Abstract
Photoactivated disinfection has a strong local antimicrobial effect. In the field of dentistry it is an emerging adjunct to mechanical debridement during endodontic and periodontal treatment. In the present study, we investigate the effect of photoactivated disinfection using riboflavin as a photosensitizer and blue LED light for activation, and compare it to photoactivated disinfection with the widely used combination of toluidine blue O and red light. Riboflavin is highly biocompatible and can be activated with LED lamps at hand in the dental office. To date, no reports are available on the antimicrobial effect of photoactivated disinfection using riboflavin/blue light on oral microorganisms. Planktonic cultures of eight organisms frequently isolated from periodontal and/or endodontic lesions (Aggregatibacter actinomycetemcomitans, Candida albicans, Enterococcus faecalis, Escherischia coli, Lactobacillus paracasei, Porphyromonas gingivalis, Prevotella intermedia and Propionibacterium acnes) were subjected to photoactivated disinfection with riboflavin/blue light and toluidine blue O/red light, and survival rates were determined by CFU counts. Within the limited irradiation time of one minute, photoactivated disinfection with riboflavin/blue light only resulted in minor reductions in CFU counts, whereas full kills were achieved for all organisms when using toluidine blue O/red light. The black pigmented anaerobes P. gingivalis and P. intermedia were eradicated completely by riboflavin/blue light, but also by blue light treatment alone, suggesting that endogenous chromophores acted as photosensitizers in these bacteria. On the basis of our results, riboflavin cannot be recommended as a photosensitizer used for photoactivated disinfection of periodontal or endodontic infections.
Introduction
Photoactivated disinfection (PAD) has proven to have a strong antimicrobial effect on a range of different microorganisms including bacteria and fungi [1–4]. PAD involves the activation of a photosensitizer (PS) by light of an appropriate wavelength, which generates reactive oxygen species (ROS) when molecular oxygen is present. During PAD, photosensitizing molecules attach to microbial cell structures and become excited to a high energy triplet state when light of an appropriate wavelength is captured by the chromophores of the PS. The triplet state PS may then react further via one or both of two pathways that lead to microbial killing: In type I reactions, triplet state PS reacts directly with microbial constituents by electron transfer. This produces radical ions which react with oxygen to generate ROS that can be detrimental to microbial membrane integrity [5, 6]. In type II reactions, triplet state PS transforms ground state molecular oxygen from the regular triplet configuration into the highly reactive singlet state. Singlet oxygen then causes oxidation of microbial constituents such as lipids, proteins and nucleic acids.
PAD is currently employed in several medical disciplines, including dentistry where it is emerging as an adjunct to mechanical procedures of biofilm debridement in the fields of endodontics and periodontology. The success of endodontic treatment is dependent on the complete removal of microorganisms from the intricate root canal system [7, 8], a condition that is not always achieved by conventional chemo-mechanical debridement [9–13]. Likewise, the outcome of periodontal therapy is dependent on the removal of pathogenic biofilms from the subgingival crevice.
For both diseases, PAD represents a promising therapeutic approach. Due to the high affinity of certain PS to bacterial membranes [14], the short half-life and the short diffusion paths of ROS [15], a strong and local disinfection can be achieved. Moreover, the unspecific mechanism of action renders the development of bacterial resistance unlikely [1, 14, 16].
Various light sources and PS are available for PAD. Most studies conducted in the field of dentistry use lasers to activate the PS [17–20], but excitation can as well be performed efficiently with less expensive conventional LED lamps [21–23]. Among the different PS employed in the field of dentistry, PAD with toluidine blue O (TBO) has been studied extensively, and it’s antimicrobial effect on oral pathogens is well documented [21–31].
Riboflavin (RFV, vitamin B2) is a promising alternative photosensitizing molecule. It is a micronutrient and an intrinsic PS which generates ROS when irradiated with blue light [32]. It is highly biocompatible and can be activated with LED lamps at hand, used for curing composite, in the dental office. Owing to its advantageous and well known toxicological and pharmacokinetic properties [33–35], RFV is labeled ‘generally recognized as safe’ by the FDA [36].
At present, literature on the effect of PAD using RFV/blue light to kill bacteria is sparse [37], and no reports focus on oral microorganisms. We therefore investigate the effect of PAD using RFV/blue light on a range of different endodontic and periodontal pathogens in planktonic suspension, and compare it to the effect of PAD using a combination of TBO and red light.
Materials and Methods
Microorganisms and culture conditions
Organisms included in the study were Aggregatibacter actinomycetemcomitans (HK915), Candida albicans (NCO 09001; = ATCC 11775), Enterococcus faecalis (DSM 20478), Escherichia coli (ATCC 11775), Lactobacillus paracasei (DSM 5622), Porphyromonas gingivalis (ATCC 33277), Prevotella intermedia (CCUG 24041) and Propionibacterium acnes (DSM 1897).
A. actinomycetemcomitans was cultivated on chocolate agar (Statens Serum Institut, Copenhagen, Denmark) in air enriched with 5% CO2; C. albicans, E. faecalis, E. coli and L. paracasei were grown on blood agar (Statens Serum Institut, Copenhagen, Denmark) under aerobic conditions; P. acnes was grown on blood agar under anaerobic conditions; P. gingivalis and P. intermedia were cultivated anaerobically on modified Columbia blood agar (Statens Serum Institut, Copenhagen, Denmark). All microorganisms were grown at 36.5°C.
Prior to experimental use, all organisms were grown in liquid culture until late exponential phase. A. actinomycetemcomitans, P. gingivalis, P. intermedia and P. acnes were cultivated in plaque medium [38]; C. albicans, E. faecalis, E. coli and L. paracasei were grown in tryptic soy broth (Scharlab, Barcelona, Spain). See S1 Table for details.
Light sources and photosensitizers
Two LED lamps were used: FotoSan 630 LAD pen, emitting light in the red spectrum with a power peak at 630 nm, and FlashMax P3 460 emitting light in the blue spectrum with a power peak at 460 nm. Relative spectral power distributions are shown in S1 Fig. Both lamps were equipped with short, cone-shaped conductive tips (planktonic tip, Ø 4 mm at the cone end) during treatment of microorganisms.
Photosensitizers used were watery solutions of TBO, used in conjunction with FotoSan 630 LAD pen, and RFV, used in conjunction with FlashMax P3 460. Both photosensitizers were prepared to a concentration of 266 μmol L-1 and stored in the dark until experimental use. Light sources and photosensitizers were provided by CMS Dental (Copenhagen, Denmark).
PAD treatment
Microorganisms were centrifuged for 5 min at 5.000 rpm and washed once in 0.9% sterile saline. For each organism, optical density measurements (Novaspec II, Pharmacia Biotech, Cambridge, England; 550 nm) were calibrated to cell counts in a microscopic counting chamber (Bürker-Türk, Glaswarenfabrik Karl Hecht “Assistent”, Sondheim/Rhön, Germany), and cell concentrations were adjusted to 107-108 mL-1 spectrophotometrically (see S1 Table).
For both photosensitizers (P) and the respective red or blue light sources (L), four different treatments were carried out on each organism. 1) PAD treatment (P+L+): Bacterial/fungal suspensions were vortexed for 30 s, after which 60 μL of bacterial suspension and an equal volume of photosensitizer solution (final concentration: 133 μmol L-1) were transferred to the lid (area: 0.64 cm2) of a sterile 1.5 mL Eppendorf tube and mixed with a sterile pipette. One minute after application of the photosensitizer, the suspension was irradiated for 1 min with the corresponding light source. During the irradiation of planktonic cells, the light tip was mounted and the cone end was held just above the suspension at the level of the lid entrance; 1 mm from the surface of the bacterial suspension. For both light sources, an energy dose of 24 J was delivered to the suspension (power: 0.4 W; fluence: 37.7 J/cm2; fluence rate: 0.63 W/cm2). 2) Negative control treatment (P-L-): Microbial suspensions were mixed with 60 μL of 0.9% sterile saline and the light source was placed in the irradiation position for 1 min without emitting light. 3) Photosensitizer alone (P+L-): Microbial suspensions and photosensitizer solution were mixed and the corresponding light source was held in irradiation position for 1 min without irradiating. 4) To test the effect of the light sources alone (P-L+), microbial suspensions and sterile saline were mixed and irradiated for 1 min. The same microbial parent suspension was used for each series of four treatments.
Immediately after treatment, samples of 100 μL were removed from the lids, subjected to serial dilution and cultivated on agar plates. Counts of colony-forming units (CFU) were determined on at least three replicate agar plates. All experiments were performed in duplicate.
To determine the effect of irradiation time on bacterial killing, suspensions of E. faecalis were subjected to PAD using different irradiation intervals. 60 μL of bacterial suspension and 60 μL of either TBO or RFV were mixed in Eppendorf lids and irradiated for 0 s, 10 s, 30 s, 1 min or 2 min with the corresponding light source. Again, the same microbial parent suspension was used. Serial dilution, plating and counting of CFU were performed as previously described. The experiments were performed in duplicate.
Statistical analysis
The CFU count data were assumed to follow a Poisson distribution with a mean inversely proportional to the dilution factor. Data from each organism were analyzed separately by Poisson regression methodology using robust standard errors to account for a possible overdispersion. For both PAD systems (RFV/blue light, TBO/red light) the effect of each treatment (P+L+, P+L-, P-L+) was expressed as a survival fraction with a 95% confidence interval relative to the negative control (P-L-). To compare PAD with RFV/blue light (PADRFV) to PAD with TBO/red light (PADTBO), an interaction term was included in the regression model. The interaction term represented the ratio of the survival fractions for PADTBO relative to the survival fraction for PADRFV. In the analysis of the time series data the treatment effect (P+L+ relative to P-L-) was also estimated by Poisson regression as a survival fraction for each PAD system and each irradiation period. All survival fractions were converted to log10 reductions. For all calculations, p-values below 0.05 were considered as statistically significant and p-values below 0.001 as statistically highly significant.
Results
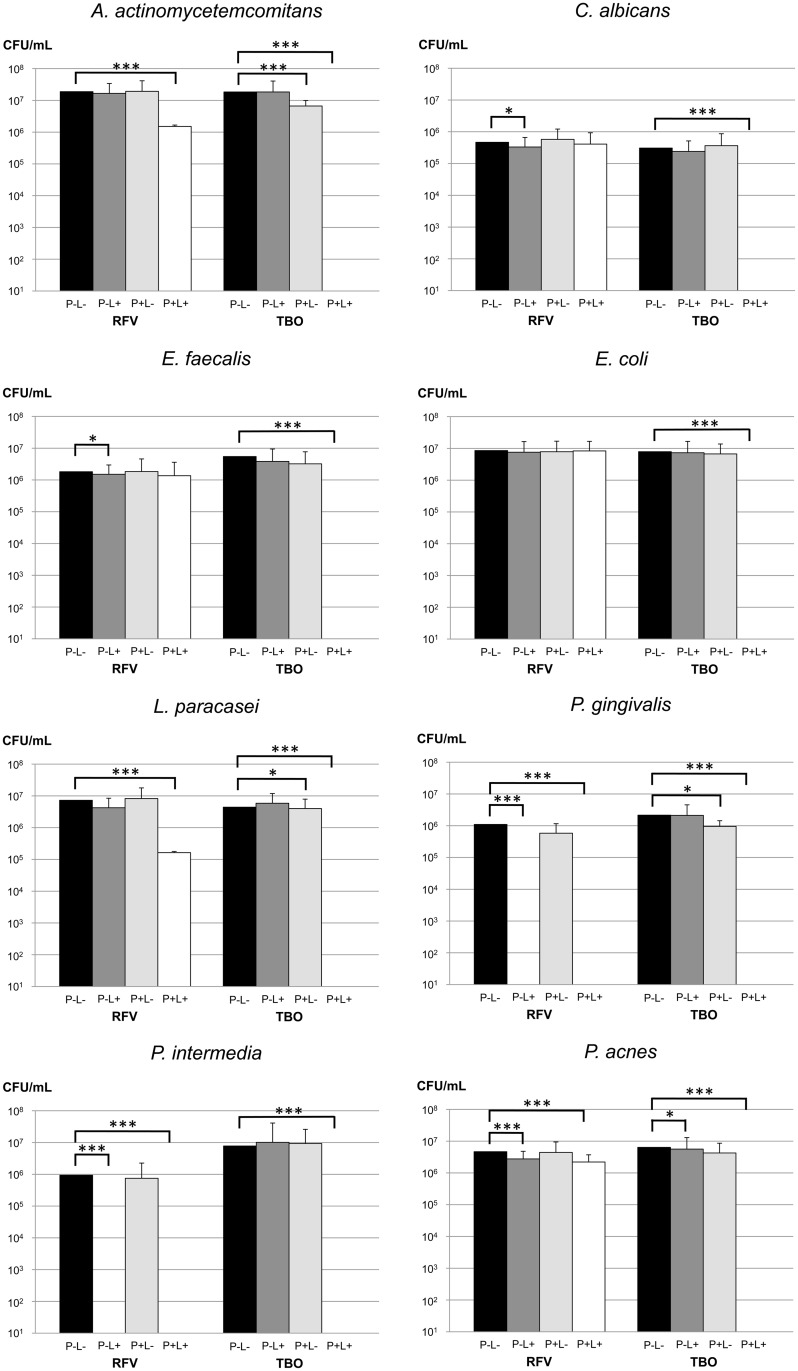
Overall, PAD using RFV/blue light (PADRFV) killed less microorganisms than PAD with TBO/red light (PADTBO). In planktonic suspensions of C. albicans, E. coli and E. faecalis, PADRFV did not reduce the amount of viable cells significantly. Moderate effects of PADRFV were observed for A. actinomycetemcomitans and L. paracasei, with mean log10 reductions of 1.11 and 1.36, respectively (p<0.001). A minor effect of PADRFV was observed for P. acnes (0.29 log10 reduction, p<0.001), but treatment with blue light alone led to a similar reduction (0.21 log10 reduction, p<0.001).
PADRFV only achieved full kills in the cases of P. gingivalis and P. intermedia (p<0.001). However, both species were also eradicated by 1 min of irradiation with blue light alone (p<0.001).
Experiments with different irradiation times showed that the effect of PADRFV on E. faecalis increased slightly with longer irradiation times. 0.22 log10 reduction was achieved after 10 s, 0.25 log10 reduction after 30 s, 0.37 log10 reduction after 1 min and 0.40 log10 reduction after 2 min.
When PADTBO was performed, total microbial killing was observed of all the species included. The effect of PADTBO was significantly stronger than the one of PADRFV for all organisms (p<0.001), except for P. intermedia and P. gingivalis, where PADRFV resulted in complete eradication, too.
Experiments with different irradiation times showed that E. faecalis was eradicated completely after 30 s of irradiation, and even 10 s of irradiation led to a 5.27 log10 reduction (p<0.001).
TBO alone had a moderate yet significant effect on A. actinomycetemcomitans (0.46 log10 reduction, p<0.001) and P. gingivalis (0.69 log10 reduction, p = 0.001). Moreover, slight but statistically significant reductions were observed for treatment with TBO alone on L. paracasei (0.06 log10 reduction, p = 0.01) and treatment with blue light alone on C. albicans (0.05 log10 reduction, p = 0.04) and E. faecalis (0.09 log10 reduction, p = 0.02). All results are presented in Fig 1, S2 Table and S2 Fig.
Fig 1. Effect of PAD using riboflavin (RFV)/blue light or toluidine blue O (TBO)/red light on selected microorganisms.
For both PAD systems, CFU/mL of all species were determined after PAD treatment (P+L+), treatment with light alone (P-L+) and treatment with the photosensitizer alone (P+L-) and compared to negative control treatment (P-L-). * p<0.05; *** p<0.001.
Discussion
On a theoretical plane, Riboflavin offers some advantages as a photosensitizer for photoactivated disinfection. It is a highly biocompatible molecule that can be used intraorally without reservation [36]. With its faint yellow color, it does not stain the hard tissues of the tooth as severely as TBO, which is advantageous in aesthetically important regions. Moreover, it can be excited with blue light emitting LED lamps at hand in the dental office. Implementing PAD with Riboflavin and blue light in dental treatments would thus require minimal effort.
It has previously been shown that RFV can be excited with blue light emitting LED lamps used for composite curing and that ROS are generated [32]. However, our study clearly shows that the antimicrobial effect of PAD with RFV and blue light cannot compete with the one of PAD using TBO and red light.
PADRFV left C. albicans, E. coli and E. faecalis unaffected, and it only had a moderate effect of less than 2 log reductions on A. actinomycetemcomitans, L. paracasei and P. acnes when compared to negative control treatment. The irradiation time series experiments performed on E. faecalis showed a tendency to a more pronounced antibacterial effect when cultures were irradiated for 2 min. Even longer irradiation times are likely to increase the antimicrobial effect of PAD with RFV and blue light, but they would be inacceptable in a dental clinical setting and were therefore not tested. PADRFV with longer exposure times might still be tested in other medical disciplines where the sites of infection are more easily accessible and treatment does not require the continuous presence of health care personnel [33, 37, 39–41].
Interestingly, PADRFV had a strong antibacterial effect on P. gingivalis and P. intermedia. Within one minute of irradiation, full kills were achieved for both organisms. However, control treatment with irradiation alone equally resulted in full kills of both species. P. gingivalis and P. intermedia belong to the black pigmented Bacteroides group that possesses the endogenous chromophores μ-oxo bisheme and hematin [42, 43]. Their respective absorption maxima being situated at 393 nm [44] and 398 nm [45], it is likely that μ-oxo bisheme and hematin acted as photosensitizers and caused bacterial killing in the experiments [46–48]. The same mechanism likely explains the moderate effect of blue light on P. acnes, which equally produces intracellular photosensitizing porphyrins, mainly coproporphyrin III [49]. All three organisms have previously been shown to be susceptible to blue light treatment. Effects observed on P. acnes were moderate [50, 51], while stronger effects, comparable to the ones we observed, were reported for treatment of P. gingivalis and P. intermedia with light doses comparable to the ones used in the present study [46, 52, 53].
By comparison, PADTBO had a strong effect on all eight investigated organisms. Full kills were achieved within the rather short irradiation time of one minute, and the time series experiments conducted with planktonic cultures of E. faecalis show that even shorter irradiation times of 30 s (full kill) or 10 s (3.7 log10 reduction) are adequate to obtain a powerful antibacterial effect. It is important to point out that an LED light source and not a laser was used for treatment. Most studies investigating the antibacterial effect of PADTBO employ lasers that cost more than LED lamps and are subjected to strong power output limitations to avoid causing damage to adjacent tissues. Comparison of treatment results from the present study with results from other studies obtained with laser light shows that LED light sources should be considered as cheaper and safer alternatives for PAD [29, 54–56].
Unlike blue light, treatment with red light alone did not result in strong reductions for any of the tested organisms. Previous studies have reported moderate effects of red light on P. acnes, P. intermedia and P. gingivalis [57, 58], but absorption by endogenous porphyrins in the red spectrum is comparatively low [43], and compared to blue light, higher energy doses are required to achieve antimicrobial effects [46].
The poor effect of PADRFV may be explained by a lower ROS production from RFV compared to TBO [32]. Moreover, the uncharged state of RFV may be unfavorable. It has been reported that cationic PS such as TBO show a better interaction with the Gram-negative cell membrane, which allow for a stronger antimicrobial effect [14]. The present study, however, did not demonstrate an inferior effect of PADRFV on Gram-negatives compared to Gram-positives.
While the effect of PADTBO on pathogens in planktonic suspension is strong, a number of problems encountered in clinical situations may compromise the effect in vivo: 1. Biofilms, and especially multi-species biofilms, are more resistant to treatment than planktonic cells [59]. 2. The propagation of light to the site of infection is hampered by local factors (deep periodontal pockets, intricate root canal anatomy). 3. The photosensitizer might be inactivated and ROS absorbed by molecules in biological fluids (blood, pus, gingival crevicular fluid, saliva). 4. Low availability of oxygen in deep periodontal pockets and in root canals might limit the production of ROS and thus the effect of PAD.
Conclusion
The present study shows that Riboflavin is not suitable as a photosensitizer for use in endodontic or periodontal therapy. Limited microbial kills of PAD using riboflavin/blue light were observed for most of the investigated species within the short irradiation times that are practicable in a dental clinical setting. Photoactivated disinfection with toluidine blue O and red light provided by an LED lamp, on the other hand, had an excellent antimicrobial effect on all investigated species and merits further research. LED light sources should be considered as cheaper and safer alternatives to lasers for photoactivated disinfection.
Supporting Information
Power peaks of the red (A) and blue (B) LED lamps match the excitation maxima of toluidine blue O (630 nm) and riboflavin (460 nm).
(TIF)
SF = survival fraction; PAD = photoactivated disinfection; RFV = riboflavin; TBO = toluidine blue O.
(TIF)
Optical densities corresponding to cell concentrations of 107-108 mL-1.
(DOCX)
P+L+ = photosensitizer, light (treatment); P+L- = photosenzitizer, no light; P-L+ = sterile saline; Log10 = log10 reduction; SF = survival fraction; CI = confidence interval; RFV = riboflavin; TBO = toluidine blue O; * = statistically significant reduction.
(DOCX)
Acknowledgments
The authors would like to thank Mogens Kilian for kind donation of bacterial strains and Bente Nyvad for fruitful discussions. Special gratitude is extended to Mette Nikolajsen for excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Calzavara-Pinton PG, Venturini M, Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. Journal of photochemistry and photobiology B, Biology. 2005;78(1):1–6. 10.1016/j.jphotobiol.2004.06.006 . [DOI] [PubMed] [Google Scholar]
- 2. Gursoy H, Ozcakir-Tomruk C, Tanalp J, Yilmaz S. Photodynamic therapy in dentistry: a literature review. Clinical oral investigations. 2013;17(4):1113–25. 10.1007/s00784-012-0845-7 . [DOI] [PubMed] [Google Scholar]
- 3. Tuite EM, Kelly JM. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. Journal of photochemistry and photobiology B, Biology. 1993;21(2–3):103–24. . [DOI] [PubMed] [Google Scholar]
- 4. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). The Journal of antimicrobial chemotherapy. 1998;42(1):13–28. . [DOI] [PubMed] [Google Scholar]
- 5. Athar M, Mukhtar H, Elmets CA, Zaim MT, Lloyd JR, Bickers DR. In situ evidence for the involvement of superoxide anions in cutaneous porphyrin photosensitization. Biochemical and biophysical research communications. 1988;151(3):1054–9. . [DOI] [PubMed] [Google Scholar]
- 6. Korytowski W, Bachowski GJ, Girotti AW. Photoperoxidation of cholesterol in homogeneous solution, isolated membranes, and cells: comparison of the 5 alpha- and 6 beta-hydroperoxides as indicators of singlet oxygen intermediacy. Photochemistry and photobiology. 1992;56(1):1–8. . [DOI] [PubMed] [Google Scholar]
- 7. Fabricius L, Dahlen G, Sundqvist G, Happonen RP, Moller AJ. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. European journal of oral sciences. 2006;114(4):278–85. 10.1111/j.1600-0722.2006.00380.x . [DOI] [PubMed] [Google Scholar]
- 8. Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. International endodontic journal. 1997;30(5):297–306. . [DOI] [PubMed] [Google Scholar]
- 9. Peters OA, Peters CI, Schonenberger K, Barbakow F. ProTaper rotary root canal preparation: effects of canal anatomy on final shape analysed by micro CT. International endodontic journal. 2003;36(2):86–92. . [DOI] [PubMed] [Google Scholar]
- 10. Siqueira JF Jr., Magalhaes KM, Rocas IN. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. Journal of endodontics. 2007;33(6):667–72. 10.1016/j.joen.2007.01.004 . [DOI] [PubMed] [Google Scholar]
- 11. Siqueira JF Jr., Rocas IN, Paiva SS, Guimaraes-Pinto T, Magalhaes KM, Lima KC. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2007;104(1):122–30. . [DOI] [PubMed] [Google Scholar]
- 12. Waltimo T, Trope M, Haapasalo M, Orstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. Journal of endodontics. 2005;31(12):863–6. . [DOI] [PubMed] [Google Scholar]
- 13. Wu MK, van der Sluis LW, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. International endodontic journal. 2003;36(3):218–24. . [DOI] [PubMed] [Google Scholar]
- 14. Maisch T, Szeimies RM, Jori G, Abels C. Antibacterial photodynamic therapy in dermatology. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2004;3(10):907–17. 10.1039/b407622b . [DOI] [PubMed] [Google Scholar]
- 15. Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: state of the art. Journal of photochemistry and photobiology B, Biology. 2005;79(2):159–70. 10.1016/j.jphotobiol.2004.11.023 . [DOI] [PubMed] [Google Scholar]
- 16. Raghavendra M, Koregol A, Bhola S. Photodynamic therapy: a targeted therapy in periodontics. Australian dental journal. 2009;54 Suppl 1:S102–9. 10.1111/j.1834-7819.2009.01148.x . [DOI] [PubMed] [Google Scholar]
- 17. Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. Journal of clinical periodontology. 2008;35(10):877–84. 10.1111/j.1600-051X.2008.01303.x . [DOI] [PubMed] [Google Scholar]
- 18. de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr., Taba M Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. Journal of periodontology. 2007;78(6):965–73. 10.1902/jop.2007.060494 . [DOI] [PubMed] [Google Scholar]
- 19. Fonseca MB, Junior PO, Pallota RC, Filho HF, Denardin OV, Rapoport A, et al. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomedicine and laser surgery. 2008;26(3):209–13. 10.1089/pho.2007.2124 . [DOI] [PubMed] [Google Scholar]
- 20. Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. Journal of endodontics. 2008;34(2):138–42. 10.1016/j.joen.2007.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eick S, Markauskaite G, Nietzsche S, Laugisch O, Salvi GE, Sculean A. Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagnosis and photodynamic therapy. 2013;10(2):156–67. Epub 2013/06/19. 10.1016/j.pdpdt.2012.12.001 . [DOI] [PubMed] [Google Scholar]
- 22. Paschoal MA, Santos-Pinto L, Lin M, Duarte S. Streptococcus mutans photoinactivation by combination of short exposure of a broad-spectrum visible light and low concentrations of photosensitizers. Photomedicine and laser surgery. 2014;32(3):175–80. Epub 2014/02/21. 10.1089/pho.2013.3656 ; PubMed Central PMCID: PMCPmc3952525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlafer S, Vaeth M, Horsted-Bindslev P, Frandsen EV. Endodontic photoactivated disinfection using a conventional light source: an in vitro and ex vivo study. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;109(4):634–41. 10.1016/j.tripleo.2009.12.027 . [DOI] [PubMed] [Google Scholar]
- 24. Dobson J, Wilson M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Archives of oral biology. 1992;37(11):883–7. Epub 1992/11/01. . [DOI] [PubMed] [Google Scholar]
- 25. Donnelly RF, McCarron PA, Tunney MM, David Woolfson A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. Journal of photochemistry and photobiology B, Biology. 2007;86(1):59–69. Epub 2006/09/12. . [DOI] [PubMed] [Google Scholar]
- 26. Haas R, Dortbudak O, Mensdorff-Pouilly N, Mailath G. Elimination of bacteria on different implant surfaces through photosensitization and soft laser. An in vitro study. Clinical oral implants research. 1997;8(4):249–54. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 27. O'Neill JF, Hope CK, Wilson M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers in surgery and medicine. 2002;31(2):86–90. Epub 2002/09/05. 10.1002/lsm.10087 . [DOI] [PubMed] [Google Scholar]
- 28. Seal GJ, Ng YL, Spratt D, Bhatti M, Gulabivala K. An in vitro comparison of the bactericidal efficacy of lethal photosensitization or sodium hyphochlorite irrigation on Streptococcus intermedius biofilms in root canals. International endodontic journal. 2002;35(3):268–74. . [DOI] [PubMed] [Google Scholar]
- 29. Williams JA, Pearson GJ, Colles MJ. Antibacterial action of photoactivated disinfection {PAD} used on endodontic bacteria in planktonic suspension and in artificial and human root canals. Journal of dentistry. 2006;34(6):363–71. 10.1016/j.jdent.2005.08.002 . [DOI] [PubMed] [Google Scholar]
- 30. Zanin IC, Goncalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. The Journal of antimicrobial chemotherapy. 2005;56(2):324–30. Epub 2005/06/29. 10.1093/jac/dki232 . [DOI] [PubMed] [Google Scholar]
- 31. Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Hofling JF, Goncalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. European journal of oral sciences. 2006;114(1):64–9. Epub 2006/02/08. 10.1111/j.1600-0722.2006.00263.x . [DOI] [PubMed] [Google Scholar]
- 32. Bouillaguet S, Wataha JC, Zapata O, Campo M, Lange N, Schrenzel J. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomedicine and laser surgery. 2010;28(4):519–25. 10.1089/pho.2009.2505 . [DOI] [PubMed] [Google Scholar]
- 33. Corbin F 3rd. Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer. International journal of hematology. 2002;76 Suppl 2:253–7. . [DOI] [PubMed] [Google Scholar]
- 34. Unna K, Greslin J. Studies on the toxicity and pharmacology of riboflavin. J Pharmacol Exp Ther. 1942;76:75–80. [Google Scholar]
- 35. Zempleni J, Galloway JR, McCormick DB. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. The American journal of clinical nutrition. 1996;63(1):54–66. . [DOI] [PubMed] [Google Scholar]
- 36.Administration USFaD. Listing of Specific Substances Affirmed as GRAS. Sec. 184.1695 Riboflavin. 2014 [cited 2015 05/02]. Available: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1695.
- 37. Thakuri PS, Joshi R, Basnet S, Pandey S, Taujale SD, Mishra N. Antibacterial photodynamic therapy on Staphylococcus aureus and Pseudomonas aeruginosa in-vitro. Nepal Medical College journal: NMCJ. 2011;13(4):281–4. . [PubMed] [Google Scholar]
- 38. Jensen SB, Loe H, Schiott CR, Theliade E. Experimental gingivitis in man. 4. Vancomycin induced changes in bacterial plaque composition as related to development of gingival inflammation. Journal of periodontal research. 1968;3(4):284–93. . [DOI] [PubMed] [Google Scholar]
- 39. Cardo LJ, Salata J, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Trypanosoma cruzi in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfus Apher Sci. 2007;37(2):131–7. 10.1016/j.transci.2007.07.002 . [DOI] [PubMed] [Google Scholar]
- 40. Eubanks LM, Dickerson TJ, Janda KD. Vitamin B2-mediated cellular photoinhibition of botulinum neurotoxin A. FEBS Lett. 2005;579(24):5361–4. 10.1016/j.febslet.2005.08.072 . [DOI] [PubMed] [Google Scholar]
- 41. Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW Jr. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28(10):706–13. 10.3928/1081597X-20120921-06 . [DOI] [PubMed] [Google Scholar]
- 42. Smalley JW, Silver J, Birss AJ, Withnall R, Titler PJ. The haem pigment of the oral anaerobes Prevotella nigrescens and Prevotella intermedia is composed of iron(III) protoporphyrin IX in the monomeric form. Microbiology (Reading, England). 2003;149(Pt 7):1711–8. Epub 2003/07/12. . [DOI] [PubMed] [Google Scholar]
- 43. Smalley JW, Silver J, Marsh PJ, Birss AJ. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. The Biochemical journal. 1998;331 (Pt 3):681–5. Epub 1998/04/30. ; PubMed Central PMCID: PMCPmc1219405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smalley JW, Birss AJ, Withnall R, Silver J. Interactions of Porphyromonas gingivalis with oxyhaemoglobin and deoxyhaemoglobin. The Biochemical journal. 2002;362(Pt 1):239–45. Epub 2002/02/07. ; PubMed Central PMCID: PMCPmc1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Itoh T, Yamada T, Kodera Y, Matsushima A, Hiroto M, Sakurai K, et al. Hemin (Fe(3+))—and heme (Fe(2+))—smectite conjugates as a model of hemoprotein based on spectrophotometry. Bioconjugate chemistry. 2001;12(1):3–6. . [DOI] [PubMed] [Google Scholar]
- 46. Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, et al. Phototargeting oral black-pigmented bacteria. Antimicrobial agents and chemotherapy. 2005;49(4):1391–6. Epub 2005/03/29. 10.1128/aac.49.4.1391-1396.2005 ; PubMed Central PMCID: PMCPmc1068628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feuerstein O, Persman N, Weiss EI. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochemistry and photobiology. 2004;80(3):412–5. Epub 2004/12/30. . [DOI] [PubMed] [Google Scholar]
- 48. Fukui M, Yoshioka M, Satomura K, Nakanishi H, Nagayama M. Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis. Journal of periodontal research. 2008;43(2):174–8. 10.1111/j.1600-0765.2007.01009.x . [DOI] [PubMed] [Google Scholar]
- 49. Lee WL, Shalita AR, Poh-Fitzpatrick MB. Comparative studies of porphyrin production in Propionibacterium acnes and Propionibacterium granulosum. Journal of bacteriology. 1978;133(2):811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. Journal of dermatological science. 2002;30(2):129–35. . [DOI] [PubMed] [Google Scholar]
- 51. Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS immunology and medical microbiology. 2003;35(1):17–24. . [DOI] [PubMed] [Google Scholar]
- 52. Song HH, Lee JK, Um HS, Chang BS, Lee SY, Lee MK. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J Periodontal Implant Sci. 2013;43(2):72–8. 10.5051/jpis.2013.43.2.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chui C, Aoki A, Takeuchi Y, Sasaki Y, Hiratsuka K, Abiko Y, et al. Antimicrobial effect of photodynamic therapy using high-power blue light-emitting diode and red-dye agent on Porphyromonas gingivalis. Journal of periodontal research. 2013;48(6):696–705. 10.1111/jre.12055 . [DOI] [PubMed] [Google Scholar]
- 54. Williams JA, Pearson GJ, Colles MJ, Wilson M. The effect of variable energy input from a novel light source on the photoactivated bactericidal action of toluidine blue O on Streptococcus Mutans. Caries research. 2003;37(3):190–3. . [DOI] [PubMed] [Google Scholar]
- 55. Qin Y, Luan X, Bi L, He G, Bai X, Zhou C, et al. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers in medical science. 2008;23(1):49–54. 10.1007/s10103-007-0454-x . [DOI] [PubMed] [Google Scholar]
- 56. Yao N, Zhang C, Chu C. Effectiveness of photoactivated disinfection (PAD) to kill enterococcus faecalis in planktonic solution and in an infected tooth model. Photomedicine and laser surgery. 2012;30(12):699–704. 10.1089/pho.2011.3216 . [DOI] [PubMed] [Google Scholar]
- 57. Konig K, Teschke M, Sigusch B, Glockmann E, Eick S, Pfister W. Red light kills bacteria via photodynamic action. Cellular and molecular biology. 2000;46(7):1297–303. . [PubMed] [Google Scholar]
- 58. Choi MS, Yun SJ, Beom HJ, Park HR, Lee JB. Comparative study of the bactericidal effects of 5-aminolevulinic acid with blue and red light on Propionibacterium acnes. J Dermatol. 2011;38(7):661–6. 10.1111/j.1346-8138.2010.01094.x . [DOI] [PubMed] [Google Scholar]
- 59. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology 2000. 2002;28:12–55. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Power peaks of the red (A) and blue (B) LED lamps match the excitation maxima of toluidine blue O (630 nm) and riboflavin (460 nm).
(TIF)
SF = survival fraction; PAD = photoactivated disinfection; RFV = riboflavin; TBO = toluidine blue O.
(TIF)
Optical densities corresponding to cell concentrations of 107-108 mL-1.
(DOCX)
P+L+ = photosensitizer, light (treatment); P+L- = photosenzitizer, no light; P-L+ = sterile saline; Log10 = log10 reduction; SF = survival fraction; CI = confidence interval; RFV = riboflavin; TBO = toluidine blue O; * = statistically significant reduction.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.