad J. Updated National Guidelines for Pediatric Tuberculosis in India, 2012. 2013; Indian Pediatr 50: 301–306. Available: http://www.indianpediatrics.net/mar2013/mar-301-306.htm [DOI] [PubMed] [Google Scholar]
- 5.Revised National Tuberculosis Control Program. TB India 2015, RNTCP Annual Status Report, Reach the Unreached, 2015; Available: http://www.tbcindia.nic.in/pdfs/tb%20india%202014.pdf
- 6. Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study, 2012; Clin Infect Dis 54: 1388–1396 10.1093/cid/cis190 Epub 2012 Apr 3 Available: http://www.ncbi.nlm.nih.gov/pubmed/22474 220 Accessed: 01 April 2014. [DOI] [PubMed] [Google Scholar]
- 7. Enarson PM, Enarson DA and Gie R. Management of tuberculosis in children in low-income countries, 2005; Int J Tuberc Lung Dis 2005;9:1299–304, Available: http://www.ncbi.nlm.nih.gov/pubmed/16466050 [PubMed] [Google Scholar]
- 8. Raj A, Singh N, Mehta PK. Gene Xpert MTB/RIF Assay: A New Hope for Extra-pulmonary Tuberculosis, 2014; IOSR Journal of Pharmacy Vol. 2, Issue 1, Jan-Feb 2012, pp. 083–089, Available: http://www.iosrphr.org/papers/v2i1/N021083089.pdf, Accessed 25 August 2014. [Google Scholar]
- 9. Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology, 2005; J Clin Microbiol. 43(9), 2005, 4357–4362, Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1234147/. Accessed 25 August 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uria G A, Azcona J M, Midde M, Naik P, Reddy S, Reddy R. Rapid Diagnosis of Pulmonary and Extrapulmonary Tuberculosis in HIV-Infected Patients Comparison of LED Fluorescent Microscopy and the GeneXpert MTB/RIF Assay in a District Hospital in India, 2012; Hindawi Publishing Corporation Tuberculosis Research and Treatment; Volume 2012, Article ID 932862, 4 pages, 10.1155/2012/932862, Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3433122/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haldar S, Bose M, Chakrabarti P, Daginawala H F, Harinath B C, Kashyap RS, et al. Improved laboratory diagnosis of tuberculosis—The Indian experience, 2011; Tuberculosis, 91(5), 2011, 414–26. Available: http://www.ncbi.nlm.nih.gov/pubmed/21764383 Accessed 25 August 2014. 10.1016/j.tube.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 12. Pai M, Flores LL, Pai N, Hubbard A, Riley LW, Colford JM. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. 2003; Lancet Infect. Dis. 3(10), 2003, 633–643. Available: http://www.stoptb.org/wg/new_diagnostics/assets/documents/Evidence_Synthesis_Handout_pos85.pdf [DOI] [PubMed] [Google Scholar]
- 13. Pai M, Flores LL, Pai N, Hubbard A, Riley L W, Colford JM. Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis, 2004; BMC Infect Dis. 4, 2004, 6. Available: http://www.biomedcentral.com/1471-2334/4/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. 2007; Health Technol Assess. 11(3), 2007, 1–196. Available: http://www.ncbi.nlm.nih.gov/pubmed/17266837 [DOI] [PubMed] [Google Scholar]
- 15. Pai M. Extrapulmonary Tuberculosis: New Diagnostics and New Policies, 2014; Indian J Chest Dis Allied Sci 2014;56:71–73, Available: http://medind.nic.in/iae/t14/i2/iaet14i2p71.pdf, Accessed 26 August 2014. [PubMed] [Google Scholar]
- 16.World Health Organization. Policy update: automated real time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/ RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children 2013; Available http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf?ua=1. Accessed 26 August 2014. [PubMed]
- 17.World Health Organization. Xpert MTB/RIF implementation manual, Technical and operational ‘how to’ practical consideration, 2014; Available: http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf?ua=1. Accessed 25 August 2014.
- 18.Central TB Division Ministry of Health and Family Welfare, New Delhi, India. Revised National TB Control Programme, Guidelines on Programmatic Management of Drug Resistant TB (PMDT) in India, 2012; Available: http://tbcindia.nic.in/pdfs/Guidelines%20for%20PMDT%20in%20India%20-%20May%202012.pdf
- 19.Revised National Tuberculosis Control Program. Standard Operating Procedure; 2014, Available: http://www.ghdonline.org/uploads/GeneXpert_SOP_Xpert_processing_EPTB_specimens_DRAFT.pdf. Accessed 27 August 2014.
- 20.Central TB Division Ministry of Health and Family Welfare, New Delhi, India. Revised National TB Control Programme, National Guidelines on diagnosis and treatment of Pediatric Tuberculosis, 2012; Available: http://tbcindia.nic.in/Paediatric%20guidelines_New.pdf
- 21. Raizada N, Sachdeva KS, Sreenivas A, Vadera B, Gupta RS, Parmar M, et al. Feasibility of Decentralised Deployment of Xpert MTB/RIF Test at Lower Level of health System in India, 2014; PLOS ONE 9(2): e89301 10.1371/journal.pone.0089301, Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0089301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance, 2010; New England Journal of Medicine, 363: 1005–1015 Available: http://www.nejm.org/doi/full/10.1056/NEJMoa0907847. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahrili R, et al. Feasibility, diagnostic accuracy, and eff effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study, 2011; Lancet 377: 1495–1505 10.1016/S01406736(11)604388 Available: http://www.ncbi.nlm.nih.gov/pubmed/21507477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M DN. Xpert ® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (Review). Chochrane Database Syst Rev, 2013; Available: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009593.pub2/pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. The diagnostic accuracy of Xpert MTB/RIF on extra pulmonary tuberculosis specimens: 2 Establishing a laboratory testing algorithm for South Africa, 2014; Journal of Clinical Microbiology, 10.1128/JCM.03553-13, Available: http://jcm.asm.org/content/early/2014/03/06/JCM.03553-13.full.pdf+html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillemann D, Gerdes SR, Boehme C, Richter E. Rapid Molecular Detection of Extrapulmonary Tuberculosis by the Automated GeneXpert MTB/RIF system, 2011; Published ahead of print 26 January 2011, 10.1128/JCM.02268-10 J. Clinical. Microbiology. April 2011. vol. 49 no. 4 1202–1205, Available: http://www.ncbi.nlm.nih.gov/pubmed/21270230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonenka U, Hofmann-Thiel S, Turaev L, Esenalieva A, Abdulloeva M, Sahalchyk E, et al. Comparison of Xpert MTB/RIF with ProbeTec ET DTB and COBAS TaqMan MTB for direct detection of M. tuberculosis complex in respiratory specimens, 2013; BMC Infectious Diseases 2013, 13:280 10.1186/1471-2334-13-280, Available: http://www.biomedcentral.com/1471-2334/13/280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eing BR, Becker A, Sohns A, Ringelmann R.Comparison of Roche Cobas Amplicor Mycobacterium tuberculosis Assay with In-House PCR and Culture for Detection of M. tuberculosis, 1998; J. Clinical Microbiology, July 1998. vol. 36 no. 7 2023–2029, Available: http://jcm.asm.org/content/36/7/2023.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloemberg GV, Voit A, Ritter C, Deggim V, Böttger EC. Evaluation of Cobas TaqMan MTB for Direct Detection of the Mycobacterium tuberculosis Complex in Comparison with Cobas Amplicor MTB, 2013; 10.1128/JCM.00142-13J Clin. Microbiol. July 2013. vol. 51 no. 7 2112–2117, Available: http://jcm.asm.org/content/51/7/2112.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gen Probe. Amplified Mycobacterium Tuberculosis Direct Test For In Vitro Diagnostic Use Available: http://www.hologic.com/sites/default/files/package%20inserts/IN0014-EN-RevP.pdf, Accessed 27 August 2014.
- 31.World Health Organization, Policy Statement: Molecular Line Probe Assays For Rapid Screening Of Patients At Risk Of Multidrug-Resistant Tuberculosis (MDR-TB), 2008; pp 6–7 Available: http://www.who.int/tb/features_archive/policy_statement.pdf
- 32.Detjen A, DiNardo A, Leyden J, Steingart K, Menzies D, Schiller I, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis, 2015; www.thelancet.com/respiratory Published online March 24, 2015, 10.1016/S2213-2600(15)00095-8, Available: http://www.thelancet.com/pb/assets/raw/Lancet/pdfs/S2213260015000958.pdf [DOI] [PMC free article] [PubMed]
- 33. Pang Y, Wang Y, Zhao S, Liu J, Zhao Y, Li H. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis (2014) Pediatr Infect Dis J. 2014;33:1047–51, Available: http://medind.nic.in/ibv/t14/i12/ibvt14i12p1007.pdf 10.1097/INF.0000000000000403 [DOI] [PubMed] [Google Scholar]
- 34. Bates M, O'Grady J, Maeurer M, Tembo J, Chilukutu L, Chabala C, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. (2013); Lancet Infect Dis. 2013 January;13(1):36–42. 10.1016/S1473-3099(12)70245-1 Epub 2012 Nov 5, Available: http://www.ncbi.nlm.nih.gov/pubmed/23134697 [DOI] [PubMed] [Google Scholar]
- 35. Patel VB, Theron G, Lenders L, Matinyena B, Connolly C, Singh R, et al. Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study, (2013) PLOS Med 10(10): e1001536 10.1371/journal.pmed.1001536, Available: https://www.plos.org/wp-content/uploads/2013/05/plme-10-10-Patel.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith LM, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extra pulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review (2014); BMC Infectious Diseases 2014, 14:709 10.1186/s12879-014-0709-7, Available: http://www.biomedcentral.com/1471-2334/14/709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA, Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert system (2012);Saudi Med J. 2012. October;33(10):1100–5, Available: http://www.ncbi.nlm.nih.gov/pubmed/23047207 [PubMed] [Google Scholar]
- 38. Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples (2011); J Clin Microbiol. 2011. October;49(10):3458–62. 10.1128/JCM.05212-11 Epub 2011 Aug 17; Available: http://www.ncbi.nlm.nih.gov/pubmed/21849695?dopt=Abstract&holding=npg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friedrich SO, Bidlingmaier FG2, Diacon HA. Xpert MTB/RIF Assay for Diagnosis of Pleural Tuberculosis (2011); J. Clin. Microbiol. December 2011. vol. 49 no. 12 4341–4342; Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232996/ 10.1128/JCM.05454-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeka AN, Sezai Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF Assay for Rapid Diagnosis of Tuberculosis and Detection of Rifampin Resistance in Pulmonary and Extrapulmonary Specimens (2011); J. Clin. Microbiol. December 2011. vol. 49 no. 12 4138–4141; Available: http://jcm.asm.org/content/49/12/4138 10.1128/JCM.05434-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raizada N, Sachdeva KS, Nair SA, Kulsange S, Gupta RS, Thakur R, et al. Enhancing TB Case Detection: Experience in Offering Upfront Xpert MTB/RIF Testing to Pediatric Presumptive TB and DR-TB Cases for Early Rapid Diagnosis of Drug Sensitive and Drug Resistant TB, 2014; PLOS ONE 9(8):e105346 10.1371/journal.pone.0105346, Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0105346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swaminathan S, Datta M, Radhamani MP, Mathew S, Reetha AM, Rajajee S, et al. A profile of bacteriologically confirmed pulmonary tuberculosis in children, 2008; Indian Pediatr. 2008. September;45(9):743–7, Available: http://www.ncbi.nlm.nih.gov/pubmed/18820380?dopt=Abstract, Accessed 06 April 2015 [PubMed] [Google Scholar]
- 43. Shah I, Chilkar S. Clinical Profile of Drug Resistant Tuberculosis in Children, 2012; Indian Pediatric Journal 2012:49:741–744, Available: http://www.indianpediatrics.net/sep2012/sep-741-744.htm [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.