Abstract
Streptococcus pneumoniae (pneumococcus) is a major human pathogen. The main pneumococcal autolysin LytA and the pneumolysin Ply are two of the bacterium’s most important virulence factors. The lytA- and ply-related genes are also found in other streptococci of the Mitis group (SMG). The precise characteristics of the lytA-related—but not the ply-related—genes of SMG and their prophages have been previously described. A search of the more than 400 SMG genomic sequences available in public databases (ca. 300 for S. pneumoniae), showed Streptococcus pseudopneumoniae IS7493 to harbor four ply-related genes, two of which (plyA and plyB) have 98% identical nucleotides. The plyA homolog of S. pseudopneumoniae is conserved in all S. pneumoniae strains, and seems to be included in a pathogenicity island together with the lytA gene. However, only nonencapsulated S. pneumoniae strains possess a plyB gene, which is part of an integrative and conjugative element. Notably, the existence of a bacterial lytA-related gene in a genome is linked to the presence of plyA and vice versa. The present analysis also shows there are eight main types of plyA−lytA genomic islands. A possible stepwise scenario for the evolution of the plyA−lytA island in S. pneumoniae is proposed.
Keywords: pneumococcus, main autolysin, pneumolysin, streptococci of the Mitis group, evolution, genomic island
Introduction
Streptococcus pneumoniae (pneumococcus) is a leading human pathogen that usually asymptomatically colonizes the mucosal surfaces of the upper respiratory tract in early childhood (Crook et al. 2004). Once carriage is established, however, S. pneumoniae may invade several sterile sites, leading to what is known as invasive pneumococcal disease. Indeed, the pneumococcus is responsible for episodes of community-acquired bacteremic pneumonia, bacteremia, and meningitis, mainly in children, the elderly, and immunocompromised patients (Henriques-Normark and Tuomanen 2013). Pneumococci are also a major cause of noninvasive diseases such as nonbacteraemic pneumonia, acute otitis media, sinusitis, and conjunctivitis. In addition to the capsular polysaccharide (CPS)—the main virulence factor of S. pneumoniae—many proteins are involved in the establishment and/or development of pneumococcal disease. These include the main autolytic enzyme LytA (a peptidoglycan hydrolase) and the pneumolysin Ply (a pore-forming toxin); both have been known for more than a century and are among the most widely studied proteins involved in pneumococcal virulence (Mitchell and Mitchell 2010; Ramos-Sevillano et al. 2015; Zahlten et al. 2015).
Pioneering work by Neufeld (1900) reported that pneumococci (but not other streptococci) rapidly “dissolve” in bile, a property still widely employed in the clinical setting (together with the optochin susceptibility test) for identifying S. pneumoniae among α-hemolytic, catalase-negative streptococcal isolates. Nowadays, however, sodium deoxycholate (Doc) is used in place of bile (Blaschke 2011). Doc-induced lysis is driven by the N-acetylmuramoyl-L-alanine amidase (NAM-amidase; EC 3.5.1.28) activity of the lytA gene product (García et al. 1986). LytA is also responsible for the characteristic autolytic behavior of the pneumococcus, the bacteriolysis caused by β-lactam antibiotics and, in cooperation with the glucosaminidase LytB, for the diplococcal morphology typical of this species (López and García 2004). LytA belongs to the amidase_2 family of proteins (whose members possess an Amidase_2 domain; PF01510), which includes Zn-dependent NAM-amidases and the peptidoglycan-recognition proteins (highly conserved pattern-recognition molecules of the immune system; Zhang et al. 2012). LytA was the first-discovered of the choline-binding family of proteins (López and García 2004). Choline-binding proteins are characterized by a choline-binding domain responsible for the binding of these proteins to the choline residues present in the teichoic and lipoteichoic acids of the bacterial surface (Sánchez-Puelles et al. 1990; Fernández-Tornero et al. 2001, 2002).
The lytA gene is located immediately downstream of the lytR–cinA–recA–dinF gene cluster in the S. pneumoniae genome (Mortier-Barrière et al. 1998). The same applies for the strain IS7493 of Streptococcus pseudopneumoniae (Shahinas et al. 2013), but not for Streptococcus mitis B6 (Denapaite et al. 2010) or Streptococcus oralis Uo5 (Reichmann et al. 2011) that lack a lytA-like gene at this position. Although the lytA gene has been considered exclusive to S. pneumoniae (Magomani et al. 2014), other, closely related streptococci (hereafter termed streptococci of the Mitis group [SMG]) and many pneumococcal and SMG prophages code for LytA-like lytic enzymes (Obregón et al. 2002; Romero et al. 2004, 2009; Llull et al. 2006). Fortunately, a number of characteristics allow for the accurate discrimination between typical pneumococcal lytA alleles (lytASpn) and SMG (lytASMG) or phage-encoded alleles (designated lytAPPH or lytASPH, standing for pneumococcal and SMG prophages, respectively; this nomenclature follows that used previously by Morales et al. [2010]). These characteristics are: 1) lytASpn alleles are 957 bp-long, whereas lytASMG (951 bp) shows characteristic signatures involving ≈100 nt positions and a distinctive 6-bp deletion (ACAGGC) between nucleotide positions 868 and 873. 2) In sharp contrast to LytASpn, the LytASMG NAM-amidases of S. pseudopneumoniae and other SMG are inhibited by 1% Doc, which explains why these bacteria are not lysed in its presence—although they still lyse in the presence of 1% Triton X-100 (Díaz et al. 1992b; Obregón et al. 2002). 3) Even if all pneumococcal prophages reported to date code for a 318 amino acid-long NAM amidase (endolysin) (957-bp lytAPPH alleles), there are significant nucleotide differences with respect to lytASpn alleles, for example, ≥12 differences exist between the first 33 nucleotides of the prophage endolysin genes (lytAPPH or lytASPH) and the bacterial alleles lytASpn and lytASMG (Llull et al. 2006). 4) All the endolysin-coding genes of phages—but none of the genuine bacterial lytA genes—are preceded by one (or two) holin/antiholin-like genes; this feature allows for easy discrimination between lytA genes of phage and bacterial origin. Interestingly, two temperate bacteriophages of S. mitis, ϕB6 and ϕHER, also code for LytASpn-like endolysins with 318 amino acid residues (Romero et al. 2004), whereas the EJ-1 inducible prophage isolated from S. mitis strain 101 harbors a gene (ejl) with the 6-bp deletion characteristic of lytASMG alleles (Díaz et al. 1992a).
Rufus Cole first reported that pneumococci synthesize an intracellular hemotoxin, the action of which is prevented by small amounts of cholesterol (Cole 1914). In fact, S. pneumoniae synthesizes a cholesterol-dependent cytolysin or CDC (pneumolysin) that is an important virulence factor for this organism (Marriott et al. 2008; Dando et al. 2014). Ply induces cell death by pore formation and toxin-induced apoptosis (Mitchell and Dalziel 2014), although there is evidence showing that a nonhemolytic Ply allele may be associated with outbreaks of invasive disease (see below). Ply prevents complement deposition on S. pneumoniae, mainly by interfering with the classical pathway (Yuste et al. 2005).
Although the pneumolysin-coding gene (ply; 1,416 bp) is relatively well conserved among pneumococcal isolates (Marriott et al. 2008), Ply alleles showing reduced or nonhemolytic activity have been described (Kirkham et al. 2006; Jefferies, Johnston, et al. 2007; Jefferies et al. 2010; Harvey et al. 2011). Moreover, as stated above for lytA, ply orthologs have been described in some S. mitis strains (mitilysin; Mly) (Jefferies, Nieminen, et al. 2007) and most S. pseudopneumoniae isolates (pseudopneumolysin; pPly) (Johnston et al. 2010). Furthermore, a Ply-related, CDC cytolysin is synthesized by several S. mitis strains (Farrand et al. 2008). This protein has been named “lectinolysin” and is nearly identical to that previously designated as Sm-hPAF (standing for “S. mitis-derived human platelet aggregation factor”) (Ohkuni et al. 1997). This name, however, is misleading because “PAF” is the accepted standard abbreviation for “platelet-activating factor”—a glyceryl ether containing phosphoglyceride (Demopoulos et al. 1979). These proteins are secreted CDCs and contain a 36 amino acid-long, clevable signal peptide (SP). This is in sharp contrast to Ply that contains no SP (Mitchell and Dalziel 2014). Moreover, and unlike other previously characterized CDCs, lectinolysin-type proteins contain an additional domain of 162 amino acid residues (the F5_F8_type C domain; PF00754) that binds to fucose and difucosylated tetrasaccharides within Lewis y (Ley) and Lewis b (Leb) antigens. In the present study the term lectinolysin (Lly) is used, reflecting its ability to bind carbohydrate.
Recently, Denapaite et al. (2010) reported the ply gene to be separated from lytA by ≈10 kb in the genome of R6 (the common laboratory strain of S. pneumoniae), and that neither gene is present in the S. mitis B6 genome. Interestingly, these authors noted that, in the R6 strain, the ply−lytA region was flanked by a ≈100 bp direct repeat (87% identity between sequences); this is designated hereafter as plREP (standing for ply−lytA REPeat). In contrast, only one copy of plREP exists in the S. mitis B6 genome, and it overlaps the termination codon of the dinF gene (smi_1838) (Denapaite et al. 2010). Interestingly, polymerase chain reaction (PCR) experiments have shown that ply- and lytA-like genes are found in some SMG strains, and Southern blotting experiments have suggested that the presence/absence of one of these genes is somehow linked with the presence/absence of the other (Kilian et al. 2008). More recently, a putative genomic island that contains pply plus lytASMG has also been found in the S. pseudopneumoniae strain IS7493 (Shahinas et al. 2013), the only complete S. pseudopneumoniae genome reported to date. Taken together, these results suggest the existence of a ply−lytA island in pneumococci and some of its closely related SMG.
To get insight into the evolutionary relationships of ply and lytA, the genomic sequences (complete or not) of more than 400 SMG were examined. Novel data on ply-related genes were found. Taken as a whole, our analyses suggest that the plyA and lytA genes of S. pneumoniae may form part of a pathogenicity island.
Materials and Methods
Bioinformatic Genomic Analysis
The pneumococcal genomic sequences were aligned with the nucleotide sequences for the plyD39 (SPD_1726) and/or lytASpn_D39 (SPD_1737) genes of strain D39 (Acc. No. CP000410) (Lanie et al. 2007). The reason for selecting this strain for comparison lies in that the corresponding genes of the reference pneumococcal strains TIGR4 and R6 are also annotated in the same file. Alignments were performed using the BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&BLAST_SPEC=MicrobialGenomes) or Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) (for multiple alignments) algorithms, employing the default parameters. The same programs were used to search for sequences similar to plREP (see above). As previously reported for the R6 strain (Denapaite et al. 2010), two copies of this repeat were found in the D39 genome (or any other pneumococcal genome known to date; see below), one located downstream of ply (coordinates 1720617−1720723) and the other overlapping the termination codon of dinF (coordinates 1730877−1730970). With the exceptions of strains NCTC 7465—the S. pneumoniae type strain—and GA02270, in which ply, lytA, and/or plREP lie in two different contigs (and which unexpectedly were found to overlap for more than 100 identical nucleotides), only continuous genomic sequences including ply, lytA and the two plREPs (241 strains) were analyzed further.
The description of the pneumococcal strains for which the genome was available in the National Center for Biotechnology Information (NCBI) BioProject database (http://www.ncbi.nlm.nih.gov/bioproject/, last accessed September 14, 2015) includes the sequence type (ST) (Enright and Spratt 1998) plus data such as serotype/serogroup. When this information was not available, the allele number and ST were searched for in the corresponding genomic sequences and assigned using the pneumococcal MLST website (http://pubmlst.org/spneumoniae/, last accessed September 14, 2015), which is hosted at the University of Oxford and has been funded by the Wellcome Trust. The serotype/serogroup was predicted by comparing the genomic sequences with those of capsular biosynthetic genes of S. pneumoniae (Bentley et al. 2006).
The ISfinder database (http://www-is.biotoul.fr, last accessed September 14, 2015) was searched for potential insertion sequences (ISs) (Siguier et al. 2006). For the prediction of protein function, the Pfam database (http://pfam.xfam.org/, last accessed September 14, 2015) was employed (Finn et al. 2014). The SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/, last accessed September 14, 2015) was used to predict SPs (Petersen et al. 2011).
Results
The genomic sequences (complete or otherwise) of 1,466 streptococcal strains (representing at least 65 species) were obtained from the NCBI (http://www.ncbi.nlm.nih.gov/; last date accessed, October 25, 2014). Of these, 404 (27.5%) correspond to SMG (supplementary table S1, Supplementary Material online). The database also contains the genomes of 304 S. pneumoniae strains, 27 of which are complete (table 1). It should be noted that the quality of the assemblies has not been ascertained here and that there are different assembly levels ranging from short-read contigs to gapless, high-quality genomes.
Table 1.
Summary of the S. pneumoniae Strain Material Examined
| Number | |
|---|---|
| Genomes in databasesa | 304 |
| Strains analyzedb | 241 |
| Serogroupsc | 21 |
| STs | 115d |
| PMEN clones | 28 (169)e |
| lytASpn/LytASpn alleles | 30/13f |
| plyASpn/PlyASpn alleles | 35/10 |
| lytASpn–plyASpn combinations | 65 |
aLast date accessed, October 25, 2014.
bOf the 304 pneumococcal genomes available in databases, those analyzed were the ones in which both the plREPs that flank lytA and plyA were located in the same contig.
cThe nonencapsulated R6 strain is not taken into account here.
dNot including nine strains showing seven new and two unknown STs.
eThe total number of strains belonging to PMEN clones, including SLVs and DLVs, are shown in parentheses.
fThe lytA30-Spn allele was not translated into its protein since it may contain a sequencing error.
To properly analyze the structure and possible evolution of the ply−lytA island, we first examined the possibility that more than one ply-related gene may exist in some S. pneumoniae strains and related SMG and found several ply-related genes in SMG to be more frequent than previously thought. S. pseudopneumoniae IS7493 would appear to represent a special case since it codes for four Ply-related proteins.
A Variety of ply-Like Genes Exist in Pneumococci and Other SMG
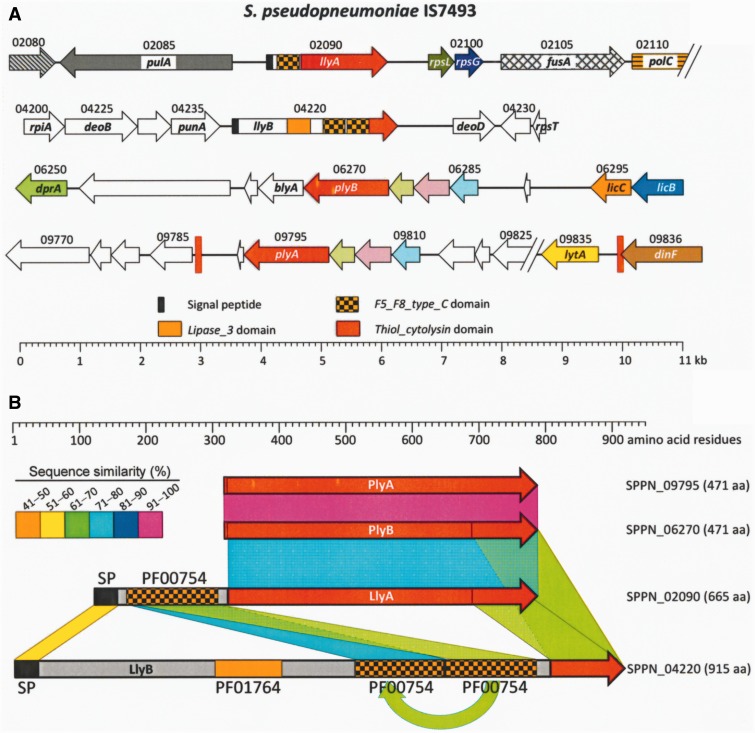
Although apparently never mentioned before (Shahinas et al. 2013), the S. pseudopneumoniae IS7493 genome contains, in addition to the pply gene (SPPN_09795; 1,416 bp; ply_A hereafter), another gene named SPPN_06270 (plyB hereafter) that shares the same length and 98% of its nucleotides with SPPN_09795 (465 identical amino acid residues) (fig. 1). The plyA and plyB genes are located far apart in the chromosome. Notably, two more S. pseudopneumoniae genes are also ply-related: SPPN_02090 and SPPN_04220. The former (1,998 bp; llyA) is proposed to encode a thiol-activated cytolysin and corresponds to the Lly protein mentioned above, whereas the latter (2,748 bp) is annotated simply as coding for a hypothetical protein. The SPPN_04220 gene product (named LlyB hereafter) contains, as does LlyA, a SP and a Thiol_cytolysin domain (PF01289). Moreover, both proteins contain one (LlyA) or two (LlyB) F5_F8_type_C domains (see above). The latter protein also harbors a Lipase_3 domain (PF01764) (fig. 1). The nucleotide sequence identity between either llyA or llyB, and plyA/plyB was ≤64%.
Fig. 1.—
Pneumolysin genes and pneumolysin-like genes in the S. pseudopneumoniae IS7493 genome (Acc. No. CP002925) and their encoded proteins. (A) Pneumolysin-related genes are indicated by red arrows. Figures over the arrows correspond to SPPN_ loci. The locations of two direct repeats are indicated by vertical red bars. (B) Diagram of pneumolysin and pneumolysin-like proteins. The Thiol_cytolysin domain of PlyA/B and LlyA/B are shown in red. Amino acid sequence similarities are indicated by colors. SP, signal peptide; PF00754, F5_F8_type_C domain ; PF01764, Lipase_3 domain.
Ply-related genes similar to llyA or llyB also exist in various SMG, but apparently they are absent in pneumococci (supplementary figs. S1 and S2, Supplementary Material online). For example, a gene ≥98% identical to llyA is present in two S. mitis strains (SK597 and SK1080) and in the six S. pseudopneumoniae strains for which draft genomic sequences are available (supplementary fig. S1A, Supplementary Material online). The llyA gene is located immediately upstream of pulA and orientated in the opposite direction. Sequence comparisons also revealed the existence of other llyA-related genes—located downstream of the parC gene—in a variety of S. mitis strains (supplementary fig. S1B, Supplementary Material online). The products of these llyA-related genes were ≥90% identical to one another, whereas they showed 70−75% amino acid sequence similarity with the LlyA proteins mentioned above (data not shown). The terms LlyA1 and LlyA2 subfamilies of proteins will be used hereafter to designate those CDCs encoded by genes located, respectively, upstream of pulA or downstream of parC.
Additional llyB-like genes were found between punA and deoD in several SMG genomes (supplementary fig. S2, Supplementary Material online). Interestingly, most, if not all S. pneumoniae strains, contained a llyB remnant (or pseudogene). In the case of the D39 strain, this remnant corresponds to genes SPD_0727 to SPD_0729, the latter being annotated as “hemolysin-related protein,” although apparently lacks cytolysin activity (Yamaguchi et al. 2009).
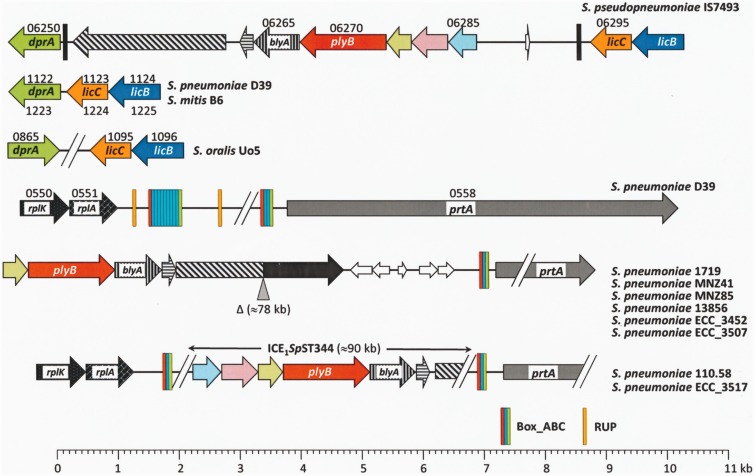
The supernumerary S. pseudopneumoniae plyB gene is located immediately upstream of blyA, a gene coding for a 248 amino acid-long protein with an Amidase_2 domain (as seen in the LytA NAM-amidase), and downstream of three genes (SPPN_06275−SPPN_06285) virtually identical to those located at an equivalent position in the plyA−lytA island (SPPN_09800, SPPN_09805, and SPPN_09810) (see below) (fig. 2). The plyB gene of S. pseudopneumoniae IS7493 resides in a 8.7 kb DNA fragment inserted between genes dprA and licC (coding for CTP:phosphocholine cytidylyl transferase) (Rock et al. 2001). These two genes are adjacent in the S. pneumoniae and S. mitis B6 genomes (but not in that of S. oralis Uo5) (fig. 2). It must be underlined that, in addition to S. pseudopneumoniae IS7493, a plyB−blyA tandem of genes exists only in a subset (group II) of nonencapsulated S. pneumoniae (non-Ec-Sp) strains (Keller et al. 2013; Valentino et al. 2014). Some non-Ec-Sp strains (group I) possess nonsense mutations in the CPS biosynthesis locus cap/cps. In contrast, group II non-Ec-Sp lack all the genes usually found in the cap/cps sequences of encapsulated S. pneumoniae isolates, but contains one or two aliB-like open reading frames (Hathaway et al. 2004). More recently, a new subset of group II non-Ec-Sp isolates containing a novel gene (named nspA or pspK) have been reported (Park et al. 2012; Salter et al. 2012). The group II non-Ec-Sp are related to a deep-branching classic lineage comprised mainly of the frequently identified ST344 and ST448 (Hilty et al. 2014). Interestingly, in pneumococci these genes are located in a chromosomal region (upstream of the prtA gene coding for a cell wall-associated serine protease [Bethe et al. 2001]) completely different to where they appear in S. pseudopneumoniae IS7493 (fig. 2). During the present work, a fully closed and annotated reference genome of a non-Ec-Sp isolate (strain 110.58; ST344) became available. Hilty et al. (2014) showed that ST344 non-Ec-Sp harbor two integrative conjugative elements (ICEs) not previously described in S. pneumoniae. A detailed analysis of the genome of strain 110.58 showed that plyB forms part of ICE1SpST344, and that this element may be partly deleted in other non-Ec-Sp pneumococcal isolates of the same (or different) ST (fig. 2).
Fig. 2.—
Diagram showing the chromosomal region containing (or not) a plyB-like gene in different SMG. The eight S. pneumoniae strains indicated at the bottom right of the figure are nonencapsulated. Thin arrows represent interrupted genes (pseudogenes). Genes sharing ≥90% identical nucleotides are represented by identical color and shading. The sequence types of the non-Ec-Sp strains shown are: 1719 and 13856, ST2996; MNZ41, ST6153; MNZ85 and ECC_3507, ST2315; ECC_3452 and 110.58, ST344; ECC_3517, ST1270. ST1270, ST2996, and ST6153 are SLVs of ST344.
In summary, our genomic screening showed the existence of a wide variety of ply-related genes with different chromosome locations in SGM species including S. pneumoniae.
Some, but Not All, Mitis Group Streptococci Possess One Copy of the plyA−lytA Repeat
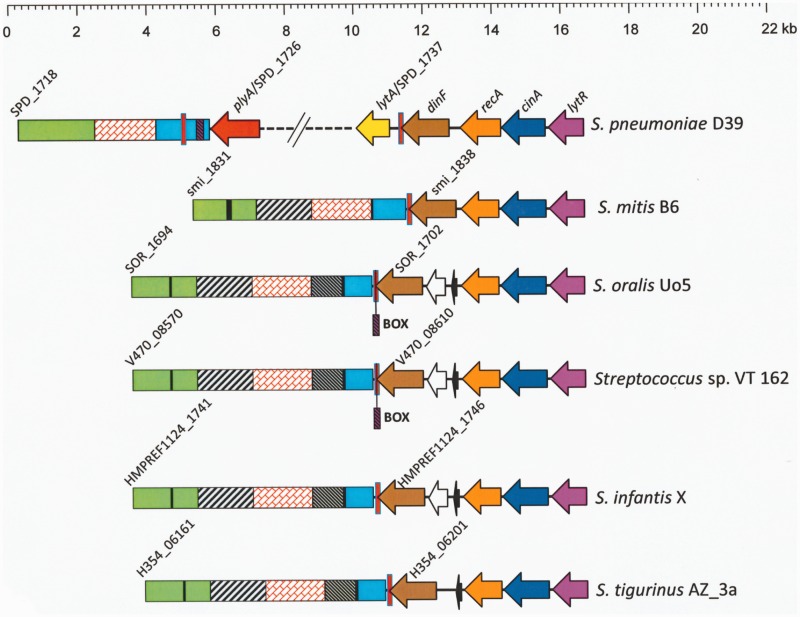
The nucleotide sequence of the plREP copy overlapping the termination codon of the dinF gene in the S. pneumoniae D39 genome (nucleotide positions 1730877−1730970 in CP000410) was used as a query sequence to search the NCBI database of complete genomes (excluding S. pneumoniae genomes) for possible recombinational events. Only four genomes (all belonging to the Streptococcus genus) rendered significant similarities (E ≤ 10−6): S. pseudopneumoniae IS7493 (E = 10−34), S. mitis B6 (E = 10−32), Streptococcus sp. VT 162 (E = 10−13), and S. oralis Uo5 (E = 10−11). A similar search performed against the database containing streptococcal draft genomic sequences (also excluding S. pneumoniae) further confirmed that the existence (cutoff E value = 10−6) of at least one plREP copy is restricted to some SMG: S. pseudopneumoniae (6 strains), S. mitis (30 strains), S. oralis (12 strains), Streptococcus tigurinus (4 strains), Streptococcus infantis (6 strains), Streptococcus dentisani (2 strains), and 21 out of 39 strains classified as Streptococcus sp. (supplementary table S1, Supplementary Material online). Interestingly, the presence of a pIREP copy at the 3′-end of dinF appears to be independent of the presence of a lytASMG gene in SMG (supplementary fig. S3, Supplementary Material online). The particular case of SMG with lytASMG genes, that is, all the sequenced strains of S. pseudopneumoniae and some S. mitis, is discussed below. All the examined strains showed well-conserved local synteny, and plREP overlapped the termination codon of a dinF-like gene, in turn located immediately downstream of the lytR−cinA−recA-like gene cluster (fig. 3). In contrast, dinF appeared located far away from the cinA−recA genes (which typically lie together and in this order) in other streptococci also harboring a dinF homolog but lacking a sequence similar to plREP (supplementary fig. S4, Supplementary Material online). The possession of a lytR-like gene in a position similar to that of S. pneumoniae appears to be uncommon in streptococci (not shown).
Fig. 3.—
Diagram showing the gene organization around the dinF gene in selected SMG lacking the lytASMG gene. The region of the genome of S. pneumoniae D39 between SPD_1718 and SPD_1741 (lytR) is shown for comparison. Genes of known function are shown with arrows pointing in the direction of transcription. The vertical red bar represents the plREP copy. Potential ISs are indicated by black bars. Regions showing ≥80% sequence similarity are represented by identical color and shading.
A plyA−lytA Island Is Present in Most, If Not All, S. pneumoniae Isolates
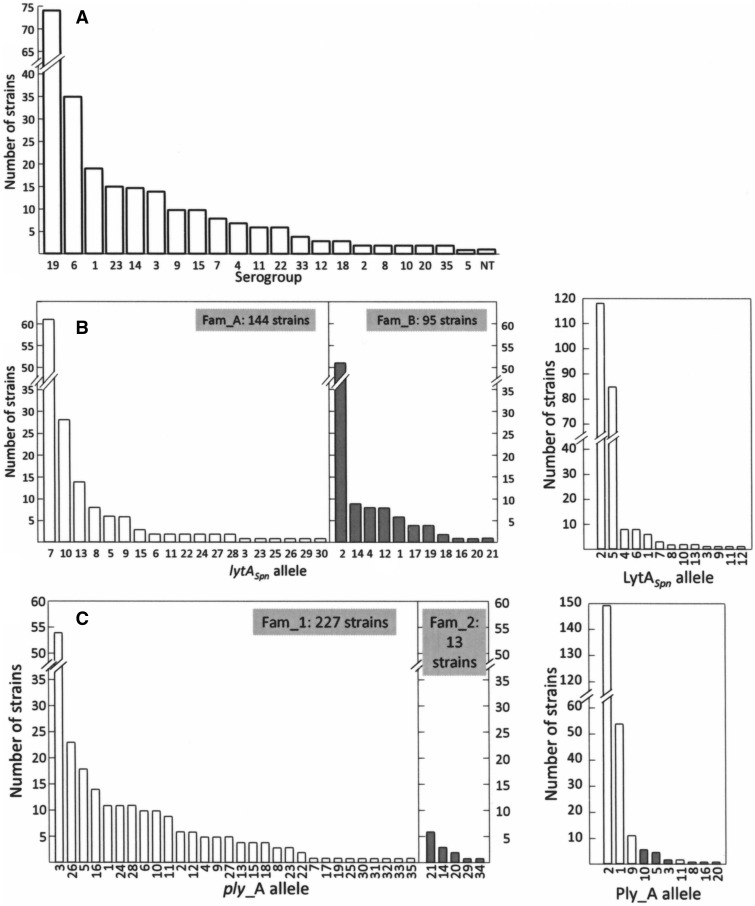
Table 1 summarizes some of the characteristics of the S. pneumoniae genomes analyzed in this study. Of the 304 genomic projects found, 241 genomic sequences (corresponding to the 21 serogroups most frequently found in clinical specimens [Grabenstein and Musey 2014]; fig. 4A) fulfilled the requirement mentioned above, that is, to have two copies of the plREP element flanking the ply and lytA genes in one contig (or in two unnoticed overlapping contigs) (supplementary table S2, Supplementary Material online). It should be noted that the 63 pneumococcal strains that did not meet the above criterion did contain, nonetheless, a copy of both ply and lytASpn genes (supplementary table S3, Supplementary Material online). In every case, the location of the ply gene corresponded to that designated as plyA above (fig. 1).
Fig. 4.—
Serogroup, and lytASpn/plyA allele distribution, among the 241 S. pneumoniae strains analyzed. (A) Serogroups. Non-Ec, nonencapsulated. (B) Alleles of lytA (left) and LytA (right) in the sample. Open and gray bars indicate Fam_A and Fam_B lytA alleles, respectively. (C) Alleles of plyA (left) and PlyA (right). Open and gray bars indicate Fam_1 and Fam_2 ply alleles, respectively.
Nearly half of the isolates examined differed in genotype (115 STs were recognized in the present study) (table 1). Further, up to 70% of the strains belonged to one of 28 (out of the possible 43) Pneumococcal Molecular Epidemiology Network (PMEN) clones. PMEN clones are resistant to one or more antibiotics in wide clinical use and dominate the population of antibiotic-resistant pneumococci. Globally susceptible clones known to be important in disease are also included in the PMEN clone data set (http://spneumoniae.mlst.net/pmen/pmen.asp, last accessed September 14, 2015).
Thirty different lytASpn alleles were found (fig. 4B and supplementary table S4, Supplementary Material online). The deduced amino acid sequences were then aligned, resulting in the identification of 13 different NAM-amidases. Similarly, 35 plyA alleles (coding for 10 PlyA cytolysins including a new allele given the preliminary designation “allele 20”; see below) were found (fig. 4C and supplementary table S5, Supplementary Material online). The lytASpn alleles were classified into two families (Fam_A and Fam_B) according to a previous proposal (Morales et al. 2010). This classification resulted from the finding that these two families of pneumococcal lytASpn alleles (but not the corresponding NAM-amidases) can be differentiated by PCR and digestion with restriction enzymes on the basis of a highly polymorphic region located around position 453 of lytASpn (taking the first nucleotide of the ATG initiation codon as position 1). It is noteworthy that alleles LytA2_Spn and LytA5_Spn together represented more than 85% of the NAM-amidases encoded by the pneumococcal strains studied in the present work (fig. 4B). Similarly, we propose a subdivision of plyA alleles: Fam_1 clustered alleles with a full length gene (1,416 bp), while the 1,410 bp alleles—which lack the nucleotides located between positions 808 and 813 (GTCAAC) encoding Val270 and Lys271—formed Fam_2 (fig. 4C). Since previous studies had assigned numbers (1 through 19) to the different PlyA alleles found (but not to the corresponding genes) (Jefferies, Johnston, et al. 2007; Jefferies et al. 2010; Harvey et al. 2011), the plyA alleles were renumbered, but the nomenclature used for their encoded proteins was maintained.
The serotype 3 SPNA45 strain (ST6934) (supplementary table S2, Supplementary Material online) appears to represent an unusual case among pneumococci in its having undergone a lengthy deletion extending from the 5′-region of plyA to the 3′-region of lytA. The origin of the SPNA45 strain is obscure although it may correspond to a serotype 3 isolate of equine origin. These equine isolates lack hemolytic activity and are Doc-insoluble (Whatmore et al. 1999). Notably, a comparison between the genomes of SPNA45 and D39 revealed that, in addition to the ply−lytA deletion, two large rearrangements occurred in the former strain (supplementary fig. S5, Supplementary Material online).
Gene Organization of the Pneumococcal plyA−lytA Island
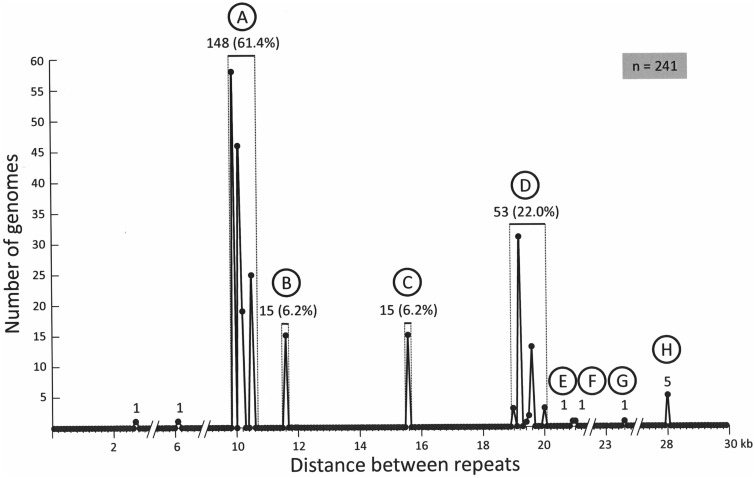
The length of the plyA−lytA island differed greatly among S. pneumoniae strains (supplementary table S2, Supplementary Material online). When the distance separating the flanking plREP was approximated to its nearest value, a bimodal distribution with peaks at ≈10 kb and ≈19 kb (together accounting for >80% of strains) was observed (fig. 5). A clear correlation was seen between length and clonal origin: the 15 strains with an 11.6 kb-long island belonged to the clonal complex formed by the PMEN global clone North Carolina6A-23 (ST376) and several of its double locus variants (DLVs) (ST1296, ST1339, ST2268, ST8207), whereas those with a 15.6-kb-long island belonged to the complex formed by the PMEN clone Spain9V-3 (ST156) and three of its single locus variants (SLVs) (ST3148, ST4026, ST4464). In addition, the five strains with the longest island (28.2 kb) were seen to be members of the clonal complex formed by the PMEN clone Maryland6B-17 (ST384) and its SLV, ST2150 (supplementary table S2, Supplementary Material online).
Fig. 5.—
Size distribution of the plyA−lytA island in 241 S. pneumoniae strains. Letters A−H correspond to the arrangements shown in figure 6.
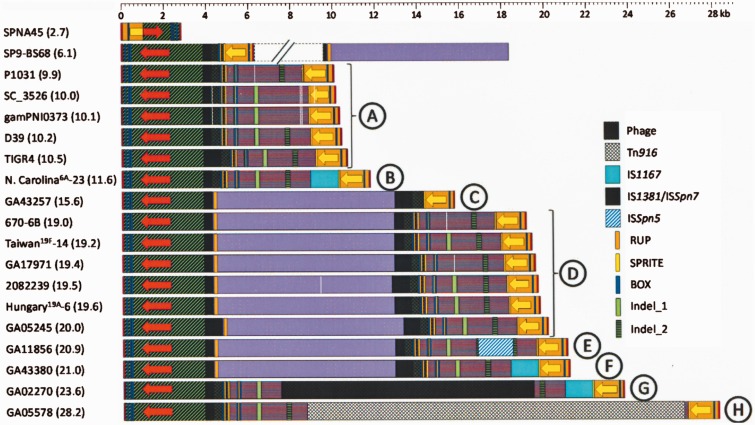
Figure 6 shows a diagram of the region encompassing the plREPs in pneumococcal genomes. Leaving out SPNA45 and SP9-BS68, eight different genomic arrangements (designated A to H) were recognized. These were clustered into two major groups. The most numerous (169 out of 241 strains; 70%) was named Gr1 and includes arrangement types A (9.9−10.5 kb), B (11.6 kb), G (23.6 kb), and H (28.2 kb); the other (Gr2) comprises types C−F. The main differences among arrangements of the same group reside in the presence/absence of different ISs (either complete or partly deleted), some insertions/deletions (indels), and/or repeat sequences (fig. 6). To be precise, the ISs present/absent were IS1167, IS1381 (also named ISSpn7), and ISSpn5. Moreover, at least one copy of the repeat sequences BOX, RUP, and SPRITE, characteristic of pneumococci (Croucher et al. 2011), was also present.
Fig. 6.—
Organization of the various pneumococcal plyA−lytA islands. The name of one representative strain of each class is shown at the left. Red and yellow arrows correspond to plyA and lytA genes, respectively. The arrows show the direction of transcription. Regions with ≥85% sequence identity are represented by identical color and shading.
The main difference seen between Gr1 and Gr2 strains resided in that the latter possesses an additional ≈8.6-kb DNA fragment (fig. 6). By way of example, this fragment is located between nucleotide positions 1,872,219 and 1,880,825 of the S. pneumoniae strain 670-6B genome (Acc. No. CP002176). The same fragment was identified in strain SP9-BS68, but is located in a contig different to that containing the plyA−lytA island. This 8.6-kb DNA fragment appears to be the result of a horizontal transfer event from an unknown source. In fact, the maximum closeness (60−70% identity) between this DNA fragment and any other sequence in the databases (last date accessed, October 25, 2014) corresponds to a fragment in the chromosome of S. suis D9, but apparently not in other S. suis isolates (supplementary fig. S6, Supplementary Material online). Moreover, sequence comparisons suggested that this DNA fragment codes for proteins that are annotated as involved in transport and related functions (supplementary fig. S6, Supplementary Material online).
The S. pneumoniae strain GA02270 (fig. 6), which was found to have a 23.6-kb island (arrangement G), is a DLV (ST1339) of the North Carolina6A-23 PMEN clone. In contrast, some members of this clone possess a shorter island (11.6 kb; arrangement B). Strain GA02270 appears to be unique in that it has integrated a potentially defective prophage (≈ 12 kb) into its type B plyA−lytA island (supplementary fig. S7, Supplementary Material online). In a similar manner, the strains with a type H arrangement (28.2 kb) differ from type A strains by the insertion of a copy of Tn916 (≈18 kb) (fig. 6). This mobile genetic element is widely distributed among important human pathogens, including Enterococcus faecalis, Clostridium difficile, Staphylococcus aureus, and S. pneumoniae, and it harbors the tetracycline resistance-conferring tet(M) gene (Santoro et al. 2014).
A search for a possible genetic link between particular plyA and lytA alleles among different arrangements gave no evidence of association. In other words, no preference for association between lytA alleles belonging to families A or B and plyA alleles of Fam_1 or Fam_2 was seen (data not shown). Nevertheless, as many of the alleles may encode identical proteins (or different proteins with similar enzymatic activity) (supplementary tables S4 and S5, Supplementary Material online), the existence of a functional association between PlyA and LytA proteins in different arrangements could not be completely discarded. Data relative to the specific activity of these proteins only exist in the case of PlyA. Actually, the S. pneumoniae strains studied here can be grouped into four categories on the basis of PlyA alleles (Jefferies, Johnston, et al. 2007): 1) alleles 1, 2, 9, and 11, with a specific activity of about 4 × 105 hemolytic units (HU) mg−1; 2) alleles 8 and 10, with slightly reduced hemolytic activity (≈105 HU mg−1); 3) allele 3, having a very reduced hemolytic activity (≈3−7 × 103 HU mg−1), and 4) allele 5 that completely lacks hemolytic activity (supplementary table S5, Supplementary Material online). It should be noted that, all together, alleles 1, 2, 9, and 11 were present in near 90% of the studied strains (215 out of 240; fig. 4C). As the enzymatic activity of the LytA alleles had not been previously determined, we decided to determine the activity of encoded NAM-amidases. The lytA genes coding for LytA alleles 1, 2, 4, 5, or 7 were cloned into the pT7-7 expression vector, overexpressed and the corresponding NAM-amidases were purified (Supplementary Material online). These four alleles correspond to approximately 90% of the studied strains (220 strains out of 239; fig. 4B). Only LytA_7 showed a slight (albeit statistically significant) increase in the specific activity of the NAM-amidase (supplementary table S4, Supplementary Material online). Unfortunately, and spite of our efforts, attempts to find any significant functional association between PlyA and LytA alleles of high/low specific activities were unsuccessful (data not shown).
As mentioned above, Ply contains no SP and autolysis caused mainly by the LytA NAM-amidase has long been recognized for its ability to rapidly release the majority of the pneumococcal hemolytic activity (Mitchell and Dalziel 2014). Early results had shown that LytA was required for optimal biofilm formation in vitro (Moscoso et al. 2006). A similar requirement for Ply has also been reported recently (Shak et al. 2013). Interestingly, the role of Ply on in vitro biofilm formation appears to be independent of its hemolytic activity, as pneumococcal strains synthesizing a nonhemolytic Ply allele (allele 5; see above) were still able to form biofilms (Shak et al. 2013). We have now confirmed that both proteins (Ply and LytA) are important for optimal biofilm formation in S. pneumoniae, although the biofilm forming capacity of a double ply lytA mutant was not significantly different to that of the single ply mutant (supplementary fig. S8, Supplementary Material online).
A plyA−lytA Island Is Also Present in Several SMG Different to S. pneumoniae
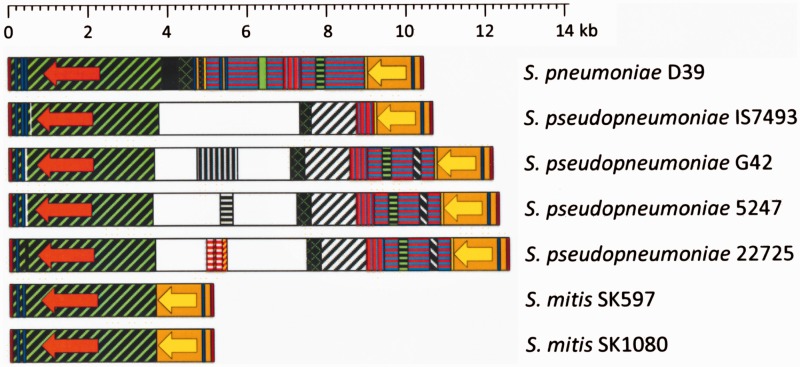
As mentioned above, all the S. pseudopneumoniae strains whose genome is known in either full (1 strain) (Shahinas et al. 2013) or draft (6 strains) version (this study), appear to code for a LytASMG NAM-amidase. However, we have now found that only a minority of S. mitis strains (3 strains out of 31) contained bona fide homologs of this gene. All these strains were found to contain a plyA homolog (nucleotide similarity ≥96%), although only four S. pseudopneumoniae and two S. mitis strains could be examined in detail since only these contained the plyA and lytASMG genes in the same contig (fig. 7). These results fully confirmed the previous proposal that the presence/absence of one of these genes is somehow linked with the presence/absence of the other (Kilian et al. 2008). S. pseudopneumoniae strains showed a plyA−lytA island arrangement more complex than that of S. mitis strains, although in both species the island was always flanked by plREP, as in S. pneumoniae. The intervening sequences found in S. pseudopneumoniae potentially encode proteins of unknown function (not shown).
Fig. 7.—
Diagram showing the plyA−lytA islands in two species of SMG. The S. pneumoniae D39 strain is shown for comparison. Red and yellow arrows correspond to the plyA and lytA genes, respectively. The arrows indicate the direction of transcription. Regions having ≥85% sequence identity are represented by identical color and shading.
A Plausible Scenario for the Evolution of the plyA−lytA Island in S. pneumoniae
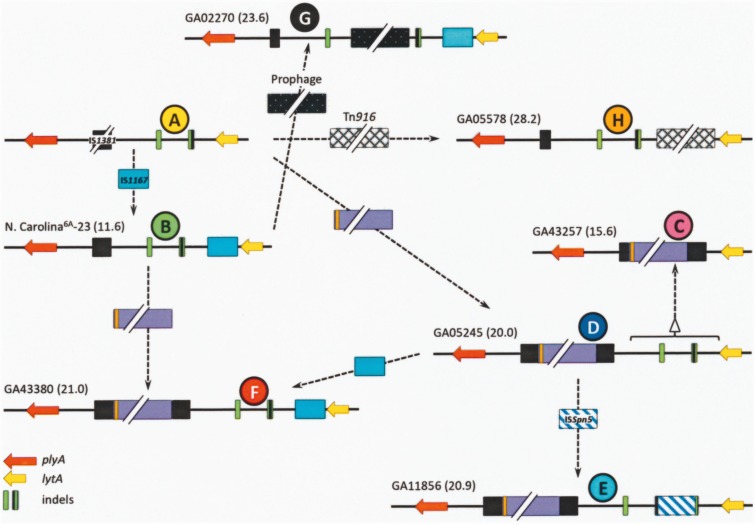
Arrangement A (ca. 10 kb-long) was assumed to represent the founder since it is by far the most widely distributed among otherwise distantly related S. pneumoniae strains, including nonencapsulated isolates (deduced using a phylogenetic tree of the core genome of 44 S. pneumoniae genomes [Donati et al. 2010], and a dendrogram based on genomic BLAST of 320 pneumococcal strains [supplementary fig. S9, Supplementary Material online]). In contrast, arrangement D appears to have arisen and/or horizontally transferred independently on several occasions. Based on these data, Figure 8 provides a possible evolutionary pathway compatible with the observed diversity of the pneumococcal plyA−lytA island. The sources of the proposed earliest island, and of the ≈8.6-kb fragment characteristic of arrangements C−F, are unknown. Additional indel events explain the formation of the rest of the island arrangements. Similar, albeit apparently independent, events may have occurred in S. pseudopneumoniae strains (fig. 7). However, the uncommon S. mitis strains with a plyA−lytA island (namely, strains SK597, 1080, and likely, SK564), were seen to possess an arrangement closely related to that of the S. pneumoniae strain SP9-BS68 (fig. 6) that may have arisen through additional genomic rearrangements.
Fig. 8.—
Possible evolutionary steps leading to the present diversity of the plyA–lytA island of S. pneumoniae. It is proposed that the starting point corresponds to an island of about 10 kb that was introduced into the pneumococcal genome from an unknown source (A). Arrangements B, G, and H may arise by insertions of IS1167 (B), a prophage (G), and/or Tn916 (H), respectively. Insertion of an ≈8.6 kb fragment (mauve bar) and successive insertions/deletions (indels) are proposed to have taken place for arrangements C−F.
Discussion
Although all the S. pneumoniae strains analyzed here contain one plyA copy, which forms an island with the lytASpn gene, most if not all of the group II non-Ec-Sp strains also harbor a supernumerary plyB gene elsewhere in their genome as part of an ICE element (ICE1SpST344) (fig. 2). While strains that lack a capsule have substantially reduced virulence in invasive infections, non-Ec-Sp—which are unaffected by current vaccines that target the pneumococcal CPS —cause up to one-third of all episodes of acute conjunctivitis outbreaks (Martin et al. 2003; Marimon et al. 2013). The reasons underlying the conjunctival tropism of NT pneumococci are not completely understood (Valentino et al. 2014). However, due to the presence of a plyB copy in their genomes, non-Ec-Sp may synthesize more Ply than encapsulated isolates. It should be remarked that the region containing the plyA promoter (Walker et al. 1987) is identical to that of plyB. Interestingly, the PlyB pneumolysin of the non-Ec-Sp 110.58 strain (and other non-Ec-Sp) is identical to allele 7 of Ply_A that displays full hemolytic activity (Jefferies, Johnston, et al. 2007) (data not shown). Experimental evidence exists to suggest that Ply-deficient bacteria show greatly reduced virulence in animal models of ocular keratitis or endophtalmitis, and that immunization with Ply showed a protective effect (Mitchell and Dalziel 2014). To the best of our knowledge, however, no study has reported either an increased production of Ply in non-Ec-Spn or that this may be the reason for a greater capacity to cause conjunctivitis.
According to the present data, genes coding for Lly-related proteins are quite common in S. mitis, uncommon in S. pseudopneumoniae, and either absent (this work) or, perhaps, seldom present (Farrand et al. 2008) in S. pneumoniae. Only a gene remnant potentially encoding a truncated protein of the LlyB type appears to be present in pneumococcal genomes (supplementary fig. S2, Supplementary Material online). It is unclear whether the extant pneumococcal pseudogene has resulted from the accumulation of mutations, or whether a gain-of-function mechanism resulted in the presence of a complete open reading frame in some SMG. Actually, only four mutations (two single nucleotide insertions plus two transition mutations) are required to convert the SpnNT_01477−01479 remnant (supplementary fig. S2, Supplementary Material online) into a potential gene coding for a 771-long Lly_B-like polypeptide in the non-Ec-Sp strain 110.58 (data not shown).
We have also found that the S. mitis strain SK597, which appears to be a mitis/oralis hybrid (Kilian et al. 2008), harbors genes encoding Lly-like proteins of the LlyA1, LlyA2, and Lly_B type (see above). It is tempting to speculate that harboring several Lly homologs might represent an advantage for virulence as the different proteins may be better adapted to particular cell types or surface receptors. S. mitis proteins of the LlyA family, and particularly those of the LlyA1 subfamily, have been shown to bind cholesterol and the human glycophosphatidylinositol-anchored CD59 (Kimberley et al. 2007) prior to triggering pore-formation (Tabata et al. 2014). The relative redundancy of lly-like genes observed in S. mitis is intriguing. CDCs are part of the membrane attack complex/perforin (MACPF)/CDC superfamily of pore-forming proteins that include the MACPF family (Gilbert 2014). For a long time it was believed that cholesterol served as the membrane receptor for CDC toxin monomers (Gilbert 2010). However, although it is known that Ply requires membrane cholesterol for its cytolytic effects, it has not been definitively shown that cholesterol functions as the cellular receptor. Further, Lly-type proteins possess an F5_F8_type_C lectin-like domain that binds to fucose and to the difucosylated tetrasaccharides Leb and Ley (Farrand et al. 2008; Feil et al. 2012), which would increase the number and types of binding receptors in the target cells. Quite unexpectedly, very recent results have revealed that Ply and streptolysin O (from Streptococcus pyogenes) also have lectin activity and bind glycans (Shewell et al. 2014).
The tendency of the pneumococcal lytA and plyA genes to persist recurrently in relative vicinity observed in this study suggests that their corresponding products might function together, forming a (patho)physiological protein network. The clustering of genes in bacteria contributes to the attainment of high local protein concentrations close to their encoding genes (because transcription and translation are simultaneous); thus, they may selectively interact even when they are not cotranscribed (Lawrence 1999; Montero Llopis et al. 2010). Previous experiments have shown that plyA and lytA are required for optimal biofilm formation in vitro (Moscoso et al. 2006; Shak et al. 2013). However, we have now found that a double ply lytA mutant did not show any additional impairment on biofilm-forming capacity, as compared with the single mutants (supplementary fig. S8, Supplementary Material online). A possible explanation may be that—in contrast with what has been a working hypothesis for many years, that is, that Ply escapes from the cell on lysis caused by the pneumococcal LytA NAM-amidase (Marriott et al. 2008)—near 50% of the Ply is localized to the cell wall compartment in a LytA-independent manner (Price and Camilli 2009).
The LuxS/autoinducer 2 quorum-sensing system is known to regulate the transcript levels of both lytA and ply genes during early biofilm formation (Vidal et al. 2011). Moreover, lytA and ply have been reported to show similar levels of expression in nasopharyngeal samples taken from colonized (but otherwise healthy) children (Sakai et al. 2013). Additional support for the coordinated expression of ply and lytA being important in disease development comes from observations made in mixed infections of influenza A virus (IAV) and S. pneumoniae. Mechanisms of pathogenesis include IAV destruction of the respiratory epithelial cells with subsequent impairment of mucociliary bacterial clearance, upregulation or exposure of receptors for pneumococcal adhesion, and IAV-induced suppression of innate and adaptive immune responses to S. pneumoniae (McCullers 2014). It has recently been reported that infection with IAV induces the release of bacteria from biofilms. These dispersed bacteria show differential virulence gene expression that results in a significantly increased ability to disseminate and cause invasive infections (Marks et al. 2013). Compared with biofilm-grown bacteria, lytA and ply are upregulated in IAV-dispersed cells (Pettigrew et al. 2014). In agreement with early results (Canvin et al. 1995), recent experimental evidence from a murine model of infection shows coordinated activity of Ply and LytA to be important in complement evasion and in the establishment of pneumococcal pneumonia and sepsis (Ramos-Sevillano et al. 2015).
The CPS is a critical virulence factor of S. pneumoniae and is generally assumed to distinguish pathogenic pneumococci from commensal SGM by its capacity to avoid phagocytosis. Most (but not all) SMG synthesize neither CPS nor other pneumococcal virulence factors (e.g., IgA1 protease) (Kilian et al. 2008). In a similar way, the present study has revealed that a plyA–lytA island is missing in ≈90% of S. mitis and completely absent in other SMG, with the only exception of S. pseudopneumoniae isolates that consistently harbor such an island (fig. 7). It should be noted, however, that the lytASMG alleles encode a NAM-amidases with reduced specific activity, that is, ≈50% of that LytASpn (Obregón et al. 2002). Therefore, our results give further support to the hypothesis that commensal streptococci, for example, SMG, gradually evolved from the pathogen S. pneumoniae by genome reduction (Kilian et al. 2008, 2014).
Compared with the core genome, genomic islands frequently have high percentage of genes coding for proteins with unknown function (Hsiao et al. 2005), and in particular, pathogenicity islands have several additional characteristics such as presence of genes encoding virulence factors, mobility genes (integrases, transposases), phage-related genes, direct repeats, and insertion sequences, that allow their potential identification in bacterial genomes (Che et al. 2014). The results presented here reveal that most of these features are also present in the plyA–lytA region and support the proposal that the plyA and lytA genes of S. pneumoniae may form part of a pathogenicity island, although further experimental and/or epidemiological work would be required to establish a real link with pathogenesis.
Supplementary Material
Supplementary figures S1−S8 and tables S1−S5 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This research was supported by grant SAF2012-39444-C01/02 from Ministerio de Economía y Competitividad (MINECO). A.J.M.-G. is the recipient of a Miguel Servet research contract funded by the Fondo de Investigación Sanitaria from the MINECO. Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES) is an initiative of ISCIII. The authors thank Eloísa Cano and Susana Ruiz for skillful technical assistance.
Literature Cited
- Bentley SD, et al. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethe G, et al. 2001. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol Lett. 205:99–104. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ. 2011. Interpreting assays for the detection of Streptococcus pneumoniae. Clin Infect Dis. 52:S331–S337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin JR, et al. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 172:119–123. [DOI] [PubMed] [Google Scholar]
- Che D, Hasan MS, Chen B. 2014. Identifying pathogenicity islands in bacterial pathogenomics using computational approaches. Pathogens 3:36–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. 1914. Pneumococcus hemotoxin. J Exp Med. 20:346–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. 2004. Pneumococcal carriage. In: Tuomanen E, Mitchell TJ, Morrison DA, Spratt BG, editors. The pneumococcus. Washington, D.C: ASM Press; p. 136–147. [Google Scholar]
- Croucher NJ, Vernikos GS, Parkhill J, Bentley SD. 2011. Identification, variation and transcription of pneumococcal repeat sequences. BMC Genomics 12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SJ, et al. 2014. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 27:691–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos CA, Pinckard RN, Hanahan DJ. 1979. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 254:9355–9358. [PubMed] [Google Scholar]
- Denapaite D, et al. 2010. The genome of Streptococcus mitis B6—what is a commensal? PLoS One 5:e9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E, López R, García JL. 1992a. EJ-1, a temperate bacteriophage of Streptococcus pneumoniae with a Myoviridae morphotype. J Bacteriol. 174:5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E, López R, García JL. 1992b. Role of the major pneumococcal autolysin in the atypical response of a clinical isolate of Streptococcus pneumoniae. J Bacteriol. 174:5508–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati C, et al. 2010. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 11:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright MC, Spratt BG. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049–3060. [DOI] [PubMed] [Google Scholar]
- Farrand S, et al. 2008. Characterization of a streptococcal cholesterol-dependent cytolysin with a Lewis y and b specific lectin domain. Biochemistry 47:7097–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil SC, et al. 2012. Structure of the lectin regulatory domain of the cholesterol-dependent cytolysin lectinolysin reveals the basis for its Lewis antigen specificity. Structure 20:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tornero C, García E, López R, Giménez-Gallego G, Romero A. 2002. Two new crystal forms of the choline-binding domain of the major pneumococcal autolysin: insights into the dynamics of the active homodimer. J Mol Biol. 321:163–173. [DOI] [PubMed] [Google Scholar]
- Fernández-Tornero C, López R, García E, Giménez-Gallego G, Romero A. 2001. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat Struct Biol. 8:1020–1024. [DOI] [PubMed] [Google Scholar]
- Finn RD, et al. 2014. Pfam: the protein families database. Nucleic Acids Res. 42:D222–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P, García JL, García E, López R. 1986. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene 43:265–272. [DOI] [PubMed] [Google Scholar]
- Gilbert R. 2014. Structural features of cholesterol dependent cytolysins and comparison to other MACPF-domain containing proteins. Subcell Biochem. 80:47–62. [DOI] [PubMed] [Google Scholar]
- Gilbert RJC. 2010. Cholesterol-dependent cytolysins. Adv Exp Med Biol. 677:56–66. [DOI] [PubMed] [Google Scholar]
- Grabenstein JD, Musey LK. 2014. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine 32:2399–2405. [DOI] [PubMed] [Google Scholar]
- Harvey RM, Ogunniyi AD, Chen AY, Paton JC. 2011. Pneumolysin with low hemolytic activity confers an early growth advantage to Streptococcus pneumoniae in the blood. Infect Immun. 79:4122–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway LJ, Meier PS, Battig P, Aebi S, Muhlemann K. 2004. A homologue of aliB is found in the capsule region of nonencapsulated Streptococcus pneumoniae. J Bacteriol. 186:3721–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques-Normark B, Tuomanen EI. 2013. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 3:a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty M, et al. 2014. Global phylogenomic analysis of nonencapsulated Streptococcus pneumoniae reveals a deep-branching classic lineage that is distinct from multiple sporadic lineages. Genome Biol Evol. 6:3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao WWL, et al. 2005. Evidence of a large novel gene pool associated with prokaryotic genomic islands. PLoS Genet. 1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies J, Nieminen L, et al. 2007. Identification of a secreted cholesterol-dependent cytolysin (mitilysin) from Streptococcus mitis. J Bacteriol. 189:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies JMC, et al. 2010. Identification of novel pneumolysin alleles from paediatric carriage isolates of Streptococcus pneumoniae. J Med Microbiol. 59:808–814. [DOI] [PubMed] [Google Scholar]
- Jefferies JMC, Johnston CH, et al. 2007. Presence of nonhemolytic pneumolysin in serotypes of Streptococcus pneumoniae associated with disease outbreaks. J Infect Dis. 196:936–944. [DOI] [PubMed] [Google Scholar]
- Johnston C, et al. 2010. Detection of large numbers of pneumococcal virulence genes in streptococci of the mitis group. J Clin Microbiol. 48:2762–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LE, et al. 2013. Draft genome sequences of five multilocus sequence types of nonencapsulated Streptococcus pneumoniae. Genome Announc. 1:e00520-00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, et al. 2008. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3:e2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, et al. 2014. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio 5:e01490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley FC, Sivasankar B, Morgan BP. 2007. Alternative roles for CD59. Mol Immunol. 44:73–81. [DOI] [PubMed] [Google Scholar]
- Kirkham L-AS, et al. 2006. Identification of invasive serotype 1 pneumococcal isolates that express nonhemolytic pneumolysin. J Clin Microbiol. 44:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanie JA, et al. 2007. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 189:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. 1999. Selfish operons: the evolutionary impact of gene clustering in prokaryotes and eukaryotes. Curr Opin Genet Dev. 9:642–648. [DOI] [PubMed] [Google Scholar]
- Llull D, López R, García E. 2006. Characteristic signatures of the lytA gene provide a rapid and reliable diagnosis of Streptococcus pneumoniae infections. J Clin Microbiol. 44:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López R, García E. 2004. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev. 28:553–580. [DOI] [PubMed] [Google Scholar]
- Magomani V, et al. 2014. Challenges of using molecular serotyping for surveillance of pneumococcal disease. J Clin Microbiol. 52:3271–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimon JM, Ercibengoa M, García-Arenzana JM, Alonso M, Pérez-Trallero E. 2013. Streptococcus pneumoniae ocular infections, prominent role of unencapsulated isolates in conjunctivitis. Clin Microbiol Infect. 19:E298–E305. [DOI] [PubMed] [Google Scholar]
- Marks LR, Davidson BA, Knight PR, Hakansson AP. 2013. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 4:e00438-00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott HM, Mitchell TJ, Dockrell DH. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 8:497–509. [DOI] [PubMed] [Google Scholar]
- Martin M, et al. 2003. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniaen . N Engl J Med. 348:1112–1121. [DOI] [PubMed] [Google Scholar]
- McCullers JA. 2014. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 12:252–262. [DOI] [PubMed] [Google Scholar]
- Mitchell AM, Mitchell TJ. 2010. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 16:411–418. [DOI] [PubMed] [Google Scholar]
- Mitchell TJ, Dalziel CE. 2014. The biology of pneumolysin. Subcell. Biochem. 80:145–160. [DOI] [PubMed] [Google Scholar]
- Montero Llopis P, et al. 2010. Spatial organization of the flow of genetic information in bacteria. Nature 466:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, et al. 2010. Evidence of localized prophage-host recombination in the lytA gene encoding the major pneumococcal autolysin. J Bacteriol. 192:2624–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier-Barrière I, de Saizieu A, Claverys J-P, Martin B. 1998. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol. Microbiol. 27:159–170. [DOI] [PubMed] [Google Scholar]
- Moscoso M, García E, López R. 2006. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol. 188:7785–7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld F. 1900. Ueber eine specifische bakteriolystische wirkung der galle. Z Hyg Infektionskrankh. 34:454–464. [Google Scholar]
- Obregón V, et al. 2002. Molecular peculiarities of the lytA gene isolated from clinical pneumococcal strains that are bile insoluble. J Clin Microbiol. 40:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni H, et al. 1997. Purification and partial characterization of a novel human platelet aggregation factor in the extracellular products of Streptococcus mitis, strain Nm-65. FEMS Immunol Med Microbiol. 17:121–129. [DOI] [PubMed] [Google Scholar]
- Park IH, et al. 2012. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. mBio 3:e00035-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. [DOI] [PubMed] [Google Scholar]
- Pettigrew MM, et al. 2014. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun. 82:4607–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KE, Camilli A. 2009. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J Bacteriol. 191:2163–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Sevillano E, et al. 2015. Pleiotropic effects of the cell wall amidase LytA on Streptococcus pneumoniae sensitivity to the host immune response. Infect Immun. 83:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann P, et al. 2011. Genome of Streptococcus oralis strain Uo5. J. Bacteriol. 193:2888–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CO, Heath RJ, Park H-W, Jackowski S. 2001. The licC gene of Streptococcus pneumoniae encodes a CTP:phosphocholine cytidylyltransferase. J Bacteriol. 183:4927–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, García E, Mitchell TJ. 2009. Development of a prophage typing system and analysis of prophage carriage in Streptococcus pneumoniae. Appl Environ Microbiol. 75:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, López R, García E. 2004. Characterization of LytA-like N-acetylmuramoyl-L-alanine amidases from two new Streptococcus mitis bacteriophages provides insights into the properties of the major pneumococcal autolysin. J Bacteriol. 186:8229–8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai F, Talekar SJ, Klugman KP, Vidal JE, for the RESPIRA PERU Group. 2013. Expression of virulence-related genes in the nasopharynx of healthy children. PLoS One 8:e67147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter SJ, et al. 2012. Variation at the capsule locus, cps, of mistyped and non-typable Streptococcus pneumoniae isolates. Microbiology 158:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Puelles JM, Sanz JM, García JL, García E. 1990. Cloning and expression of gene fragments encoding the choline-binding domain of pneumococcal murein hydrolases. Gene 89:69–75. [DOI] [PubMed] [Google Scholar]
- Santoro F, Vianna ME, Roberts AP. 2014. Variation on a theme; an overview of the Tn916/Tn1545 family of mobile genetic elements in the oral and nasopharyngeal streptococci. Front Microbiol. 5:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinas D, et al. 2013. Comparative genomic analyses of Streptococcus pseudopneumoniae provide insight into virulence and commensalism dynamics. PLoS One 8:e65670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shak JR, et al. 2013. Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. mBio 4:e00655-00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewell LK, et al. 2014. The cholesterol-dependent cytolysins pneumolysin and streptolysin O require binding to red blood cell glycans for hemolytic activity. Proc Natl Acad Sci U S A. 111:E5312–E5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34:D32–D36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata A, et al. 2014. The diversity of receptor recognition in cholesterol-dependent cytolysins. Microbiol Immunol. 58:155–171. [DOI] [PubMed] [Google Scholar]
- Valentino MD, et al. 2014. Unencapsulated Streptococcus pneumoniae from conjunctivitis encode variant traits and belong to a distinct phylogenetic cluster. Nat Commun. 5:5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JE, Ludewick HP, Kunkel RM, Zähner D, Klugman KP. 2011. The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect Immun. 79:4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Allen RL, Falmagne P, Johnson MK, Boulnois GJ. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae . Infect. Immun. 55:1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmore AM, et al. 1999. Molecular characterization of equine isolates of Streptococcus pneumoniae: natural disruption of genes encoding the virulence factors pneumolysin and autolysin. Infect Immun. 67:2776–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, et al. 2009. Nrc of Streptococcus pneumoniae suppresses capsule expression and enhances anti-phagocytosis. Biochem Biophys Res Commun. 390:155–160. [DOI] [PubMed] [Google Scholar]
- Yuste J, Botto M, Paton JC, Holden DW, Brown JS. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol. 175:1813–1819. [DOI] [PubMed] [Google Scholar]
- Zahlten J, et al. 2015. Streptococcus pneumoniae-induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J Infect Dis. 211:1822–1830. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Long QX, Xie J. 2012. Roles of peptidoglycan recognition protein (PGRP) in immunity and implications for novel anti-infective measures. Crit Rev Eukar Gene Expr. 22:259–268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.