Abstract
BACKGROUND
Few African countries separate blood donations into components; however, demand for platelets (PLTs) is increasing as regional capacity to treat causes of thrombocytopenia, including chemotherapy, increases. Namibia introduced single-donor apheresis PLT collections in 2007 to increase PLT availability while reducing exposure to multiple donors via pooling. This study describes the impact this transition had on PLT availability and safety in Namibia.
STUDY DESIGN AND METHODS
Annual national blood collections and PLT units issued data were extracted from a database maintained by the Blood Transfusion Service of Namibia (NAMBTS). Production costs and unit prices were analyzed.
RESULTS
In 2006, NAMBTS issued 771 single and pooled PLT doses from 3054 whole blood (WB) donations (drawn from 18,422 WB donations). In 2007, NAMBTS issued 486 single and pooled PLT doses from 1477 WB donations (drawn from 18,309 WB donations) and 131 single-donor PLT doses. By 2011, NAMBTS issued 837 single-donor PLT doses per year, 99.1% of all PLT units. Of 5761 WB donations from which PLTs were made in 2006 to 2011, a total of 20 (0.35%) were from donors with confirmed test results for human immunodeficiency virus or other transfusion-transmissible infections (TTIs). Of 2315 single-donor apheresis donations between 2007 and 2011, none of the 663 donors had a confirmed positive result for any pathogen. As apheresis replaced WB-derived PLTs, apheresis production costs dropped by a mean of 8.2% per year, while pooled PLT costs increased by an annual mean of 21.5%. Unit prices paid for apheresis- and WB-derived PLTs increased by 9 and 7.4% per year on average, respectively.
CONCLUSION
Namibia’s PLT transition shows that collections from repeat apheresis donors can reduce TTI risk and production costs.
In sub-Saharan Africa, most blood transfusions are performed with whole blood (WB), and fewer than half of countries report separating any collected blood units into components.1 Reasons for reliance on WB transfusions include transportation, inventory management and cold chain challenges, inadequate storage capacity, clinicians’ inexperience in the appropriate use of blood components, and limited financial resources to support blood component production facilities.2–7 In developed countries, platelet (PLT) transfusions are most frequently indicated for hematologic and oncologic conditions complicated by thrombocytopenia.8–10 While obstetric hemorrhage, malaria-related anemia, and trauma have historically accounted for most transfusions in sub- Saharan Africa, increased numbers of patients with hematologic conditions and other malignancies are expected due to improved diagnostic and clinical capacity.11–15 As blood banking capabilities in the region continue to develop, there has been growing emphasis on the preparation of blood components, including PLTs, to effectively manage thrombocytopenia due to malignancy or chemotherapy-related complications.16,17
In Namibia, a medium human development index country in southern Africa with a high human immunodeficiency virus (HIV) burden, the Blood Transfusion Service of Namibia (NAMBTS), a nonprofit organization that funds the majority of its operations through a cost-recovery system, is the only entity authorized to collect, process, and distribute blood and blood components, including PLTs. Nearly all transfusions in the country occur with components rather than WB. NAMBTS only collects blood from voluntary, nonremunerated blood donors (VNRDs) and prepares PLTs from repeat VNRDs who have made at least two previous donations, a group considered at lowest risk for HIV infection.18,19
The country has relatively small numbers of cancer diagnoses annually. Between 2006 and 2011, the Cancer Association of Namibia documented 13,652 new cases of adult cancers (unpublished data, Cancer Association of Namibia). Patients have historically been referred to neighboring South Africa for treatment;20–22 however, increasingly, therapy for hematologic and other malignancies is provided in Namibia.20 To meet the national demand for PLTs, NAMBTS initially prepared WB-derived PLTs using the buffy coat method.23 In 2007, however, NAMBTS procured apheresis equipment and began single-donor apheresis PLT collections. The decision to transition from WB-derived PLTs pooled from several donors to single-donor apheresis PLTs was driven by concern about Namibia’s high HIV adult (ages 15–49) population prevalence, which ranged from an estimated 15.7% in 2002 and 2003 to an estimated 13.5% in 2010 and 2011,24 and a desire to reduce the risk of transfusion-transmitted infections (TTIs) associated with exposure to multiple donors.25
While Namibia’s annual production of PLTs is small compared to industrialized countries, the evolution of NAMBTS’s PLT collection and production practices may provide useful lessons for other countries in the region considering implementing or expanding PLT production. This assessment of Namibia’s transition to apheresis PLT collections provides strong evidence of the increased safety of highly regular apheresis donors, a rare group of repeat blood donors in sub-Saharan Africa, and quantifies and describes the current demand for PLTs in an African health care system. Finally, investments in apheresis PLT production in Namibia have had important cost and sustainability implications for Namibia, which may be relevant to other countries in the region.
MATERIALS AND METHODS
Approval was obtained from the Namibian Ministry of Health and Social Services (MOHSS) before data collection. Because data were collected as part of routine public health program activities, the project was exempted from review by an institutional review board by the Centers for Disease Control and Prevention in Atlanta, Georgia.
Study sites and data collection
Demographic, clinical, and laboratory data are routinely collected on all NAMBTS blood donors, including the type of collection (WB or apheresis), infectious disease screening results, the number of previous donations, and the number and type of components produced from each collection. Some limited information on transfusion recipients, including diagnosis and patient demographics, is regularly recorded on blood request forms, which were last updated in 2007. Blood donor, laboratory screening, and PLT production data are stored in an electronic, NAMBTS-designed SQL database, which also captures information on blood requests from facilities.
To describe the transition of PLT production from WB collections to apheresis and to calculate the prevalence of TTIs among the two groups of PLT donors, a data set containing information on donor demographics, donation type (WB or apheresis), and TTI results was extracted from the database for all donations between January 1, 2006, and December 31, 2011. The safety of WB-derived and apheresis PLT collections was further documented by the incidence of TTI between WB and apheresis donors. An additional assessment of safety was determined by estimating the residual risk of HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) transmission via transfusion attributable to both product types using a previously described incidence rate/window period model.26 For this study, the incidence rates and residual risk of transfusion-transmitted HIV, HBV, and HCV could not be calculated for donations between 2006 and 2011 because information on interdonation intervals and person-years of observation were not available for the incidence rate estimate calculation. However, since donor demographics remained stable from 2006 and 2013, and there were no changes to donor recruitment, deferral criteria, or the TTI screening algorithm, an alternative, representative, period (February 1, 2012–June 30, 2013) for which data on interdonation intervals were available was used to calculate residual risk. Because NAMBTS had stopped production of pooled PLT units by 2012, the residual risk of transmission among WB donors was modeled using the former eligibility criteria for WB donations to be selected for pooled PLT production. (To be selected for pooled PLT production, a WB donor must have had three previous TTI-negative donations recorded in the NAMBTS database, at least one of which must have occurred within the previous 6 months.)
Pooled adult PLT doses were prepared by combining five WB-derived PLT units resulting in a product with a mean volume of 275 to 300 mL and expected PLT count of more than 240 × 109/L. A pediatric dose is a single, unpooled, WB-derived PLT unit with a mean volume of 50 to 60 mL and a PLT count of more than 55 × 109/L. Adult apheresis doses were collected from a single donor in 200- to 300-mL bags using apheresis equipment (Haemonetics MCS+9000, Haemonetics Corp., Braintree, MA). Each apheresis dose had an expected PLT count of more than 300 × 109/L. All PLTs were collected and produced at the NAMBTS blood center in Windhoek.
All TTI screening was performed by the South African National Blood Service, which uses an algorithm combining immunoassays and individual-donation nucleic acid tests (ID-NAT) for HIV, HBV, and HCV screening and confirmatory testing and Treponema pallidum hemagglutination assay (TPHA) for syphilis screening.27 An infected donor was defined as a PLT donor with a repeat-reactive serologic test plus a confirmed ID-NAT for HIV, HBV or HCV, or a positive TPHA result.
Blood request data, including underlying patient diagnoses, are captured by blood banks nationally and returned on a routine basis to NAMBTS headquarters. For this study, the total number of all PLT units requested and issued to health care facilities nationally were extracted for all years (2006–2011). To describe national demand for PLTs, 4 years (2007–2011) of electronic PLT request records that included patient diagnosis information were reviewed. The standardized national blood request form requires physicians to enter a diagnosis for each patient, but physicians are not required to use a standard set of diagnostic codes or terms. For this analysis, more than 200 individual diagnoses entered by physicians were reviewed by one of the coinvestigators (SB) and grouped into 20 broad diagnostic categories defined by the WHO International Classification of Disease system (ICD-10).28
To evaluate the impact of introducing apheresis collections on unit prices charged to public sector hospitals via the NAMBTS cost-recovery system, invoice data were extracted from an electronic accounting system used by NAMBTS. The analysis was limited to the public sector since MOHSS-operated facilities accounted for a mean of 77% of all PLT units issued each year. Prices are set annually by NAMBTS in consultation with the MOHSS and are derived from a costing algorithm that divides the sum of all production costs (including waste) minus any non–cost-recovery revenue (e.g., external grants) by the total number of PLT units produced each year.29 This model assigns fixed costs across all of the product categories and accounts for differences in the amount of labor, utilities, and other variables required to produce different types of blood components (e.g., red blood cells or buffy coat pooled PLTs). In this way, unit costs associated with “pooled PLTs” reflect the additional inputs related to the PLT extraction and pooling processes, whereas “whole blood” unit costs do not. Pooled PLT costs and prices were analyzed for 2006 to 2011. Because the accounting database does not match the calendar year, 2008 was the first full year for which apheresis unit costs and prices were available for analysis. Similarly, because cost data reflect actual prices paid for goods and services during each year, all analyses were conducted using unadjusted Namibian dollars. Mean annual US dollar exchange rates are provided in Table 1.
TABLE 1.
Change in PLT production costs and unit prices, Namibia, 2006 to 2011
| Type of PLT unit (type of donation) | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Mean annual change (%) |
|---|---|---|---|---|---|---|---|
| Adult WB-derived (five pooled WB donations) | |||||||
| Production cost (N$ per unit)* | 1,933.40 | 2,677.54 | 1,986.10 | 2,786.30 | 3,213.96 | 4,475.78 | 21.5 |
| Sales price (N$ per unit) | 2,433.00 | 2,433.00 | 2,676.80 | 2,945.00 | 3,181.00 | 3,467.30 | 7.4 |
| Pediatric WB-derived (one WB donation) | |||||||
| Production cost (N$ per unit) | 385.35 | 536.56 | 397.93 | 6.7 | |||
| Sales price (N$ per unit) | 404.00 | 404.00 | 444.80 | 5.0 | |||
| Adult apheresis (single-donor donation) | |||||||
| Production cost (N$ per unit) | 3,034.83 | 2,738.58 | 1,822.47 | 2,159.73 | −8.2 | ||
| Sales price (N$ per unit) | 2,676.80 | 2,945.00 | 3,181.00 | 3,467.30 | 9.0 | ||
| Pediatric apheresis (single-donor donation) | |||||||
| Production cost (N$ per unit) | 349.46 | 453.32 | 358.96 | 614.86 | 26.7 | ||
| Sales price (N$ per unit) | 444.80 | 489.40 | 529.00 | 576.70 | 9.0 | ||
During the study period, the annual Namibian dollar–US dollar exchange rate averaged N$7.5 to the US dollar.
Data were stratified by sex, age, type of donation, and year. Descriptive statistics were calculated with computer software (Microsoft Excel, Microsoft Corp., Seattle, WA; Stata 13.1, StataCorp., College Station, TX). National population estimates were drawn from the Namibian Central Bureau of Statistics.30
RESULTS
Donor demographics
Between 2006 and 2011, NAMBTS produced WB-derived PLT units from 4173 WB donors. Of these, 2473 (59%) were males; the mean age was 40.1 years (range, 16–73 years). From 2007 to 2011, a total of 663 donors made apheresis PLT donations. Of these, 399 (60.2%) were males; the mean age was 43.2 years (range, 19–66 years).
From 2006 to 2011, a total of 5761 WB donations from 4173 donors were converted into PLT units. Among this donor pool, the mean number of donations per donor per year was 1.2 (range, 1.5 in 2006 to 1 in 2011). By contrast, among apheresis PLT donors, a mean of 5.0 donations were made per year between 2007 and 2011 (range, 2.9 in 2007 to 6.7 in 2010). From 2006 to 2011, of 5761 WB donations from which PLT units were derived, 20 (0.3%) were from donors with laboratory confirmed results for one or more TTIs (Table 1). Of these, 12 donations (60%) were from male donors. No confirmed TTIs were detected among 663 apheresis PLT donors between 2007 and 2011 (Table 1).
Incidence rate and residual risk
Between February 1, 2012, and June 30, 2013, the alternative period for which person-years of exposure were available for all donor records, 903 apheresis and 17,330 eligible WB donations were made in Windhoek. Among the 17,330 WB donations that met the eligibility criteria to be selected for buffy coat pooled PLT production, HIV, HBV, and HCV infections were confirmed by ID-NAT in 10 (0.06%), two (0.01%), and zero (0.0%) donations, respectively. None of the 903 single-donor apheresis donations had an ID-NAT confirmed-positive result for any of the three viral markers (Table 2). Incidence rates per 10,000 donations and residual risk estimates for each marker and the two donation groups are shown in Table 2.
TABLE 2.
Disease prevalence among WB (pooled) and single-donor apheresis PLT donations, Namibia, 2007 to 2011*
| Confirmed TTI prevalence | ||||||
|---|---|---|---|---|---|---|
| Donation type | Number of donations |
Confirmed HIV |
Confirmed HBV |
Confirmed HCV |
Confirmed TPHA |
Total confirmed TTI |
| WB | 5761 | 5 (0.1%) | 4 (0.1%) | 5 (0.1%) | 6 (0.1%) | 20 (0.3%) |
| Single-donor apheresis† | 663 | 0 (0.0%) | (0.0%) | (0.0%) | (0.0%) | 0 (0.0%) |
Data are reported as number (%).
Single-donor apheresis donations began in 2007.
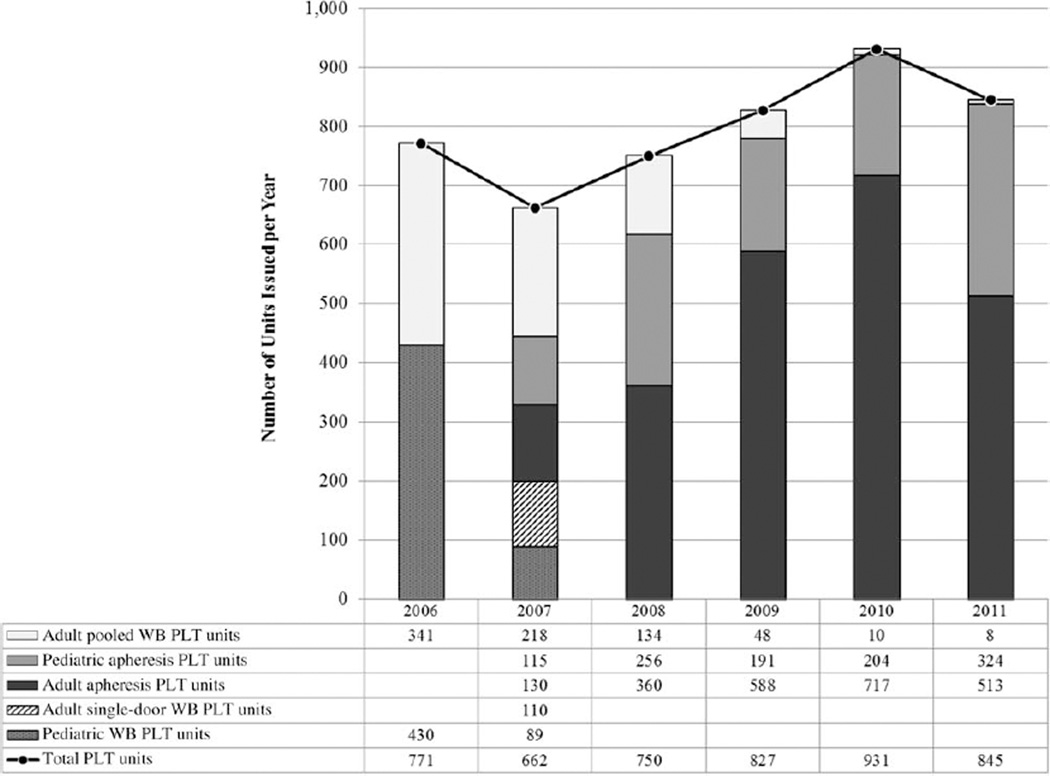
In 2006, NAMBTS issued 771 WB-derived PLT doses (341 pooled adult doses, 430 pediatric doses). Apheresis PLT collections were introduced in 2007. During that year, NAMBTS issued 241 WB-derived PLT doses (218 pooled adult doses, 23 pediatric doses) and 245 apheresis PLT doses (130 adult, 115 pediatric). WB-derived pooled units were almost eliminated by 2011, when only eight pooled PLT units were issued nationally. By 2011, NAMBTS produced and issued 837 apheresis PLT doses (324 pediatric units, 513 adult units). As a proportion of all PLT units issued per year, single-donor apheresis doses accounted for 37% (245/662) of all PLT doses issued in 2007, but 99.1% (837/845) of all PLT doses issued in 2011 (Fig. 1). Although 39 health care facilities (including 29 public hospitals) requested PLTs between 2006 and 2011 (annual range, 20–25 facilities per year), 64.2% of all requests were from two large public, tertiary referral hospitals in the capital, Windhoek.
Fig. 1.
PLT units issued by type, Namibia, 2006 to 2011.
Patient diagnosis data from blood request forms were routinely captured by the NAMBTS information system starting in 2007. Between 2007 and 2011, a total of 4015 PLT doses were issued to hospitals nationally (Fig. 1). Of these, 1199 (30%) were given to patients with ICD-10 diagnoses associated with “malignant neoplasms” (C00–C97) and “aplastic anemia” (D50–D64), which collectively accounted for the highest proportion of all diagnoses.
The cost to produce an adult WB-derived pooled PLT dose increased by a mean of 21.5% per year between 2006 and 2011 (Table 1). As apheresis donations accounted for an increasing proportion of all PLT units produced by NAMBTS, production costs for adult apheresis doses declined by a mean of 8.2% per year between 2008 and 2011 (Table 1). During the study period, unit prices for WB-derived doses and apheresis doses increased by a mean of 7.4 and 9.0% per year. By 2011, as the production of WB-derived PLTs fell to nearly zero, nonapheresis PLTs cost more to produce than the cost-recovery price.
DISCUSSION
Investments in apheresis technology allowed NAMBTS to improve the availability and, likely, the safety of PLTs in Namibia by collecting PLTs from a core population of reliable, high-frequency, repeat apheresis donors. While the incidence and residual risk estimates presented are derived from a more recent time period, we believe that they are an accurate proxy for the situation between 2006 and 2011. This is because donor selection policies and TTI screening algorithms were unchanged between the two periods, as were underlying TTI prevalence rates in the donor population. As such, these findings add to an already solid evidence base supporting repeat VNRD as the safest blood donors in settings with high population burdens of HIV and other TTI.31–33 Namibia’s experience also highlights the potential for blood services in resource limited settings in sub-Saharan Africa to support the broader health care sector to treat patients with hematologic and oncologic diseases and match demand as population increases (Namibia’s population grew by an estimated 9.7% between 2006 and 201130). The investments made in Namibia allowed for incremental reductions in PLT unit production costs, but this experience raises important questions about consumers’ ability or willingness to pay, as well as the need for additional funding to sustain current apheresis PLT production capacity, and expand PLT availability beyond the capital.
The lessons learned in Namibia present several important points for consideration by blood services operating elsewhere in the region, particularly those seeking to expand the availability of transfusion therapies to treat a broader range of clinical conditions, including hematologic and oncologic diseases. First, the findings of this study demonstrate that the size and safety of the PLT supply in a sub-Saharan African setting can be increased quickly and efficiently. However, decisions to develop PLT production capacity must be made in conjunction with an expansion of overall health care services beyond the traditional drivers of transfusion demand in sub-Saharan Africa (e.g. HIV, malaria, peripartum hemorrhage). Planners must consider logistic challenges related to PLT storage requirements and the short shelf life of PLT units, which may limit their utility in areas with limited distribution infrastructure. As demonstrated in this study, while the numbers of PLT units available nationally increased incrementally, most patients requiring PLT transfusions (e.g., oncology and hematology) continue to be referred to two large public hospitals in Windhoek, the same city where the PLTs were manufactured. Expanding clinical services, including PLT availability, to population centers in the northern, more resource-constrained part of the country, will be an ongoing challenge for NAMBTS and health care planners in Namibia. Looking ahead, Namibia’s MOHSS has already indicated that it will expand oncology services elsewhere in the country. In 2010, for example, radiotherapy services were expanded at the regional referral hospital in Oshakati, a city approximately 700 km north of Windhoek.34
Second, planning for PLT production must include extensive training of clinicians (on appropriate component use), collection staff (on apheresis collection machines), laboratory workers (on apheresis unit handling and quality control), and donor mobilization teams (to educate donors on the difference between WB and apheresis donations). Finally, particularly in areas where apheresis PLT production is being considered, reliance on apheresis equipment will require investments in maintenance and contingency planning for periods when the equipment may be off-line. By 2011, PLT production in Namibia was highly dependent on two Haemonetics apheresis machines. Although apheresis donors could agree to return to WB donations in the event that the machines were unavailable, contingency plans must also consider ongoing training for laboratory staff to ensure the quality of pooling procedures in facilities that no longer routinely produce pooled units.
Consideration should also be given to the long-term sustainability of PLT production systems, especially given the high per unit costs observed in this study. In Namibia, the investments that allowed NAMBTS to expand PLT production were substantially subsidized by grants from the US President’s Emergency Plan for AIDS Relief (PEPFAR). However, despite these grants, PLT unit prices remained high in Namibia, a reflection of the blood service’s need to recover a growing proportion of costs as the PEPFAR subsidy declined. While Namibia’s upper middle income economy has been able to support this cost structure, similar costs would likely make PLT therapy prohibitive elsewhere in the region, despite substantial PEPFAR investments in strengthening blood services.35,36 As PEP-FAR funding for blood safety scales down in Namibia and other parts of the region, mobilizing adequate domestic resources will become a greater challenge, as will the need to sustain the reduced apheresis production costs observed in this study. Domestic and international program and policy planners in Namibia and similar settings should continue to pay close attention to costs and pricing to ensure continued availability of blood and blood components and to ensure that cost-recovery systems remain a viable option for financial sustainability. An increased focus on understanding costs and the impact of reductions in external funding on health care service delivery has also been included in proposed US legislation to reauthorize the PEPFAR initiative through 2018.37 Supplemental revenue sources may also be required, particularly since a cost-recovery-only model may not be feasible in many sub-Saharan African countries. For example, to augment its non–cost-recovery revenue base, NAMBTS exports plasma to South Africa for fractionation. Other countries in the region may explore similar possibilities, but in countries that currently fund national blood transfusion services through government budgets and external donor support, expansion of PLT production may be precluded by the substantial resource mobilization requirements, as well as limited capacity to consume PLTs in the health care system.
This study is subject to the following limitation. Because there is currently no system in Namibia to track the use of blood products within health care facilities, blood request and issuance data are considered a proxy for patient demand. While the figures presented here are likely reasonable estimates of actual PLT demand in public and private sector hospitals and clinics, they do not capture instances where PLTs were not available to fill a request or the needs of patients who cannot access a health care facility. As a result, these findings likely represent an incomplete estimate of PLT demand and clinical capacity to treat conditions requiring PLTs.
In conclusion, Namibia’s experience with growing clinical capacity for PLTs and reduced production costs after the introduction of apheresis technology are certainly positive signs. But in more resource-limited settings in the region, a longer-term approach than that observed in Namibia may be required before similar gains can be realized.
TABLE 3.
Estimated incidence rate and residual risk of HIV, HCV, and HBV transmission through transfusion of WB-derived pooled PLT units and single-donor apheresis PLT units, Namibia, February 1, 2012, to June 30, 2013
| Test | Number of donors |
Number of donations |
ID-NAT confirmed cases |
Prevalence (%) |
Incidence rate per 10,000 donations |
Person- years (PY)* |
Incidence rate (IR) per PY |
Residual risk (%)†‡ |
Pooled residual risk (%)§ |
|---|---|---|---|---|---|---|---|---|---|
| HIV | |||||||||
| Single-donor apheresis | 129 | 903 | 0 | 0.0 | 0.0 | 100.8 | 0.0000 | 0.000 | |
| Eligible WB donations | 6140 | 17,330 | 10 | 0.06 | 5.8 | 3,929.5 | 0.0025 | 0.003 | 0.016 |
| HCV | |||||||||
| Single-donor apheresis | 129 | 903 | 0 | 0.0 | 0.0 | 100.8 | 0.0000 | 0.000 | |
| Eligible WB donations | 6140 | 17,330 | 0 | 0.0 | 0.0 | 3,930.9 | 0.0000 | 0.000 | 0.000 |
| HBV | |||||||||
| Single-donor apheresis | 129 | 903 | 0 | 0.0 | 0.0 | 100.8 | 0.0000 | 0.000 | |
| Eligible WB donations | 6140 | 17,330 | 2 | 0.01 | 1.2 | 3,930.4 | 0.0005 | 0.002 | 0.010 |
Person-time was calculated as the difference between the date of the current donation and the date of the previous donation. This difference was then scaled to years by dividing it by 365.25. Each interval was defined based on the current donation type (i.e., single-donor apheresis or eligible WB donation). Intervals ending in ID-NAT confirmed cases were divided by two. If the previous donation date occurred before the start of the study period, the date of the previous donation was set to February 1, 2012.
Residual risk = IR × (WP/365.25).
Window periods based on ID-NAT values reported for the Procleix Ultrio assay (Novartis Diagnostics/Grifols, Emeryville, CA; available at: http://www.novartisdiagnostics.com/products/procleixassays/ultrio-plus.shtml): 4.7 (HIV), 2.2 (HCV), and 14.9 days (HBV).
Pooled residual risk = 1 – (1 – residual risk)5; pooled residual risk estimate was calculated because five WB-derived PLT units were pooled for each adult transfusion dose.
ACKNOWLEDGMENTS
The authors thank NAMBTS staff in the apheresis collection clinics and the laboratory and hemovigilance departments for their contributions to collecting and managing the data presented in this report. JPP, SVB, and RW participated in study design, study supervision, data collection, and data interpretation; CSS, MP, MM, BvF, AM, RWS, and DL participated in data interpretation. All authors participated in writing the manuscript and approved the final content; and JPP had full access to all of the data and takes responsibility for the accuracy of the data analysis.
This project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- ID-NAT
individual-donation nucleic acid test
- MOHSS
Ministry of Health and Social Services
- NAMBTS
Blood Transfusion Service of Namibia
- PEPFAR
President’s Emergency Plan for AIDS Relief
- TPHA
Treponema pallidum hemagglutination assay
- TTI(s)
transfusion-transmitted infection(s)
- VNRB(s)
voluntary, nonremunerated blood donor(s)
- WB
whole blood
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.World Health Organization. Brazzaville (Congo): WHO; 2007. Status of blood safety in the WHO African region: report of the 2004 survey. [Google Scholar]
- 2.Kouriba B, Diarra A, Coulibaly MD, et al. Mali Med. Vol. 25. French: 2010. Quality assurance set-up in a blood transfusion facility: the experience of the National Blood Transfusion Center of Mali; pp. 23–28. [PubMed] [Google Scholar]
- 3.Kajja I, Kyeyune D, Bimenya GS, et al. Bottlenecks of blood processing in Uganda. Transfus Med. 2010;20:329–336. doi: 10.1111/j.1365-3148.2010.01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Arewa OP. One year clinical audit of the use of blood and blood components at a tertiary hospital in Nigeria. Niger J Clin Pract. 2009;12:429–433. [PubMed] [Google Scholar]
- 5.Tagny CT, Mbanya D, Tapko JB, et al. Blood safety in sub-Saharan Africa: a multi-factorial problem. Transfusion. 2008;48:1256–1261. doi: 10.1111/j.1537-2995.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 6.Diakite M, Diawara SI, Tchogang NT, et al. Transfus Clin Biol. Vol. 19. French: 2012. Knowledge and attitudes of medical personnel in blood transfusion in Bamako, Mali; pp. 74–77. [DOI] [PubMed] [Google Scholar]
- 7.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev. 2012;26:164–180. doi: 10.1016/j.tmrv.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi H, Lowe D, Dobson P, et al. National comparative audit of the use of platelet transfusions in the UK. Transfus Clin Biol. 2007;14:509–513. doi: 10.1016/j.tracli.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Simon TL, Synder EL, Stowell CP, et al. Rossi’s principles of transfusion medicine. West Sussex (UK): Wiley-Blackwell; 2009. [Google Scholar]
- 10.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132–150. doi: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119:5078–5087. doi: 10.1182/blood-2012-02-387092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lackritz EM, Ruebush TK, 2nd, Zucker JR, et al. Blood transfusion practices and blood-banking services in a Kenyan hospital. AIDS. 1993;7:995–999. doi: 10.1097/00002030-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Mundy CJ, Bates I, Nkhoma W, et al. The operation, quality and costs of a district hospital laboratory service in Malawi. Trans R Soc Trop Med Hyg. 2003;97:403–408. doi: 10.1016/s0035-9203(03)90070-8. [DOI] [PubMed] [Google Scholar]
- 14.Natukunda B, Schonewille H, Smit Sibinga CT. Assessment of the clinical transfusion practice at a regional referral hospital in Uganda. Transfus Med. 2010;20:134–139. doi: 10.1111/j.1365-3148.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 15.Bugge HF, Karlsen NC, Oydna E, et al. A study of blood transfusion services at a district hospital in Malawi. Vox Sang. 2013;104:37–45. doi: 10.1111/j.1423-0410.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva: World Health Organization; 2005. (WHO) aide-memoire for national health authorities: safe blood components. [cited 2013 Jun 6]. Available from http://www.who.int/bloodsafety/processing/who_eht_05_01_en.pdf. [Google Scholar]
- 17.Petti CA, Polage CR, Quinn TC, et al. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 18.Kimani D, Mwangi J, Mwangi M, et al. Blood donors in Kenya: a comparison of voluntary and family replacement donors based on a population-based survey. Vox Sang. 2011;100:212–218. doi: 10.1111/j.1423-0410.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- 19.Government of the Republic of Namibia. Windhoek (Namibia): Ministry of Health and Social Services; 2010. Standards for the practice of blood transfusion in Namibia. [Google Scholar]
- 20.Stefan DC, Shalongo S, Ribeiro R. Twinning in paediatric oncology—an African experience. S Afr Med J. 2012;102:28–29. [PubMed] [Google Scholar]
- 21.Wessels G, Hesseling PB. Incidence and frequency rates of childhood cancer in Namibia. S Afr Med J. 1997;87:885–889. [PubMed] [Google Scholar]
- 22.Wessels G, Hesseling PB. Unusual distribution of childhood cancer in Namibia. Pediatr Hematol Oncol. 1996;13:9–20. doi: 10.3109/08880019609033368. [DOI] [PubMed] [Google Scholar]
- 23.Devine DV, Howe D. Processing of whole blood into cellular components and plasma. ISBT Sci Ser. 2010;5:78–82. [Google Scholar]
- 24.Government of the Republic of Namibia. Global AIDS response progress reporting 2012: monitoring the 2011 political declaration on HIV/AIDS. 2012 [cited 2014 Oct 24]. Available from: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_NA_Narrative_Report[1].pdf.
- 25.Hillyer KL. Apheresis blood component collections. In: Shaz BH, Hillyer CD, Zimring JC, Abshire TC, editors. Transfusion medicine and hemostasis. Burlington (MA): Elsevier; 2009. pp. 33–36. [Google Scholar]
- 26.Glynn SA, Kleinman SH, Wright DJ, et al. International application of the incidence rate/window period model. Transfusion. 2002;42:966–972. doi: 10.1046/j.1537-2995.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen M, Lelie N, Sykes W, et al. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion. 2009;49:1115–1125. doi: 10.1111/j.1537-2995.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Geneva: World Health Organization; 1994. International statistical classification of diseases and related health problems 10th revision. [cited 2012 Aug 15]. Available from: http://apps.who.int/classifications/icd10/browse/2010/en. [Google Scholar]
- 29.Pitman JP, Bocking A, Wilkinson R, et al. The impact of external donor support through the U.S. President’s Emergency Plan for AIDS Relief on the cost of red cell concentrate in Namibia, 2004–2011. Blood Transfus. 2014;23:1–8. doi: 10.2450/2014.0122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Government of the Republic of Namibia. Windhoek (Namibia): Central Bureau of Statistics; 2006. Population projections. [Google Scholar]
- 31.Diarra A, Kouriba B, Baby M, et al. HIV, HCV, HBV and syphilis rate of positive donations among blood donations in Mali: lower rates among volunteer blood donors. Transfus Clin Biol. 2009;16:444–447. doi: 10.1016/j.tracli.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Bruhn R, Lelie N, Custer B, et al. Prevalence of human immunodeficiency virus RNA and antibody in first-time, lapsed, and repeat blood donations across five international regions and relative efficacy of alternative screening scenarios. Transfusion. 2013;53:2399–2412. doi: 10.1111/trf.12299. [DOI] [PubMed] [Google Scholar]
- 33.Namululi BA, Guerrieri C, Dramaix M. Med Sante Trop. Vol. 22. French: 2012. Impact of method of recruitment of blood donors on the prevalence of HIV and HBV in Bukavu, DR Congo; pp. 69–74. [DOI] [PubMed] [Google Scholar]
- 34.International Atomic Energy Agency. Vienna: International Atomic Energy Agency; 2011. India contributes to the fight against cancer in Namibia. [cited 2013 Sep 26]. Available from http://cancer.iaea.org/newsstory.asp?id=97. [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Progress toward strengthening national blood transfusion services—14 countries, 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1577–1582. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) Progress toward strengthening blood transfusion services—14 countries, 2003–2007. MMWR Morb Mortal Wkly Rep. 2008;57:1273–1277. [PubMed] [Google Scholar]
- 37.PEPFAR Stewardship and Oversight Act of 2013. Amendment to the United States Leadership 8 Against HIV/AIDS, Tuberculosis, and Malaria Act of 9 2003 (22 U.S.C. 7611(f)(1)) In: U.S. Senate, editor. Washington (DC): United States Senate; 2013. [Google Scholar]