Abstract
Objective
Despite data linking Attention-deficit/Hyperactivity Disorder (ADHD) and adult binge eating, there are limited data in children with loss of control eating. We examined inhibitory control in children with loss of control eating syndrome (LOC-ES) and its association with ADHD.
Method
79 children (8–14 years) over the 5th weight percentile were recruited, irrespective of LOC eating or ADHD status. The Eating Disorder Examination for Children and the Standard Pediatric Eating Episode Interview assessed LOC-ES. ADHD diagnosis was determined by the Schedule for Affective Disorders and Schizophrenia for children and Conners-3 (Parent Report) DSM-IV Scales of Inattention and/or Hyperactivity (T score>65). The Go/No-Go Task and the Behavior Regulation Inventory of Executive Function parent report (BRIEF) assessed impulse control.
Results
Odds of LOC-ES were increased 12 times for children with ADHD (adjusted odds ratio [aOR] =12.68, 95% confidence interval [CI] =3.11, 51.64, p<0.001), after adjusting for BMI z-score and relevant covariates. Children had 1.17 times higher odds of reporting LOC-ES with every 5% increase in Go/No-Go Commission Rate (aOR= 1.17, CI=1.01, 1.36, p<0.05) and 1.25 times higher odds of reporting LOC-ES with every 5 unit T-score increase in BRIEF Inhibit Scale (aOR=1.25, CI=1.04, 1.50, p<0.05).
Discussion
Children with ADHD had significantly greater odds of LOC-ES compared to children without ADHD. Children with LOC-ES had significantly greater impulse control deficits on performance-based neuropsychological assessments and on parent reports than children without LOC-ES. These findings suggest a need to investigate possible shared mechanisms such as impulse control deficits, among children with LOC-ES and ADHD.
Keywords: Binge eating, Attention Deficit/Hyperactivity Disorder, Impulsivity, Eating Disorder, Obesity
Attention Deficit/Hyperactivity Disorder (ADHD) is a highly prevalent disorder that has been associated with pediatric obesity.1 Impulsivity is one of the defining features of ADHD. Neurobehavioral studies have demonstrated an association between impulsivity and pediatric obesity.2 One hypothesized factor that could link ADHD and obesity may be disinhibited eating behavior, such as binge eating,3 which has been found to partially mediate the association between ADHD and obesity among adults.4 Although impulsivity has been associated with adult binge eating disorder (BED),5,6 less data are available regarding the role of impulsivity in children who engage in binge eating behavior, perhaps because few children meet criteria for BED.7
Loss of control (LOC) eating is a form of pediatric disinhibited or binge eating that involves the subjective experience of being unable to control what or how much is being consumed.8 Since it is difficult to determine what constitutes a large amount of food in growing children of different ages, the term LOC eating is often used in childhood when either unambiguously large amounts of food (objective large binge episode) or ambiguously large amounts of food (subjectively large binge episode) are reported.9 LOC eating is associated with increased weight gain and psychological health consequences.10 Loss of control eating disorder describes a cluster of symptoms that includes associated behavioral and emotional features of children who report this form of disinhibited eating7, and may represent a child presentation of BED. Children who fit putative criteria for loss of control eating disorder will be referred to as loss of control eating syndrome (LOC-ES).
Impulsivity may be present with impaired impulse control and response inhibition deficits.11 Impulse control deficits have been described in the pediatric ADHD literature as a core feature of the disorder.12 Children with ADHD show deficits in response inhibition on experimental paradigms, including the Go/No-Go (GNG) task12, on skeletomotor tasks and are rated by parents as having impaired impulse control.13 It is conceivable that impulse control deficits similar to those seen in ADHD may also be at play in LOC-ES.14 Given literature describing an association between ADHD and obesity in children,15 it is conceivable that LOC-ES could possibly influence the relationship between ADHD and obesity.3,16 In a community sample, a significant association was found between children with ADHD and binge eating behavior and the findings were compatible with the hypothesis that binge eating partially mediated the association between ADHD and BMI-Z scores, although the data were cross-sectional and could not be used to infer causality.3 We can delineate several possible theoretical mechanisms for the comorbidity of ADHD and LOC-ES, which could: 1. Reflect the random base rates of LOC-ES and ADHD in the population. 2. Result from an underlying common risk factor (such as impulsivity) leading to symptoms 3. Reflect symptom overlap in these disorders, with ADHD presenting a behavioral form of impaired impulse control and LOC-ES presenting an eating-based form of impaired impulse control.
Impaired impulse control and impaired executive function are hypothesized mechanisms of disinhibited eating behavior.17 There are mixed data regarding impulse control in adults with binge eating, with some suggestion that executive dysfunction might contribute to binge episodes particularly when women are experiencing stress,18 and others showing no such relationship.19 Inconsistent findings may be due, in part, to different types of experimental tasks used. However, emerging data suggest deficits in response suppression and executive dysfunction among adults with BED.20
Obese children have also been shown to be more impulsive, with poorer impulse control and response inhibition than leaner children,2,21 raising the question of whether there is also an association between impulse control deficits and LOC-ES in children who have difficulty controlling food intake. To our knowledge, there has only been one published study of LOC eating and neurobehavioral task-assessed impulsivity in youth; which found greater response inhibition deficits among those individuals with LOC eating, but only in response to negative mood induction and not during neutral states.14 However, that study excluded adolescents with both LOC eating and ADHD, hindering further examination between the two conditions.
The goal of the present study was to examine the associations between ADHD, LOC-ES and impulse control deficits in children. Given associations in the literature among ADHD, impulsivity and pediatric obesity, we hypothesized that children with ADHD would be more likely to present with LOC-ES. Moreover, based on evidence of response inhibition deficits in both obese children and children with ADHD, we hypothesized that response inhibition deficits would be associated with LOC-ES. Further, we hypothesized that children with LOC-ES (and elevated BMI z-scores) would exhibit higher odds of ADHD diagnoses than controls without LOC-ES. We also aimed to assess whether the data would support the hypothesis that LOC-ES mediates the relationship between ADHD and increased BMI z-score.
METHOD
Participants
Children and adolescents (ages 8–14 years) were recruited. Participants were referred from the community, through pediatric mental health clinics, medical clinics and flyers on public bulletin boards. Children with body mass index (BMI) greater than the 5th percentile were eligible, which included both children with and without LOC-ES. Participants were excluded for past or current anorexia or bulimia nervosa, schizophrenia, autism, bipolar disorder or substance abuse. An abbreviated Full Scale Intelligence Quotient of at least 70 was required, based on the two-subtest version of the Wechsler Abbreviated Scale of Intelligence.22 Participants were excluded if taking antipsychotic or steroid medications. Buproprion, stimulants, or alpha-2 adrenergic agonists were permitted if administered at a stable dose for 30 days. Socioeconomic status (income and Hollingshead Four Factor Index of social status)23 was collected.
Measures
Diagnostic assessments
The Schedule for Affective Disorders and Schizophrenia for school-age children-Present and Lifetime Version (K-SADS-PL)24 is a semi-structured diagnostic interview, used to diagnose past and current child and adolescent psychiatric comorbidities.
ADHD symptoms were assessed using the Conners-3 Parent Rating Scale-Revised (CRPS)25 via the long parent report form. ADHD diagnosis required a K-SADS-PL diagnosis of current ADHD and a T-score over 65 on either or both of the Conners-3 DSM-IV-TR Scales (Inattentive and/or Hyperactive-Impulsive).
The Eating Disorder Examination26 adapted for Children (ChEDE) is a semi-structured interview that assesses eating-disordered behavior in children 8 to 14 years of age. The ChEDE has excellent inter-rater reliability and discriminant validity (Cohen’s kappa of 1.00 for eating episodes).27 The ChEDE generates three categories of eating episodes: objective binge episodes (objective overeating with LOC), subjective binge episodes (consumption of an ambiguously large amount of food with LOC) and objective overeating (overeating without LOC). The Standard Pediatric Eating Episode Interview (SPEEI)9 was administered to determine the context as well as behavioral and emotional aspects of eating episodes. LOC-ES presence was determined based on previously published putative criteria7 for loss of control eating disorder, using the ChEDE and the SPEEI. We examined the presence of LOC-ES (rather than simply LOC episodes) in order to capture behavioral correlates of LOC eating in children with associated emotional symptoms (such as ‘eating in response to negative affect’), which are similar to those in adult BED diagnoses.28,29
Executive function, including impulsivity, was assessed by the parent report of the Behavior Regulation Inventory of Executive Function (BRIEF).30 Parents rate their child’s behavior on a 3-point Likert scale (sometimes, never, often), and eight scales are obtained (including inhibit and emotional control subscales). Higher ratings indicate greater impairment. Mean internal consistency ratings using the BRIEF Parent Form have been reported as ranging from .82 to .98.13 The inhibit and the emotional control subscales of the BRIEF overlap with ADHD criteria. The BRIEF was found to measure different elements of impulse control than a computerized performance test31, and the BRIEF has been shown to capture executive dysfunction in clinical groups when performance-based measures alone have not.13 Thus, both the BRIEF and neurobehavioral tasks were used to assess impulse control in our study.
Neuropsychological Assessments
The Go/No-Go neurobehavioral task is a computerized task using E Prime software.32 The computer screen flashed green and red spaceships. Participants were told to press the spacebar in response to green ships only. Cues appeared on the screen for 300 msec and were presented once every 1,800 msec (fixed 1,500-ms inter-stimulus interval). Cues were weighted towards green spaceships at a ratio of 3:1 and the task lasted 8 minutes. Commissions were defined as pressing the space bar after the presentation of a red ship. Children with disorders involving inhibitory control such as ADHD have been shown to produce significantly more commission errors than controls.12 The percentage of commission errors (commission rate), reflecting response inhibition, was the primary variable of interest; percentage of omission errors (omission rate), and coefficient variability were also measured.
The Conflicting Motor Response Task measures motor inhibition.33 Participants were told: “If I show you my finger, you show me your fist; if I show you my fist, you show me your finger.” The task requires the child to inhibit the prepotent tendency to mimic the examiner. The variable of interest relevant is the total number of correct trials (maximum score=48).
Procedures
This study was approved by the local Institutional Review Board at the Johns Hopkins Medical Institutions. Written consent was obtained from at least one parent or legal guardian, with the participants’ written assent. Assessments included neurobehavioral tasks, followed by diagnostic parent and child assessments, parent- and self-report forms. Weight and height were measured using a regularly calibrated balance-beam scale and stadiometer; BMI z-scores were calculated.34 Diagnostic assessments were performed by an experienced board-certified child and adolescent psychiatrist and neuropsychological assessments were supervised by an experienced clinical psychologist.
Statistical Analyses
LOC-ES and ADHD were defined as dichotomous variables (rated “Yes or No”; “Yes” for disorder presence; “No” for disorder absence or subthreshold symptoms). All other variables were continuous except where indicated. We split the sample into 3 groups based on their BMI z-scores and LOC-ES status (non-overweight or obese, overweight/obese without LOC-ES, and overweight/ obese with LOC-ES). Examination of demographic data and comparisons across the 3 LOC/weight groups was performed using Chi-square or Fisher’s exact tests and one-way analysis of variance (ANOVA). Scheffé’s method was used to look at pair-wise differences after a significant ANOVA test while accounting for multiple comparisons. Odds ratios for LOC-ES and ADHD diagnosis were calculated using a logistic regression model with impulsivity and neuropsychological measures as main predictors. All models included adjustment for age, sex, race, and BMI z-score, in keeping with previous studies.21 Behavioral tasks, including the Go-No/Go analyses were also adjusted for stimulant medication use.
We examined the relationship between the LOC/weight group and ADHD measures [Inattention, Hyperactivity-Impulsivity (Conners’ Parent Scale T-Scores), Inhibit and Emotional Control scales (BRIEF Parent Form T-Score]. These group indicators were used as predictors in linear regression models for Conners’ and BRIEF measures and a logistic regression model for ADHD diagnosis. Assumptions of the linear regression were checked. To account for non-Gaussian residuals, bias-corrected confidence intervals are reported based on 500 bootstrapped samples of the estimated beta coefficients. To assess LOC-ES as a potential mediator, we used the MacArthur approach to regress LOC-ES on ADHD, BMI z-score on LOC-ES, BMI-z score on ADHD, and the interaction between ADHD and LOC-ES.35 ADHD was the independent variable, BMI z-score was the dependent variable and LOC-ES was a mediator. Statistical significance threshold was set at p-values less than 0.05. All statistical analyses were performed using STATA 11.36
RESULTS
Participant Characteristics
Detailed demographic characteristics of the sample by weight and LOC-ES status are presented in Table 1. Eighty participants were recruited; one participant with IQ less than 70 was excluded. The mean number of LOC eating episodes was 9.16 (SD=15.77). As expected, when comparing across the three groups (non-overweight/obese, no LOC-overweight/obese and LOC-overweight/obese), there was a significant difference in BMI z-scores (p<0.0001). The three children with LOC-ES who were non-overweight/obese were included in the non-overweight/obese group.
Table 1.
Participant Characteristics by Loss of Control Eating Disorder (LOC-ES) and Weight Status
| Weight/LOC-ES categories
|
Total (n=79) | p-value | |||
|---|---|---|---|---|---|
| Non-overweight/obese (n=25) | no LOC-ES overweight/obese (n=19) | LOC-ES overweight/obese (n=35) | |||
| Age, mean (SD) | 10.7 (2.0) | 11.9 (1.8) | 10.6 (1.8) | 11.0 (1.9) | 0.04*1 |
| BMI Z-score, mean (SD) | 0.2 (0.5) | 1.9 (0.5) | 2.2 (0.5) | 1.5 (1.0) | <0.0001*2 |
| Hollingshead, mean (SD) | 38.3 (19.8) | 37.1 (14.6) | 32.7 (13.2) | 35.5 (15.9) | 0.366* |
| Sex, n (%) | 0.881*** | ||||
| Male | 14 (56.0) | 9 (47.4) | 18 (51.4) | 41 (51.9) | |
| Female | 11 (44.0) | 10 (52.6) | 17 (48.6) | 38 (48.1) | |
| Race, n (%) | 0.918*** | ||||
| Non-White | 13 (52.0) | 10 (52.6) | 20 (57.1) | 43 (54.4) | |
| White | 12 (48.0) | 9 (47.4) | 15 (42.9) | 36 (45.6) | |
| Ethnicity, n (%) | 0.922*** | ||||
| Non-Hispanic | 22 (88.0) | 16 (84.2) | 29 (82.9) | 67 (84.8) | |
| Hispanic | 3 (12.0) | 3 (15.8) | 6 (17.1) | 12 (15.2) | |
| Income, n (%) | 0.071*** | ||||
| < $20000 | 10 (40.0) | 6 (31.6) | 11 (31.4) | 27 (34.2) | |
| $20000-$39999 | 1 (4.0) | 4 (21.1) | 10 (28.6) | 15 (19.0) | |
| $40000-$59999 | 1 (4.0) | 4 (21.1) | 7 (20.0) | 12 (15.2) | |
| $60000-$79999 | 2 (8.0) | 2 (10.5) | 2 (5.7) | 6 (7.6) | |
| $80000-$99999 | 3 (12.0) | 2 (10.5) | 2 (5.7) | 7 (8.9) | |
| >= $100000 | 8 (32.0) | 1 (5.3) | 3 (8.6) | 12 (15.2) | |
| Stimulant use, n (%) | 0.028*** | ||||
| No | 21 (84.0) | 13 (68.4) | 18 (51.4) | 52 (65.8) | |
| Yes | 4 (16.0) | 6 (31.6) | 17 (48.6) | 27 (34.2) | |
One way analysis of variance (ANOVA)
Chi-square test of association
Fisher’s exact test
- test for LOC-ES overweight/obese vs. No LOC-ES overweight/obese, p-value = 0.053
- significant test for normal weight vs. both overweight groups, p<0.0001
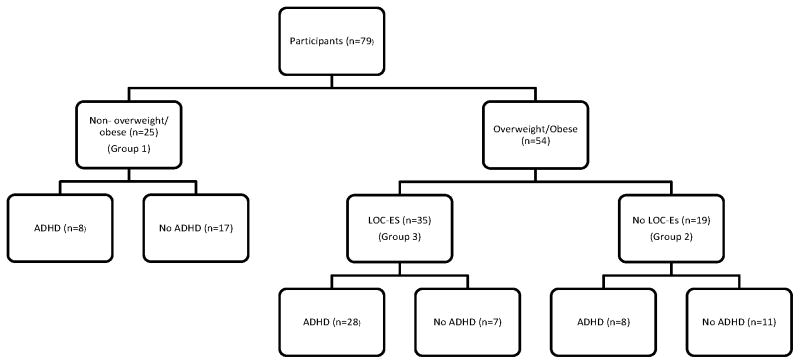
Children with ADHD had significantly greater BMI z-score than non-ADHD children (p=0.006), and children with LOC-ES had significantly greater BMI-z score than children without LOC (p<0.001). Among children with ADHD, 70.5% (31/44) had LOC-ES diagnosis, compared to 20% (7/35) of children without ADHD (p<0.001) (see Figure 1). Thirty-four percent (27/79) of participants took stimulant medication, including 59% (26/44) of children with ADHD and 50% (19/38) of children with LOC-ES. Of children on stimulants, 59.3% (16/27) took short-acting stimulants, 55.6% (15/27) were taking long- acting stimulants and 14.8% (4/27) took both. When stratified by ADHD status, there was no significant association between LOC-ES and stimulant use.
Figure 1.
Loss of Control Eating Syndrome (LOC-ES), Overweight/Obese and Attention Deficit/Hyperactivity Disorder (ADHD) Status of Participants
ADHD = Attention Deficit/Hyperactivity Disorder
LOC-ED = Loss of Control Eating Syndrome
Association between ADHD and LOC-ES
The adjusted odds of LOC-ES were 12.68 times higher in children with ADHD, compared to those without ADHD (adjusted odds ratio [aOR]: 12.68, 95% Confidence Interval [CI] = 3.11, 51.64, p<0.001). A 5-point higher T-score on the Conners-3 Inattentive subscale was associated with 1.39 times higher odds of LOC-ES (aOR 1.39, CI = 1.14, 1.70, p=0.001). A 5-point higher Hyperactivity/Impulsivity subscale T-score (aOR 1.41, CI = 1.15, 1.73, p=0.001) was associated with a significantly greater likelihood of LOC-ES. These odds ratios are adjusted for sex, age, race (white vs. non-white), & BMI z-score.
Association between Response Inhibition and LOC-ES
The associations between parent ratings of impulse control deficits (BRIEF Inhibit scale) and LOC-ES status (aOR 1.25, CI = 1.04, 1.50, p=0.019), and between parent ratings of emotional control deficits (BRIEF Emotional Control scale) and LOC-ES (aOR 1.35, CI = 1.07, 1.70, p=0.011) were both significant.
In terms of performance-based neuropsychological assessment, the adjusted odds of having LOC-ES was 1.17 times higher with every 5 percentage point increase in Go/No-Go commission error rate (aOR 1.17, CI = 1.00, 1.36, p=0.045). A 5-percentage point increment in Go/No-Go omission rate (aOR=1.09, CI = 0.73, 1.63, p=0.673) and a 5-percentage point increment in Go/No-Go coefficient variability (aOR=0.95, CI = 0.78, 1.15, p=0.589) were not significantly associated with LOC-ES. The Conflicting Motor Response Task Performance was associated with LOC-ES (OR 0.91, CI = 0.83, 0.99, p<0.05) but did not remain significant once adjusted for sex, race, age, stimulant medication and BMI z-score (aOR 0.79, CI =0.45, 1.38, p=0.408), for every 5 point increment. These odds ratios are adjusted for sex, age, race, BMI z-score, and stimulant use.
Groups based on BMI z-score and LOC-ES: Association with ADHD
Relationships between groups based on their BMI z-score and LOC status and ADHD measures are presented in Table 2. Group 1 included 25 children who were not overweight/ obese. Group 2 included 19 children without LOC-ES but who were overweight/obese. Group 3 included 35 children with LOC-ES who were overweight/ obese (see Figure 1). In the logistic regression model, Group 3 membership was associated with higher odds of ADHD diagnosis (aOR = 10.44 for Group 3 vs. Group 1, CI=2.96, 36.75, p<0.001). This group also had higher Conners’ (Inhibit; Hyperactivity) and BRIEF (Inhibit; Emotion control) scores compared to Group 1, after adjusting for age and sex (all p<0.001). The overweight/obese children with LOC-ES had 7 times the odds of ADHD diagnosis versus those who were overweight/obese without LOC-ES. Those participants with OW/OB and LOC-ES had 13 points higher on the Conners’ Inattentive Scale as well as the Hyperactive-Impulsive Scale than OW/OB children (without LOC-ES).
Table 2.
Relationship between Group Assignment by BMI z-score and LOC-ES and ADHD Measures
| ADHD Diagnosisa | Conners’ Inattentive† | Conners’ Hyperactive- Impulsive† | BRIEF Inhibit†† | BRIEF Emotional Control†† | |
|---|---|---|---|---|---|
| Group | Adjusted Odds Ratio (95% CI) | Regression Coefficient (95% CI)b | Regression Coefficient (95% CI)b | Regression Coefficient (95% CI)b | Regression Coefficient (95% CI)b |
| OW/OB + LOC-ES vs. non-OW/OB | 10.44*** (2.96, 36.75) | 20.70*** (12.63, 28.63) | 19.49*** (10.52, 28.06) | 13.99*** (5.94, 21.10) | 14.59*** (8.01, 21.15) |
| OW/OB + NO LOC-ES vs. non-OW/OB | 1.43 (0.39, 5.33) | 6.96 (−3.29, 67.07) | 6.57 (−3.17, 16.29) | 7.43 (−1.95, 18.30) | 8.74* (0.01, 17.24) |
| OW/OB + LOC-ES vs. OW/OB + NO LOC-ES | 7.29* (1.88, 28.17) | 13.73* (2.88, 22.33) | 12.91* (3.73, 21.71) | 6.56 (−2.64, 14.77) | 5.86 (−2.68, 13.50) |
OW=overweight; OB= obese; LOC-ES=Loss of control eating disorder; ADHD=Attention Deficit-Hyperactivity Disorder; CI=Confidence interval; vs= versus
KSADS (Kiddie-SADS-Present and Lifetime Version) diagnosis plus either >65 on either Conners DSM-IV-TR Scale (Inattentive or hyperactive-impulsive)
Bias-corrected confidence intervals based on 500 bootstrapped samples, adjusted for age and sex
Conners’= Conners-3 Parent Form DSM-IV-TR ADHD (Inattentive or Hyperactive-Impulsive subscale T-scores)
BRIEF=Behavior Rating Inventory of Executive Function (Inhibit or Emotional Control Scale T-scores)
p<0.05;
p<0.001
ADHD, LOC-ES and BMI z-score
Mediation Analysis
LOC-ES mediated the association between ADHD and BMI z-score. There was a statistically significant association between ADHD and BMI z-score (β= 0.63, CI = 0.18, 1.07, p<0.01) and LOC-ES and BMI z-score (β= 1.04, CI = 0.65, 1.44, p<0.001). ADHD was significantly associated with LOC-ES (OR= 9.54, CI = 3.33, 27.30, p<0.001). After adjusting for LOC-ES, sex, age, and race in addition to adding an interaction term between LOC-ES and ADHD, the main effect of LOC-ES was statistically significant (β= 1.36, CI = 0.59, 2.13, p<0.01), suggesting that LOC-ES mediates the association between ADHD and BMI. After adjusting for LOC-ES, the association between ADHD and BMI z-score was attenuated (β= 0.34, CI = −0.28, 0.95, p=0.28); consistent with the hypothesis that LOC-ES mediates the association between ADHD and BMI z-score.
DISCUSSION
The findings from this cross-sectional study support our hypothesis that children with ADHD diagnoses had significantly increased odds of LOC-ES compared to children without ADHD. Children with LOC-ES were also significantly more likely than those without LOC-ES to manifest deficits in response inhibition, measured by both performance-based measures and by parental ratings. Specifically, children with LOC-ES exhibited greater commission rates on the Go/No-Go task, indicating increased impulsive errors and decreased impulse control. Additionally, children with LOC-ES were rated as significantly more impulsive on the BRIEF parent report form (Inhibit and Emotional Control scales).
Children with ADHD diagnoses were significantly more likely to present with LOC-ES than children without ADHD diagnoses. Further, OW/OB children with LOC-ES had seven times the odds of ADHD diagnoses versus OW/OB without LOC-ES. Additionally, symptoms of inattention and hyperactivity/ impulsivity on the Conners-3 (and not only full-criteria ADHD diagnoses) were associated with LOC-ES. This pattern suggests an overlap of behavioral and eating symptom presentations. It is possible that children with ADHD and LOC-ES reflect a subgroup of ADHD children with overlapping behavioral symptoms and disordered eating symptoms. Alternately, it is possible that children with both ADHD and LOC-ES have a shared underlying risk factor (such as a genetic predisposition to become impulsive). Future studies are needed to explore these theoretical possibilities which are beyond the scope of this study. It is also noteworthy that the current study did not find significant differences in motor impulsivity after adjusting for relevant covariates. It is possible that the Go/No-Go task is more sensitive to the inhibitory control difficulties associated with LOC eating behaviors37 or that the Go/No-Go task requires more of an active cognitive suppression of the impulse than the Conflicting Motor Response Task. It is also possible that unlike ADHD, LOC-ES may not have a prominent motoric component to poor response inhibition.
Our second hypothesis, that children with LOC-ES were significantly more likely to manifest deficits in response inhibition on behavioral tasks than those without LOC-ES, was also supported. Consistent with our findings, behavioral tasks in adults have demonstrated deficits in response inhibition compared to non-binge eating controls.38 Impulsive behavior was also reported in a study of women with BED, suggesting a possible separate “behavioral phenotype of obesity”.17 In adolescents, differences in response inhibition have been found among eating disordered subtypes;39 however that study did not examine binge eating alone in the absence of purging. We are aware of only one other pediatric study that specifically examined behaviorally-assessed impulsivity and binge eating behavior. In that study, there was no group difference in impulsivity without negative mood induction.14 However in our sample the participants were somewhat younger, which may have influenced results given typical improvements in impulse control as children mature.40 Taken together, two pediatric studies have supported an association between behaviorally-assessed response inhibition deficits and binge eating, however, important distinctions include: 1. The first study included purging behavior.39 2. The second study14 only found differences in behaviorally-assessed impulsivity during negative mood states. This study examined LOC-ES in children (who fit putative criteria for loss of control eating disorder rather than solely LOC eating episodes), replicating some of the adult BED literature that showed an association of BED with behaviorally-assessed impulsivity.
Observational parental report ratings of impulse control deficits, and not only behavioral tasks, were associated with higher odds of children presenting LOC-ES. Studies with adults have inconsistently suggested this association between impulse control deficits and eating behavior. For example, a recent study of obese adults (with and without BED) did not identify differences in executive function; however unlike the present study, there was not any non-overweight control group.19 Another study involving only young women reported greater behavioral impulsivity when experiencing negative affect.18 It is possible that that our results may have differed as a function of inclusion of children of both sexes, rather than adults. One other pediatric study examined self-report assessed impulsivity and LOC eating, but did not find differences in impulsivity. Our findings might have differed in part because our study examined somewhat younger more diverse racially-diverse participants than the present study. Additionally, this study examined criteria-defined LOC-ES in contrast to focusing specifically on the presence of LOC eating episodes. Secondary analyses in that study by Hartmann41 suggested that 8 children with some symptoms of ADHD and LOC eating episodes had higher self-reported impulsivity.41 It is difficult to draw conclusions given the small number of children; however, consistent with our study, it raises the question whether a subgroup of obese children with ADHD symptoms and LOC eating might exhibit impulse control differences.
Our data tend to support the hypothesis that LOC-ES may mediate the relationship between ADHD and BMI-z score; however, we cannot infer causality due to the cross-sectional nature of this study. Further, mediation cannot be formally assessed in this study. A large epidemiological study of adults reported that ADHD and obesity were partially mediated by BED.4 A recent cross-sectional study also suggested that binge eating behavior may partially mediate the relationship between ADHD and BMI among children in pediatric mental health clinics.3 Future prospective pediatric studies are required to establish temporality. Children with LOC-ES who have ADHD might represent a more severe behavioral subgroup, reflecting both behavioral and eating-based symptoms or underlying common risk factors. Addressing inhibitory control in children who have LOC-ES and ADHD could also present a possible treatment target for obesity prevention and treatment.39 Further, ADHD may serve not only as a marker for more severe eating symptomatology but could impede the treatment of LOC-ES and binge eating behaviors.5
Limitations include the cross-sectional design that precludes us from establishing any causal links or inferences regarding how impulsivity, ADHD and LOC-ES are related temporally. We also cannot formally assess mediation due to the cross-sectional design of this study, and we caution that no causal conclusions may be made based on these cross-sectional data. Moreover, since we aimed to reflect a real-world community sample with comorbidities, we permitted the use of stimulant medications in the context of unchanged dose in the prior 30 days to the study visit. It is possible, however, that stimulants could have affected the behavioral tasks. Therefore, we adjusted for stimulant use in analyses of the behavioral tasks. We could not calculate interrater reliability given that only one rater, a board certified experienced Child and adolescent psychiatrist, performed the ChEDE. Although the SPEEI has been shown to be valid in the literature and was developed by experienced researchers in disordered eating and obesity fields for a multicenter study, there is no published reliability data. This study examined the presence of LOC-ES rather than the number of episodes of loss of control eating in an attempt to more closely replicate the adult literature examining binge eating disorder, ADHD and impulsivity (rather than the number of binge eating episodes); however, it is unclear if LOC-ES (which fits LOC eating disorder putative criteria) confers additional diagnostic value, which might be a focus of future studies. Lastly, we did not perform pubertal staging of the children; however, we adjusted for age (a proxy for puberty) in our analyses.
Strengths of our study include the use of both behavioral tasks and self-report forms to assess different aspects of general impulsivity, including response inhibition as well as collateral information from parents. Furthermore, the BRIEF appears to measure different elements of impulse control than a computerized performance test,31 highlighting the importance of using both behavioral tasks as well as self-report forms to assess impulsivity. An additional strength of this study is that it includes a control group of overweight children without LOC-ES and non-overweight children. This study extends prior work examining impulse control and binge eating to include younger school-aged children, children of both sexes as well as children with ADHD. Additional strengths include the use of interview-based diagnostic assessments, measured height and weight and a racially diverse sample of children.
This study contributes to our understanding of LOC eating by identifying a significant association between ADHD in children with LOC-ES, as well as a significant association between impulse control deficits and LOC-ES. Furthermore, these data support the notion that LOC-ES may be a relevant construct in youth. Taken together, these findings suggest that examining issues of response control related to LOC-ES in ADHD should be considered with the goal of understanding both eating-specific and behavioral aspects of impulse control. Screening for ADHD or targeting impulse control may inform treatment and future research into treatment strategies for LOC-ES. Longitudinal studies should examine the underlying phenomenology of LOC-ES and explore possible shared mechanisms between ADHD, impulse control deficits and LOC eating.
Acknowledgments
This study was supported by the U.S. National Institutes of Health, National Institute of Mental Health, Grant K23MH083000 (S.P. Reinblatt), P30HD24061, Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR) and the NIH Roadmap for Medical Research. The authors thank Cynthia M. Bulik, PhD, Constantine (Kostas) Lyketsos, MD and Gwenn Smith, PhD for helping with the development of this manuscript. Statistical experts were Drs. Yenokyan and Leoutsakos. Dr. Riddle received aripiprazole from Bristol Myers Squibb for a NIH-sponsored study. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of USUHS or the U.S. Department of Defense; they also do not necessarily represent the official view of NCRR or NIHD.
References
- 1.Khalife N, Kantomaa M, Glover V, Tammelin T, Laitinen J, Ebeling H, et al. Childhood attention-deficit/hyperactivity disorder symptoms are risk factors for obesity and physical inactivity in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:425–436. doi: 10.1016/j.jaac.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Nederkoorn C, Coelho JS, Guerrieri R, Houben K, Jansen A. Specificity of the failure to inhibit responses in overweight children. Appetite. 2012;59:409–413. doi: 10.1016/j.appet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Reinblatt SP, Leoutsakos JM, Mahone EM, Forrester S, Wilcox HC, Riddle MA. Association between binge eating and attention-deficit/hyperactivity disorder in two pediatric community mental health clinics. Int J Eat Disord. 2014 doi: 10.1002/eat.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagoto SL, Curtin C, Lemon SC, Bandini LG, Schneider KL, Bodenlos JS, et al. Association between adult attention deficit/hyperactivity disorder and obesity in the US population. Obesity (Silver Spring) 2009;17:539–544. doi: 10.1038/oby.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazar BP, Suwwan R, de Sousa Pinna CM, Duchesne M, Freitas SR, Sergeant J, et al. Influence of attention-deficit/hyperactivity disorder on binge eating behaviors and psychiatric comorbidity profile of obese women. Compr Psychiatry. 2013 doi: 10.1016/j.comppsych.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. 2007;40:727–732. doi: 10.1002/eat.20441. [DOI] [PubMed] [Google Scholar]
- 7.Tanofsky-Kraff M, Marcus MD, Yanovski SZ, Yanovski JA. Loss of control eating disorder in children age 12 years and younger: Proposed research criteria. Eating Behav. 2008;9:360–365. doi: 10.1016/j.eatbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, et al. A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol. 2011;120:108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol. 2007;75:901. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psychol. 2004;72:53. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: Relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- 12.Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, et al. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- 13.Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or tourette syndrome. Arch Clin Neuropsychol. 2002;17:643–662. [PubMed] [Google Scholar]
- 14.Hartmann AS, Rief W, Hilbert A. Impulsivity and negative mood in adolescents with loss of control eating and ADHD symptoms: An experimental study. Eat Weight Disord. 2013;18:53–60. doi: 10.1007/s40519-013-0004-4. [DOI] [PubMed] [Google Scholar]
- 15.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: Results from a national sample. Pediatrics. 2008;122:e1–6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 16.Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: A risk model for obesity. Obes Res. 2004;12:929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- 17.Schag K, Teufel M, Junne F, Preissl H, Hautzinger M, Zipfel S, et al. Impulsivity in binge eating disorder: Food cues elicit increased reward responses and disinhibition. PLoS One. 2013;8:e76542. doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly NR, Bulik CM, Mazzeo SE. Executive functioning and behavioral impulsivity of young women who binge eat. Int J Eat Disord. 2013;46:127–139. doi: 10.1002/eat.22096. [DOI] [PubMed] [Google Scholar]
- 19.Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, et al. Cognitive function in morbidly obese individuals with and without binge eating disorder. Compr Psychiatry. 2012;53:490–495. doi: 10.1016/j.comppsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity. 2013;21:367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braet C, Claus L, Verbeken S, Van Vlierberghe L. Impulsivity in overweight children. Eur Child Adolesc Psychiatry. 2007;16:473–483. doi: 10.1007/s00787-007-0623-2. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 23.Hollingshead AB. Four factor index of social status. 1975 [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised conners’ parent rating scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 26.Fairburn C, Cooper Z. In: The eating disorder examination. Fairburn CG, Wilson GT, editors. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 27.Watkins B, Frampton I, Lask B, Bryant-Waugh R. Reliability and validity of the child version of the eating disorder examination: A preliminary investigation. Int J Eat Disord. 2005;38:183–187. doi: 10.1002/eat.20165. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental health disorders: DSM-5. 5. Washington DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 29.Marcus MD, Kalarchian MA. Binge eating in children and adolescents. Int J Eat Disord. 2003;34 (Suppl):S47–57. doi: 10.1002/eat.10205. [DOI] [PubMed] [Google Scholar]
- 30.Gioia GA. BRIEF: Behavior rating inventory of executive function: Professional manual. Psychological Assessment Resources. 2000 [Google Scholar]
- 31.Dennison BA, Boyer PS. Risk evaluation in pediatric practice aids in prevention of childhood overweight. Pediatr Ann. 2004;33:25–30. doi: 10.3928/0090-4481-20040101-09. [DOI] [PubMed] [Google Scholar]
- 32.Ryan M, Martin R, Denckla MB, Mostofsky SH, MAHONE E. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahone E, Powell SK, Loftis CW, Goldberg MC, Denckla MB, Mostofsky SH. Motor persistence and inhibition in autism and ADHD. Journal of the International Neuropsychological Society. 2006;12:622–631. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- 34.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United states. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 35.Churma Kraemer H, Kieman M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the baron & kenny and MacArthur approaches. Health Psychology. 2008;27:S101. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stata Corp. Stata statistical software: Release 11. 2009 [Google Scholar]
- 37.O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balodis IM, Grilo CM, Kober H, Worhunsky PD, White MA, Stevens MC, et al. A pilot study linking reduced fronto–Striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int J Eat Disord. 2013 doi: 10.1002/eat.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lock J, Garrett A, Beenhakker J, Reiss AL. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann AS, Rief W, Hilbert A. Laboratory snack food intake, negative mood, and impulsivity in youth with ADHD symptoms and episodes of loss of control eating. where is the missing link? Appetite. 2012;58:672–678. doi: 10.1016/j.appet.2012.01.006. [DOI] [PubMed] [Google Scholar]