Abstract
Approach and avoidance constitute a basic dimension of all animal behavior. A large literature documents approach and avoidance elicited by specific sensory stimuli, yet comparatively little is known about default approach biases when stimulus information is reduced. The amygdala is well known to contribute to approach and avoidance behaviors in response to specific sensory stimuli, and here we test whether the amygdala’s role might extend to situations where stimulus information is reduced. A novel task asked three rare patients with bilateral amygdala lesions to make approach-related judgments about photos of faces when intact, and with all internal facial features occluded. Direct comparisons of these stimuli isolated a stimulus-independent bias. The patients showed a greater tendency than controls to default to rating occluded faces as more approachable than whole faces. These findings suggest that the amygdala’s role in approach behavior extends beyond responses to specific stimuli.
Keywords: amygdala, approach, avoidance, face-processing, Urbach-Wiethe disease, lesion, ambiguity
From single-celled organisms to humans, all mobile species exhibit approach-avoidance behavior. In humans, approach-avoidance behavior is regulated by motivation and influenced by emotion (Elliot, Eder, & Harmon-Jones, 2013); at a more primitive level, it is related to instinctive defensive behaviors (Blanchard, Griebel, Pobbe, & Blanchard, 2011; McNaughton & Corr, 2004).
While basic, approach-avoidance behavior shows large individual differences. Whereas some people would walk into and explore an unfamiliar dark room, others would pause and gather more information, and some might even flee. What accounts for this behavioral variability? Prior experience might sway one’s response, but the example situation offers little information, and may not have been encountered previously. Nevertheless, a behavioral tendency will be observed. The amygdala is a brain structure known for its role in memory, learning, and emotion, and implicated in psychiatric disorders including anxiety. We investigate what role the amygdala might play in regulating stimulus-independent behavior, termed a “default bias.” A default approach bias may be normal in certain contexts; here, we ask whether amygdala lesion patients exhibit an abnormally large approach tendency to low-information stimuli.
An abnormal tendency to approach others and normally threatening stimuli in amygdala lesioned monkeys (Klüver & Bucy, 1939), rodents (Choi & Kim, 2010), and humans (Feinstein, Adolphs, Damasio, & Tranel, 2011; Kennedy, Glaescher, Tyszka, & Adolphs, 2009) points to the amygdala as being important in regulating approach-avoidance behavior. However, the basis of this approach tendency is unclear.
On the one hand, the bias may be specifically tuned for certain stimuli: much of what we know about the amygdala’s contribution to cognition and behavior has come from studies investigating faces. Single-unit amygdala response selectivity has been found for faces in humans (Rutishauser et al., 2011) and monkeys (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007), and fits with the known connectivity of the amygdala with anterior temporal neocortex (Amaral, Price, Pitkanen, & Carmichael, 1992), a region containing face-selective cells (Perrett, Rolls, & Caan, 1982; Tsao, Freiwald, Tootell, & Livingstone, 2006). Lesions of the human amygdala can result in a remarkably specific impairment in recognizing fear (Adolphs, Tranel, Damasio, & Damasio, 1994; Adolphs et al., 1999; Broks et al., 1998) and trustworthiness from faces (Adolphs, Tranel, & Damasio, 1998).
On the other hand, the amygdala might contribute to stimulus-independent baseline or default biases, similar to the “tonic influence on behavior” theorized over two decades ago (Amaral, Price, Pitkänen, & Carmichael, 1992). In amygdala lesion patients, some preliminary evidence for a general approach bias includes a propensity to approach other people or potentially dangerous situations, regardless of context (Feinstein, et al., 2011; Kennedy, et al., 2009).
The amygdala’s roles in a face-specific or default approach bias are not mutually exclusive. Trustworthiness and approachability judgments to whole faces in SM, a widely-tested amygdala lesion patient, already hint at two distinct processes. The first: a deviation from normal judgments that increases as faces become less trustworthy (i.e., an inability to process facial cues to untrustworthiness). The second: a more uniform positive bias across all faces irrespective of their perceived trustworthiness (i.e., a global bias towards trust; Adolphs et al., 1998; cf. Supplemental Figure 3). In the present study, we attempt to disentangle these two components, while also making two improvements: using more than a single case-study and controlling for regression-to-the-mean.
One mechanism by which a default bias could be achieved is through sensitivity to ambiguity. Between detecting a dangerous stimulus and launching a defensive response, the amygdala, in conjunction with the cortex, must contextually assess potential danger (Davis & Whalen, 2001). When signals are ambiguous, the amygdala may increase vigilance and predictive information by lowering sensory thresholds (Whalen, 2007). Sensitivity to ambiguity has been implicated across species; in mice and humans, temporal unpredictability in stimulus presentation elicits anxious behavior and amygdala activity (Herry et al., 2007). At a sufficiently long stimulus duration to permit appraisal (van der Zwaag, Da Costa, Zürcher, Adams Jr, & Hadjikhani, 2012), ambiguous fearful faces with direct gaze elicit greater fMRI amygdala activation than unambiguous averted-gaze fearful faces (Adams, Gordon, Baird, Ambady, & Kleck, 2003). Individual variation in state anxiety correlates with amygdala BOLD response to potentially ambiguous neutral faces (Somerville, Kim, Johnstone, Alexander, & Whalen, 2004). To our knowledge, we are the first to explore whether human amygdala lesions produce an abnormal approach tendency as a function of ambiguity.
We tested the hypothesis that amygdala damage produces a stimulus-independent default bias by directly contrasting approach-related judgments for two sets of otherwise identical face stimuli, one set modified such that the inner part of the face is erased (cf. Figure 1a). Participants made trust and threat judgments, which are known to be processed relatively automatically from faces (Willis & Todorov, 2006). In the real world, distance (Sinha, Balas, Ostrovsky, & Russell, 2006), accessories (scarves, sunglasses), and objects naturally occlude facial features. In both our whole and occluded stimulus conditions, the same external-facial cues (hairline, shape) were available, but facial feature information was only available in the whole condition. We operationalized a default occluded-approach bias as a tendency, within-subject, to judge the low-information occluded face as more trustworthy and less threatening than the corresponding whole face.
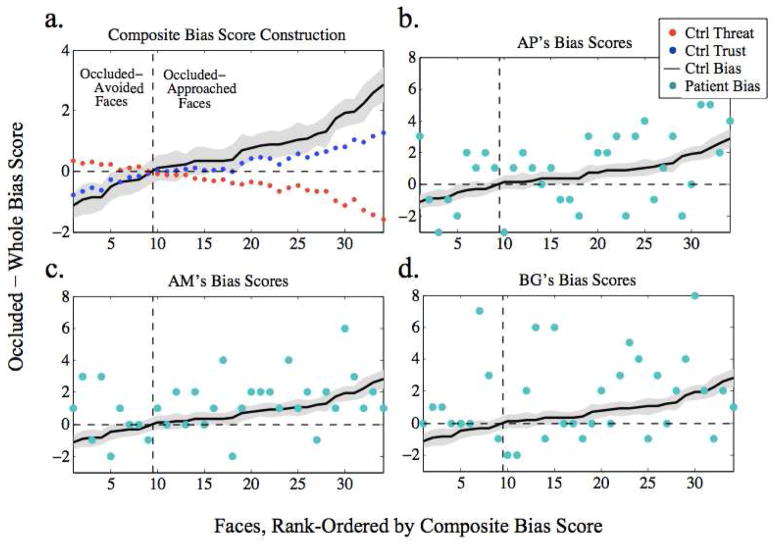
Figure 1.
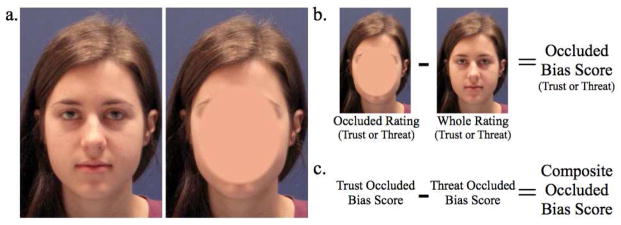
Stimuli and construction of bias scores. Panel a. Sample whole (left) and occluded (right) facial images; occluded face stimuli contained less information than whole face stimuli. Panel b. Occluded bias scores were calculated by subtracting the whole from occluded rating for each face. Positive occluded bias trust scores indicate a tendency to find the occluded face more trustworthy; negative occluded bias threat scores indicated a face was less threatening in the occluded condition. Panel c. Composite occluded bias scores combined trust and threat occluded bias scores, subtracting threat bias scores from trust bias scores so that the two measures had the same directionality: faces with larger positive composite bias scores were more approachable (more trustworthy and less threatening) in the occluded condition.
Materials and Methods
Participants
Amygdala patients
Selective bilateral damage to the human amygdala is extremely rare, but can arise from the genetic disease, Urbach-Wiethe disease (Hofer, 1973). Three women with bilateral amygdala calcification lesions (Supplemental Figure 1) caused by Urbach-Wiethe disease were tested. Two patients, AM and BG, are identical twin sisters from rural southern Germany. They were 36 years of age at testing, are married with children, and have been in full-time employment since they received their 13 years of education in Germany. The third patient, AP, is American, was 27 years of age at testing, and has worked since she obtained her Bachelor’s degree. All three patients have an IQ in the average range (HAWIE-R: AM, 101; BG, 96; WASI: AP, 98), (Becker et al., 2012). Their lesions are all similarly symmetric and confined to the amygdala (AM, 1.12 cubic centimeter bilaterally; BG, 1.15 cc; AP, 0.71 cc). The damage includes complete ablation of the basolateral amygdala with minor damage of other amygdaloid regions, including anterior and ventral cortical regions at the rostral level and lateral and medial parts of the central nucleus and amygdalo-hippocampal area at the caudal level (Supplemental Figure 1). All amygdala patients were tested individually in the laboratory.
Healthy comparison subjects
81 age, gender, and education-matched controls with no current mental health diagnosis were tested. Of the control group, 61 participants were American (mean age: 30.5 ± 8.0 years) and 20 were German (mean age: 35.1 ± 6.1 years). Americans were recruited through Amazon’s Mechanical Turk and Germans were recruited through emails forwarded to acquaintances of the authors’ German colleagues. All control participants completed the experiment using Qualtrics, an online survey-hosting platform, under conditions that were otherwise identical to the in-lab tests completed by the lesion patients. All participants, lesion patients and controls, gave informed consent/assent in accordance with a protocol approved by the Caltech IRB.
Sample size
For our normal controls, we tested a sample size (N=81) exceeding ones that have been investigated in approach/withdrawal in the past (N=46, e.g., Adolphs et al., 1998). With respect to the patients with amygdala lesions, these have in the past typically been reported as single case studies; here we report three. We present their results both individually and as a small group, and use bootstrap analyses to compare them statistically to the control group.
Stimuli
The stimulus set consisted of 34 high-resolution color images (16 females and 18 males between 20 to 50 years of age) showing essentially neutral facial expressions. Images were taken with the same camera, at the same angle with controlled lighting and in front of the same plain background. After image capture, images were luminance matched on each RBG channel, using the SHINE toolbox (Willenbockel et al., 2012). The 34 original images (“Whole”) were used to create a second set of images, which were oval-masked so that there were no inner facial features (“Occluded”), (Figure 1). All 68 images were resized such that the inter-ocular distance for all images was constant. All faces were unfamiliar to the participants.
Experimental Design
For each face, participants indicated whether or not they found the person threatening or trustworthy. Participants rated all images in a self-paced manner on a six-point scale (“Strong No”, “No”, “Weak No”, “Weak Yes”, “Yes”, “Strong Yes”). The directionality of the rating scale was counterbalanced across participants. All occluded face stimuli were presented first. Preferably presentation order would be counterbalanced or randomized; here, counterbalancing was not possible with a patient sample of 3 and, without a much larger stimulus set, it would have been difficult to control for memory effects with randomization. While our design therefore includes a presentation order confound, the same confound is present across participant groups and therefore does not affect our main question of interest – whether within-subject shifts in ratings between stimulus conditions differ between participant groups. Within the constraint of presenting occluded stimuli first, the trust and threat judgments were presented in a randomized order. Each judgment (threat or trust) comprised a block, in which all 34 images (occluded or whole) were presented, also in randomized order. For the German participants, the entire experiment was translated into German: ‘threatening’ was translated as ‘bedrohlich’ (synonyms: menacing, ominous); ‘trustworthy’ was translated as ‘zuverlässig’ (synonyms: reliable, trustworthy - note that this translation was probably more ambiguous than that used for threat). Translations were independently verified by five bilingual German/English speakers.
Analysis
Rescaling German participants’ scores
The rescaling discussed in this section does not affect the approach biases discussed below, which constitute our main effect of interest. Those biases are comprised of within-subject rating shifts, which are not affected by group-level manipulations applied uniformly to individuals’ scores. This rescaling only affects separate reports of ratings for each stimulus condition (2: whole, occluded) by judgment (2: trust, threat), cf., Table 2 and Figure S2.
Table 2.
Bootstrapped control samples exceeding mean and individual patient values.
| Judgment | Stimuli | Controls Exceeding Patients (%) | Control Mean (95% CI) | AP | AM | BG | Summary |
|---|---|---|---|---|---|---|---|
| Threat | Whole faces | 19.9 | [3.2, 3.4] | 3.3 | 2.7 | 3.2 | AP & BG same, but AM lower |
| Occluded faces | 4.7 | [2.8, 3.1] | 2.6 | 2.2 | 2.1 | All lower | |
| Occluded bias | 12.1 | [−0.5, −0.3] | −0.7 | −0.4 | −1.1 | AP and BG lower; AM not different, but already impaired for whole faces | |
|
| |||||||
| Trust | Whole faces | 23.7 | [3.1, 3.3] | 3.1 | 2.6 | 4.6 | AP normal; AM low; BG high |
| Occluded faces | 3.9 | [3.3, 3.5] | 3.3 | 3.4 | 5.0 | AP and AM normal; BG very high | |
| Occluded bias | 8.9 | [0.1, 0.3] | 0.2 | 0.8 | 0.4 | AP normal; AM and BG high | |
|
| |||||||
| Composite Bias | All faces | 7.6 | [0.4, 0.8] | 0.9 | 1.2 | 1.6 | All higher |
| Occluded-avoided faces | 1.9 | [−0.8, −0.4] | 0.2 | 0.4 | 1.2 | All higher | |
| Occluded-approached faces | 15.8 | [0.8, 1.2] | 1.2 | 1.5 | 1.7 | AP normal; AM and BG higher | |
Note. Occluded bias defined as occluded minus whole face rating. Composite bias defined as occluded trust bias minus occluded threat bias. Occluded-avoided faces have a negative average composite bias score; occluded-approached faces have a positive average composite bias score. For threat, the percentage of lower bias scores were counted; for trust and composite bias, the percentage of higher scores were counted.
To control for cultural and language differences between the two nationalities, we minimally rescaled all German ratings with a fixed bias offset. The average American trust rating across all faces (whole and occluded) and all control participants was subtracted from the same average rating for German control participants; the value of this difference between the two groups was subtracted from every single German rating, including those of the two German lesion patients. German threat ratings were rescaled following the same procedure. Rescaling was small, and likely simply reflects differences in language (N.b. trustworthiness, for which the German translation was likely more ambiguous than that for threat, had a larger absolute rescaling factor). German trust ratings were rescaled by 0.429 and threat ratings by −0.188. Since there were no mean differences between American and German controls after rescaling (Supplemental Figure 2), we pooled these control groups in all subsequent analyses.
Comparing patients to controls: general approach
Comparisons of the three lesion patients to controls was driven by asking how the three lesion patients would compare to three people randomly drawn from the general population. This comparison was estimated by building a bootstrapped population estimate from 100,000 bootstrap samples of the average rating of three randomly sampled controls, and calculating the proportion of this bootstrap distribution that lay in the tail of the distribution that exceeded the three patients’ average rating.
Defining occluded-approach bias
We defined a bias score (Figure 1b), for each face, as the occluded minus the whole face rating given to that face. Positive shifts in trust judgments and negative shifts in threat judgments represented an occluded-approach bias.
Determining consistency of approach bias across judgments
To determine the consistency of the directionality of an approach bias across the two judgments, plots of mean control trust and threat bias scores for each face were overlaid (cf. Figure 2a), demonstrating that trust and threat occluded biases moved together in a consistent fashion. Each face’s threat bias scores were subtracted from its trust bias scores to form composite occluded-bias scores (Figure 1c) for each participant. Mean negative “occluded-avoided” composite bias scores indicated faces that were avoided in the occluded condition, receiving higher threat and lower trust ratings than in the whole condition. Mean positive “occluded-approached” composite bias scores indicated faces that received lower threat and higher trust ratings in the occluded condition.
Figure 2.
Composite bias score defined and compared to patients’ scores. Panel a. Overlaid plots of controls (N=81) mean occluded minus whole difference score (y-axis) for each face for trust (blue) and for threat (red) show that faces that were not trusted in the occluded condition also tended to be found threatening relative to the whole-face condition. This consistent bias contributed to the formation of a composite bias score (black; 95% CI in gray) defined by adding the trust and reversed threat bias scores for each face. Face stimuli (x-axis) rank-ordered according to composite bias score. Faces with a negative bias score tended to be avoided in the occluded relative to whole condition (or, conversely, approached more in the whole face condition), while faces with a positive bias score tended to be approached more in the occluded face condition. Panels b–d. Controls’ mean composite bias score (black; 95% CI in gray), with individual patients’ scores overlaid in teal demonstrate a trend for patient bias scores to exceed controls’ scores. Ctrl=controls.
Testing difference in occluded-approach bias in patients
Each individual patient’s occluded bias scores were qualitatively compared to those of controls by overlaying their individual bias score for each face on a 95% confidence interval plot of the mean control bias score for each face, with higher scores indicating an approach bias in each patient.
The group of patients’ occluded bias scores were quantitatively compared to a bootstrapped distribution of controls’ ratings as described above for (1) all faces, (2) for faces with occluded-avoided (negative occluded bias) scores and (3) for faces with occluded-approached (positive occluded bias) scores. To remove any statistical dependency between the face-classification and comparison of control and patient scores, faces were reclassified as occluded-avoided or occluded-approached on each bootstrap iteration according to the mean bias score of the 78 controls who were not randomly sampled on that bootstrap iteration.
Testing difference in “positivity” bias in occluded and whole faces
To disentangle the contributions of occluded and whole face ratings to an approach bias, we separately defined a “positivity” approach bias (cf. (Norris, Gollan, Berntson, & Cacioppo, 2010)) in whole and occluded faces, as the trust minus threat rating for each whole or occluded face. More positive scores indicated faces that were approached with a “positivity” bias (more trustworthy than threatening) and more negative scores indicated faces that were avoided (more threatening than trustworthy). Bootstrap resampling compared patients’ and controls’ positivity bias for whole and for occluded faces.
Results
Overview
Results are fully detailed below, and have been summarized in Table 1 to quickly orient readers to our findings.
Table 1.
Results Summary. Main findings listed alongside relevant Results source.
| Finding | Source |
|---|---|
| A composite occluded minus whole bias score, combining trust and threat ratings to the same face is valid: faces with an occluded-approach bias for trust ratings tended to have an occluded-avoid bias for threat ratings and vice versa. | Figure 2a |
| Patients’ composite occluded minus whole bias scores tend to be more positive (occluded-approach bias) than controls’ scores for each face. | Figure 2b–d |
| Bootstrap analysis comparing 3 randomly sampled controls’ mean composite occluded bias score to the patients’ actual mean score shows a stronger occluded-approach tendency in the patients for all faces, especially for faces controls tended to avoid in the occluded condition. | Figure 3 |
| Parametric visualization of the bias shown in Figure 3. To test whether patients’ bias results from greater difficulty in rating occluded faces, we generated synthetic patient bias scores derived from actual whole-face ratings and random occluded-face ratings, which did not explain their bias. | Figure 4 |
| Bootstrap comparison of controls’ and patients’ general “positivity” (trust minus threat) bias for whole and occluded faces separately shows (1) that all participants had a bias to approach occluded faces more than whole faces, but (2) also confirms this bias was greatly enhanced in the patients. | Figure 5 |
| Full reporting of patients’ individual scores and 95% CI of mean patient scores for each judgment X stimulus type. | Table 2 |
Lesion Patients’ Composite Bias Scores Relative to Controls
We sought to demonstrate a general approach bias in amygdala lesion patients by exploring whether they tended to approach occluded stimuli more than whole face stimuli, relative to controls.
Before comparing the patients’ and controls’ occluded bias, we first confirmed the validity of a composite bias score. Controls’ trust and threat bias scores moved together in an expected manner (Figure 2a), such that faces that tended to be rated as more trustworthy (blue dots) when occluded were also rated as less threatening (red dots) when occluded, and vice-versa. Therefore, for each subject, the threat bias score for each face was subtracted from its trust bias score, combining biases in the two judgments into a composite bias score (black line), with negative scores denoting faces avoided in the occluded condition and positive scores denoting faces approached in the occluded condition.
The patients’ bias scores (teal dots) for each face were compared to the pooled controls’ mean bias scores (black line) (Figure 2b–c): overall, their bias scores indicated a heightened tendency to approach faces in the occluded condition (this bias was not alternatively driven by a heightened tendency to avoid faces in the whole condition, see below and Table 2). Each patient’s mean bias score was higher than those of controls (M = 0.59, SD = 0.76): AP’s mean score was 0.46 SD higher than controls’, AM’s 0.85 SD, and BG’s 1.28 SD.
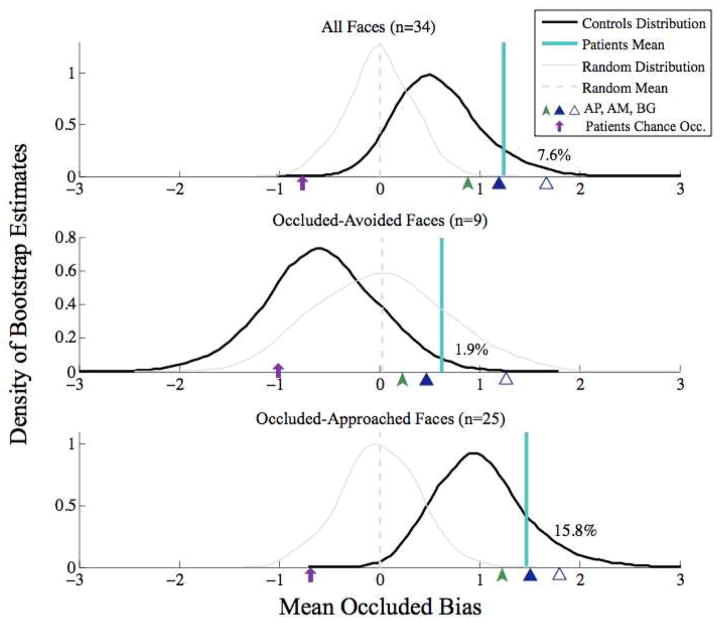
Further confirming the finding that patients have a general approach bias to rate occluded faces as more trustworthy and less threatening than whole faces, our bootstrap analysis showed that the patients’ composite bias scores (patient average shown as teal line in Figure 3) were indeed higher than controls’ scores (distribution shown by black line in Figure 3), with 7.6% of control bootsamples exceeding the mean patient bias score. In addition to this bootstrap analysis, the control and patient group averages were strongly distinct (d =1.13), with nonoverlapping 95% CIs [0.43, 0.76] and [0.90, 1.59], respectively. This difference from controls was weaker for those faces that controls also had a bias to approach in the occluded condition (bottom panel of Figure 3) (15.8% of control bootsamples exceeded the patient mean, and group averages were only moderately distinct (d = 0.74), with 95% CIs for controls [0.84, 1.20] and patients [1.19, 1.74]). As expected, this difference was, however, most pronounced for those faces that controls had a tendency to avoid in the occluded condition (middle panel of Figure 3 - 1.9% of control bootsamples exceeded the patient mean, and group averages were strongly distinct (d = 1.53), with 95% CIs for controls [−0.81, −0.37] and patients [0.04, 1.22]).
Figure 3.
Mean patient occluded bias scores compared to bootstrapped mean control bias scores. Density plot estimates (black) of the bootstrap distribution of three randomly-sampled controls’ mean occluded-bias scores for all faces (top), “occluded-avoided” faces as classified by control ratings (middle), and “occluded approached” faces (bottom), with the three patients’ actual mean bias score overlaid in teal and the individual patients’ mean scores indicated by green and blue arrows on the x-axis. To remove statistical dependency between our classification of “occluded-avoided” and “occluded-approached” faces and our comparison of 3 randomly sampled controls to the patients, for each bootstrap iteration, occluded-avoided and occluded-approached faces were classified according to the 78 remaining controls. Percent of bootstrap control estimates with a higher occluded bias rating is indicated on each plot, demonstrating that patients’ occluded-approach bias scores tended to be higher than controls’ scores. To test against these reported effects being driven by regression to the mean, bootstrapped estimates of 3 patients with random bias scores (built from raw trust and threat scores evenly distributed from 1 to 6) are overlaid in gray with their mean value overlaid in a pale blue dashed line. Demonstrating that the patients’ deviation from controls was driven by their occluded ratings, an average synthetic patient bias rating given their actual whole face scores and chance occluded scores is indicated on each plot with a purple arrow.
To test against the possibility that these effects were driven by regression to the mean (that is, that the amygdala lesion patients simply produced noisier, more random, ratings of the faces), bootstrapped estimates of 3 simulated patients with random bias scores (built from raw trust and threat scores evenly distributed from 1 to 6) were overlaid (grey lines) on the actual controls’ bootstrapped distributions (black lines) in Figure 3. Adding noise to the control data strengthened the separation of the controls’ and patients ratings for all faces (top panel of Figure 3), and occluded-avoided faces alone (bottom panel), as the random distribution (grey line) moved below the actual distribution (black line) for controls, increasing the separation between controls and the patients’ mean (teal line). While this increased separation was not observed for the occluded-avoid faces, the bias in the patients measured by our bootstrap analysis was strong with only 1.9% of randomly sampled control mean bias scores exceeding the actual patients’ mean bias score; in tandem with the effect of noise on the other groupings of faces, the possibility of regression-to-the-mean is unlikely as an explanation for the patients’ impairment.
To support the conclusion that this effect was driven by a specific bias in rating occluded faces relative to whole faces, we next derived synthetic difference ratings by subtracting actual whole face ratings from chance occluded-face patient ratings (i.e., 3.5, with a fixed bias offset for the German patients): these (indicated by purple arrows in Figure 3) tended to be lower than controls’ bias scores, once again going in a direction opposite to that seen for the actual ratings given by the amygdala lesion patients.
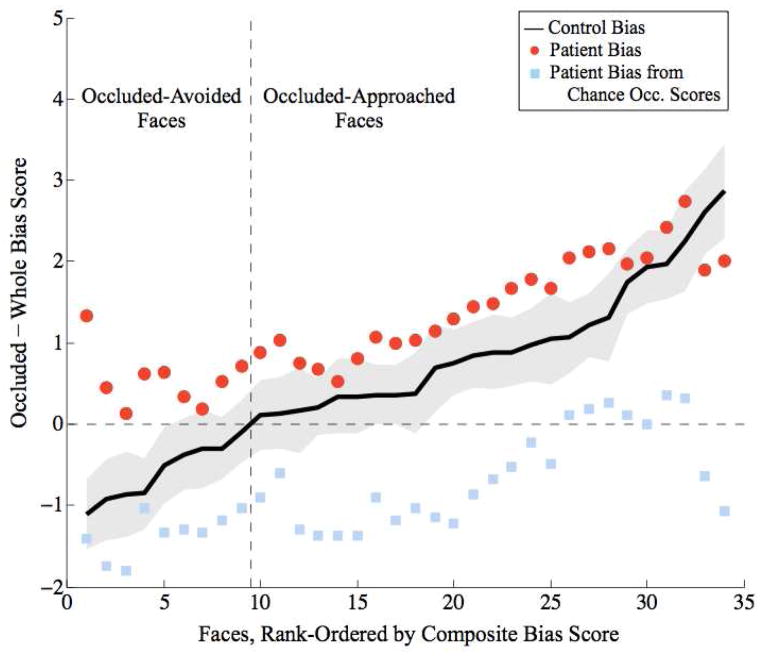
Binary classification of faces as occluded-avoided (middle panel in Figure 3) or occluded-approached (bottom panel) indicated that the patients’ occluded approach bias was strongest for faces avoided by controls in the occluded condition. We visualized how the patients differed from controls for each face by overlaying a 95% CI plot of controls’ rank-ordered mean bias scores (black line with grey area in Figure 4) with a plot of the patients’ mean score for each face (red dots), smoothed with a 10-face moving average to improve visualization of the general parametric trend. While the patients tended to have a higher bias scores than controls across all faces, the patients’ bias scores became less distinct from controls’ as a function of the controls’ occluded-bias scores. To again support the hypothesis that this effect was driven by a specific deficit in rating occluded faces, we also plotted synthetic ratings derived by subtracting actual whole-face patient ratings from chance occluded-face ratings (blue dots in Figure 4). These synthetic ratings did not positively deviate from controls’ ratings as did the patients’ difference ratings - in fact, they were lower - indicating that the patients’ deviation from controls’ scores were indeed driven by abnormal, but consistent, occluded-face ratings: the bias we report.
Figure 4.
Parametric bias visualization. Overlaid plots of controls’ (N=81) mean composite bias score (y-axis, black; 95% CI in gray) and patients’ (N=3) mean composite bias score (red circles; smoothed with a 10 face moving average, with a step size of one face). The patients all tended to have a higher approach bias than controls, meaning they tended to approach occluded faces more than controls. Face stimuli (x-axis) rank-ordered according to mean control composite bias score. Synthetic patient ratings (light-blue squares, also smoothed with a 10 face moving average), indicate patient bias scores given actual whole-face ratings and chance occluded-face ratings; these scores test the hypothesis that the deviation of patients’ bias scores from controls’ scores was driven by abnormal occluded-face ratings; since chance occluded ratings did not exceed controls’ bias scores, the effect was driven by the patients’ tendency to approach occluded faces more than whole faces.
Trust-Threat “Positivity” Bias in Whole and Occluded Stimuli Alone
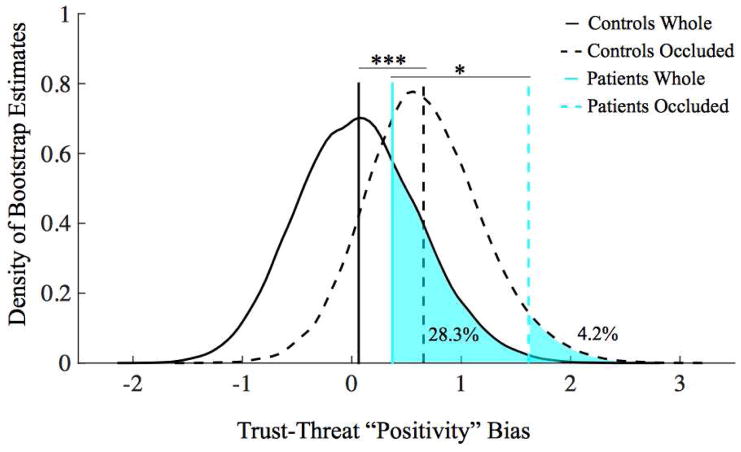
Separate examination of an approach-related “positivity” bias (positive for faces with stronger trust than threat ratings) for whole and occluded faces separately revealed a default positive approach bias to occluded but not whole faces in both controls and patients (Figure 5). While a shift between whole positivity ratings (M=0.063) and occluded positivity ratings (M=0.656) was present in controls (p=5.90×10−10), this shift in positivity bias for occluded faces was markedly greater in the patients (M =0.373 for whole vs. M =1.618 for occluded faces, p=0.0199).
Figure 5.
Bootstrap comparison of patients and controls approach-related “positivity” biases. Density plot estimates of three randomly sampled controls’ mean positivity bias (black lines), and the actual patients’ mean positivity bias (blue lines) for whole (solid lines) and occluded (dashed lines) faces. Patients and controls both had a greater approach-related positivity bias for occluded than whole faces, but the magnitude of the shift was greater for the patients, who were also most distinct from controls for the occluded faces specifically. *p<0.05; ***p<0.001.
Results Separated by Judgment and Stimulus Type
Across comparisons, an occluded approach bias was defined as a tendency to give both higher trust (“Yes,” trustworthy) and lower threat (“No,” not threatening) ratings to occluded faces than to whole faces. While a composite approach bias was the most comprehensive way to describe our results, separate trust and threat biases obviously contribute to this construct. We thus also compared the lesion patients’ mean trust and threat occluded biases separately to bootstrapped control distributions, alongside patient performance for whole and occluded faces alone (Table 2), confirming that the patients’ observed approach bias was driven by abnormal ratings of occluded faces.
As a group, the patients’ whole-face ratings were not different from controls’ ratings, which was somewhat unexpected given a previous finding in amygdala lesion patient SM (Adolphs et al., 1998), which showed that SM gave abnormally high trustworthiness judgments to whole faces (albeit a different set of faces than the ones used in our present study). To verify that this discrepancy from SM’s behavior in our amygdala lesion patients was not driven by differences in the stimulus sets, we tested two of our patients (AM and BG) also on the original stimuli from that experiment with SM (Adolphs et al., 1998). While AM and BG tended to deviate from controls, especially for the faces controls avoided most (Supplemental Figure 3), they only exhibited a weak trend towards the prior finding in SM. Unfortunately, SM was not available for testing in our new task, and AP was not available for testing on the original task used by Adolphs et al. (1998).
Discussion
A significantly enhanced approach-related bias, relative to controls, was uncovered in three rare patients with selective bilateral amygdala lesions by comparing ratings of faces in a whole and occluded condition. A default bias was demonstrated by the patients’ greater willingness to approach a face (i.e., less threatening and more trustworthy ratings) in the low-information occluded condition.
Returning to our example situation of walking into a dark room, the normal response to an ambiguous situation is risk-assessment (Blanchard et al., 2011) - given insufficient information to determine whether a threat is present, one should pause and gather more information before proceeding; our patients’ ratings indicated that they would simply enter the room; at the other end of the spectrum, anxious individuals might flee the dark room before gathering further evidence. Future experiments exploring individual differences (e.g., trait/state anxiety, perceived dominance, history of exposure to physical/social threat or betrayal) will be important to both validate our task and determine what factors beyond amygdala damage relate to heightened approach tendencies. It is worth noting that in our sample a few control individuals had an approach bias similar to that of the patients, emphasizing the importance of future studies to determine the cause of these individual differences.
In humans, given a lack of stimulus information, an exploratory tendency may normally promote a default approach bias, the “positivity offset” in the Evaluative Space Model (Norris et al., 2010), similar to our observed shift in “positivity” approach ratings between the occluded and whole face conditions (Figure 5). This occluded positivity bias was observed both in controls and patients, but enhanced in the patients.
While patients gave stronger approach ratings than controls, they were not completely indiscriminate: their judgments differed more in degree than direction (Table 2). Future work should test how their enhanced approach bias extends to (1) other classes of degraded stimuli (including non-linguistic tasks to better facilitate cross-cultural comparison), and (2) the real world. Showing abnormal proxemic (i.e, personal space) behavior and a tendency to approach real threatening stimuli (e.g., snakes) in these three patients, as has already been done in patient SM (Feinstein et al., 2011; Kennedy et al., 2009), would further corroborate a default approach bias. Confirmed in preliminary testing, at least BG has abnormally small personal space and fear responses (D.P. Kennedy, J. Feinstein, & R. Adolphs, personal communication). Testing participants’ actual behavior is crucial – compensatory processing may allow them to give more “correct” explicit ratings: for example, although SM abnormally approached actual snakes without showing any fear, beforehand, she verbally insisted that she “hates” snakes and “tries to avoid them” (Feinstein, Adolphs, Damasio, & Tranel, 2011).
Differences amongst the amygdala lesion patients need to be resolved. Amygdala damage can prompt two distinct approach processes - a default bias, as well as a face-specific bias - both of which can operate simultaneously. Removing facial feature information from facial stimuli allowed us to challenge participants to indicate a default bias while working within the general category of facial stimuli. Across all participants, responses to facial features were variable and the patients were similar to controls. SM’s whole face ratings had been different from controls (Adolphs, et al., 1998); this deviation is in line with the heterogeneity of impairments reported in bilateral amygdala damage (Adolphs et al., 1999; Hamann et al., 1996; Siebert, Markowitsch, & Bartel, 2003), and likely reflects compensatory processing (Becker et al., 2012; Scheele et al., 2012). SM’s impairment for whole faces hints at progressive amygdala damage/impairment - expected in Urbach-Wiethe disease (Appenzeller et al., 2006).
While patient differences in a face specific deficit need to be further explored and explained based on precise anatomical differences, the present study focused on isolating a stimulus-independent shift, which will clearly interact with responses to facial features. Sometimes, “good” facial features (determined idiosyncratically) helped occluded-avoided faces; sometimes “bad” features harmed occluded-approached faces. However, across the entire stimulus set, a general occluded-approach bias could be observed.
Mechanistically, the patients’ approach bias may relate to a specific cautionary deficit, related to disrupted vigilance (Davis & Whalen, 2001; Whalen, 2007). This viewpoint is anatomically compatible with the amygdala launching a defensive behavioral response to coincident sensory and contextual danger signals, conveyed via the temporal and prefrontal cortices, respectively (Freese & Amaral, 2009).
However, the patients’ approach bias can be explained by a more general mechanism of amygdala function. A general role in processing saliency/self-relevance (Cunningham & Brosch, 2012; Harrison & Adolphs, 2015; Sander, Grafman, & Zalla, 2003) is compatible with a wider array of known amygdala activity. The amygdala contributes to negative and positive reinforcement (Murray, Izquierdo, & Malkova, 2009), and processes positively and negatively valenced stimuli (Anderson et al., 2003; Hamann, Ely, Hoffman, & Kilts, 2002). In rats (Hatfield, Han, Conley, Gallagher, & Holland, 1996) and nonhuman primates (Izquierdo & Murray, 2007; Málková, Gaffan, & Murray, 1997), basolateral amygdala lesions interfere with reinforcer devaluation, such that an animal will indiscriminately approach devalued food items, similar to our patients’ default approach bias. Hypothetically, the basolateral nucleus, damaged in our patients, updates the self-relevant value of a stimulus (Murray et al., 2009).
A saliency/relevance explanation binds our default bias finding with prior findings in amygdala lesion patients: amygdala lesions do not preclude the ability to experience fear - indeed, CO2 inhalation can induce fear and panic in amygdala lesion patients (Feinstein et al., 2013), but instead inhibit proper orienting to stimuli (Spezio, Huang, Castelli, & Adolphs, 2007), which often results in a diminished ability to experience (Feinstein et al., 2011) or recognize (Adolphs et al., 2005) fear. Proper orienting can recover this ability: in SM, fear is correctly identified following explicit top-down instruction to look at the eyes (Adolphs et al., 2005).
Our finding of an enhanced default positivity bias suggests a further role for the amygdala in setting a default on what is potentially relevant or salient, normally preventing us from approaching situations that may be threatening, while simultaneously permitting exploration. In our patients, this balance is shifted. Similarly, in psychiatric disorders featuring dis-regulation of the amygdala (e.g., anxiety disorders (Davis, 1992; Etkin & Wager, 2007) and autism (Baron-Cohen et al., 2000; Castelli, Frith, Happe, & Frith, 2002; Dalton et al., 2005)), stimuli are not correctly evaluated, from shifted baseline biases as well as under- or over-weighting the threat, social importance, or relevance of stimuli.
In summary, contrasting judgments of occluded and whole faces, we uncovered a stimulus-independent approach bias following bilateral amygdala damage. Future directions include (1) testing for a default approach or avoidance bias in psychiatric disorders for which the amygdala is implicated, as well as (2) developing implicit tests of an approach bias to circumvent potential compensatory mechanisms and (3) devising tests to provide a clearer mechanistic account of our findings.
Supplementary Material
Acknowledgments
This research was funded by grants from the National Institutes of Mental Health [grant P50MH094258], the Gordon and Betty Moore Foundation, and the German Research Foundation (DFG).
We thank: Dirk Scheele, Yoan Mihov, Ronnie Bryan, Mike Tyszka, Julien, and especially the patients for their assistance.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Author Contributions: L.A. Harrison and R. Adolphs developed the study concept and design. Testing and data collection were performed by L.A. Harrison and R. Hurlemann. L.A. Harrison performed the data analysis and interpretation under the supervision of R. Adolphs. L.A. Harrison drafted the manuscript, and R. Adolphs provided critical revisions. All authors approved the final version of the manuscript for submission.
Contributor Information
Laura A. Harrison, Division of Engineering and Applied Sciences, Computation and Neural Systems Program, California Institute of Technology, Pasadena, California
Rene Hurlemann, Department of Psychiatry, University of Bonn, Germany.
Ralph Adolphs, Division of Humanities and Social Sciences and Division of Biology, California Institute of Technology, Pasadena, California.
Bibliography
- Adams RB, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300(5625):1536–1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young A, Calder A, Anderson A, Damasio AR. Recognition of Facial Emotion in Nine Subjects with Bilateral Amygdala Damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical Organization of the Primate Amygdaloid Complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss, Inc; 1992. pp. 1–66. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Appenzeller S, Chaloult E, Velho P, de Souza EM, Araujo VZ, Cendes F, Li LM. Amygdalae calcifications associated with disease duration in lipoid proteinosis. Journal of Neuroimaging. 2006;16:154–156. doi: 10.1111/j.1552-6569.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, Hurlemann R. Fear processing and social networking in the absence of a functional amygdala. Biological Psychiatry. 2012;72:70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neuroscience and Biobehavioral Reviews. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac C, Hadley D. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Choi J, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21(1):54–59. [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Eder AB, Harmon-Jones E. Approach-avoidance motivation and emotion: convergence and divergence. Emotion Review. 2013;5:308–311. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Wemmie JA. Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience. 2013;16:270–272. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Current Biology. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the Primate Amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York: Guilford Press; 2009. pp. 3–42. [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Stefanacci L, Squire LR, Adolphs R, Tranel D, Damasio H, Damasio A. Recognizing facial emotion. Nature. 1996;379:497. doi: 10.1038/379497a0. [DOI] [PubMed] [Google Scholar]
- Harrison L, Adolphs R. The Amygdala and Social Perception. In: Toga AW, editor. Brain Mapping. Waltham: Academic Press; 2015. pp. 91–96. [Google Scholar]
- Hatfield T, Han J, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. The Journal of Neuroscience. 1996;16(16):5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer PA. Urbach-Wiethe disease (lipoglycoproteinosis; lipoid proteinosis; hyalinosis cutis et mucosae). A review. Acta dermato-venereologica Supplementum. 1973;53:1–52. [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. The Journal of Neuroscience. 2007;27(5):1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology & Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. The Journal of Neuroscience. 1997;17(15):6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A, Malkova L. Amygdala Function in Positive Reinforcement: Contributions from Studies of Nonhuman Primates. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York, NY: The Guilford Press; 2009. pp. 82–104. [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biological Psychology. 2010;84(3):422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurons responsive to faces in the monkey temporal cortex. Experimental Brain Research. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Tudusciuc O, Neumann D, Mamelak A, Heller AC, Ross IB, Adolphs R. Single-unit responses selective for whole faces in the human amygdala. Current Biology. 2011;21:1654–1660. doi: 10.1016/j.cub.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Scheele D, Mihov Y, Kendrick KM, Feinstein JS, Reich H, Maier W, Hurlemann R. Amygdala lesion profoundly alters altruistic punishment. Biological Psychiatry. 2012;72:e5–e7. doi: 10.1016/j.biopsych.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Sinha Pawan, Balas Benjamin, Ostrovsky Yuri, Russell Richard. Face recognition by humans: Nineteen results all computer vision researchers should know about. Proceedings of the IEEE. 2006;94(11):1948–1962. [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spezio L, Huang PS, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. Journal of Neuroscience. 2007;27:3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag Wietske, Da Costa Sandra E, Zürcher Nicole R, Adams Reginald B, Jr, Hadjikhani Nouchine. A 7 tesla FMRI study of amygdala responses to fearful faces. Brain Topography. 2012;25(2):125–128. doi: 10.1007/s10548-012-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Willenbockel V, Sadr J, Fiset D, Horne G, Gosselin F, Tanaka JW. Controlling low-level image properties: the SHINE toolbox. Behavior Research Methods. 2012;42:671–684. doi: 10.3758/BRM.42.3.671. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: Making up your mind after a 100-ms exposure to a face. Psychological Science. 2006;17:592–598. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.