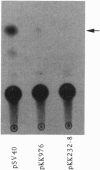
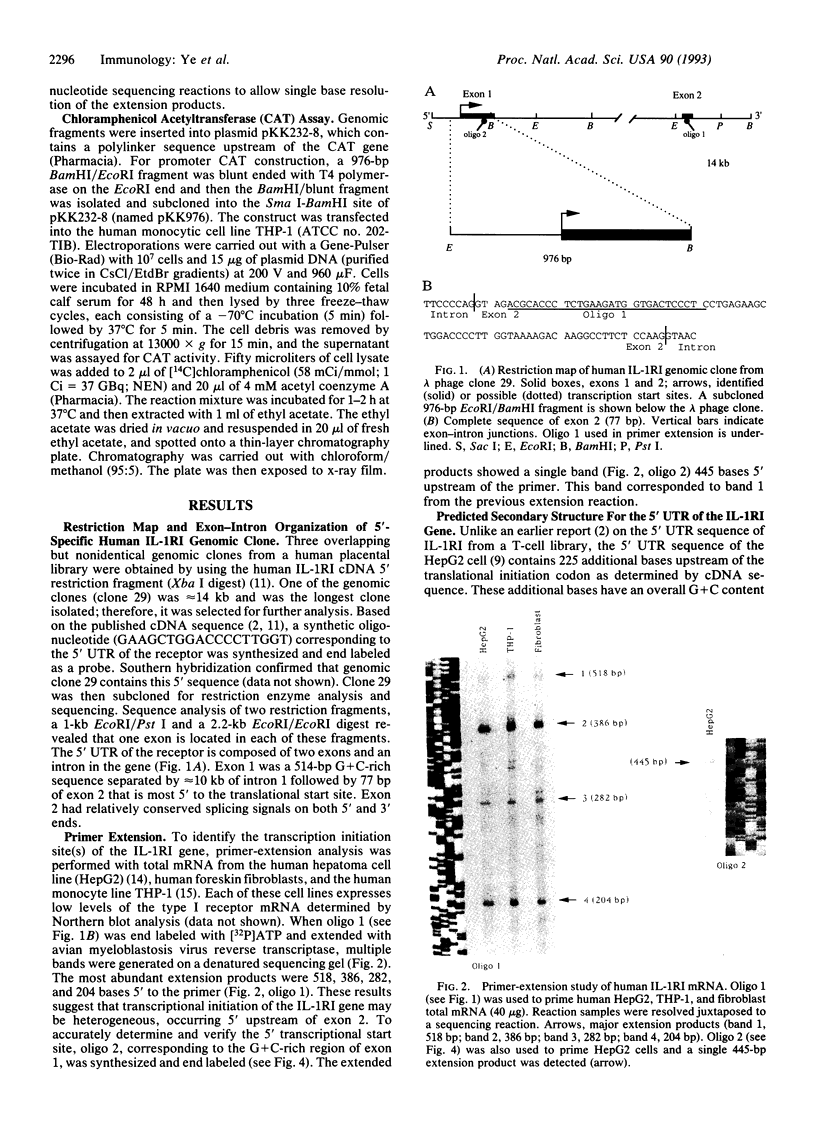
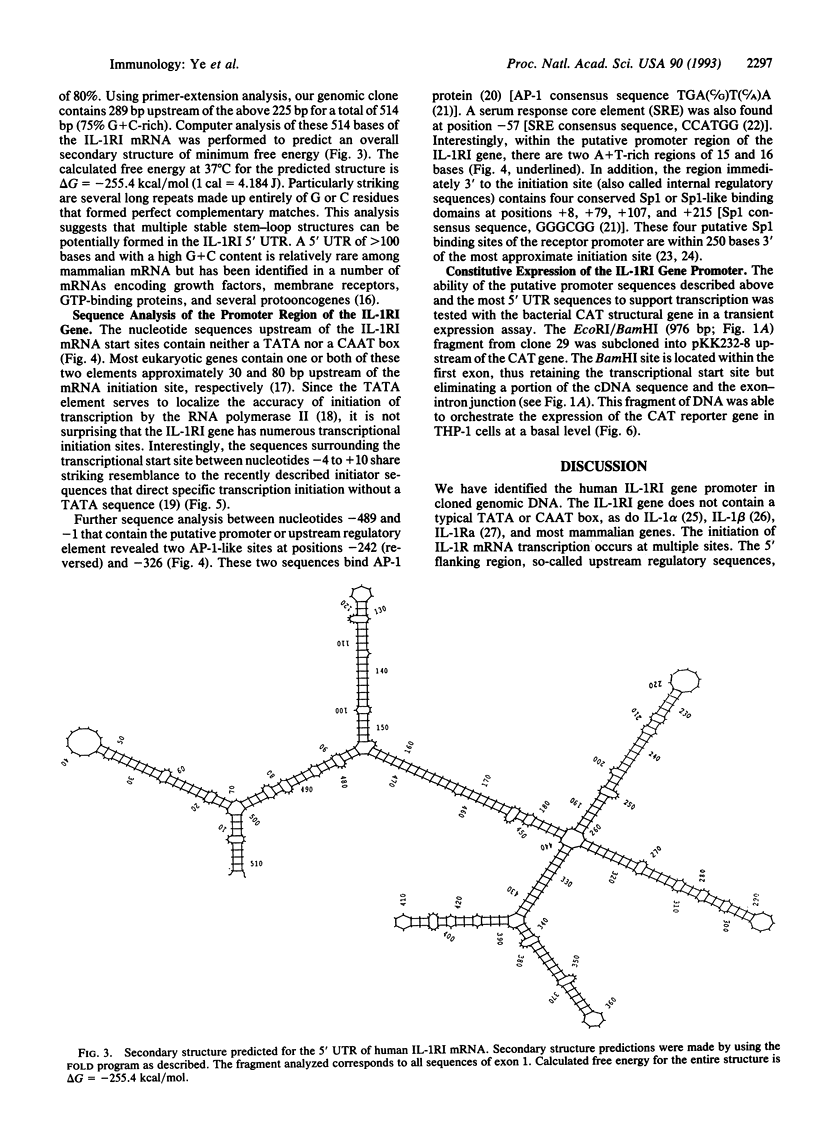
Abstract
To better understand the role of interleukin 1 (IL-1) and its receptor in disease, we have isolated a genomic clone of the human IL-1 type I receptor and have identified the promoter region. There are multiple transcriptional initiation sites as demonstrated by primer extension. DNA sequence analysis shows that the promoter region contains neither a TATA nor a CAAT box; however, the 5' upstream regulatory elements contain two AP-1-like binding sites. The internal regulatory sequences found immediately downstream to the 5' transcriptional start site contain four Sp1 binding domains and have a high G+C content of 75%. This portion of the 5' untranslated region of the mRNA can form stable secondary structure as predicted by computer modeling. Base pairs -4 to + 10 share striking resemblance to an initiator sequence that directs basal expression of certain TATA-less genes-e.g., terminal deoxynucleotidyltransferase in lymphocytes. The IL-1 receptor promoter directs basal expression of chloramphenicol acetyltransferase in transiently transfected cells. Overall, the promoter of the IL-1 type I receptor gene resembles that of constitutively expressed genes that have housekeeping- and/or growth-related functions. The constitutive nature of the promoter may account for this gene being expressed at low levels in diverse cell types. Our finding sheds more understanding into the mechanisms governing the regulation of the IL-1 receptor in health and disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Chou H. S., Loh D. Y. A conserved sequence in the T-cell receptor beta-chain promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3551–3554. doi: 10.1073/pnas.85.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E., Shimada F., Uzawa H., Mori M., Ebina Y. Characterization of the promoter region of the human insulin receptor gene. Evidence for promoter activity. J Biol Chem. 1987 Nov 25;262(33):16186–16191. [PubMed] [Google Scholar]
- Chiou W. J., Bonin P. D., Harris P. K., Carter D. B., Singh J. P. Platelet-derived growth factor induces interleukin-1 receptor gene expression in Balb/c 3T3 fibroblasts. J Biol Chem. 1989 Dec 25;264(36):21442–21445. [PubMed] [Google Scholar]
- Chua A. O., Gubler U. Sequence of the cDNA for the human fibroblast type interleukin-1 receptor. Nucleic Acids Res. 1989 Dec 11;17(23):10114–10114. doi: 10.1093/nar/17.23.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. D., Collins K. L., Gandy M. S., Webb A. C., Auron P. E. Genomic sequence for human prointerleukin 1 beta: possible evolution from a reverse transcribed prointerleukin 1 alpha gene. Nucleic Acids Res. 1986 Oct 24;14(20):7897–7914. doi: 10.1093/nar/14.20.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke D. W., Bankert L. A., Roberts C. T., Jr, LeRoith D., Casella S. J. Analysis of the human type I insulin-like growth factor receptor promoter region. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1113–1120. doi: 10.1016/0006-291x(91)90654-p. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Gallis B., Overell R. W., McMahan C. J., DeRoos P., Ireland R., Eisenman J., Dower S. K., Sims J. E. T-cell interleukin 1 receptor cDNA expressed in Chinese hamster ovary cells regulates functional responses to interleukin 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3045–3049. doi: 10.1073/pnas.86.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Sims J. E., Cerretti D. P., Bird T. A. The interleukin-1 system: receptors, ligands and signals. Chem Immunol. 1992;51:33–64. [PubMed] [Google Scholar]
- Dynan W. S., Sazer S., Tjian R., Schimke R. T. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986 Jan 16;319(6050):246–248. doi: 10.1038/319246a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Fukui T., Ohue M., Nomura H., Yamada M., Nakamura S. Complete nucleotide sequence of the gene for human interleukin 1 alpha. Nucleic Acids Res. 1986 Apr 25;14(8):3167–3179. doi: 10.1093/nar/14.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin A. M., Pawar S., Marth J. D., Perlmutter R. M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988 Aug;8(8):3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Robb R., Wong W. L., Horuk R. HepG2 cells predominantly express the type II interleukin 1 receptor (biochemical and molecular characterization of the IL-1 receptor). Cytokine. 1992 Jan;4(1):18–23. doi: 10.1016/1043-4666(92)90031-l. [DOI] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Freeman N., George D. L. Structural and functional characterization of the promoter region of the mouse c-Ki-ras gene. Mol Cell Biol. 1987 Jul;7(7):2592–2596. doi: 10.1128/mcb.7.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Merlino G. T., Pastan I. Promoter region of the human Harvey ras proto-oncogene: similarity to the EGF receptor proto-oncogene promoter. Science. 1985 Dec 20;230(4732):1378–1381. doi: 10.1126/science.2999983. [DOI] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Wells R. D. Effects of neighboring DNA homopolymers on the biochemical and physical properties of the Escherichia coli lactose promoter. I. Cloning and characterization studies. J Biol Chem. 1982 Nov 10;257(21):12954–12961. [PubMed] [Google Scholar]
- Koch K. C., Ye K., Clark B. D., Dinarello C. A. Interleukin 4 (IL) 4 up-regulates gene and surface IL 1 receptor type I in murine T helper type 2 cells. Eur J Immunol. 1992 Jan;22(1):153–157. doi: 10.1002/eji.1830220123. [DOI] [PubMed] [Google Scholar]
- Koromilas A. E., Lazaris-Karatzas A., Sonenberg N. mRNAs containing extensive secondary structure in their 5' non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 1992 Nov;11(11):4153–4158. doi: 10.1002/j.1460-2075.1992.tb05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Leader length and secondary structure modulate mRNA function under conditions of stress. Mol Cell Biol. 1988 Jul;8(7):2737–2744. doi: 10.1128/mcb.8.7.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau N. R., St John T. P., Weissman I. L., Wolf S. C., Silverstone A. E., Baltimore D. Cloning of terminal transferase cDNA by antibody screening. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5836–5840. doi: 10.1073/pnas.81.18.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Buzard G. A dictionary of transcription control sequences. DNA Seq. 1990;1(1):3–11. doi: 10.3109/10425179009041342. [DOI] [PubMed] [Google Scholar]
- McMahan C. J., Slack J. L., Mosley B., Cosman D., Lupton S. D., Brunton L. L., Grubin C. E., Wignall J. M., Jenkins N. A., Brannan C. I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991 Oct;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J., Witte O. N. The 5' noncoding region of the human leukemia-associated oncogene BCR/ABL is a potent inhibitor of in vitro translation. Mol Cell Biol. 1989 Nov;9(11):5234–5238. doi: 10.1128/mcb.9.11.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orencole S. F., Dinarello C. A. Characterization of a subclone (D10S) of the D10.G4.1 helper T-cell line which proliferates to attomolar concentrations of interleukin-1 in the absence of mitogens. Cytokine. 1989 Nov;1(1):14–22. doi: 10.1016/1043-4666(89)91044-2. [DOI] [PubMed] [Google Scholar]
- Palecek E. Local supercoil-stabilized DNA structures. Crit Rev Biochem Mol Biol. 1991;26(2):151–226. doi: 10.3109/10409239109081126. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Sehgal A., Patil N., Chao M. A constitutive promoter directs expression of the nerve growth factor receptor gene. Mol Cell Biol. 1988 Aug;8(8):3160–3167. doi: 10.1128/mcb.8.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. F., Jr, Eidlen D., Brewer M. T., Eisenberg S. P., Arend W. P., Gutierrez-Hartmann A. Human IL-1 receptor antagonist promoter. Cell type-specific activity and identification of regulatory regions. J Immunol. 1992 Sep 15;149(6):2000–2007. [PubMed] [Google Scholar]
- Spriggs M. K., Lioubin P. J., Slack J., Dower S. K., Jonas U., Cosman D., Sims J. E., Bauer J. Induction of an interleukin-1 receptor (IL-1R) on monocytic cells. Evidence that the receptor is not encoded by a T cell-type IL-1R mRNA. J Biol Chem. 1990 Dec 25;265(36):22499–22505. [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K., Koch K. C., Clark B. D., Dinarello C. A. Interleukin-1 down-regulates gene and surface expression of interleukin-1 receptor type I by destabilizing its mRNA whereas interleukin-2 increases its expression. Immunology. 1992 Mar;75(3):427–434. [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]