Abstract
Exposure to ionizing radiation is associated with a long term risk of health effects including cancer. Radiation exposure to the U.S. population from cardiac imaging has increased markedly over the past three decades. Initiatives to reduce radiation exposure have focused on the tenets of appropriate study “justification” and “optimization” of imaging protocols. This article reviews ways to optimally reduce radiation dose across the spectrum of cardiac imaging.
Keywords: Ionizing radiation, cardiovascular imaging, optimization, justification, stochastic risk
Introduction
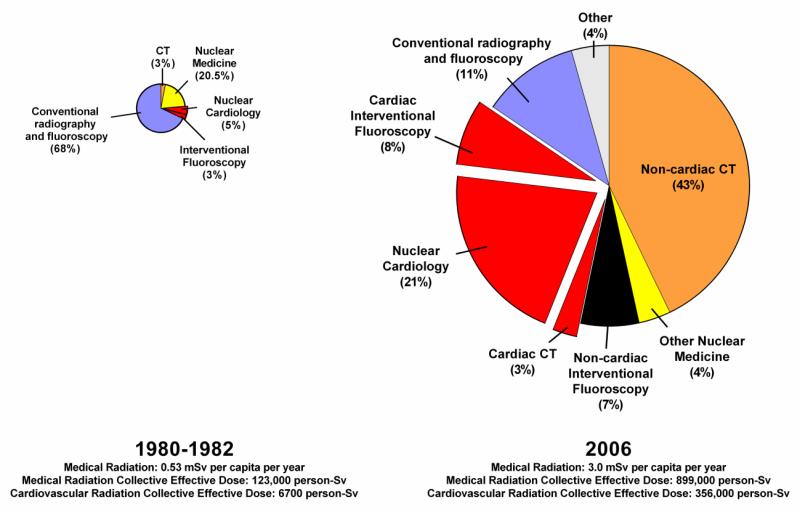
Over the preceding three decades, the U.S. population has seen an estimated seven-fold increase in annual medical imaging ionizing radiation exposure.1 Cardiac imaging procedures are a major contributor to population radiation exposure in the U.S., collectively accounting for nearly one-fifth of the cumulative radiation dose and approximately 40% of the cumulative dose from medical imaging procedures (Figure 1).1-3 In its 2007 report, the International Commission on Radiologic Protection (ICRP) noted that cardiologists frequently receive inadequate training in radiation protection.4 Fortunately, this is beginning to change and an increased focus on radiation safety by the cardiology community has led to advances in technology, imaging protocols and the development of appropriate use criteria to limit radiation exposure. The purpose of this article is to provide an overview of ionizing radiation during medical imaging including dosing metrics, risk estimation, and strategies to reduce dose and/or mitigate radiation risk during cardiovascular procedures.
Figure 1.
Medical imaging radiation exposure to the U.S. population
How is radiation dose measured?
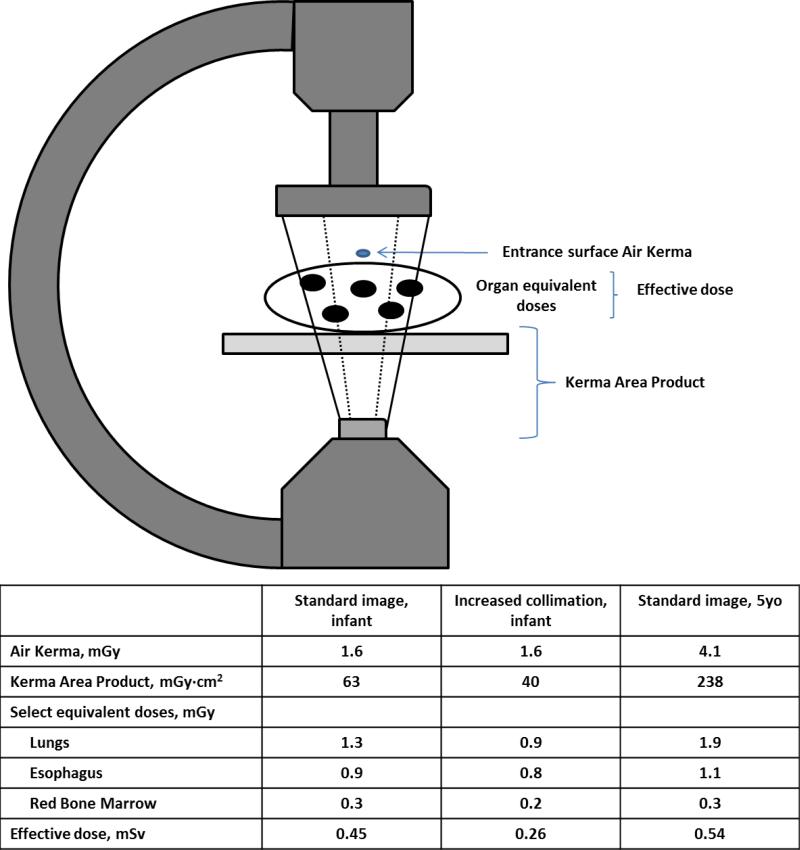
Radiation dose is a complex topic and there are a slew of different measures that quantify various aspects of radiation (Table 1). Figure 2 demonstrates how some of these different dose measures used during fluoroscopy will vary depending on the various aspects of radiation dose that are being evaluated. Similarly for other imaging modalities, including CT and nuclear medicine scans, different metrics might be useful depending on the dosing scenario. This review focuses largely on the long-term consequences associated with radiation exposure to various organ and tissue structures. In this respect, the fundamental dose quantity is the absorbed dose, reflecting the concentration of energy deposited in a tissue or organ. However, more commonly reported is the effective dose, a whole-body quantity that weights organ absorbed doses to reflect their relative effects from radiation and to reflect the type of radiation used.
Table 1.
Common radiation dose metrics
| Metric | Description | Unit |
|---|---|---|
| Radioactivity | Atom decay/time | curie (Ci) becquerel (Bq) |
| Exposure | Total charge of ions traveling through air | roentgen (R) |
| Kerma: Sum of kinetic energy of charged particles liberated per unit mass | ||
| Metrics Reflecting Kinetic Energy | - Incident air kerma: Kerma to air from an incident X-ray beam at the patient/phantom surface without backscatter included | gray (Gy) |
| - Entrance surface air kerma: Kerma to air at the patient/phantom surface including backscatter | gray (Gy) | |
| - Air kerma-area product: Integral of air kerma over the area of the xray beam (independent of distance) | Gy-m2 | |
| - Air kerma-length product: Integral of air kerma over a line of length | Gy-m | |
| Absorbed dose | Radiation deposited in tissue per unit weight | gray (Gy) |
| Metrics Reflecting Biologic effects |
-Equivalent dose: Multiplies dose with a radiation weighting factor to account for relative biological effect. -Effective dose: Weighted sum of equivalent doses. Accounts for biological effectiveness and tissue sensitivity by multiplying equivalent dose with a tissue sensitivity weighting factor |
sievert (Sv) |
Figure 2.
A fluoroscopy example illustrating how different dose metrics may vary depending on the imaging. The doses in the table were obtained from 1 minute of fluoroscopic imaging of anthropomorphic phantoms representing a small infant and a larger child. When comparing doses in the infant using a standard (solid lines) versus more collimated (dashed lines) image, there is no difference in surface Air Kerma (a point estimate of dose). However, Kerma Area Product is reduced due to a smaller imaging field of view. Organ equivalent doses represent absorbed doses to individual organs. The esophagus dose has not changed substantially because it is in the center of the imaging field of view and is less affected by peripheral collimation. However, lung doses are reduced by 30% due to restricted field of view. Effective dose is more significantly reduced because lungs are high sensitivity organs more heavily weighted in effective dose calculations. In the older phantom, increased body mass requires higher emitted doses (Air Kerma and Kerma Area Product). However increased tissue attenuation limits organ absorption and effective dose is only marginally higher.
Effective dose is often used for comparison of long-term risks between modalities or across imaging protocols because effective dose values can be readily compared when different tissue structures are exposed or when comparing whole versus partial body exposure scenarios. These comparisons are possible because effective dose is calculated using tissue weighting factors that are published by the ICRP and reflect estimates of tissue sensitivity to radiation. For example, breast, lung, stomach, colon or bone marrow are more heavily weighted (weighting factors [wt] = 0.12) because radiation related tumorigenesis is more likely in these organs than in less sensitive organs such as brain, skin, bone surface or salivary glands (all with weighting factors [wt] = 0.01). Intermediate weighting factors are assigned to gonads (0.08), liver, esophagus, thyroid and bladder (all 0.04). To calculate effective dose, organ specific equivalent doses are multiplied by these weighting factors and then summed. The weighting factors reflect our current understanding of relative risk to differing tissues. However, they have changed over time as evidence of tissue sensitivity has evolved.4 It is also important to note that the weighting factors represent rounded values reflecting multiple factors averaged over both genders and all age ranges. They are not intended to predict risk to an individual patient but rather to approximately characterize the radiation burden to a typical individual from a given procedure and protocol. Another major limitation of effective dose is that it is not readily attainable in patient care scenarios because organ absorbed doses must be measured, typically using phantoms or with simulation software.
How much radiation from medical imaging?
Effective dose is typically reported in units of millisieverts (mSv). In its 2009 report, the National Council on Radiation Protection (NCRP) estimated an average annual exposure to an individual in the U.S. of 6.2 mSv;1 approximately half from medical imaging procedures with the other half from background sources, predominantly radon.5 For frame of reference, a single antero-posterior chest radiograph typically requires 0.02 mSv of effective dose. Figure 2 demonstrates estimated contributions from various medical imaging sources to the U.S. population radiation exposure and compares current estimates with the 1987 NCRP report.6 Annual medical exposures have increased by more than seven-fold over the preceding three decades with most of the increases from CT, interventional fluoroscopy and nuclear medicine scans. Cardiac imaging annual exposures have increased more than 50-fold and now account for almost 40% of the medical imaging radiation exposure to the U.S. population.
What is the risk to patients?
Ionizing radiation involves charged particles containing enough energy to displace electrons and break chemical bonds.7 Any cell or molecule can be damaged; however, this review is primarily focused on the long term risk of stochastic effects, most importantly iatrogenic cancer. Cancer is believed to result from misrepair of DNA damage. The extent of DNA damage is proportional to the ionizing radiation exposure and this mechanistic relationship underlies the linear, no threshold model of cancer induction following exposure. Although not without controversy, this model is the most widely accepted for purposes of radiological protection, with several critically important implications. First no dose of ionizing radiation, no matter how low, is considered risk free. Second repetitive doses of ionizing radiation increase risk, which is proportionate to the cumulative dose.7 Epidemiologically-based studies support this association between low dose ionizing radiation and life-time cancer risk. Although the risk to any given patient from any given imaging study is low, at the population level the cumulative burden of disease may be substantial.8
Atomic bombs and Nuclear Workers
The most robust and widely cited epidemiologic study is the Life Span Study (LSS).9,10 This cohort study, now with over 40 years of follow up, was designed to understand the relationship between radiation exposure and health outcomes in survivors of the Hiroshima and Nagasaki atomic bombings. The LSS cohort includes nearly 30,000 bomb survivors who, due to distance from the bomb hypocenter, were exposed to low doses (all < 100mSv, mean 29mSv) of ionizing radiation. When compared to a cohort of bomb survivors with minimal exposure (colon doses < 5mGy), the excess relative risk of solid cancers was estimated to increase by 2.0% including rates of excess common cancers of 2.0% for colon, 2.3% for lung, 4.3% for female breast, 3.9% for thyroid, 1.8% for ovary, 1.3% for liver and 1.9% for nervous system including brain. Further data have been garnered from the 15-Country Study of Cancer Risk in Radiation Workers in the Nuclear Industry.11,12 This study evaluated 407,391 workers individually monitored for external radiation at 154 different facilities with a total follow-up of 5.2 million person years. The mean overall cumulative recorded dose was 19.4 mSv and 90% of workers received < 50 mSv. The relative risk for all cancers excluding leukemia was increased by 97% per Sv (95% CI 14%-197%) and was higher than the risk estimates from the LSS cohort. However, a major limitation of this study was the inability to account for important confounders such as smoking and diet.
CT scans
In more recent years, two important epidemiologic studies have evaluated risk to children/adolescents undergoing CT scans. Pearce and colleagues linked cancer incidence data from the United Kingdom National Health Service Central Registry to compiled hospital records data documenting past performance of CT scans.13 The cohort included 178 604 young patients (< 22 years of age) without previous cancer diagnoses who were examined with CT between 1985 and 2009. The risk for leukemia was increased by 3.6% per mGy (95% CI 0.5% - 12.0%) and by 2.3% per mGy (95% CI 1.0% - 4.9%) for brain tumors. These estimates translate into a 10-year risk of approximately one excess case of leukemia and one excess brain tumor per 10 000 children exposed to a 10 mGy CT scan (assuming exposure before the age of 10 years). In a similar analysis, Mathews et al. linked the Australian Cancer Database and National Death Index to the Australian Medicare system electronic medical record to estimate cancer risk to patients exposed to CT scans during childhood and/or adolescence between 1985 and 2006.14 The cohort included almost 9 million people including 680,211 exposed to a CT scan with a mean length of follow-up of 9.5 years for the exposed group. For all types of cancer combined, incidence was 24% greater for the exposed versus unexposed group. Approximately one out of every 1800 CT scans was followed by an excess cancer.
While these studies raise concern, critiques have questioned the biologic plausibility as risks for some cancers were substantially greater than those estimated from the LSS. Moreover, retrospective studies such as these can be confounded by reverse causation, the possibility that CT scans were performed because of a proven or suspected cancer diagnosis.15 To account for this, both studies excluded cancer diagnoses occurring in close proximity to the CT scan. However, critics question whether the one to five year time lags were adequate. Nonetheless, when considered along with other epidemiologic studies and basic science data, there is increasingly convincing evidence of a small yet real increased risk of cancer induction following low dose ionizing radiation exposure.
Are certain populations at increased risk?
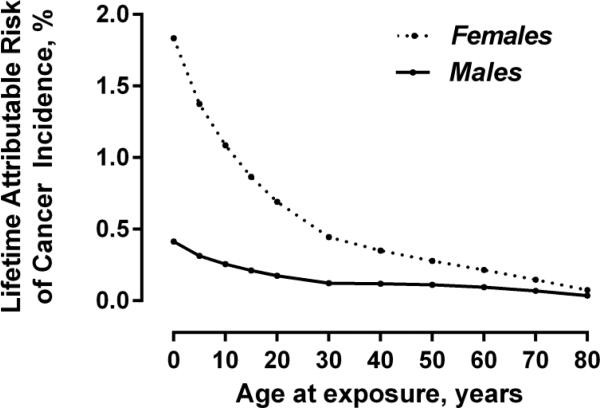
On the basis of epidemiologic studies, combined with mathematical modeling, basic science data and expert consensus, a series of National Academy of Sciences committees on the Biological Effects of Ionizing Radiation (known by the acronym BEIR) have published cancer risk estimates for males and females based on age at exposure.7 The most recent BEIR risk estimates highlight the substantially increased risk to females due to the risk of breast cancer, and to the young due to the increased sensitivity of rapidly dividing cells as well as the typical time-lag between exposure and cancer onset. Figure 3 illustrates the relative differences, projecting the lifetime attributable risk of cancer for males and females based on age at exposure to a non-gated coronary CT angiogram. Although not strictly accurate, we have kept the organ doses constant across the various age ranges to demonstrate the effect of age and gender on exposure-related risk. For a given set of organ doses, a female infant has an estimated four-fold increased lifetime attributable risk of cancer when compared to a male infant. Similarly, estimated risks are two to four-fold greater for an exposure occurring at age 20 years versus age 60 years.
Figure 3.
Estimated cancer lifetime attributable risk: effect of gender and age at exposure. Organ doses used for these risk estimations were based on previously published organ doses for coronary CT angiography performed in adults, for a scan with an estimated effective dose of 18 mSv. 32 In this figure organ doses were not changed across age ranges so as to demonstrate the relative effect of age and gender on exposure-related risk.
How to reduce radiation burden during cardiac imaging?
The principles of justification and optimization form the backbone of medical imaging dose reduction recommendations.2 Justification means that a medical procedure should only be performed when the anticipated clinical benefits exceed all anticipated risks, including radiation risk. For individual patients the long-term risks associated with radiation exposure are extremely low. Nonetheless, according to established appropriate use criteria,16-18 a significant percentage (from ~5% to > 45% depending on the study and imaging modality) of cardiac imaging studies are not justified.19-23 Quality improvement initiatives including automated point-of-order decision support tools20, education/peer-group feedback19, and web-based instruments and interaction23 have all been shown to significantly reduce the number of inappropriate cardiac studies. Approaches such as these offer a promising means to substantially reduce cumulative dose to the U.S. population.
Optimization means that the radiation dose to the patient is suitable for the medical purpose, and radiation that is clinically unnecessary or unproductive is avoided.24 Over the preceding decade there have been tremendous advances in medical imaging dose reduction strategies. The remainder of this review will focus on the best means to optimize radiation dose during: 1) fluoroscopically guided procedures, 2) myocardial perfusion imaging, and 3) cardiac CT.
Fluoroscopically guided procedures
Radiation dose from fluoroscopically guided cardiac procedures varies widely depending on the complexity of the procedure. Table 2 provides some dose range estimates for some of the more common fluoroscopically guided procedures. Typically, coronary angiography requires effective doses in the range of 5-10mSv while percutaneous coronary interventions require doses in the range of 15-25mSv.25 More complex procedures, including complex interventions in children, chronic total occlusion coronary interventions and complex radiofrequency ablation, can require doses exceeding 100 mSv. Moreover, many patients require repeated procedures and the cumulative exposures to these higher risk patient populations are far greater.26
Table 2.
Typical Effective Doses (ED) for cardiac procedures*
| Modality | Protocol | Typical ED (mSv) |
|---|---|---|
| Fluoroscopy | Diagnostic coronary angiography | 2-20 |
| Fluoroscopy | Percutaneous coronary intervention | 5-57 |
| Fluoroscopy | TAVR, transapical approach | 12-23 |
| Fluoroscopy | TAVR, femoral approach | 33-100 |
| Fluoroscopy | Diagnostic electrophysiology study | 0.1-3.2 |
| Fluoroscopy | Radiofrequency arrhythmia ablation | 1-25 |
| Coronary CT angiography | Helical without tube current modulation | 8-30 |
| Coronary CT angiography | Helical with tube current modulation | 6-20 |
| Coronary CT angiography | Prospectively triggered axial | 0.5-7 |
| Coronary CT angiography | High pitch helical | <0.5-3 |
| Pre-TAVR CT angiography | Coronary (multiphase) and chest/abdomen/pelvis | 5-50 |
| Coronary CT angiography | Calcium score | 1-5 |
| SPECT | Dual isotope: 3.5 mCi 201TI rest/30 mCi sestamibi stress | 23 |
| SPECT | Dual isotope: 3.5 mCi 201TI rest/30 mCi tetrofosmin stress | 22 |
| SPECT | 30mCi 99mTc sestamibi rest/30mCi 99mTc sestamibi stress | 18 |
| SPECT | 15mCi 99mTc sestamibi rest/45mCi 99mTc sestamibi stress | 17 |
| SPECT | 15mCi 99mTc tetrofosmin rest/45mCi 99mTc tetrofosmin stress | 14 |
| SPECT | 30mCi 99mTc tetrofosmin rest/30mCi 99mTc tetrofosmin stress | 14 |
| SPECT | 10mCi 99mTc sestamibi rest/30mCi 99mTc sestamibi stress | 11 |
| SPECT | 10mCi 99mTc tetrofosmin rest/30mCi 99mTc tetrofosmin stress | 9 |
| SPECT | 30mCi 99mTc sestamibi stress only | 8 |
| PET | 10 mCi 18F FDG | 7 |
| PET | 50mCi 82Rb rest/50 mCi 82Rb stress | 4 |
| SPECT | 10mCi 99mTc sestamibi stress only | 2.7 |
| SPECT | 10mCi 99mTc tetrofosmin stress only | 2.3 |
| PET | 15mCi 13N ammonia rest/15 mCi 13N ammonia stress | 2 |
Adapted with permission from Einstein et al. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 63 (15) 2014.44
Dose reduction strategies during fluoroscopy fall into two broad but overlapping categories – technological advances and operator dependent techniques. Technological advances over the preceding decades have included automated exposure control systems, spectral beam shaping features, pulsed fluoroscopy, flat panel detectors, last image hold, fluoro-loops and fluoro-save features. Different fluoroscopy systems employ variations of these features. The physics can be complex and it is often difficult to know which systems perform superiorly to others. Although a comprehensive review is beyond the scope of this manuscript, excellent prior reviews are available.27 Table 2 summarizes some of the more impactful operator dependent dose minimization techniques and their estimated effect on overall dose.24
Cardiac CT
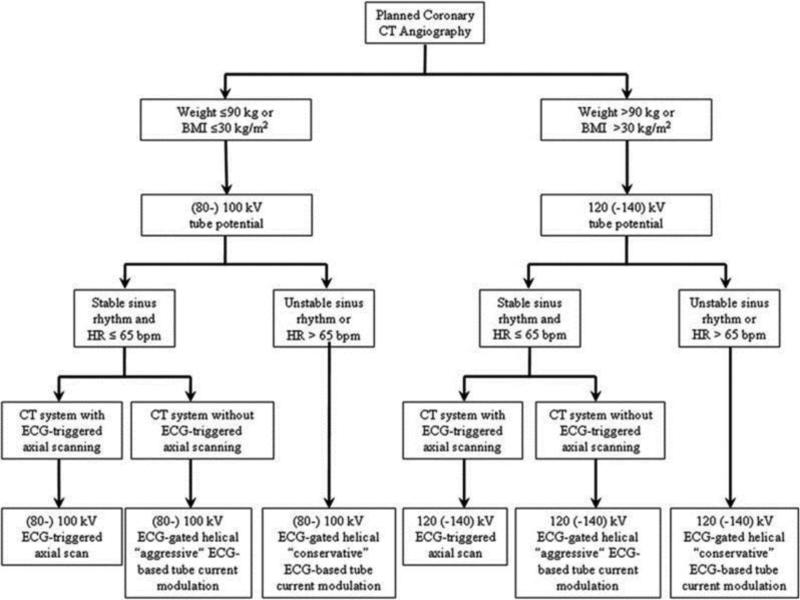
Cardiac CT encompasses several distinct procedures, including coronary artery calcium scoring (coronary calcium scans), coronary CT angiography, pulmonary vein CT angiography, myocardial CT perfusion, and CT attenuation correction of nuclear cardiology image data.28 Dose ranges for cardiac CT scans can vary widely. For example, a typical helical coronary CT angiogram might require anywhere from 6 to 30 mSv depending on the imaging approach. With advanced imaging techniques and equipment, these doses can be lowered even further, potentially to less than 1 mSv (Table 2). This next section and Figure 4 summarize guidelines for dose optimization during cardiac CT from the Society of Cardiovascular Computed Tomography.29
Scan modes (gating) – for moving structures such as the coronary arteries, optimal imaging requires timing of data acquisition to the cardiac cycle and evaluation of images obtained at times of low motion. ECG-gating can be performed retrospectively, with the patient exposed to radiation throughout the cardiac cycle. Retrospective ECG-gated helical scanning is very robust; however, only data from the cardiac phases with the least motion are used for image interpretation and thus a substantial portion of the data acquired is not utilized clinically. More recently, prospective ECG-triggered axial scanning has emerged as a lower dose alternative to retrospective gating protocols. In prospective scanning, imaging is limited to a defined phase of the R-R interval, thereby minimizing beam time. In a multicenter trial randomizing 400 subjects with low heart rates to axial versus helical scanning protocols for coronary angiography, prospective-axial scanning reduced radiation dose by 69% (effective dose reduced from 11.2 ± 5.9 mSv to 3.5 ± 2.1 mSv, p<0.001) without compromising image quality.30 A disadvantage of prospectively gated protocols is the greater potential for image blurring in patients with either arrhythmias or faster (> 65- 70 beats per minute) heart rates. New software techniques such as automated arrhythmia rejection methods may increase utility of some of these protocols. Other technologic improvements including wide detector arrays and prospective ECG-triggered high pitch helical scans can reduce acquisition times and therefore reduce radiation dose.
Tube potential – expressed in units of kilovolts (kV), represents the electrical potential applied across the x-ray tube to accelerate electrons towards the imaging target. Higher tube potentials increase the speed at which the electrons travel and therefore the penetrance or degree to which the x-rays have passed through the body. Reducing the tube potential will reduce radiation dose but also increases image noise. Lower tube potentials (i.e. 70-100kV) can be used in non-obese adults and in the vast majority of children/adolescents, while higher tube potentials (120 and rarely 135 or 140 kV) are needed in obese patients. Radiation dose is approximately proportionate to the square of the tube potential so the dose to a patient undergoing a typical coronary CT angiogram performed at 120 kV (estimated effective dose of 21 mSv) could be reduced by at least 31% (~ 14.6mSv) if performed with a tube potential of 100 kV and by at least 44% (~9.4mSv) if performed at 80 kV (assuming no other imaging parameters are altered).
Tube current – expressed in units of milliamperes (mA) or milliampere-seconds (mAs) represents the number of electrons accelerated across the x-ray tube. There is a linear relationship between tube current and radiation dose but lowering tube current will also increase image noise. ECG-synchronized tube current modulation may be used in helical scans to down-regulate the tube current during the anticipated “less critical” components of the cardiac cycle. Because the data are still acquired, images that require less resolution (e.g. functional evaluation such as measurement of ventricular volumes) can still be compiled but at a lower dose. While not as effective in lower dose as prospectively-triggered scan, these tube current modulation protocols can reduce total dose during coronary artery evaluation by up to 40%.31 For example, in one study, doses during CT coronary angiography with and without tube current modulation were estimated to be reduced from 21 mSv to 14 mSv in females and from 15 mSv to 9 mSv in males.32 Similar to tube potential, tube current can be selected to reflect patient body habitus – thinner patients require less current as there is less tissue attenuation.
Pitch – during helical scanning, pitch represents the ratio of table travel per gantry rotation. An increase in pitch results in less overlap between images with a proportional reduction in dose. In general, overlap is required to avoid gaps in the data but the amount depends on several factors including the patient's heart rate and the gantry rotation time. The latest generation of dual-source CT technology uses a second tube/detector system to fill data gaps; accordingly, the pitch can be increased with no overlap (i.e. pitch > 1). For this scanner, ECG-triggered scanning can be performed at pitches > 3 which can reduce radiation dose potentially to below 1mSv for a typical coronary CT angiogram.
Scan length – minimizing scan length and optimizing imaging to the desired region of interest represent some of the simplest ways to reduce dose. For example, the extra ~ 10cm of scan length that is required to image the ascending aorta and aortic arch during coronary CT angiography can increase dose by more than 30-60% and should only be used when the extra imaging is clinically indicated.32
Iterative reconstruction algorithms – these algorithms use iterative mathematical models to predict projection data and thereby enhance the image reconstruction process. Various algorithms and approaches exist but when optimally implemented, they reduce image noise and permit scanning at lower tube current and potential, thereby reducing overall radiation dose.
Figure 4.
Society of Cardiovascular Computed Tomography algorithm for radiation dose optimization in coronary CT angiography
Myocardial perfusion imaging
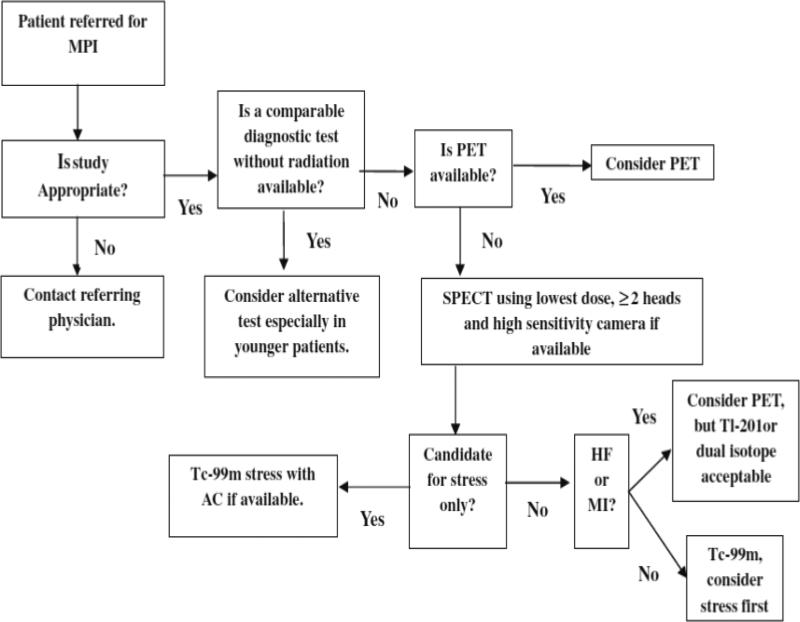
MPI is the single medical test with the highest radiation burden to the population of the U.S.33 MPI accounts for > 10% of the cumulative effective dose to the American population from all sources, excluding radiotherapy.1 Moreover, multiple testing with MPI is common and one study demonstrated that almost 1/3rd of patients undergoing MPI received >100 mSv of cumulative effective dose, a level at which there is little controversy over the potential for increased cancer risks.34 This next section and Figure 5 summarize recommendations from the American Society of Nuclear Medicine for optimizing MPI to reduce radiation exposure.35 For perspective, Table 2 provides estimates of typical doses from various MPI protocols.
Tracers and imaging protocols: Radiotracers significantly affect dose. PET tracers have a shorter half-life than SPECT tracers and impart a lower effective dose. However PET scanning equipment is more expensive and not always readily available. For SPECT scanning, Tc-99m-based SPECT protocols (sestamibi and tetrofosmin) offer lower patient radiation exposure than Tl-201 (stress/redistribution and stress/reinjection) or dual-isotope (Tl-201 rest/Tc-99m stress) protocols. Using a stress only protocol, when appropriate, reduces dose compared to combined stress and rest protocols.36 In general, if stress imaging is completely normal, there is no added utility to the rest protocol. The use of attenuation correction or prone imaging can increase the normalcy rate of stress imaging, by correcting for attenuation artifacts, and thereby facilitate the performance of stress-only imaging, hence reducing radiation dose.
Software: Iterative reconstruction algorithms, as in CT, reduce image noise. This improves image quality allowing use of lower administered activity (mCi) and thus lower dose. Other software-based approaches including resolution recovery and noise compensation can be combined with iterative reconstruction to further reduce activity and dose. Resolution recovery models the physics and geometry of the emission and detection processes while noise compensation optimizes the signal-to-noise ratio based on the desired resolution and smoothness of the final image.37 In one study, SPECT imaging with wide beam reconstruction, an iterative algorithm including resolution recovery, half dose radiotracer protocols actually had superior image quality than standard dose protocols without wide beam reconstruction.38
Hardware: Solid-state detector-based SPECT cameras, when compared to conventional sodium iodide cameras, produce a more discrete output signal by directly (e.g. cadmium-zinc-telluride [CZT] semiconductor detectors) or indirectly (e.g. cesium iodide) converting gamma rays into electrons. Several cameras incorporate multiple solid-state detectors with detector geometry optimized for cardiac imaging, resulting in greater count sensitivity with improved energy and spatial resolution. Camera designs positioning detectors closer to the chest wall and/or using “cardiocentric orbits” as opposed to the 180° “body-centered” orbits of more conventional cameras, improve efficiency and thus can be used to decrease radiation dose. Another promising development is the use of custom-designed collimators that more precisely focus on the myocardium. These and other manufacturer-specific hardware designs are beyond the scope of this article but are excellently described in several previous reviews. 37,39 Several studies have demonstrated the effectiveness of these newer camera designs in facilitating lower dose imaging protocols. In the MILLISIEVERT study, an ultra-low-dose imaging protocol (3.5mCi 99mTc sestamibi, effective dose: ~1.15mSv) using high efficiency cameras with multiple solid state CZT detectors was compared to a standard low-dose protocol (7-13mCi 99mTc sestamibi, effective dose: ~2.39 mSv) using conventional Anger SPECT cameras. The unique design of this study allowed blinded image comparisons of the two imaging protocols performed in the same patient. Overall image quality was superior with the ultra-low-dose protocol.40 In a separate study of patients presenting to the emergency department with chest pain, a stress-first protocol (with same-day rest imaging only when indicated) was employed using high efficiency cameras (5 mCi 99mTc-tetrofosmin). Overall, 69/100 patients required stress-only imaging with an average effective dose of 0.99 mSv (2.22 mSv over all patients). Taken together, these two studies have demonstrated the feasibility and utility of ultra low dose imaging protocols performed with high efficiency cameras.41
Figure 5.
American Society for Nuclear Imaging algorithm for radiation dose optimization in MPI
Conclusions and future directions
For the foreseeable future, procedures involving ionizing radiation will remain a vital part of cardiovascular practice; despite the great benefits of these procedures, there are inherent risks to patients and the estimated public health burden is substantial. Therefore, the cardiology community must continue to strive to minimize radiation burden but without compromising diagnostic accuracy or procedural safety. Immediate gains are achievable with improved study justification and by optimizing existing imaging protocols. System-based approaches including education initiatives, dose monitoring programs and point-of-order decision support tools have all proven successful at reducing the number of “unjustified” studies. Wider scale implementation of the most successful of these initiatives could have a marked population-wide impact.19,20,23,42,43 Moreover, there is a need for initiatives to establish reasonable benchmarks, to facilitate provider feedback reporting and to more accurately gauge long-term patient risk. It also remains critical to achieve further technological advances and to ensure that these advances are both cost effective and available to the broader population.24 Beyond technology, other paradigms such as therapies that limit free-radical induced DNA damage offer further potential but are currently in very early stages of development. Perhaps there will come a time when radiation is no longer an important part of medical practice. Until then, dose optimization should remain a priority for patients, providers, institutions, regulatory agencies and all other stakeholders.
Table 3.
Dose optimization strategies for fluoroscopically guided procedures
| Technique | Typical dose reduction | Comments |
|---|---|---|
| Establish a dose monitoring program | +++ (>50%) | Awareness is the most important variable in dose reduction43 |
| Beam time | +++ (> 50%) | Pulsed fluoroscopy allows imaging for a shorter number of pulses per second and also at a shorter pulse width. Frame rates of 15,10,7.5 or even 5 frames/second reduce beam time substantially compared to continuous fluoroscopy (30-35 frames/sec) |
| Object to image distance | + (15-25%) | A raised receptor will capture fewer x-rays and prompt auto-exposure controls to increase radiation to maintain exposure. |
| Camera magnification | + (15-25%) | Incremental increases in camera magnification (e.g. 7.5 to 5 inch) increase the delivered dose by up to 30% although the dose will be delivered to a smaller area. |
| Collimation | + (15-25%) | Avoids unnecessary radiation to organs outside the region of interest. Dose/risk reduction depends on the extent of collimation and the sensitivity of organs excluded from the radiation field. |
| Camera angulation | ++ (25-50%) | With oblique camera angles the radiation beam passes through more tissue prompting auto-exposure controls to increase dose. |
| Image acquisition | ++ (25-50%) | Compared to conventional image intensifiers, flat panel detectors limit image degradation and provide higher image quality at lower doses. This is most noticeable at higher magnification because image intensifier systems require a 3- to 4-fold increase in dose for increased magnification versus 1.3-2-fold dose increases for flat panel detectors.25,45 |
| Fluoroscopy modes (low versus normal contrast) | ++ (25-50%) | International Electrotechnical Commission standards require that fluoroscopy systems include a “low” contrast mode with half the dose of the “normal” mode.46 |
+ mild, ++ moderate, +++ substantial potential for dose reduction
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding groups.
Dr. Hill is supported in part by a grant KL2TR001115-02 from The National Center for Advancing Translational Sciences of the National Institutes of Health, and a grant from the Mend A Heart Foundation. Dr. Einstein is supported in part by grant R01 HL10971 from the National Heart Lung and Blood Institute and by a Herbert Irving Associate Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States (NCRP Report No 160) National Council on Radiation Protection and Measurements. Bethesda, Md.: 2009. [Google Scholar]
- 2.Cousins C, Miller DL, Bernardi G, Rehani MM, Schofield P, Vano E, et al. ICRP PUBLICATION 120 : Radiological protection in cardiology. Ann ICRP. 2013;42(1):1–125. doi: 10.1016/j.icrp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119(7):1056–65. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 4.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37(2-4):1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Schauer DA, Linton OW. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, medical exposure--are we doing less with more, and is there a role for health physicists? Health Phys. 2009;97(1):1–5. doi: 10.1097/01.HP.0000356672.44380.b7. [DOI] [PubMed] [Google Scholar]
- 6.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States : recommendations of the National Council on Radiation Protection and Measurements. The Council; Bethesda, MD: 1987. [Google Scholar]
- 7.Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. National Academies Press; Washington, DC: 2006. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; Nuclear and Radiation Studies Board, Division on Earth and Life Studies, National Research Council of the National Academies. [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiation research. 2007;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 10.Einstein AJ. Beyond the bombs: cancer risks of low-dose medical radiation. Lancet. 2012;380(9840):455–7. doi: 10.1016/S0140-6736(12)60897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331(7508):77. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation- related cancer risks. Radiation research. 2007;167(4):396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 13.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56(22):1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014. 63(4):380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Chinnaiyan KM, Peyser P, Goraya T, Ananthasubramaniam K, Gallagher M, Depetris A, et al. Impact of a continuous quality improvement initiative on appropriate use of coronary computed tomography angiography. Results from a multicenter, statewide registry, the Advanced Cardiovascular Imaging Consortium. J Am Coll Cardiol. 2012;60(13):1185–91. doi: 10.1016/j.jacc.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Lin FY, Dunning AM, Narula J, Shaw LJ, Gransar H, Berman DS, et al. Impact of an automated multimodality point-of-order decision support tool on rates of appropriate testing and clinical decision making for individuals with suspected coronary artery disease: a prospective multicenter study. J Am Coll Cardiol. 2013;62(4):308–16. doi: 10.1016/j.jacc.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 21.Hendel RC, Cerqueira M, Douglas PS, Caruth KC, Allen JM, Jensen NC, et al. A multicenter assessment of the use of single-photon emission computed tomography myocardial perfusion imaging with appropriateness criteria. J Am Coll Cardiol. 2010;55(2):156–62. doi: 10.1016/j.jacc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Doukky R, Hayes K, Frogge N, Balakrishnan G, Dontaraju VS, Rangel MO, et al. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation. 2013;128(15):1634–43. doi: 10.1161/CIRCULATIONAHA.113.002744. [DOI] [PubMed] [Google Scholar]
- 23.Saifi S, Taylor AJ, Allen J, Hendel R. The use of a learning community and online evaluation of utilization for SPECT myocardial perfusion imaging. JACC Cardiovascular imaging. 2013;6(7):823–9. doi: 10.1016/j.jcmg.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Fazel R, Gerber TC, Balter S, Brenner DJ, Carr JJ, Cerqueira MD, et al. Approaches to enhancing radiation safety in cardiovascular imaging: a scientific statement from the american heart association. Circulation. 2014;130(19):1730–48. doi: 10.1161/CIR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 25.Pantos I, Patatoukas G, Katritsis DG, Efstathopoulos E. Patient radiation doses in interventional cardiology procedures. Curr Cardiol Rev. 2009;5(1):1–11. doi: 10.2174/157340309787048059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JN, Hornik CP, Li JS, Benjamin DK, Jr., Yoshizumi TT, Reiman RE, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130(2):161–7. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin PJ. Technical advances of interventional fluoroscopy and flat panel image receptor. Health Phys. 2008;95(5):650–7. doi: 10.1097/01.HP.0000326336.40775.94. [DOI] [PubMed] [Google Scholar]
- 28.Weigold WG, Abbara S, Achenbach S, Arbab-Zadeh A, Berman D, Carr JJ, et al. Standardized medical terminology for cardiac computed tomography: a report of the Society of Cardiovascular Computed Tomography. Journal of cardiovascular computed tomography. 2011;5(3):136–44. doi: 10.1016/j.jcct.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. Journal of cardiovascular computed tomography. 2011;5(4):198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, et al. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovascular imaging. 2012;5(5):484–93. doi: 10.1016/j.jcmg.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113(10):1305–10. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 32.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Jama. 2007;298(3):317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 33.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–57. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Einstein AJ, Weiner SD, Bernheim A, Kulon M, Bokhari S, Johnson LL, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA. 2010;304(19):2137–44. doi: 10.1001/jama.2010.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2010;17(4):709–18. doi: 10.1007/s12350-010-9244-0. [DOI] [PubMed] [Google Scholar]
- 36.Depuey EG, Mahmarian JJ, Miller TD, Einstein AJ, Hansen CL, Holly TA, et al. Patient-centered imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19(2):185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 37.DePuey EG. Advances in SPECT camera software and hardware: currently available and new on the horizon. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19(3):551–81. doi: 10.1007/s12350-012-9544-7. quiz 85. [DOI] [PubMed] [Google Scholar]
- 38.DePuey EG, Ata P, Wray R, Friedman M. Very low-activity stress/high-activity rest, single-day myocardial perfusion SPECT with a conventional sodium iodide camera and wide beam reconstruction processing. J Nucl Cardiol. 2012;19(5):931–44. doi: 10.1007/s12350-012-9596-8. [DOI] [PubMed] [Google Scholar]
- 39.Slomka PJ, Patton JA, Berman DS, Germano G. Advances in technical aspects of myocardial perfusion SPECT imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2009;16(2):255–76. doi: 10.1007/s12350-009-9052-6. [DOI] [PubMed] [Google Scholar]
- 40.Einstein AJ, Blankstein R, Andrews H, Fish M, Padgett R, Hayes SW, et al. Comparison of image quality, myocardial perfusion, and left ventricular function between standard imaging and single- injection ultra-low-dose imaging using a high-efficiency SPECT camera: the MILLISIEVERT study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(9):1430–7. doi: 10.2967/jnumed.114.138222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Einstein AJ, Johnson LL, DeLuca AJ, Kontak AC, Groves DW, Stant J, et al. Radiation Dose and Prognosis of Ultra-Low-Dose Stress-First Myocardial Perfusion SPECT in Patients with Chest Pain Using a High-Efficiency Camera. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(4):545–51. doi: 10.2967/jnumed.114.150664. [DOI] [PubMed] [Google Scholar]
- 42.Sawdy JM, Kempton TM, Olshove V, Gocha M, Chisolm JL, Hill SL, et al. Use of a dose-dependent follow-up protocol and mechanisms to reduce patients and staff radiation exposure in congenital and structural interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2011;78(1):136–42. doi: 10.1002/ccd.23008. [DOI] [PubMed] [Google Scholar]
- 43.Raff GL, Chinnaiyan KM, Share DA, Goraya TY, Kazerooni EA, Moscucci M, et al. Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA. 2009;301(22):2340–8. doi: 10.1001/jama.2009.814. [DOI] [PubMed] [Google Scholar]
- 44.Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, et al. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 2014;63(15):1480–9. doi: 10.1016/j.jacc.2013.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nickoloff EL. AAPM/RSNA physics tutorial for residents: physics of flat-panel fluoroscopy systems: Survey of modern fluoroscopy imaging: flat-panel detectors versus image intensifiers and more. Radiographics. 2011;31(2):591–602. doi: 10.1148/rg.312105185. [DOI] [PubMed] [Google Scholar]
- 46.International Electrotechnical Commission Publication 61267 ed2.0 International Standard: Medical diagnostic X-ray equipment - Radiation conditions for use in the determination of characteristics. 2005 Accessible online at http://www.iec.ch/dyn/www/f?p=103:22:0::::FSP_ORG_ID:1362.